Abstract
VSV-IFNβ-NIS is a novel recombinant oncolytic vesicular stomatitis virus (VSV) with documented efficacy and safety in preclinical murine models of cancer. To facilitate clinical translation of this promising oncolytic therapy in patients with disseminated cancer, we are utilizing a comparative oncology approach to gather data describing the safety and efficacy of systemic VSV-IFNβ-NIS administration in dogs with naturally occurring cancer. In support of this, we executed a dose-escalation study in purpose-bred dogs to determine the maximum tolerated dose (MTD) of systemic VSV-hIFNβ-NIS, characterize the adverse event profile, and describe routes and duration of viral shedding in healthy, immune-competent dogs. The data indicate that an intravenous dose of 1010 TCID50 is well tolerated in dogs. Expected adverse events were mild to moderate fever, self-limiting nausea and vomiting, lymphopenia, and oral mucosal lesions. Unexpected adverse events included prolongation of partial thromboplastin time, development of bacterial urinary tract infection, and scrotal dermatitis, and in one dog receiving 1011 TCID50 (10×the MTD), the development of severe hepatotoxicity and symptoms of shock leading to euthanasia. Viral shedding data indicate that detectable viral genome in blood diminishes rapidly with anti-VSV neutralizing antibodies detectable in blood as early as day 5 postintravenous virus administration. While low levels of viral genome copies were detectable in plasma, urine, and buccal swabs of dogs treated at the MTD, no infectious virus was detectable in plasma, urine, or buccal swabs at any of the doses tested. These studies confirm that VSV can be safely administered systemically in dogs, justifying the use of oncolytic VSV as a novel therapy for the treatment of canine cancer.
Introduction
Oncolytic virotherapy is a rapidly evolving field in anticancer therapy, with numerous agents under preclinical investigation and in clinical trials worldwide (Russell and Peng, 2007). Vesicular stomatitis virus (VSV), a naturally oncolytic rhabdovirus, is being engineered to develop potent new cancer therapies with desirable features, including enhanced safety and therapeutic utility (Barber, 2004; Naik and Russell, 2009). VSV expressing human interferon-β (IFNβ) and the sodium-iodide symporter (NIS) protein is a novel recombinant oncolytic virus developed as a systemically deliverable therapy that specifically replicates in and destroys disseminated cancer. VSV-IFNβ-NIS possesses documented efficacy and safety in preclinical murine models of cancer, specifically multiple myeloma (Naik and Russell, 2009; Naik et al., 2012b). An attenuated, recombinant form of the Indiana strain of VSV, this virus possesses the IFNβ gene, to exert an IFN-mediated protective effect in noncancerous tissues and to stimulate cross-priming of T cells during VSV infection (Obuchi et al., 2003; Naik et al., 2012a). The NIS gene insert encodes for the NIS protein, allowing noninvasive nuclear medicine imaging of virally infected cells. Toxicology data collected in rats and rhesus macaques established a safe starting dose for intratumoral delivery of VSV-hIFNβ to support clinical evaluation of this agent in humans with relapsed hepatocellular carcinoma (Jenks et al., 2009).
The main preclinical emphasis to date has centered on systemic delivery of VSV-hIFNβ-NIS to murine models of multiple myeloma. This body of research demonstrates susceptibility of human and murine myeloma cells to the virus and defines an efficacious dose of virus in both immune-compromised and immune-competent mice bearing myeloma xenografts or with syngeneic disease, respectively (Naik and Russell, 2009; Naik et al., 2012b). These studies demonstrate the importance of species specificity with respect to the IFNβ gene and subsequent activation of tumor-specific T cells for eradication of residual disease. However, variability in the response to therapy and uncertainty regarding attribution of clinical toxicity argues strongly for a naturally occurring model that is amenable to whole-body clinical imaging and serial sample collections of blood, tumor, and bone marrow.
Rationale for Canine Clinical Study
Many factors influence the clinical translation of oncolytic viruses, including but not limited to, characterization and attribution of toxicity, virus shedding and safety, and development of methods for optimal patient selection and monitoring. To date, progress in this area has relied heavily upon xenograft or transgenic murine models that may not accurately recapitulate heterogeneous human cancers, or provide opportunities to accurately monitor and investigate clinical toxicities or virus shedding resulting from such therapies. A complementary approach is the field of comparative oncology, where naturally occurring cancers in immune-competent pet dogs are studied and included in the traditional drug-development pathway (Paoloni and Khanna, 2007). This approach provides the opportunity to test novel anticancer strategies in order to ask critical questions regarding which factors can predict response to therapy and thus assist the physician/scientist in their design of human clinical trials.
Data describing the safety and efficacy of high doses of virus administered systemically in dogs with naturally occurring cancer are needed to inform clinical trial design for humans with disseminated malignancies. Many types of spontaneous canine cancers are accepted models for their human counterparts. The dog's physical size allows serial large-volume biologic sample collections to examine viral shedding, and their inherent tumor heterogeneity allows correlation of tumor- and patient-related factors to clinical outcomes.
In order to confidently proceed with a clinical trial in pet dogs with naturally occurring cancer, we have executed a dose-escalation study in purpose-bred dogs. This study was designed to determine the maximum tolerated dose (MTD) of VSV-hIFNβ-NIS, characterize the adverse event profile of this virus in dogs, and describe routes and duration of viral shedding in healthy, immune-competent dogs.
Objectives
A rapid dose-escalation study in purpose-bred Beagle dogs was performed to identify the adverse event profile and the MTD after systemic delivery of VSV-hIFNβ-NIS. The objectives of this clinical study were to (i) describe the expected and unexpected adverse events that occur with intravenous (IV) administration of VSV-IFNβ-NIS in a rapid-dose escalation study; (ii) define the viral shedding routes and duration after IV administration of VSV-hIFNβ-NIS via serial biologic sample collection; and (iii) define virus pharmacokinetics and antiviral immune response after IV administration of VSV-IFNβ-NIS to healthy dogs.
Study Design
Briefly, selected dogs were treated with a single IV dose of VSV-hIFNβ-NIS at 10-fold dose escalations beginning at 108 TCID50. One dog was enrolled at each dose and monitored for toxicity applying standardized toxicity-grading criteria (VCOG-CTCAE). If no dose-limiting toxicity (DLT) occurred, escalation to the next dosing cohort would occur. If a dog experienced a DLT of grade 3 or higher, two additional dogs were enrolled in the cohort. If 2/3 or 3/3 dogs experienced DLT, the escalation scheme was halted and the MTD was determined to be the preceding dose. Dogs were monitored within an ABSL2 housing unit for 30–45 days for adverse events, along with serial biologic sampling to monitor physiological changes, routes and duration of viral shedding, and antiviral immunity in response to virus administration (Table 1). Table 2 is a summary of studied dogs' vital characteristics.
Table 1.
Schema for Toxicity Study Evaluating Systemic VSV-hIFNβ-NIS Administration in Normal Dogs Including a Study Schedule for Observation and Sample Collection to Monitor Clinical Toxicities, Virus Pharmacokinetics, Shedding, and Antiviral Immune Responses
| Time point (days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monitoring | <0 | 1 | 3 | 5 | 7 | 10 | 15 | 21 | 30 | 45 | |
| Clinical symptoms | Continual | ||||||||||
| Virus pharmacokinetics | • | ||||||||||
| Labwork: CHEM/CBC/COAG/urinalysis | • | • | • | • | • | • | • | • | • | • | |
| Shedding: Blood, urine, buccal swabs, feces | qRT-PCR | • | • | • | • | • | • | • | • | • | • |
| IVR | • | • | • | • | • | • | • | • | • | • | |
| Immune response: Anti-VSV antibodies IgG/IgM | PRN | • | • | • | • | • | • | • | • | • | • |
| ELISA | • | • | • | • | • | • | • | • | • | • | |
Dots in vertical direction indicate single IV dose of VSV-IFNβ-NIS; dots in horizontal direction indicate acute and chronic toxicity monitoring.
CBC, complete blood count; CHEM, blood chemistry; COAG, blood coagulation; ELISA, enzyme-linked immunosorbent assay; IVR, infectious virus recovery; PRN, plaque reduction neutralization; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; VSV-hIFNβ-NIS, vesicular stomatitis virus expressing human interferon-beta and the sodium iodide symporter.
Table 2.
Summary of Clinical Symptoms and Adverse Events After a Single Systemic Dose of VSV-IFNβ-NIS at 10-Fold Dose Escalations in Normal Dogs
| ID | Dose (TCID50) | Sex | Summary of clinical symptoms | |
|---|---|---|---|---|
| 1 | C12033 | 1.00E+08 | M | No significant adverse events Lesions: bilateral oral lesions (mild, day 1) LFT: mild elevation in ALP (still WNL) LYMPH: mild lymphopenia, day 1 only COAG: elevation in PTT (day 1 to present) Other: UTI, mild scrotal dermatitis, monocytosis day 1 |
| 2 | C12035 | 1.00E+09 | M | No significant adverse events Lesions: bilateral oral lesions (mild, days 5–24) LFT: WNL, abnormal ALP throughout LYMPH: slight reduction in lymph day 1, but WNL COAG: elevation in PTT (day 1 to present) Other: mild scrotal dermatitis |
| 3 | C12028 | 1.00E+10 | M/C | No significant adverse events GI: unconfirmed vomiting in first 24 hr Lesions: none TEMP: temperature increase to 102°F till day 3 LFT: slight elevation in ALT (still WNL) and AST, day 1 LYMPH: lymphopenia, day 1 only COAG: mildly reduced PT (day 5 only), normal PTT Other: monocytosis days 3 and 5, UTI |
| 4 | C11029 | 1.00E+10 | M/C | No significant adverse events Lesions: none LFT: mild elevation in ALP, ALT (WNL), elev. AST (day 1) LYMPH: WNL COAG: WNL Other: elevated CK (day 1 only) |
| 5 | C12040 | 1.00E+11 | F/S | Dose limiting toxicity Euthanized ∼8 hr after VSV administration GI: severe vomiting, diarrhea TEMP: initially elevated temperature, followed by hypothermia LFT: elevated ALT, AST, ALP LYMPH: severe lymphopenia COAG: abnormal PT, PTT |
C, castrated; F, female; GI, gastrointestinal symptoms; LFT, liver function tests; LYMPH, lymphocytes; M, male; S, spayed; TEMP, temperature; WNL, within normal limits.
Dogs were monitored continuously on the day of virus administration (day 0). Dogs were fasted overnight before virus administration but fed a normal meal the evening of virus administration. Vital signs (rectal temperature, heart and respiratory rates) and general attitude/demeanor were recorded every 1–2 hr or as dictated by the dogs' clinical condition. Appetite and presence of any gastrointestinal upset (e.g., vomiting, diarrhea) was noted. On every day of study, the dogs' vital signs, overall attitude and appetite, and physical examination findings were recorded. Body weight was recorded at least once every 48 hr.
On the day of virus administration, serial 1 ml aliquots of blood were collected at 10, 30, 60, 90, 120, 240, and 360 min after IV administration to determine viral stability in the peripheral circulation. During the study, blood was drawn via Vacutainer technique for a complete blood count, serum biochemistry and electrolyte panel, and coagulation panel as indicated (Table 1). Urinalysis was collected via sterile urinary catheter. All laboratory data were performed at the University of Tennessee College of Veterinary Medicine Clinical Pathology and Microbiology Laboratories. Oral mucosal swabs were collected for detection of shed VSV in saliva. Additional aliquots of blood, serum, and plasma were collected for viral shedding and immune response assays. Feces were collected as naturally voided for detection of shed virus. All study procedures were approved by U.S. Department of Agriculture/Animal and Plant Health Inspection Service and the University of Tennessee–Knoxville's Institutional Animal Care and Use Committee and Institutional Biosafety Committee. Detailed methods and analyses are described in the supplementary materials and methods section at www.liebertpub.com
Summary of Data
Clinical toxicity and adverse events
The data indicate that systemic VSV-hIFNβ-NIS is well tolerated in dogs at doses of 108, 109, and 1010 TCID50, where 1010 is the designated MTD. A summary of clinical findings is provided in Table 2. The main expected adverse events were mild to moderate fever, self-limiting nausea and vomiting, lymphopenia, and oral vesicular lesions. Clinical labwork indicated slight and transient changes, including mild elevation in liver function tests (ALT, AST, ALP) and mild reduction in lymphocytes, though values were within or close to normal range. The development of bacterial urinary tract infection and scrotal dermatitis can likely be attributed to study-related procedures such as repeated use of sterile urinary catheters to obtain urine samples.
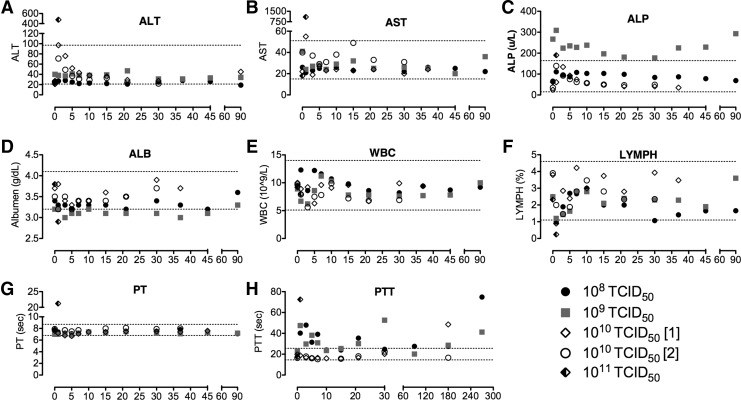
Three dogs received 1011 TCID50 intravenously, 10-fold the MTD at separate times. Two dogs, C11041 and C11042, received a viral dose of 1011 TCID50, which inadvertently contained significant endotoxin contamination. Within minutes of injection of virus, both dogs experienced significant vomiting and diarrhea with fever. The more severely affected dog was euthanized 30 hr after virus administration, while the other dog subsequently improved with symptomatic therapy and was humanely euthanized on day 30. Clinical labwork indicated hepatotoxicity, abnormal clotting, and lymphopenia (not shown). A third dog, receiving 1011 TCID50 (endotoxin-free) virus intravenously, developed severe gastrointestinal distress and fever beginning ∼3 hr after IV virus administration that did not respond to supportive fluid therapy, leading to significant dehydration and subsequent inability to regulate body temperature. The dog was humanely euthanized ∼9 hr after IV injection. Labwork at the time of euthanasia indicated, as previously observed in the two dogs treated with (endotoxin contaminated) 1011 TCID50 VSV, severely compromised liver function (elevated ALT, ALP, AST) and clotting (prolonged prothrombin [PT] and partial thromboplastin time [PTT]), along with diminished white blood cell count (Fig. 1).
FIG. 1.
(A–H) Clinical profiles of selected blood parameters over time after administration of a single systemic dose of VSV-hIFNβ-NIS in normal dogs. Blood was collected by jugular venipuncture to monitor changes in clinical chemistry, coagulation, and hematology. ALB, albumen; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LYMPH, lympocytes; PT, prothrombin time; PTT, partial thromboplastin time; VSV, vesicular stomatitis virus; WBC, white blood cell count.
Clinicopathologic and histopathologic data
A summary of clinical laboratory abnormalities is provided in Table 2. Unexpected adverse events included prolongation of PTT in some of the treated dogs and severe hepatocellular injury and symptoms of shock in one dog receiving (endotoxin-free) 1011 TCID50 (10×the MTD), ultimately requiring euthanasia. The prolonged PTT in dogs receiving the MTD was suspected to be caused by development of antiphospholipid antibodies (the so-called lupus anticoagulant phenomenon) and is currently under investigation (Triplett, 1998). Figure 1H demonstrates the changes in PTT in 4 dogs for which follow-up data are available.
The two dogs receiving endotoxin-contaminated virus at a dose of 1011 TCID50 had clinical laboratory data consistent with acute hepatocellular injury, with one dog developing overt disseminated intravascular coagulation as evidenced by cutaneous petechial hemorrhage, prolonged PT and PTT, and decreased platelet count. Necropsy and histological analysis of tissues indicated congestion and hepatocellular necrosis in liver. The other dog was humanely euthanized on day 30. Necropsy indicated mild diffuse hepatocellular cytoplasmic vesiculation and notable testicular damage. The testicular damage in this dog was deemed attributable to fever secondary to endotoxemia, not direct VSV-mediated toxicity. To further confirm these findings, two intact male dogs (C12033 and C12035) were neutered after completion of the study period, approximately 8 weeks after virus administration. Neither dog had any histopathologic evidence of testicular damage.
The third dog treated with 1011 TCID50 intravenously developed GI distress ∼2 hr after IV VSV administration, and was humanely euthanized because of deteriorating symptoms, including hypothermia, severe vomiting, and diarrhea. Clinical laboratory data indicated acute hepatocellular injury, hypoglycemia, marked hemoconcentration consistent with dehydration (Fig. 1A and B), and possible disseminated intravascular coagulation. Histological analysis of tissues indicated marked congestion of hepatic sinusoids and congested vasculature in various organs suggestive of vascular collapse associated with cytokine induced shock.
Because of the severity of the symptoms after administration at 1011 TCID50 intravenously, we concluded that DLT manifested primarily in the form of severe hepatotoxicity, disseminated intravascular coagulation, and acute gastrointestinal toxicity. Thus, 1010 TCID50 VSV-hIFNβ-NIS was the designated systemic MTD in dogs.
Viral pharmacokinetics
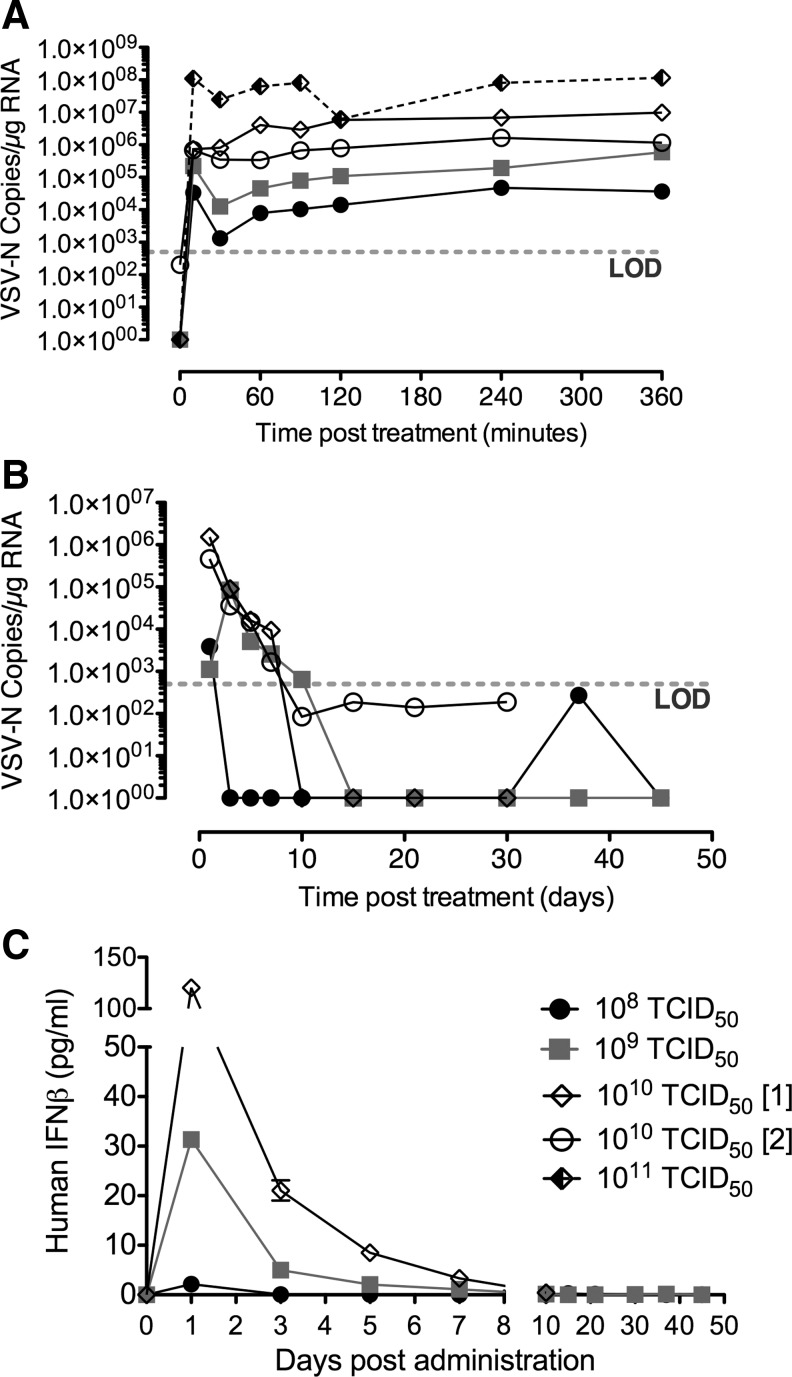
Virus pharmacokinetics after IV administration of escalating doses of VSV-hIFNβ-NIS was monitored for 3 hr after delivery. At 10, 30, 60, 90, 120, 240, and 360 min after IV administration, whole blood was collected and stored for subsequent RNA isolation to detect VSV genome copies by qRT-PCR. Viral genome copies detected in blood of VSV-treated dogs are shown in Fig. 2A.
FIG. 2.
Virus pharmacokinetics, persistence, and gene expression in blood were monitored after systemic administration of VSV-hIFNβ-NIS in normal dogs. qRT-PCR results (A and B) indicated viremia in the blood. Data were calculated for copy number of VSV-N RNA per microgram of total RNA isolated from whole blood. Limit of detection was 500 copies of VSV-N mRNA per 0.2 mg of RNA. (A) VSV-N gene copies in whole blood during first 3 hr after systemic VSV administration. (B) Long-term persistence of VSV-N gene copies in whole blood. (C) Viral expression of human interferon-beta in serum of normal dogs measured by ELISA. ELISA, enzyme-linked immunosorbent assay; LOD, limit of detection; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; VSV-N, VSV nucleocapsid.
Tenfold dose escalations resulted in correspondingly higher viral genome copies detected in the blood after systemic administration. There was slight transient decrease of viral genome copies in the blood in the first 10 min, after which VSV genomes copies were stable in the blood for the first 3 hr after systemic VSV delivery.
Viremia and viral gene expression
Virus persistence in blood after IV administration of escalating doses of VSV-hIFNβ-NIS was monitored over the 30–45-day study period. Viral genome copies detected in blood of VSV-treated dogs to indicate long-term viral persistence are shown in Fig. 2B. The data indicate that significant genome copies of the VSV-N gene were still detectable at 24 hr after IV VSV-hIFNβ-NIS administration, peaking at ∼106 copies detectable in dogs receiving the MTD of 1010 TCID50 VSV-hIFNβ-NIS. Detectable viral genome in blood diminished rapidly with the number of copies being at or below the limit of detection by day 10 after IV VSV delivery.
To further evaluate if the detection of genome copies correlates with infectious virus in blood, plasma samples from two dogs treated at the MTD (1010 TCID50) were tested using an infectious virus recovery (IVR) assay (by overlay on susceptible BHK cells). The results indicated that there was no detectable infectious virus in the plasma even 24 hr after IV VSV delivery at the MTD (not shown), and qRT-PCR of RNA isolated from plasma indicated low levels of viral genomes detected in plasma till day 10, after which measurable genome copies fell below the limit of detection (not shown). These findings suggest that systemically administered virus is associated with blood cells, and further studies are being carried out to detect both infectious virus and virus genomes in PBMCs isolated from whole blood.
VSV-hIFNβ-NIS encodes for the human IFNβ gene. In order to evaluate viral gene expression in normal dogs, we measured human IFNβ in the serum of dogs after IV VSV delivery. Serum collected during the study was evaluated by ELISA against human IFNβ. Results of this assay are shown in Fig. 2C. Human IFNβ was detectable in serum, peaking at day 1 after IV VSV delivery, where peak expression increased in a dose-dependent manner, with low levels detectable up to day 7 after VSV administration at the MTD.
Viral shedding
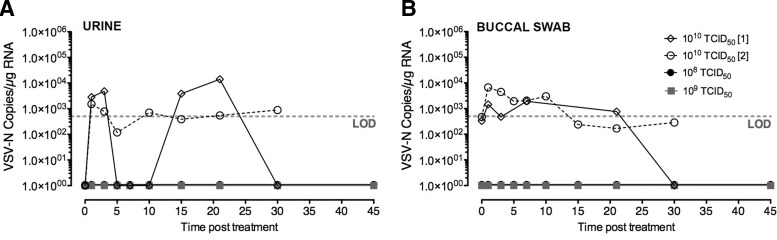
In order to determine routes and duration of viral shedding, biologic samples, including urine, buccal swabs, and feces, were collected (Table 1). These samples were processed to isolate RNA to detect viral genome copies by qRT-PCR and for infectious virus by IVR on susceptible BHK cells where possible. No infectious virus was detected in urine or buccal swabs collected during the study period, even in dogs treated at MTD (1010 TCID50). No viral genomes were detected in urine samples from dogs treated at the two lower dose levels, while low levels were detectable in the urine of dogs treated at the MTD during the study period (Fig. 3A).
FIG. 3.
Detection of virus shedding in biologic samples. Biologic samples, including urine, buccal swabs, and feces, were collected at time points indicated (in days) after systemic administration of VSV-hIFNβ-NIS in normal dogs. RNA from biologic samples were analyzed by qRT-PCR to detect VSV-N gene copies, indicating that low levels of viral gene were detectable in (A) urine cell pellets and (B) buccal swabs of dogs treated with VSV at the maximum tolerated dose. No VSV-N gene copies were detectable in feces, and no infectious virus was detectable in urine or buccal swabs.
Buccal swabs were collected at specified time points during the study (Table 1) for detection of viral genome copies. In addition to the scheduled buccal swab collections, additional swabs of mild oral lesions were collected for qRT-PCR and IVR assay from dog C12033, which received 108 TCID50 and developed bilateral oral lesions.
No viral genomes were detected in the buccal swabs of dogs treated at the lower dose levels, including swabs of the mild oral lesions. Low levels of VSV genome copies were detected in buccal swabs of dogs treated at the MTD (Fig. 3B). No virus genome was detected in RNA isolated from any of the dogs' fecal samples collected during the study period.
Antiviral immune response
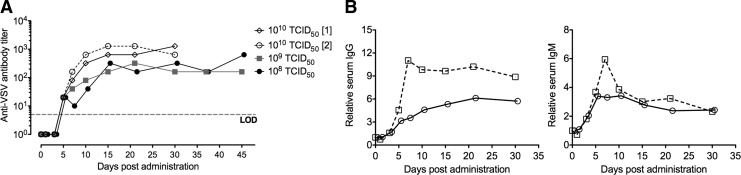
In the United States, there is a low rate of exposure to VSV in the general population (Roberts et al., 1999). This is also true of dogs, which generally are not exposed to VSV infections. This is a key feature in the use of VSV as a potential systemic cancer therapy, as preexisting immunity to an oncolytic virus greatly diminishes viral persistence in blood and ability of the virus to reach and replicate in cancer cells. The time taken for generation of antiviral antibodies to neutralize infectious virus after IV delivery of VSV impacts (a) virus safety and (b) the oncolytic period where infectious virus can replicate in cancer cells. Antiviral antibodies against VSV were detected by plaque reduction neutralization assay. The data indicate that after systemic administration of VSV-hIFNβ-NIS, neutralizing antibodies against VSV are rapidly generated and detectable by day 5. Virus dose does not affect the time for detection of neutralizing antibodies (5 days at all doses tested), but may affect the maximum detectable titer of antibodies in serum where ∼103 antibody titer was detected after IV administration of VSV-hIFNβ-NIS at the MTD (Fig. 4A). Serum from treated dogs was further analyzed to detect immunoglobulin levels after IV VSV delivery at MTD, specifically canine IgG and IgM. These results indicate that, after systemic VSV delivery, there is a steady elevation of IgG and IgM levels in serum, peaking at ∼7 days.
FIG. 4.
Monitoring antiviral immune responses. Adaptive immune response against systemically administered VSV-hIFNβ-NIS was monitored by measuring anti-VSV neutralizing antibodies in serum collected at the indicated times. (A) Anti-VSV neutralizing antibodies in serum were detected by plaque reduction neutralization assay. Anti-VSV antibody titer indicates the minimum serum dilution that did not protect Vero cells from in vitro VSV infection. (B). Anti-VSV IgG and IgM antibodies were detected in serum by ELISA. Values are shown relative to baseline (pretreatment) values. IgG, immunoglobulin G; IgM, immunoglobulin M.
Acute in vivo toxicity
In two dogs, euthanasia and necropsy were performed because of life-threatening toxicity. In the first dog, we believe that toxicity was caused partially by endotoxin contamination of the test article that contributed to acute gastrointestinal toxicity causing dehydration and disseminated intravascular coagulation (Flatland et al., 2010) and virus-related hepatotoxicity. In the second dog, toxicity was attributed to severe hepatotoxicity and hypovolemic shock. Necropsy results confirmed the antemortem clinical diagnoses made via clinical laboratory data and physical examination.
Biodistribution
Necropsy examination carried out on day 30 in one dog yielded tissue for detection of residual viral genome via qRT-PCR. Results are shown in Table 3, indicating low levels of the VSV-N gene detectable in the adrenal gland, kidney, spleen, and testes.
Table 3.
Viral Biodistribution After Systemic Recombinant Vesicular Stomatitis Virus Administration
| Day 30 necropsy | |
|---|---|
| Organ | Copies VSV-N/μg RNA |
| Adrenal gland | 585 |
| Bladder | BLD |
| Bone marrow | BLD |
| Brain (left) | BLD |
| Brain (right) | BLD |
| Cervical spinal cord | BLD |
| Colon | BLD |
| CSF | BLD |
| Eye | BLD |
| Heart | BLD |
| Jejunum | BLD |
| Kidney | 5,005 |
| Liver | BLD |
| Lumbar spinal cord | BLD |
| Lung | BLD |
| Mesenteric lymph node | No sample |
| Optic nerve | BLD |
| Oral mucosa gross lesion | BLD |
| Pancreas | BLD |
| Quadriceps | BLD |
| Salivary gland | BLD |
| Sciatic nerve | BLD |
| Spleen | 990 |
| Stomach | BLD |
| Testes | 10,375 |
Presence of VSV-N RNA in organ samples by qRT-PCR analysis for a single dog treated at 1011 TCID50 humanely euthanized at day 30 after systemic VSV-hIFNβ-NIS administration.
BLD, below limit of detection for assay (<500 copies/0.2 μg RNA reaction); VSV-N, VSV nucleoprotein.
Discussion and Conclusions
This study provides the first robust description of clinical toxicities, viral shedding patterns, and anti-VSV immune responses after systemic VSV administration in healthy immune-competent dogs. The data collected in this preclinical study indicate that 1010 TCID50 of this recombinant oncolytic virus is well tolerated and represents a logical starting dose for clinical treatment of spontaneous canine malignancies. Adverse events observed in this study were consistent and attributable to either study-related procedures (e.g., urinary tract infection) or virus-induced hepatotoxicity. Sequestration of intravenously delivered virus in liver leads to hepatotoxicity and release of inflammatory cytokines and tissue factors (Muruve et al., 1999). Hepatotoxicity may stem either from virus particle toxicity previously described after IV administration of adenoviral vectors (Lieber et al., 1997; Muruve et al., 1999; Shayakhmetov et al., 2004) or from cytotoxic infection of hepatocytes. Mild lymphopenia and self-limiting fever are nonspecific symptoms of viral infection. Some dogs developed mild oral mucosal blister-like lesions, but no infectious virus, nor viral genomes could be isolated from these lesions and their appearance was not consistent among treated dogs. At an administered dose of 10-fold of MTD, viral sequestration in the liver, and rapid release of tissue factors and inflammatory cytokines likely lead to the observed symptoms of severe clotting abnormalities, vascular collapse, and hypotension.
The prompt development of neutralizing antibodies within 7 days of viral exposure is reassuring from a biosafety standpoint. VSV is an agricultural pathogen, for which natural outbreaks can cause significant economic impact on cattle, swine, and equine populations. The data presented herein demonstrate the low likelihood that clinical use of VSV-hIFNβ-NIS represents a significant threat of outbreak in a veterinary setting.
Little is known about the dog as a host for VSV, where very early reports of intentional laboratory infection of healthy dogs via a variety of routes (intracranial and IV) resulted in no clinical signs of illness (Kowalczyk and Brandly, 1954). One important finding in our work is the lack of neurotoxicity observed in dogs receiving the recombinant VSV expressing human IFNβ. Neurotoxicity was seen in BALB/c mice receiving 1010 VSV-mIFNβ intravenously or 109 VSV-mIFNβ intrahepatically, and in some rats receiving 109 VSV-hIFNβ intrahepatically (Jenks et al., 2009). It is possible that dogs may be resistant to the neurotoxicity of VSV, but additional studies in larger groups of dogs would be needed to confirm this observation. Additionally, recombinant VSV expressing two genes (in this case IFNβ and NIS) is slightly attenuated compared with VSV expressing a single gene with respect to maximum virus yield (Naik et al., 2012b), which may contribute to reduced neurotoxicity.
Dogs in this study that developed prolonged PTT did so without other evidence of coagulopathy, which is most consistent with development of antiphopsholipid antibodies. In the 4 dogs for which data are available, the PTT elevations appear within the first 1–7 days after administration of virus and were persistent at 3- and 9-month follow-up examinations in 1/4 and 2/4 dogs, respectively. This phenomenon has been documented to occur in dogs with noninfectious diseases and in a subset of healthy Bernese Mountain Dogs (Nielsen et al., 2011). PTT elevations were seen transiently in rats receiving 109 TCID50 of VSV-hIFNβ virus intrahepatically, but the direct administration of virus into the liver could directly interfere with coagulation factor production in this organ.
We also plan to carry out additional studies evaluating the toxicity and efficacy of VSV expressing canine IFNβ in cancer-bearing dogs. The biologic activity of human IFNβ in the dog is largely unknown, although canine and human IFNβ molecules share approximately 60% sequence homology. Preliminary studies show that human IFNβ has reduced biological activity in canine MDCK cells compared with canine IFNβ, measured by its ability to protect from subsequent infection with VSV-GFP (data not shown). Treatment with VSV expressing canine IFNβ may allow systemic administration of greater than 1010 TCID50 VSV-IFNβ-NIS and/or provide the direct antitumor benefits of canine IFNβ in pet dogs with naturally occurring cancers.
A particularly interesting finding in this study was the persistence of viral genome copies detectable in whole blood up to 10 days, coupled with persistent detection of human IFNβ in serum up to 7 days, after systemic VSV administration. IFNβ has a relatively rapid half-life in blood (∼5 hr) (Salmon et al., 1996). While no infectious virus was recovered from plasma samples of the treated dogs, it is possible that systemically administered VSV is rapidly associated with PBMCs, and limited virus replication in PBMCs leads to the detectable persistent IFNβ expression. This has prompted a detailed analysis of virus distribution in PBMCs versus plasma after systemic virus delivery that can potentially impact virus safety as well as virus localization to tumor and therapeutic outcome after systemic oncolytic therapy.
Overall, this study provides strong evidence that systemic VSV therapy is well tolerated in dogs, highlights the probable treatment-related toxicities and safety, and has prompted additional studies that will provide insight into factors affecting systemic oncolytic therapy, including evaluation of virus localization in blood, imaging studies to monitor virus biodistribution, and the role of canine IFNβ in determining virus toxicity and efficacy. The findings support the initiation of veterinary studies evaluating systemic VSV therapy in tumor-bearing pet dogs that will provide critical data for clinical development of VSV as human cancer therapy.
Supplementary Material
Acknowledgments
This work was supported by a Discovery translation program grant from Mayo Clinic, and a grant from the University of Tennessee Center of Excellence in Livestock Diseases and Human Health.
Author Disclosure Statement
The authors S.N., K.-W.P., M.J.F., and S.J.R. are cofounders of Omnis Pharma, a biotech company developing VSV oncolytic therapies for cancer.
References
- Barber G.N. (2004). Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 17, 516–527 [DOI] [PubMed] [Google Scholar]
- Flatland B., Fry M.M., Leblanc C.J., and Rohrbach B.W. (2010). Leukocyte and platelet changes following low-dose lipopolysaccharide administration in five dogs. Res. Vet. Sci. 90, 89–94 [DOI] [PubMed] [Google Scholar]
- Jenks N., Myers R., Greiner S.M., et al. (2009). Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum. Gene Ther. 21, 451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T., and Brandly C.A. (1954). Experimental infection of dogs, ferrets, chinchillas and hamsters with vesicular stomatitis virus. Am. J. Vet. Res. 15, 98–101 [PubMed] [Google Scholar]
- Lieber A., He C.Y., Meuse L., et al. (1997). The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71, 8798–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve D.A., Barnes M.J., Stillman I.E., and Libermann T.A. (1999). Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10, 965–976 [DOI] [PubMed] [Google Scholar]
- Naik S., and Russell S.J. (2009). Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin. Biol. Ther. 9, 1163–1176 [DOI] [PubMed] [Google Scholar]
- Naik S., Nace R., Barber G.N., and Russell S.J. (2012a). Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-beta. Cancer Gene Ther. 19, 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Nace R., Federspiel M.J., et al. (2012b). Curative one-shot systemic virotherapy in murine myeloma. Leukemia 26, 1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L.N., Wiinberg B., Kjelgaard-Hansen M., and Kristensen A.T. (2011). The presence of antiphospholipid antibodies in healthy Bernese Mountain Dogs. J. Vet. Intern. Med. 25, 1258–1263 [DOI] [PubMed] [Google Scholar]
- Obuchi M., Fernandez M., and Barber G.N. (2003). Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77, 8843–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoloni M.C., and Khanna C. (2007). Comparative oncology today. Vet. Clin. North Am. Small Anim. Pract. 37, 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Buonocore L., Price R., et al. (1999). Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73, 3723–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J., and Peng K.W. (2007). Viruses as anticancer drugs. Trends Pharmacol. Sci. 28, 326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P., Le Cotonnec J.Y., Galazka A., et al. (1996). Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J. Interferon Cytokine Res. 16, 759–764 [DOI] [PubMed] [Google Scholar]
- Shayakhmetov D.M., Li Z.Y., Ni S., and Lieber A. (2004). Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 78, 5368–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett D.A. (1998). Many faces of lupus anticoagulants. Lupus 7Suppl. 2, S18–S22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.