Abstract
Background:
Screening rates for colorectal cancer (CRC) are increasing nationwide including Tennessee (TN); however, their up-to-date status is unknown. The objective of this study is to determine the trends and characteristics of TN adults who are up-to-date status with CRC screening during 2002-2008.
Methods:
We examined data from the TN Behavioral Risk Factor Surveillance System for 2002, 2004, 2006 and 2008 to estimate the proportion of respondents aged 50 years and above who were up-to-date status with CRC screening, defined as an annual home fecal occult blood test and/or sigmoidoscopy or colonoscopy in the past 5 years. We identified trends in up-to-status in all eligible respondents. Using multivariable logistic regression models, we delineated key characteristics of respondents who were up-to-date status.
Results:
During 2002-2008, the proportion of respondents with up-to-date status for CRC screening increased from 49% in 2002- 55% in 2006 and then decreased to 46% in 2008. The screening rates were higher among adults aged 65-74 years, those with some college education, those with annual household income ≥$35,000 and those with health-care access. In 2008, the respondents who were not up-to-date status with CRC screening included those with no health-care coverage (adjusted odds ratio [OR] 0.46, 95% confidence interval [CI] 0.33-0.63), those aged 50-54 years (OR 0.62, 95% CI 0.46-0.82) and those with annual household income <$25,000 (OR 0.65, 95% CI 0.52-0.82).
Conclusions:
TN adults who are up-to-date status with CRC screening are increasing, but not across all socio-demographic subgroups. The results identified specific subgroups to be targeted by screening programs, along with continued efforts to educate public and providers about the importance of CRC screening.
Keywords: Behavioral risk factor surveillance system, colorectal cancer, tenncare, tennessee, up-to-date screening status
INTRODUCTION
Colorectal cancer (CRC), defined as the neoplasm of colon and rectum, contributes to significant morbidity and mortality in the United States (US). It ranks second in most commonly diagnosed cancers and cancer deaths among older adults in the US.[1,2] In 2008, 142,950 people (73,183 men and 69,767 women) in the US were diagnosed with CRC and 52,857 people (26,933 men and 25,924 women) died from the disease.[3] Among all existing prevention strategies, the most effective strategy in reducing the morbidity and mortality from cancer is screening.[4,5] Screening tests identify individuals with precancerous lesions including adenomatous polyps that are asymptomatic and amenable to cure at an early age, thereby preventing them from progressing to invasive cancer. In addition, screening for CRC has been identified to be highly impact and cost-effective in general population.[6,7,8,9] It has been found that if all adults with ages 50 years and above were screened for CRC regularly, approximately 10,000 additional deaths could be prevented at an expenditure of $11,900 per life year annually.[10] In comparision to other prevention strategies such as risk factors reduction and increased diagnostic and treatment measures, few modeling studies demonstrated screening for CRC as the most effective strategy with impact greater than others.[11,12]
The key to reduce the incidence and mortality of CRC is regular screening, beginning at 50 years age. In March 2008, the American Cancer Society, the US Multisociety Task Force on CRC and the American College of Radiology recommended that all adults 50 years and older should be screened for CRC regularly.[13,14,15] In October 2008, the US Preventive Services Task Force (USPSTF) updated the 2002 recommendations for CRC screening to include adults aged only 50-75 years.[16] Routine CRC screening is not recommended in adults aged 76 years and above except on an individual basis.[4] Multiple modalities of CRC screening tests have been recommended: An annual fecal occult blood test (FOBT), flexible sigmoidoscopy (FS) or double contrast barium enema every 5 years, a combination of FS every 5 years with FOBT every 3 years or colonoscopy every 10 years. Despite strong effectiveness, expert group recommendations and multiple screening modalities, CRC screening rates remain far below compared with rates of other screening procedures like mammography for breast cancer, prostate-specific antigen screening for prostate cancer and pap smear screening for cervical cancer.[17,18]
The screening rates for CRC were low during the 1990s; however, recent reports indicated moderate increase in screening rates during the 2000s, with rates currently leveling off.[19] During 2002-2008, the percentage of adults who reported FOBT screening within the past 12 months or lower endoscopy within the past 10 years increased from 53.8% in 2002-64.2% in 2008.[20] This signifies that the incidence and mortality rates for CRC are decreasing with screening rates increasing nationwide. Similar patterns were identified in Tennessee (TN), but not at a similar rate. In 2008, the age-adjusted incidence rate for CRC in both males and females is high (47.6/100,000) for TN when compared to that of US (45.5/100,000). Moreover, the mortality rate due to CRC in both males and females is 18.7/100,000, which is higher than the national average of 16.7/100,000. Several studies have been conducted to estimate the CRC screening rates in TN by gender,[21] race including African Americans,[22] health literacy,[23] and response to colonoscopy;[24] however, until date, no study has been conducted to identify the trends of up-to-date status with CRC screening among Tennesseans. Identifying trends and factors associated with up-to-date status with CRC screening among Tennesseans will assist public health professionals and health care providers in earlier detection of cancer, reduce the incidence and mortality from the disease and improve the quality-of-life by providing support and resources. Moreover, disparities across demographic subgroups continue to play a vital role in screening for cancers, especially among certain racial/ethnic minority populations, those without health insurance or health-care access, those with lower household income and those with less education that are necessary to be evaluated.[20,25,26,27] Therefore, it is important to identify such populations who are less up-to-date with CRC screening and in need of support and resources to improve the performance of CRC screening and reduce the incidence and mortality from the cancer. In this study, we used the TN Behavioral Risk Factor Surveillance System (BRFSS), a state representative data, to not only identify trends in up-to-date status with CRC screening in TN adults, but also identify key factors associated with such status; thereby, scarce resources could be diverted toward those needy populations.
METHODS
We used the TN BRFSS to identify trends and key factors associated with up-to-date status with CRC screening in TN. BRFSS is a multistage, random-digital dialing, state-based telephonic health survey for adult US residents 18 years and older to collect information on risk behaviors, clinical prseventive health practices and health-care access primarily related to chronic diseases and injury.[28,29] The BRFSS survey questionnaire consists of approximately 80 core questions with additional optional modules for topics including the questions for CRC screening.[30] Individual states have the option to supplement these additional modules based on the assessment and data needs of their respective states. During the time of data collection in November 2010, additional modules for CRC screening questions were mandatory core item in the TN BRFSS conducted in the years 2002, 2004, 2006 and 2008. TN BRFSS conducted 3207, 3782, 4416 and 5024 interviews during the study years 2002, 2004, 2006 and 2008 respectively. Questions for CRC screening status were posed to 9,172 participants who were aged 50 years and above and are eligible for CRC screening. The survey response rates of all eligible adults with telephones in TN were calculated using the Council of American Survey Research Organization method by taking the percentage of complete and partial interviews out of an estimate of the total households. The survey response rates in the study ranged from 55.4% in 2008-75.8% in 2002.
Measures
During the 4 years, the interviewers asked TN BRFSS participants four questions related to their CRC screening status. They were asked whether they had ever been screened for CRC either with sigmoidoscopy/colonoscopy or a home FOBT and if so, when they received their screening [Table 1]. In 1999, the endoscopy questions were revised to reflect the evidence regarding colonoscopy and proctoscopy. Therefore, the participants were asked about their screening with “sigmoidoscopy/colonoscopy” instead of “sigmoidoscopy/proctoscopy.” Furthermore, in 2008, a new question has been added to the BRFSS questionnaire to differentiate between the sigmoidoscopy or colonoscopy tests that the survey participants underwent. This additional question has been added to the new CRC screening guidelines identifying either sigmoidoscopy during the past 5 years or colonoscopy during the past 10 years.[14] In this study, we defined the up-to-date status with CRC screening for those individuals who were screened for home FOBT in the past 12 months and/or a sigmoidoscopy or colonoscopy in the past 5 years. Although the updated screening guidelines in 2008 restricted the age category to 50-75 years age, we included all survey respondents aged 50 years and above for uniformity in data analysis. Moreover, we restricted including the colonoscopy screening data during the past 5 years for uniformity in the data analysis; although, the updated guidelines stated colonoscopy screening test during the past 10 years. The Institutional Review Board at East Tennessee State University approved the research study.
Table 1.
2008 behavioral risk factor surveillance system questions and their responses

Data analysis
For each year, 2002, 2004, 2006 and 2008, we calculated the proportion of respondents who were up-to-date status with screening for CRC along with socio-demographic characteristics of the respondents including age, gender, race, education and annual household income. Those participants who either did not respond or who responded “do not know/not sure” or “refused” to the questions [Table 1] were not included. In concordance with the screening guidelines, the responses of survey participants aged 49 and younger were dropped from the study. To identify key factors of up-date status with CRC screening, we conducted a multivariable logistic regression analyses for the 4 years distinctly. Adjusted odds ratios (ORs) along with 95% confidence intervals (CI) were reported. A 2-sided 5% significance level was used for all statistical inferences. SAS 9.2 (SAS Institute, Cary, NC, USA) was used for data analysis.
RESULTS
Table 2 presents the proportion of all respondents that were up-to-date status with CRC screening in TN. The proportion of survey respondents 50 years old or older who reported a home FOBT in the past 12 months and/or sigmoidoscopy/colonoscopy in the past 5 years increased from 48.6% (95% CI: 46.0, 51.1) in 2002-55.2 (95% CI: 53.3, 57.1) in 2006 and then decreased to 46.3% (95% CI: 44.6, 48.0) in 2008.
Table 2.
Frequency and proportion of Tennessee respondents 50-75 years who were up-to-date status with colorectal cancer screening, 2002-2008
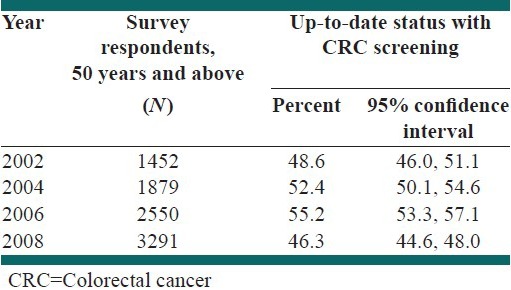
Table 3 presents trends in up-to-date status with CRC screening among survey respondents by socio-demographic characteristics. During 2002-2006, there were increasing trends in up-to-date status among both males and females, all age categories (50-54, 55-64, 65-74 and 75 years and above), Caucasians and African Americans, those with any type of education, those with household income below $50,000 and those having access to health-care. Subsequently the proportion of adults with up-to-date status with CRC screening decreased during 2006-2008 similar to trends in the overall study population. In addition, for those who did not have health insurance and those with household income $50,000 and above, the trend in up-to-date status was negative, then positive and finally negative during 2002-2008. Males were more up-to-date status with CRC screening than females, except during 2006. Those aged 65-74 years, those having more than college level education, those with household income ≥$35,000 and those having access to health-care were more up-to-date status compared to other respective categories.
Table 3.
Trends in up-to-date status* with screening of colorectal cancer by socio-demographics in Tennessee, 2002-2008
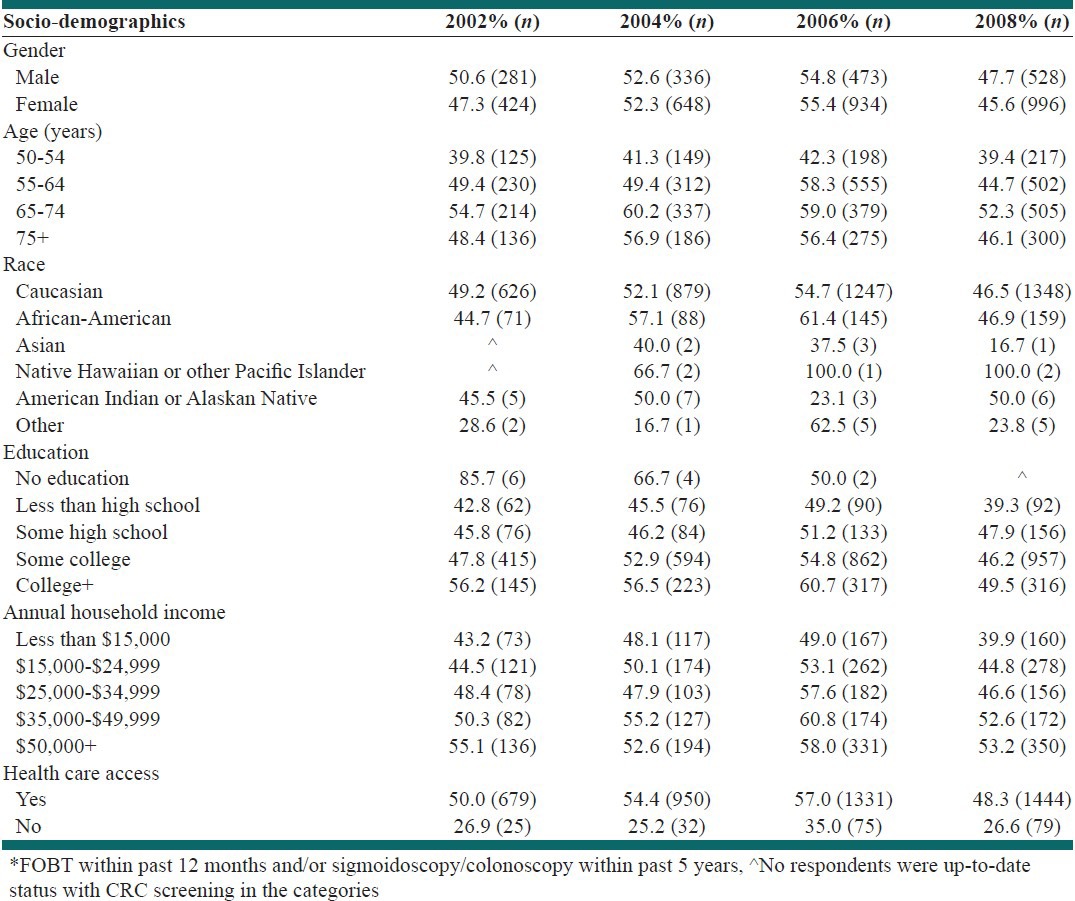
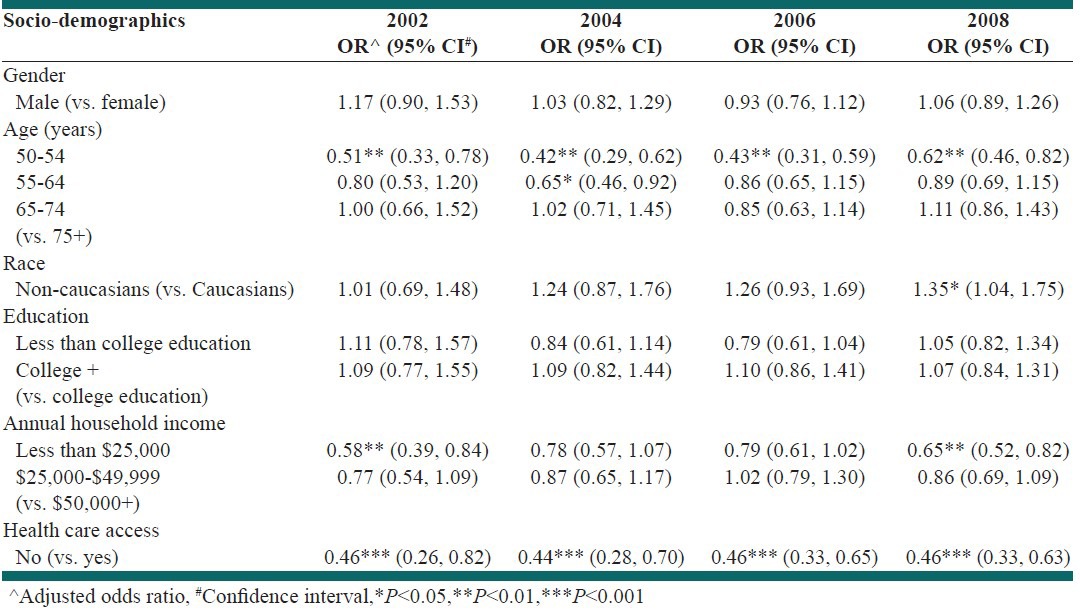
Table 4 identifies, the key socio-demographic factors associated with up-to-date status with CRC screening in TN. The adjusted estimates identified respondents in ages 50-54 years as less likely to be up-to-date status with screening for CRC compared with adults aged 75 years and above during 2002-2008 (2002: Adjusted OR 0.51, 95% CI: 0.33-0.78; 2004: OR 0.42, 95% CI: 0.29-0.62; 2006: OR 0.43, 95% CI: 0.31-0.59; 2008: OR 0.62, 95% CI: 0.46-0.82). Moreover, the adjusted estimates revealed that individuals who did not have access to health-care were approximately 50% less likely to be up-to-date status with screening for CRC than those who had access (2002: OR 0.46, 95% CI: 0.26-0.82; 2004: OR 0.44, 95% CI: 0.28-0.70; 2006: OR 0.46, 95% CI: 0.33-0.65; 2008: OR 0.46, 95% CI: 0.33-0.63). Furthermore, individuals with annual household income less than $25,000 were less likely to be up-to-date status with CRC screening for the years 2002 (OR 0.58, 95% CI: 0.39-0.84) and 2008 (OR 0.65, 95% CI: 0.52-0.82) respectively. In contrast, during the year 2008, it was found that non-Caucasians were 35% (OR 1.35, 95% CI: 1.04-1.75) more likely to be up-to-date status with CRC screening in comparison to Caucasians. Although statistically insignificant, males were more likely to be up-to-date status with CRC screening than females. Similarly, individuals having more than a college education were more likely to be up-to-date status with CRC screening compared to those with a college education.
Table 4.
Determinants of up-to-date status with colorectal cancer screening in Tennessee, 2002-2008
DISCUSSION
We found that the proportion of TN BRFSS survey respondents with up-to-date screening status for CRC were below than that of national rates. In 2002, 52% of US adults aged 50-75 years were up-to-date with screening, which is defined as FOBT in the past year or a lower endoscopy in the past 10 years,[1] compared to 48.6% adults in TN. Similarly, in 2008, approximately 64% of adults aged 50-75 years were up-to-date with CRC screening nationwide,[1] in comparison to 46.3% of adults in TN. The lower screening rates in Tennesseans might potentially be related to low socio-economic status and limited resources for health screenings. In reference to the 2000 US Census Bureau Data where 19% of US adults had less than high school education, 16% had annual household incomes below $15,000 and 12.4% lived below poverty level, approximately 24%, 19% and 13.5% of Tennesseans had less than high school education, annual household income below $15,000 or lived below poverty level respectively.[31] Although the up-to-date screening rates for CRC in TN rates were below the national rates, we found that there was an increase in percentage of TN respondents with up-to-date screening for CRC during 2002-2006. This could be attributed to significant nationwide and state health promotion efforts to encourage screening tests for CRC for the adult population in TN.[32,33] While the CRC screening rates increased during 2002-2006, the rates decreasedduring 2006-2008 (55.2-46.3%). The decrease in screening rates during this period might not be real and could be attributed to a number of factors such as the definition of up-to-date screening status for CRC used in this study versus the new updated screening guidelines by the USPSTF in 2008,[14] changes in BRFSS sampling procedures by regional health departments and changes to TN Medicaid reform in 2005.[34]
We found higher rates of up-to-date status with CRC screening among individuals with high levels of education, high annual household income or having a health insurance. These findings regarding the relationships between some socio-demographic characteristics and screening patterns were in consistent with results from prior studies.[35,36,37] In 2008, the TN BRFSS respondents aged 50-54 years had low rates of up-to-date screening compared to those aged 75 years and above and individuals with low annual household incomes had lower rates of up-to-date screening status compared to those with higher levels of annual household income. Similarly, rates of up-to-date screening status for insured or those who have access to health-care were almost twice as high among those with no health-care access. These findings indicate that current public health education and awareness programs to promote CRC screening may not be reaching these sub-group populations, which may subsequently lead these adults to progress to invasive cancer. The lack of education and promotion initiatives is also supplemented by poor access to health-care or insurance or lack of income to pay for screening tests. The differences in up-to-date status with CRC screening across socio-demographic groups can be reflected upon the disparities as stated above and addressed at individual, community and policy levels. At the individual level, it is important that all Tennesseans receive at least some education that will not only improve their quality-of-life, but also contribute to their annual household income and increased access to health-care. At the community and policy levels, the public health professionals, community workers and policy makers should effectively communicate the importance of screening, campaign for increasing education and awareness and advocate for state-funded resources for all unemployed or uneducated Tennesseans, thereby increasing screening rates. Thus, a collective action by everyone, such as “TN Cancer Coalition Network” is necessary to reduce the disparities among populations, increasing up-to-date status with CRC screening and reducing the burden of CRC in TN.[38]
Non-Caucasians, especially African-Americans are diagnosed at an advanced stage of CRC than Caucasians and have higher mortality rates than Caucasians. These disparities may be attributed in part to low rates of screening.[39,40] However, in this study, we found that in 2008, non-Caucasians are more associated with up-to-date status with CRC screening than Caucasians as identified in previous studies.[41] Although the finding is encouraging with more than a quarter of African-Americans being up-to-date status with screening, the lack of significance and fluctuations during 2000-2008 could be attributed to sampling errors, changes in the definition of up-to-date CRC screening status and family history of cancer or other risk factors that might have increased their perception toward benefits of screening and thereby contributing to increased screening rates. Instead, efforts to educate and promote the screening for CRC among non-Caucasians, especially African-Americans, should continue as these subgroup populations have higher rates of diagnoses and mortality in advanced stages of cancer. In addition, although not significant, we identified that proportion of males who is up-to-date status with CRC screening is higher than that of females, similar to earlier studies that reported higher prevalence of screening test among males than females.[42,43,44] Moreover, greater use of FOBT has been reported by females while men favored endoscopy more often.[45] It is noteworthy that several factors need to be considered while addressing the gender gap in screening rates in terms of preference, complications and efficacy of a screening modality, effective health communication, level of comfort, frequency, time and cost; thereby, future health education and promotion efforts could be targeted to deal with such factors while addressing the gender differences.
The study is subject to merits and limitations and as per our knowledge it is the first investigation to identify trends and characteristics of TN adults associated with up-to-date status with CRC screening. We utilized the TN BRFSS survey data, a state representative data to conduct this study; therefore, the study findings can be generalizable to the entire population. Although the study has significant strengths and draws important conclusions, limitations do exist. First, the updated screening guidelines for CRC in 2008 to identify individuals screened for sigmoidoscopy within the past 5 years and colonoscopy within the past 10 years, along with changes in Tenn Care reforms and sampling procedures may have resulted in lower screening rates in 2008. The extent to which these changes may have affected the results remains unclear and need further evaluation. The definition of up-to-date screening status for individuals as either colonoscopy or sigmoidoscopy within the past 5 years may underestimate the actual percentage of those who are up-to-date, since individuals who had a colonoscopy within the past 6-10 years are in compliance with current guidelines. Moreover, we cannot distinguish the use of CRC screening test for either diagnostic or screening procedure from the BRFSS questions and responses, possibly resulting in under/overestimation of the actual screening rates. Second, the TN BRFSS survey is cross-sectional in nature; hence, no causal relationships can be established. Third, the TN BRFSS survey is a telephone-based survey; therefore, responses are limited to individuals who owned home telephones. The survey response rates are low and the respondents may have answered differently than those who either did not own a telephone or chose not to participate, a measure of non-respondent bias. Another limitation is recall bias as the responses of survey participants are self-reported and may not accurately reflect the actual screening status. However, previous studies identified a fair-to-good agreement between self-reports and medical records.[46,47] Finally, other influencing factors or confounders such as transportation, accessibility to health education and screening initiatives, physician recommendations for CRC screening, individuals with the family history for CRC, patient compliance with sigmoidoscopy/colonoscopy screening procedures are not taken into consideration, which may affect the accuracy of these up-to-date screening status estimates in TN adults.
CONCLUSION
Although the CRC screening rates in TN are lower than the national rates, the percentage of TN adults who are up-to-date status with CRC screening is increasing. While this is an encouraging finding, many adults aged 50 years and above are still not up-to-date with current guidelines and some socio-demographic groups such as the uninsured, those aged 50-54 years, those with household income less than $25,000 have particularly low rates for up-to-date status with CRC screening. Therefore, there is a need for public health awareness programs to promote screening for the public, especially targeted toward subgroup populations, who had low percentages of respondents with up-to-date status and public health education for health-care providers to promote and encourage the screening for CRC thereby improving the quality-of-life among adults in TN.
ACKNOWLEDGMENTS
We would like to thank the Division of Vital Statistics at TN Department of Health, Nashville, TN for providing the data for the study and Drs. James L. Anderson and Toni H. Bounds at Department of Biostatistics and Epidemiology, College of Public Health, East Tennessee State University for their valuable input.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Vitalsigns: Colorectal cancer screening among adults aged 50-75 years-United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–12. [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2008. [Accessed 2012 Oct 15]. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/2008cafffinalsecuredpdf.pdf .
- 3.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999-2008 incidence and mortality web-based report. Atlanta, GA, Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. 2012. [Accessed 2012 Oct 15]. Available at http://apps.nccd.cdc.gov/uscs/
- 4.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: A targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman D. Cost-effectiveness of colonoscopy in screening for colorectal cancer; cost-effectiveness of screening colorectal cancer in the general population. Gastrointest Endosc. 2001;54:537–8. doi: 10.1067/mge.2001.114328. [DOI] [PubMed] [Google Scholar]
- 8.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: Health impact and cost effectiveness. Am J Prev Med. 2006;31:80–9. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 10.Maciosek MV, Coffield AB, Flottemesch TJ, Edwards NM, Solberg LI. Greater use of preventive services in U.S. health care could save lives at little or no cost. Health Aff (Millwood) 2010;29:1656–60. doi: 10.1377/hlthaff.2008.0701. [DOI] [PubMed] [Google Scholar]
- 11.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US multi-society task force on colorectal cancer, and the American college of radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 14.McFarland EG, Levin B, Lieberman DA, Pickhardt PJ, Johnson CD, Glick SN, et al. Revised colorectal screening guidelines: Joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology. 2008;248:717–20. doi: 10.1148/radiol.2483080842. [DOI] [PubMed] [Google Scholar]
- 15.Yee J, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, et al. ACR Appropriateness Criteria on colorectal cancer screening. J Am Coll Radiol. 2010;7:670–8. doi: 10.1016/j.jacr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–41. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 17.Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J Public Health Manag Pract. 2009;15:200–9. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- 18.Swan J, Breen N, Graubard BI, McNeel TS, Blackman D, Tangka FK, et al. Data and trends in cancer screening in the United States: Results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–81. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph DA, King JB, Miller JW, Richardson LC. Centers for disease control and prevention (CDC). Prevalence of colorectal cancer screening among adults: Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:51–6. [PubMed] [Google Scholar]
- 20.Rim SH, Joseph DA, Steele CB, Thompson TD, Seeff LC. Centers for disease control and prevention (CDC). Colorectal cancer screening-United States, 2002, 2004, 2006, and 2008. MMWR Surveill Summ. 2011;60:42–6. [PubMed] [Google Scholar]
- 21.Peterson NB, Murff HJ, Ness RM, Dittus RS. Colorectal cancer screening among men and women in the United States. J Womens Health (Larchmt) 2007;16:57–65. doi: 10.1089/jwh.2006.0131. [DOI] [PubMed] [Google Scholar]
- 22.Patel K, Hargreaves M, Liu J, Kenerson D, Neal R, Takizala Z, et al. Factors influencing colorectal cancer screening in low-income African Americans in Tennessee. J Community Health. 2012;37:673–9. doi: 10.1007/s10900-011-9498-8. [DOI] [PubMed] [Google Scholar]
- 23.Peterson NB, Dwyer KA, Mulvaney SA, Dietrich MS, Rothman RL. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007;99:1105–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Murff HJ. ACP Journal Club. Colorectal cancer screening with fecal immunochemical testing had higher adherence than screening with guaiac-based FOBT. Ann Intern Med. 2010;153:JC4–5. doi: 10.7326/0003-4819-153-8-201010190-02005. [DOI] [PubMed] [Google Scholar]
- 25.Ayanian JZ. Racial disparities in outcomes of colorectal cancer screening: Biology or barriers to optimal care? J Natl Cancer Inst. 2010;102:511–3. doi: 10.1093/jnci/djq089. [DOI] [PubMed] [Google Scholar]
- 26.Callcut RA, Kaufman S, Stone-Newsom R, Remington P, Mahvi D. Gender disparities in colorectal cancer screening: True or false? J Gastrointest Surg. 2006;10:1409–17. doi: 10.1016/j.gassur.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Crawford ND, Jones CP, Richardson LC. Understanding racial and ethnic disparities in colorectal cancer screening: Behavioral risk factor surveillance system, 2002 and 2004. Ethn Dis. 2010;20:359–65. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system survey data and documentation. United states department of health and human services. [Accessed 2012 Oct 15]. Available at http://www.cdc.gov/brfss/data_documentation.htm .
- 29.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Soz Praventivmed. 2001;46(Suppl 1):S3–42. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Behavioral risk factor surveillance system survey questionnaires. U S Department of health and human services. [Accessed 2012 Oct 15]. Available at http://www.cdc.gov/brfss/questionnaires.htm .
- 31.US Bureau of the Census. American Community Survey Office, 2000 US Bureau of the Census. 2000. [Accessed 2013 Apr 10]. Available at http://censtats.census.gov/data/TN/04047.pdf .
- 32.Lairson DR, Chang YC, Bettencourt JL, Vernon SW, Greisinger A. Estimating development cost for a tailored interactive computer program to enhance colorectal cancer screening compliance. J Am Med Inform Assoc. 2006;13:476–84. doi: 10.1197/jamia.M2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: Results from HINTS. J Health Commun. 2006;11(Suppl 1):181–90. doi: 10.1080/10810730600639190. [DOI] [PubMed] [Google Scholar]
- 34.Important Tenn Care Changes. Tenn Med. 2005;98:365. 368. [Google Scholar]
- 35.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21:132–7. doi: 10.1016/s0749-3797(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 36.Meissner HI, Potosky AL, Convissor R. How sources of health information relate to knowledge and use of cancer screening exams. J Community Health. 1992;17:153–65. doi: 10.1007/BF01324404. [DOI] [PubMed] [Google Scholar]
- 37.Potosky AL, Breen N, Graubard BI, Parsons PE. The association between health care coverage and the use of cancer screening tests. Results from the 1992 National Health Interview Survey. Med Care. 1998;36:257–70. doi: 10.1097/00005650-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Tennessee Cancer Coalition. Reducing the burden of cancer in Tennessee. [Accessed 2013 Apr 10]. Available at http://tncancercoalition.org/
- 39.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: A SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–93. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 40.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in Medicare beneficiaries. Cancer. 2004;100:418–24. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 41.Kim JA, Porterfield D, Gizlice Z. Trends in up-to-date status in colorectal cancer screening, North Carolina, 1998-2002. N C Med J. 2005;66:420–6. [PubMed] [Google Scholar]
- 42.Brawarsky P, Brooks DR, Mucci LA. Correlates of colorectal cancer testing in Massachusetts men and women. Prev Med. 2003;36:659–68. doi: 10.1016/s0091-7435(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 43.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 44.Etzioni DA, Ponce NA, Babey SH, Spencer BA, Brown ER, Ko CY, et al. A population-based study of colorectal cancer test use: Results from the 2001 California Health Interview Survey. Cancer. 2004;101:2523–32. doi: 10.1002/cncr.20692. [DOI] [PubMed] [Google Scholar]
- 45.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 46.Mandelson MT, LaCroix AZ, Anderson LA, Nadel MR, Lee NC. Comparison of self-reported fecal occult blood testing with automated laboratory records among older women in a health maintenance organization. Am J Epidemiol. 1999;150:617–21. doi: 10.1093/oxfordjournals.aje.a010060. [DOI] [PubMed] [Google Scholar]
- 47.Hall HI, Van Den Eeden SK, Tolsma DD, Rardin K, Thompson T, Hughes Sinclair A, et al. Testing for prostate and colorectal cancer: Comparison of self-report and medical record audit. Prev Med. 2004;39:27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]


