Abstract
Background:
India is one of the high tuberculosis (TB)-burden countries in the world. Resistance to anti-tuberculosis (anti-TB) drugs has already become an important and alarming threat in most of the regions worldwide. India ranks second in the world in harbouring multi-drug resistant cases (MDRTB). Prevalence of MDR-TB mirrors the functional state and efficacy of TB control programmes and realistic attitude of the community towards implementation of such programmes. The most important risk factor in the development of MDRTB is improper implementation in the guidelines in the management of TB, and high rate of defaults on the part of the patients. The study was carried out to evaluate the drug resistance pattern to first line anti-TB drugs in Northern Karnataka region, India.
Materials and Methods:
A prospective study was conducted at J. N. Medical College and its associated Hospitals, Belgaum. Between January 2011 and December 2012, 150 sputum samples of suspected pulmonary TB patients based on the history were examined for the AFB culture by Lowenstein-Jensen (LJ) culture technique. A total of two early morning samples were collected for the smear [Ziehl-Neelsen (ZN) staining] and culture methods. It was observed that ZN staining for AFB was positive in 113 patients (75%), while AFB culture by LJ medium yielded growth in 66 cases (44%). Thus, a total of 66 AFB culture-positive samples by LJ medium were subjected for AFB drug-sensitivity testing (DST). DST was done for Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA), Ethambutol (EMB) and Streptomycin (SM) after isolation by using the resistance proportion method.
Results:
A total of 66 AFB culture-positive specimens, 20 (30.3%) cases were sensitive to all the five drugs while 46 (69.7%) cases showed resistance to one or more drugs. Among these, the resistance to rifampicin was highest (80.4%), while resistance to isoniazid, pyrazinamide, ethambutol and streptomycin were observed to be 60%, 58.7%, 52.1% and 63%, respectively. It was also observed that, resistance to all five drugs was highest (39.18%). MDR isolates were obtained in 52.2% of the cases. Illiteracy, low socio-economic status, previous history of TB and alcoholism were found to have statistically significant association for the development of MDR.
Conclusions:
The prevalence of drug resistance in the present study was observed to be 69.7%. More than half of the cases were multi-drug resistant. The most common resistant pattern observed in this study was resistance to all the first-line drugs. Therefore, during initiation of new case proper explaining and completion of the treatment is very important to avoid the development of future drug resistance in the society.
Keywords: Drug susceptibility testing, drug-resistance surveillance, MDR, multi-drug resistance, pulmonary tuberculosis
INTRODUCTION
Tuberculosis continues to be a major health problem worldwide despite the fact that the causative agent was discovered more than 100 years ago. In 2010, there were 2-2.5 million new cases accounting for one quarter of the total cases worldwide.1 The impact of tuberculosis (TB) can be devastating, especially in developing countries suffering from high burdens of both TB and human immunodeficiency virus (HIV) infections. According to WHO, each year an estimated 9.4 million new cases of TB are detected leading to nearly 2 million deaths. In India, the disease is estimated to kill 500,000 individuals per year; about 1,000 everyday; and one per minute. Multidrug-resistant tuberculosis (MDR-TB) has been a topic of growing interest in the last decade. The exact magnitude of the problem of resistance to anti-tuberculosis drugs worldwide was not known till 1994-1997 when the global project on anti-tuberculosis drugs resistance surveillance was initiated by World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (IUAT-LD). There is rising trend of drug-resistant TB in different parts of the world, India being next only to China, both contributing to more than 50% of global MDR-TB cases. Frequency of MDR-TB is less than 3% in new cases and 12-50% among retreatment cases as per the recent studies, and this is on rise.2 During the last decade, there has been an increase in reported incidences of drug resistance in Category II cases, particularly among those treated irregularly, or with incorrect regimens and doses, with incidence of MDR-TB varying from 12 to 17%.3 Drug resistance in TB is a matter of great concern for TB control programmes since there is no cure for some multidrug-resistant strains of Mycobacterium tuberculosis. Prevalence of MDR-TB mirrors the functional state and efficacy of TB control programmes and realistic attitude of the community towards implementation of such programmes. There is concern that these strains could spread around the world, stressing the need for additional control measures, such as new diagnostic methods, better drugs for treatment, and a more effective vaccine. MDR-TB, defined as resistance to at least rifampicin (RIF) and isoniazid (INH), is a compounding factor for the control of the disease, since patients harbouring MDR strains of M. tuberculosis need to be entered into alternative treatment regimens involving second-line drugs that are more costly, more toxic and less effective.
In all countries and especially those where the number of cases of TB is rising rapidly because of association with HIV, the development and spread of resistant strains of TB is a serious concern. Hence, the present study was undertaken to assess the drug resistance and sensitivity patterns to first line anti-tuberculosis drugs in newly diagnosed as well in treated cases of pulmonary TB and to assess the risk factors responsible for the development of drug resistance in Northern parts of Karnataka.
MATERIALS AND METHODS
The study was carried out at Department of Pulmonary Medicine, KLES Dr. Prabhakar Kore Hospital and Medical Research Centre, Belgaum between January 2011 and December 2012. Patients diagnosed with sputum smear-positive pulmonary TB were included in the study.
After obtaining informed consent, a standard questionnaire containing all the relevant information was completed for each patient. A detailed history relevant to TB such as time and duration, regime of treatment was recorded in the predesigned data sheet. A patient was confirmed sputum smear positive if at least one of two sputum samples were positive for acid-fast bacilli (AFB) by Ziehel Neelson (ZN) method. Patients confirmed as having pulmonary TB for the first time and without any history of previous anti-tuberculosis (for up to 1 month) treatment were considered as new (or initial) cases and any drug resistance associated to them were reported as initial resistance. Patients with previous anti-tuberculosis treatment lasting more than 1 month were considered as a treated case, and any drug resistance based on them were reported as acquired resistance. Drug sensitivity testing was carried on specimens that grew M. tuberculosis on culture in drug-containing LJ medium.
MDR-TB was defined as isolates being resistant to at least RIF and INH. H37Rv strain was used as standard control. The ethical clearance was obtained from the Institutional Ethical Board of JNMC.
Statistical analysis
The data obtained was coded and entered into the Microsoft excel worksheet. The categorical data was expressed as rates, ratios and percentages. Continuous data was expressed as mean ± standard deviation. The data was analyzed using Chi-square test (with Yate's correction wherever appropriate) and Fischer's exact test.
RESULTS
A total of 150 sputum smear patients with pulmonary TB were included in the study. Out of these, 113 specimens (75%) were found to be positive for AFB by ZN method. The AFB cultures on LJ medium were positive in 66 patients (44%). Rest of the cultures did not reveal any growth or were reported as contamination. The baseline characteristics of these culture positive 66 patients are given in detail in Table 1. Most of the patients were from lower socio-economic status and in the productive age group. About two-third patients had prior history of TB. Twenty-one patients (31.8%) had history of smoking, while 32 patients (43.5%) had history of alcohol consumption. Another 14 patients had history of smoking as well as alcohol consumption, thus predisposing them for development of TB. Only 13 patients (19.7%) had contact history of TB. The resistance rate in the present study was observed in 46 patients (69.7%). Rest of the 20 patients (30.3%) showed sensitivity to all the drugs tested. Among these, six patients (9.1%) had primary drug resistance, while rest of 40 patients (90.9%) had acquired drug resistance.
Table 1.
Baseline characteristics of the patients (n = 66)
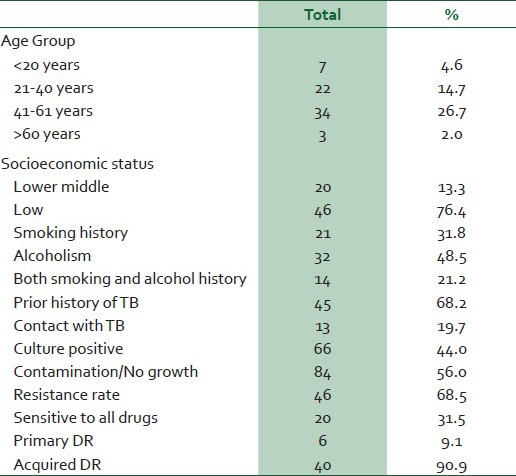
The sensitivity to ethambutol was highest (22.7%), while it was least to rifampicin (6.5%) [Table 2]. Thus, the resistance to rifampicin was highest (80.4%), while resistance to isoniazid, pyrazinamide, ethambutol and streptomycin were 60%, 58.7%, 52.1% and 63%, respectively. In the present study, it was observed that, resistance to all five drugs was highest (39.18%, n=18), while it was lowest to two drugs (6.5%) [Table 3]. Resistance to one drug, three drugs, four drugs were (17.9%), (17.9%) and (8.7%), respectively. MDR isolates were obtained in 24 patients (52.2%).
Table 2.
Resistance pattern of MTB to first-line anti-tuberculosis drugs among the 46 drug-resistant strains
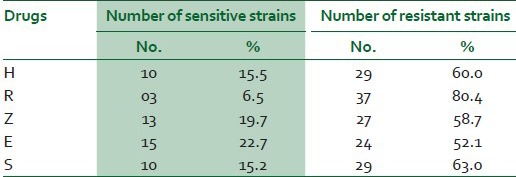
Table 3.
Resistance pattern of 46 drug-resistant strains of MTB to first-line anti-tubercular drugs
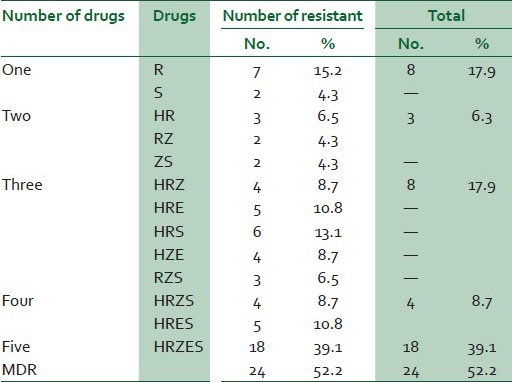
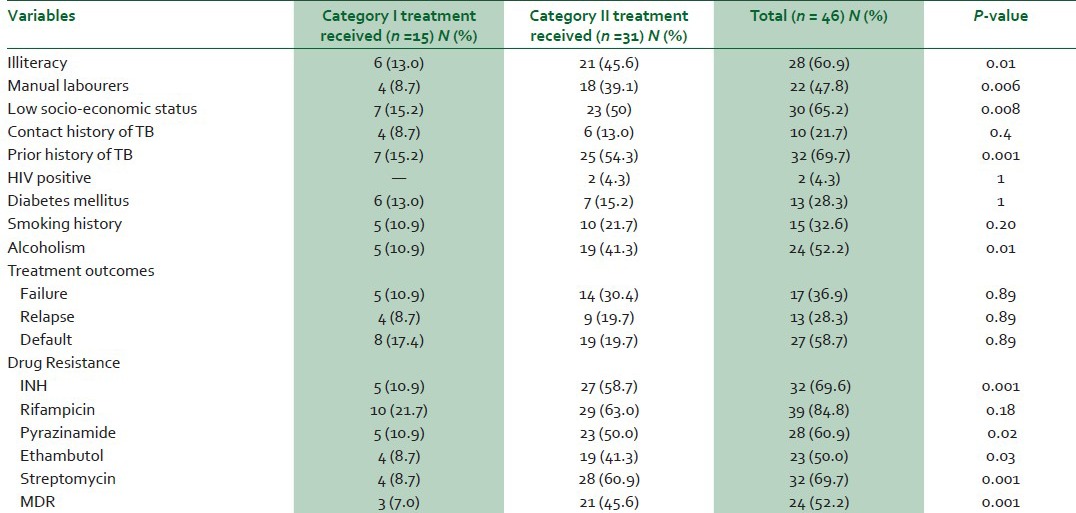
Table 4 shows analysis of various risk factors for the development of drug resistance in TB. It was observed that illiteracy (P = 0.01), low socio-economic status (P = 0.008), manual labourers (P = 0.006), past history of PTB (P = 0.001) and alcoholism (P = 0.01) were found to have statistically significant association for the development of MDR. It was also observed that individual and MDR patterns was found to be significantly high in patients who had received five drug regimen as compared to the patients on four drugs (HRZE) and two drug regimens (HR).
Table 4.
Risk factors and drug resistant patterns (n = 46)
DISCUSSION
India is one of the high-burden TB country in the world accounting for nearly 20% of the global incidence constituting 9.4 million cases and ranks second in harbouring MDR-TB cases in the world.4 MDR-TB is a compounding factor for the control of the disease. Most of the problem of MDR-TB is a man-made problem; every effort should be taken to prescribe the adequate regimen for the prescribed duration of the therapy. Since the pattern of drug resistance varies from place to place and at different periods of time it is important to know the drug-resistance pattern in a particular area to formulate an effective regime.
In the present study, totally 150 smear-positive sputum samples were subjected for culture, out of which only 66 (44.3%) samples had growth at end of 8 weeks. The remaining 47 samples (41.6%) who were sputum AFB positive were observed to be AFB-culture negative as it did not revealed any growth. In a study done at Sindh5 out of 890 sputum-positive PTB cases, growth was seen in 285 (32.02%) patients, while another study done in Tehran6 revealed 548 specimens (35.22%) culture positive among 1556 sputum-positive PTB cases. In our study, culture rates obtained were better than those obtained at other centers, but negative cultures are common and these can be due to variety of reasons: In patients receiving treatment, the organisms may have lost their ability to grow on culture media and be practically dead, patients being treated with a rifampicin-containing regimen often become culture-negative by about the third week of treatment, although they may still be sputum smear-positive: Bacilli are dead or non-viable, environmental factors may also contribute to culture negativity.
In the present study, drug sensitivity testing (DST) was carried for all first-line anti-tubercular drugs -INH, RIF, PZA, EMB and SM. It was observed that 20 specimens (30.3%) were sensitive to all the drugs while resistance to one or more drugs was seen in remaining 46 69.7%) of the cases. Multi-drug-resistant strains were obtained in 24 (52.2%) isolates. Mono resistance and poly resistance was found in 8 (17.9%) and 4 (8.7%) isolates, respectively.
The prevalence of primary/initial drug resistance observed in different studies from India varies from 7.9% to 27.7 %.7 Similarly the prevalence of acquired drug-resistance ranges from 60% to 85% in Indian studies.8,9 Recently one study10 from Hyderabad has reported MDR-TB in 28% of the cases, while another 42% of the cases were having polydrug resistance. Even though these studies do not really reflect the overall status of drug-resistance problem in India, the message from these studies is clear that there is high level of drug resistance seen with M. tuberculosis.
In our study, the prevalence of primary drug resistance observed was 9.1%, a rate found to be similar in many other Indian studies.7 In another study done at Bangalore city, primary drug resistance was found to be 27.7%.2 The overall rate of acquired drug resistance in the present study is 90.9 %. The high prevalence of acquired drug resistance (ADR) observed in this study is comparable to the rate of 60% to 85% from different studies.8,9 In a study done in Gujarat,11 ADR was observed in 58.67% of the cases. Similar prevalence of high acquired drug resistance has been observed in various other studies.12 The high rate of acquired drug resistance observed in these studies as well as in the present study reflects the absence of good effective national TB-control programme and irregular/improper use of anti-TB drugs during recent years, which have led to accumulation and multiplication of drug-resistant strains.
A total of 13 different drug-resistant patterns were obtained after testing 46 strains of M. tuberculosis isolates. The commonest pattern observed was SHERZ (39.1%). The overall combined resistance to at least two or more drugs was observed to be 41.67%. Although higher, similar findings have been observed in other studies as well. In a study done at Bangalore13 it was observed to be 65.2%, while another study has observed it to be 56.5%.14 The high rate of drug resistance to the first-line drugs reflects the absence of good effective national TB-control programme and irregular/improper use of anti-TB drugs during recent years, and effective control measures should be in place to control this menace.15,16
Various risk factors were analyzed to evaluate the association of development of drug resistsance and risk factors. It was observed that the previous history of pulmonary TB was one of the strongest risk factor in development of DR tuberculosis (P < 0.001). Low socio-economic status along with history of alcohol abuse and illiteracy contributed significantly for the development of MDR-TB in the present study. Similar findings were noted in a studies done in India.17,18,19 The high prevalence of MDR-TB is due to poor compliance in TB which is directly related to illiteracy and financial burden on the family.19
The relationship between previous anti-TB treatment and drug resistance has been clearly described.20 These previously treated patients often constitute a very heterogenous group including those who experience relapse after default, and those who start receiving a re-treatment regimen after having experienced previous treatment failure. If a patient has had previous treatment, even an adequate one, it still represents a risk factor for MDR-TB.20 A treatment can be considered inadequate because it was discontinued too early, or because it was taken irregularly, but continued. The selection of resistant mutants occurs due to inadequate treatment, resulting in acquired mono-resistant TB. Novel mutations in a growing population will eventually lead to MDR-TB, if the inadequate treatment continues.21 Inadequate treatment can be defined as direct or indirect mono-therapy, and this can be related to the health professional, to the drug, or to the patient himself. If the treatment is irregular, the number of bacterial death and bacterial growth cycles will be greater, giving more opportunities for individual mutations of different independent genes to accumulate.
Our study findings have shown categorically that alcohol ingestion had direct effect on development of MDRTB (P = 0.011). This is an expected finding, since alcoholism has been associated with high-treatment defaults and poor treatment outcome among patients with TB. Alcohol abuse/dependence was associated with an eight-fold increase in drug resistance.22 Similarly in another study, alcoholism also has shown to be as one of the risk factors for development of MDR-TB.23 Another risk factor in the development of MDR-TB is coexisting HIV infection. A sub-Saharan study done in Africa has recorded HIV- seroprevalance rates of 50-70% in patients with TB.24 According to current hypotheses, HIV increases the chances of transmission of MDR-TB rather than leading to an inadequate treatment.25 However, in some East European countries, MDR rates are double the normal in HIV-positives cases.26 Therefore, the HIV-positive status and MDR-TB may be events independent of each other in our study as all the cases developed MDR following inadequate treatment. Diabetes was present in 19.4% patients in present study. The overall importance of diabetes mellitus as a risk factor for MDRTB is still largely unidentified. It is not certain whether people with diabetes are more likely to develop MDR-TB.27 More studies are required in this regard.
While many patients from our study are likely to access the RNTCP for TB treatment, the seeking of care from private practitioners by many others and the constant shopping for treatment between the public and private sectors cannot be ignored. The contribution to MDR-TB levels from aberrant treatment practices in the unregulated private sector has been suspected for long and makes it likely that the levels of MDR-TB revealed by this study are an underestimate.28
Our study has several limitations. First, there is potential bias in estimation of drug resistance in previously treated cases. Although a detailed history was obtained from the patients, assignment of the patient to new or retreatment categories was based upon the information given by patients and may not have been accurate, therefore, we cannot exclude the possibility of misclassification. Second, the population on which the study was conducted had presented to our referral center. Hence the exact prevalence of primary drug resistance may not applicable to the general population as a whole. Third, we studied only patients with documented positive cultures, not those with negative cultures or those from whom no culture was obtained. Fourth, nonviable specimens were more likely to be resistant than viable specimen on testing by other methods. Fifth, these data are based on very small numbers of patients. The results presented should be interpreted with caution and our observations may not be generalisable to the entire country. However, the results remain important since the increasing level of drug resistance among mycobacterial isolates in our population is alarming.
In conclusion, close monitoring of drug-resistance patterns in confirmed cases of MDRTB isolates is required to formulate different regimens as per the drug sensitivity pattern. Hence early diagnosis of MDR-TB and treatment under supervision by formulating appropriate regimens based on resistance patterns is the key to success in treating MDR-TB cases. Drug resistance is major problem in treatment of pulmonary TB. More surveillance and immediate therapeutic interventions are necessary to combat the threat of MDR-TB.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: World Health Organization; 2011. Global tuberculosis control: WHO report. [Google Scholar]
- 2.Mahadev B, Kumar P, Agarwal SP, Chauhan LS, Srikantaramu N. Surveillance of drug resistance to anti-tubercular drugs in distrcts of Hooghly in West Bengal and Mayurbhanj in Orissa. Indian J Tuberc. 2005;52:5–10. [Google Scholar]
- 3.New Delhi: Directorate General of Health Services Ministry of Health and Family Welfare; 2012. TB India 2012. Revised National TB Control Programme — Annual Status Report. [Google Scholar]
- 4.World Health Organization. [Last accessed on 2013 Oct 24]. Available from: http://www.whoindia.org/en/section3/section123.htm .
- 5.Khoharo HK, Shaikh IA. Drug resistance patterns in pulmonary tuberculosis. J Pak Med Assoc. 2011;61:229–32. [PubMed] [Google Scholar]
- 6.Shamaei M, Marjani M, Chitsaz E, Kazempour M, Esmaeili M, Farnia P, et al. First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran–eight years of surveillance. Int J Infect Dis. 2009;13:e236–40. doi: 10.1016/j.ijid.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Prasad R. Current MDR status. Indian J Tuberc. 2005;52:121–31. [Google Scholar]
- 8.Baldev R, Gupta KB. Changing pattern of acquired drug resistant in patients of pulmonary tuberculosis. Lung India. 1993;10:135–8. [Google Scholar]
- 9.Janmeja AK, Raj B. Acquired drug resistance in tuberculosis in Harayana, India. J Assoc Physicians India. 1998;46:194–8. [PubMed] [Google Scholar]
- 10.Kandi S, Prasad SV, Sagar Reddy PN, Reddy VC, Laxmi R, Koppu D, et al. Prevalence of multi-drug resistance among retreatment pulmonary tuberculosis cases in a tertiary care hospital, Hyderabad, India. Lung India. 2013;30:277–9. doi: 10.4103/0970-2113.120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AR, Agarwal SK, Shah KV. Study of drug resistance in previously treated tuberculosis patients in Gujarat, India. Int J Tuberc Lung Dis. 2002;6:1098–101. [PubMed] [Google Scholar]
- 12.Rawat J, Sindhwani G, Juyal R, Dua R. Five-year trend of acquired antitubercular drug resistance in patients attending a tertiary care hospital at Dehradun. Lung India. 2009;26:106–8. doi: 10.4103/0970-2113.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Guidelines for the management of drug resistant tuberculosis. WHO. 1997. [Last accessed on 2013 Oct 20]. Available from: http://whqlibdoc.who.int/hq/1997/WHO_TB_96:210_(Rev1).pdf .
- 14.Nagaraja C, Shashibhushan BL, Asif M, Manjunath PH, Sagar C. Pattern of drug-resistance and treatment outcome in multidrug-resistant pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2012;54:23–6. [PubMed] [Google Scholar]
- 15.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. WHO Publication; 2012. Epidemiology of antituberculosis drug resistance 2002–07: An updated analysis of the Global project on anti-Tuberculosis. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Mathuria JP, Singh SK, Gulati AK, Anupurba S. Anti-tubercular drug resistance in four healthcare facilities in North India. J Health Popul Nutr. 2011;29:583–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, et al. Surveillance of anti-tuberculosis drug resistance in the world: An updated analysis, 2007-2010. Bull World Health Organ. 2012;90:111–9D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deodhar L, Priya M, Sweta C. Drug resistance in tuberculosis. Bombay Hosp J. 1999. [Last accessed on 2013 Oct 20]. cited in 1999. Available from: http://www.bhj.org/journal/1999_4103_july99/original_253.htm .
- 19.Tanrikulu AC, Hosoglu S, Ozekinci T, Abakay A, Gurkan F. Risk factors for drug resistant tuberculosis in southeast Turkey. Trop Doct. 2008;38:91–3. doi: 10.1258/td.2007.070131. [DOI] [PubMed] [Google Scholar]
- 20.Suarez-Garcia I, Rodriguez-Blanco A, Vidal-Perez JL, Garcia-Viejo MA, Jaras-Hernandez MJ, Lopez O, et al. Risk factors for multi-drug resistant tuberculosis in a tuberculosis unit in Madrid, Spain. Eur J Clin Microbiol Infect Dis. 2009;28:325–30. doi: 10.1007/s10096-008-0627-y. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Vazquez E, Esteban J, de Gorgolas M, Fernández Guerrero ML. Infection by resistant mycobacterium tuberculosis in a hospital population. A longitudinal study of incidental cases at the Fundación Jiménez Díaz. Rev Clin Esp. 1999;199:564–8. [PubMed] [Google Scholar]
- 22.Snider DE, Jr, Long MW, Cross FS, Farer LS. Six-months isoniazid-rifampin therapy for pulmonary tuberculosis. Am Rev Respir. 1984;129:573–9. [PubMed] [Google Scholar]
- 23.Fleming MF, Krupitsky E, Tsoy M, Zvartau E, Brazhenko N, Jakubowiak W, et al. Alcohol and drug use disorders, HIV status and drug resistance in a sample of Russian TB patients. Int J Tuberc Lung Dis. 2006;10:565–70. [PMC free article] [PubMed] [Google Scholar]
- 24.Spellman CW, Matty JK, Weis SE. A survey of drug- resistant mycobacterium tuberculosis and its relationship with HIV infection. AIDS. 1998;12:191–5. doi: 10.1097/00002030-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 25.McCray E, Onorato IM. The interaction of human immunodeficiency virus and multidrug resistant Mycobacterium tuberculosis. In: Bastian I, Portaels F, editors. Multidrug-resistant tuberculosis. Netherlands: Kluwer Academic Publishers; 2000. pp. 45–57. [Google Scholar]
- 26.World Health Organization. Geneva: World Health Organization; 2008. Anti tuberculosis drug resistance in the world fourth global report: The WHO/IUATLD global project on anti tuberculosis drug resistance surveillance. [Google Scholar]
- 27.Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue chest service, 1987 to 1997. Chest. 2001;120:1514–9. doi: 10.1378/chest.120.5.1514. [DOI] [PubMed] [Google Scholar]
- 28.Uplekar M, Juvekar S, Morankar S, Rangan S, Nunn P. Tuberculosis patients and practitioners in private clinics in India. Int J Tuberc Lung Dis. 1998;2:324–9. [PubMed] [Google Scholar]


