Abstract
Fungal spores are known as one of the important bioparticles causing allergic manifestation in human beings. Hence, knowledge of season and prevalence of the airborne allergens to which the patients are exposed is a prerequisite for proper diagnosis and treatment of allergic disorders in hypersensitive individuals. Keeping this in view, aerial survey was performed in the atmosphere of Rohtak city for 2 consecutive years (March 2008–February 2010), using a volumetric petri plate sampler. A total of 45 fungal spore types were recorded during the survey period. In the present study, February–April and July–November were identified as the peak seasons for Rohtak city. Cladosporium was the main contributor to the total fungal load with 25.14% followed by Alternaria (18.05%), Aspergillus niger (7.66%), Curvularia (5.31%), and Epicoccum (5.29%). Fifteen dominant viable fungal spore types were represented in the form of a fungal calendar. An attempt has also been made to assess the allergenicity of some of the fungal types recorded from the atmosphere of Rohtak city. The magnitude of variations observed in markedly positive skin reactions (2+ and above) varied from 17.3 to 2.3%. Penicillium oxalicum showed a markedly positive reaction in maximum number of patients (26; 17.3%) followed by Rhizopus nigricans (23; 15.3%). ELISA was performed with the sera of patients showing markedly positive skin reactions and the sera were classified into four groups based on percent binding. The majority of the sera showed 0–15% binding to different antigenic extracts, while sera showing >60% binding were least in number. Greater than 30% binding was observed against antigens of Rhizopus nigricans, Epicoccum purpurascens, Penicillium oxalicum, Curvularia lunata, Aspergillus flavus, Candida albicans and Neurospora sitophila. The concordance between positive skin reaction and serum-specific IgE antibodies ranged from 16.7 to 69.2%.
Keywords: Aerobiology, asthma, allergens, bioassay, fungal calendar, fungus, hypersensitivity, immunoassay, immunoglobulin E, seasonal variations
The biodiversity of fungi is immense in both an outdoor and indoor environment. They are prevalent in all climates and in every geographical area and contribute a major part of suspended bioparticulate matter of the air. They are the causative agents of hypersensitivity in susceptible individuals, associated with conditions such as bronchial asthma, allergic rhinitis, allergic bronchopulmonary mycoses, hypersensitivity pneumonitis, and atopic dermatitis.1–8 It has been estimated that nearly 20–30% of the world population suffers from allergic ailments.9–11 A preliminary study performed about 30 years ago in India reported that 9.2% of the Indian population suffer from fungal allergy.12 During the last 30–40 years, numbers of allergic patients have shown an exponential increase and the graph continues to rise. The escalating trends are caused by increased exposure to sensitizing allergens and lesser stimulation of our immune system during critical periods of its development.13 In addition, urbanization, industrialization, environmental pollution, and change in lifestyle are some other factors contributing toward increase in these disorders.14–16
India with divergent geoclimatic zones is a hub of biodiversity. The prevailing climatic conditions favor the growth and sporulation of fungi. As a result, airborne concentration of fungal propagule tends to be high. Information on airborne fungi and prevalence of sensitization to fungal allergens is of paramount importance for diagnosis and therapeutic management of allergic diseases. A great deal of work has been performed in different ecogeographic regions of the country with regard to fungal allergens.8,12,17–25 However, not much information is available from Haryana, a northern state of India, because it has not been explored with regard to dominant aeroallergens.
The present study was therefore undertaken to conduct an aeromycological survey in a suburban area (Rohtak) of Haryana, India, with an aim of achieving the following goals: (i) to understand the aerial spectrum of fungal diversity of Rohtak, (ii) to prepare a fungal spore calendar of the sampling site, (iii) to determine the prevalence of skin reactivity to fungal antigenic extracts among local patients suffering from nasobronchial allergy, and (iv) to compare skin-prick tests (SPTs) and specific-serum IgE tests used in the diagnosis of fungal sensitization. The fungal spore calendar constructed in our study provided information on the intensity of dominant spores in different months of the year and was be useful in understanding the fungal spore load in the atmosphere of Rohtak city. The information obtained on prevalence of skin test reactivity to fungal antigens in the local patients will assist in making people aware about the avoidance measures to control consequent health hazards. The study gains its importance because it is first of its kind in the region and is of great value to the local clinicians in the management of allergic ailments.
MATERIALS AND METHODS
Aerial Investigation
The present study was undertaken in Rohtak city, which is located in the southeastern part of Haryana state. It lies between 28°40′/29°05′ N latitudes and 76°13′/76°51′ E longitudes and has an area of 100.57 km2 according to the Surveyor General of India. The city harbors a population of 373,133 people (2011 census) with a population density of 466 persons/km2.
Aerial surveys were performed for 2 consecutive years (March 2008–February 2010) at human height (1.8 m) using a personal volumetric petri plate sampler. The sampler is portable, compact, battery-power operated, 10 cm in height, and 8 cm in diameter with a flow rate of 10 L/min. The sampler was operated between 10:00 A.M. to 12:00 P.M. daily for 10 minutes to collect samples from a fixed site situated in the center of the city. Fungal spores were collected directly onto a 90-mm Petri dish containing Sabouraud's agar medium through a sieve plate having 100 holes of 1-mm diameter each. The exposed Petri plates were incubated at 27 ± 2°C for 2–3 days for the development of fungal colonies.
Fungal colonies were identified to the lowest taxonomic rank possible based on their morphological characteristics such as color, size, shape, and other features of mycelia and spores. Different atlases and published literature were used for authentic identification.26–28 Fungal colony counts obtained were expressed as colony forming units (CFU) per cubic meter of air sampled.
Skin-Prick Test
SPT was performed on 150 patients with respiratory allergy attending the Department of Pulmonary and Critical care, Post Graduate Institute of Medical Sciences, University of Health Sciences, Rohtak. All of the patients with respiratory discomforts were clinically investigated and their medical history was noted. The patients were advised not to take antihistamines and tranquilizers (for at least 48 hours) and other sympathomimetic drugs (for at least 18–12 hours) before the skin test. It was also ensured that the patients had not received corticosteroids for the last 3 weeks. Prior informed written consent was obtained from all of the subjects. Patients <15 and >60 years of age were excluded from the skin test.
A total of 17 fungi predominant in the atmosphere of Rohtak city were used for SPT based on their availability and reported allergenicity. The study was approved by the Ethics Committee of the concerned institute. The consent of each patient was also obtained before skin testing. Sterilized antigenic extracts in 50% glycerinated buffer were procured from All Cure Pharmaceutical Co. (Bahadurgarh, Haryana, India). Fifty percent glycerinated phosphate buffer (1:10) and histamine dichloride (1 mg/mL) were also obtained as negative and positive controls, respectively. A drop of sterile antigenic extract was placed on the precleaned volar surface of the forearm of the subjects. A prick was made through the drop into the skin with the help of a 26G needle and excess antigen was wiped out with the help of a tissue paper. The skin response was measured after 20 minutes with respect to the wheal and/or erythema produced. The results of SPT were examined and classified into four grades as per criteria given by Shivpuri1: wheal size same as negative control = negative; wheal size twice the size of negative control = 1+; wheal size >3–5 mm = 2+, and >5 mm = 3+. To ensure that each antigenic extract was devoid of nonspecific irritants, a skin test was also performed on 20 healthy nonatopic volunteers.
Serum Samples
Venous blood was collected from patients showing markedly positive skin reaction (≥2+) to different antigenic extracts. Blood was allowed to clot for ∼1 hour at room temperature. The expressed serum was separated and centrifuged at 2000 × g for 5 minutes for sedimentation of erythrocytes. The sera obtained were stored at −20°C for immunoassay. Serum samples were also collected from healthy volunteers and treated as control.
Enzyme-Linked Immunosorbent Assay
The presence of the specific IgE antibodies in the sera of patients against different fungal antigenic extracts was determined as per the procedure outlined by Sepulveda et al.29 ELISA titer plates (NUNC 96-well microtiter plate; Tarsons, U.K.) were coated with 100 μL of antigenic extract (200 μg/mL) and incubated overnight at 4°C. Plates were then washed three times with 0.05 M of PBS (0.0 5 M of potassium dihydrogen phosphate, 0.05 M of dipotassium hydrogen phosphate, and 0.8% sodium chloride, pH 7.4) at 15-minute intervals. To block the unbound sites, 100 μL of 1% bovine serum albumin in PBS was added to each well. After incubation for 2 hours at 37°C, washing was repeated as mentioned previously. Patient's sera diluted to 1:10 with PBS containing 0.05% bovine serum albumin was then added to each well and incubated overnight at 4°C. Washing was repeated with PBS containing 0.1% Tween-20 PBS and incubated with enzyme-labeled (alkaline phosphatase) anti-human IgE (1:1000) for 4 hours at 37°C and enzyme assay was determined using p-nitrophenol phosphate (1 mg/mL) in glycine buffer containing 0.001 M of magnesium chloride and zinc chloride. Reaction was terminated at the end of 45 minutes with 50 μL of 5 N of sodium hydroxide solution. Optical density was taken at 405 nm. Serum from normal volunteers was also assayed against different antigenic extracts.
RESULTS
Aerial Fungal Spectrum
In the present investigation, 45 fungal spore types belonging to 30 genera and 17 species were identified. The annual counts of fungal types obtained during the 2-year survey and their average percent contribution are given in Table 1.
Table 1.
Total airborne fungal types identified (their CFU/m3 and average percent contribution)
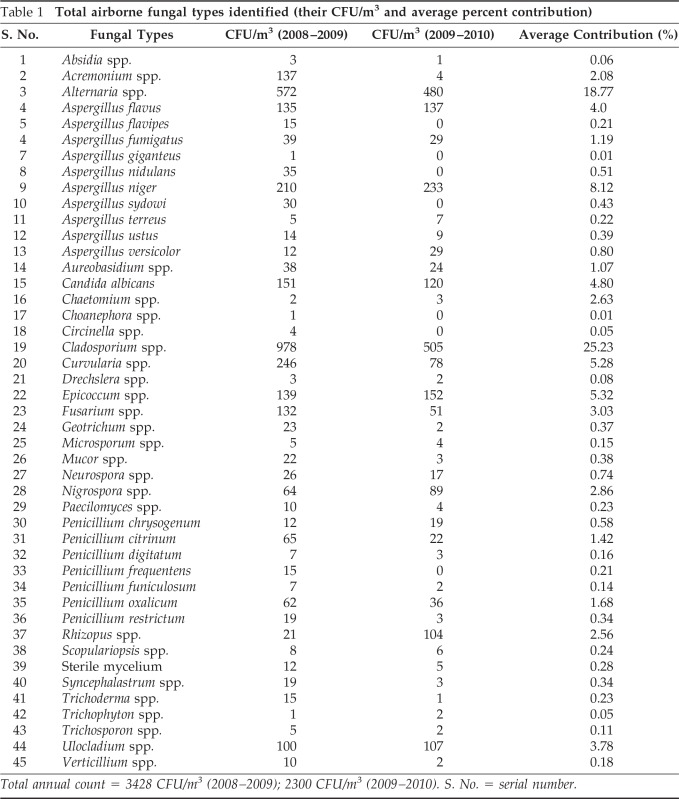
Total annual count = 3428 CFU/m3 (2008–2009); 2300 CFU/m3 (2009–2010). S. No. = serial number.
Cladosporium was the main contributor to the total fungal load with 25.14% followed by Alternaria (18.05%), Aspergillus niger (7.66%), Curvularia (5.31%), and Epicoccum (5.29%). Other important contributors were Aspergillus flavus (4.96%), Candida albicans (3.78%), Ulocladium (3.72%) and Fusarium (3.02%). Types other than these contributed <3% to the total air spora. The total viable spore count was higher (3428 CFU/m3) in the 1st year when compared with spore catch (2300 CFU/m3) recorded in the 2nd year of survey (Fig. 1).
Figure 1.
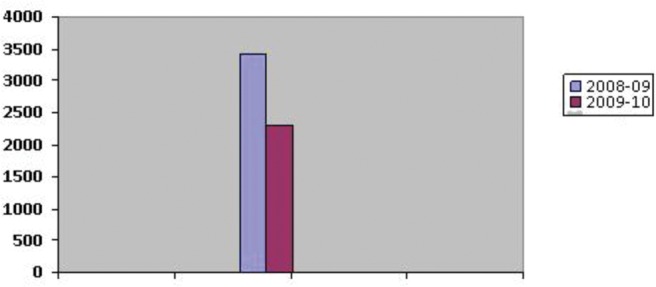
Year-to-year variations of total fungal spore types.
Seasonal Dynamics
The fungal spore types were encountered from the atmosphere of Rohtak city throughout the survey period. However, two peaks were evident: (i) February–April (spring) and (ii) July–November (autumn; Fig. 2). Candida albicans, Nigrospora, Penicillium citrinum, Epicoccum and Ulocladium contributed significantly during the first season whereas species of Aspergillus, Curvularia, Fusarium, and Penicillium were the major contributors in autumn.
Figure 2.
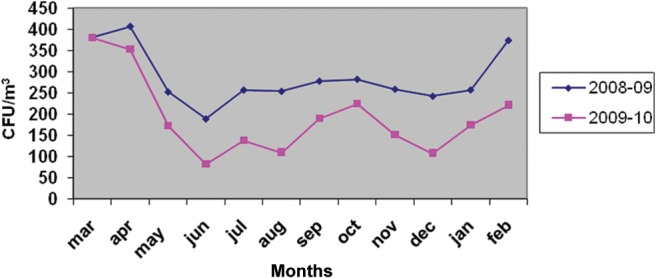
Seasonal variations of total viable fungal spore types.
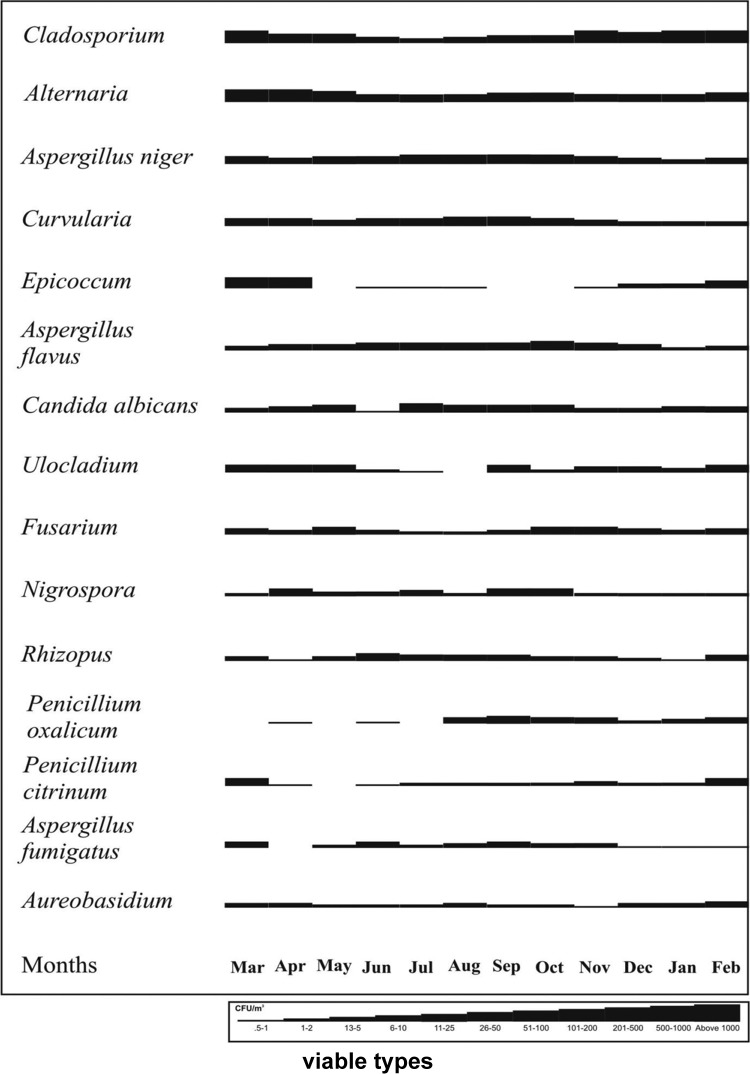
Based on the average values of viable fungal spores obtained during the 2-year investigation, a fungal calendar was constructed for 15 dominant types (Fig. 3). Cladosporium was recorded throughout the investigation period in varying concentrations with an increase from November to February. Lowest catch was observed in the month of July. Alternaria showed a season from February to May with a peak in the month of April. The major season for A. niger, A. flavus, and Curvularia was from July to October. January 2009 was the month that recorded lowest catch of A. niger. A very short and distinct season from March to April was observed for Epicoccum. Poor catch was exhibited from November to May by Curvularia and Epicoccum spores.
Figure 3.
Fungal calendar of dominant types prevalent in the atmosphere of Rohtak city.
Monsoon (July–August) was the favorable season for the spores of C. albicans. The peak season for Ulocladium was from February to May with highest catch in the month of May. Fusarium spp. exhibited in the early winter season, i.e., October–November. However, no definite seasonal pattern was observed for Nigrospora spp. and Rhizopus spp. and their spores were encountered throughout the survey period. Penicillium oxalicum was prevalent from July to November with a peak in August. P. citrinum, on the other hand, showed a peak from January to March. Aureobasidium was recorded in all of the months with a higher catch from December to April.
Bioassay
The overall results of SPT showing 1+ to 4+ reactions to 17 antigenic extracts are given in Table 2. The magnitude of variations observed in markedly positive skin reactions (2+ and above) varied from 17.3 to 2.3%. P. oxalicum showed a markedly positive reaction in maximum number of patients (26; 17.3%) followed by Rhizopus nigricans (23; 15.3%). Antigenic extracts of both Epicoccum purpurascens and Fusarium solani elicited skin reactions in 12% of the patients whereas A. flavus caused positivity in 11.3% of patients. Curvularia lunata and Neurospora sitophila also showed positive response (10.7%), whereas 9.3% of patients showed positivity to Trichoderma, Helminthosporium, and Nigrospora oryzae. Antigenic extract of other fungi elicited 2+ and above reactions in 6.7–8.7% of the patients. Four antigenic extracts, viz., Aspergillus fumigatus, C. albicans, Cladosporium herbarum, and Mucor mucedo showed positivity in 8% of cases. Of all of the antigenic extracts tested, minimum allergenicity (3.3%) was shown to Alternaria solani.
Table 2.
SPT reactions (on 150 patients) to 17 fungal antigens
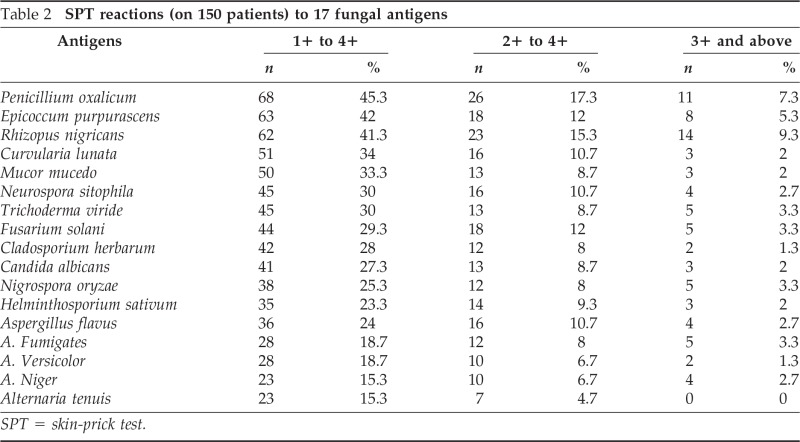
SPT = skin-prick test.
The percentage of patients showing highly positive response (3+ and above) varied from 1.3 to 8.7%. Maximum sensitization (8.7%) was shown by R. nigricans followed by P. oxalicum (7.3%). N. sitophila, E. purpurascens, and Trichoderma viride exhibited skin response in 4.0% of cases. Four antigens, viz., A. flavus, A. fumigatus, Fusarium solani, and N. oryzae showed positive reaction in 3.3% of cases whereas 2.7% of the cases showed positive reaction to the antigenic extract of A. niger.
Immunoassay
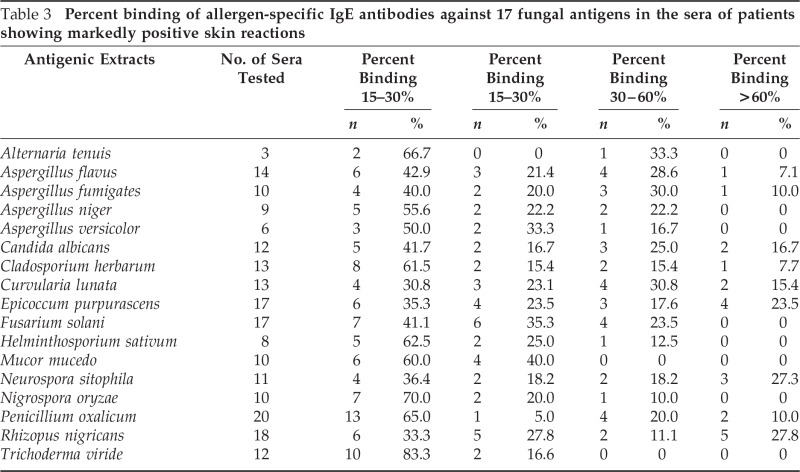
Of 26 patients showing markedly positive skin reactions (2+ and above) to any one of the 17 antigenic extracts used, only 20 consented for in vitro test. ELISA was thus performed with the sera of these patients for the presence of specific IgE antibodies against the antigens to which they showed skin positivity. Sera of five healthy volunteers were also screened. The mean optical density values obtained against each antigen for patients and normal sera are shown in Table 2. The sera was classified into four groups based on percent binding, viz., 0–15%, 15–30%, 30–60%, and >60% (Table 3). The majority of the sera showed 0–15% binding to different antigenic extracts, and sera showing >60% binding were least in number. Greater than 30% binding was observed against antigenic extract of R. nigricans, E. purpurascens, P. oxalicum, C. lunata, A. flavus, C. albicans, and N. sitophila. However, none of the sera tested showed >30% binding to the antigenic extract of M. mucedo and T. viride.
Table 3.
Percent binding of allergen-specific IgE antibodies against 17 fungal antigens in the sera of patients showing markedly positive skin reactions
Correlation between Skin Test Positivity and ELISA Results
To study concordance between the two methods, percent correlation was calculated between the results obtained by SPT (2+ and above) and ELISA (>15% binding). The concordance between bioassay and immunoassay varied from 16.7% (T. viride) to 69.2% (C. lunata). More than 50% concordance was observed against antigenic extracts of R. nigricans, N. sitophila, E. purpurascens, F. solani, A. fumigatus, and A. flavus (Table 4).
Table 4.
Correlation of ELISA with SPT
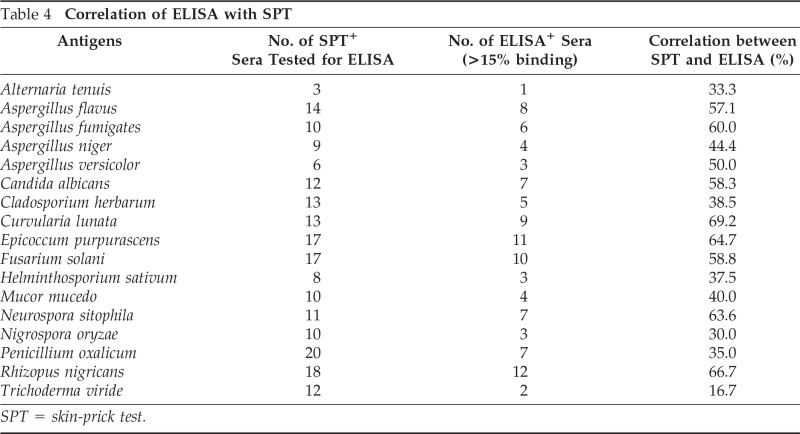
SPT = skin-prick test.
DISCUSSION
Aerial spectrum of Rohtak city exhibited rich fungal diversity with 20 genera belonging to class Deuteromycetes, 6 to Zygomycetes, 3 to Ascomycetes, and 1 to mycelia sterilia. The dominance of Deuteromycetes in the airborne fungal flora has also been reported from other geographical regions of the country such as Orissa, Rajasthan, and Uttar Pradesh.28,30,31 In the present study, 10 species of Aspergillus were recorded. However, from Delhi a nearby station only six species of Aspergillus have been reported: A. flavus, A. fumigatus, Aspergillus nidulans, Aspergillus ochraceus, Aspergillus sydowi, and Aspergillus versicolor.32 In our study, four additional species were observed: Aspergillus flavipes, Aspergillus giganteus, A. niger, Aspergillus terreus, and Aspergillus ustus. However, A. ochraceus, which was reported from Delhi, was not encountered by us.
Total fungal colonies recorded were high during the 1st year of investigation when compared with the 2nd year. Cladosporium spp., Curvularia spp., C, albicans, and Fusarium spp. contributed the maximum to the 1st year catch. A. niger, Epicoccum spp., Ulocladium spp., and Nigrospora spp. were significant contributors during the 2nd year of survey. However, Alternaria spp. and A. flavus were encountered in both of the years. Low spore catch in our results during the 2nd year may be attributed to rapid industrialization and urbanization of the city, which led to a decrease in the vegetative cover and, hence, less availability of decaying matter especially for the growth of saprophytic fungi. Year-to-year variations in aerial fugal spectrum have also been reported from other ecozones of the country.8,33
Spring and autumn are the two seasons for airborne fungi of Rohtak city. Rich vegetation amounting to high organic matter along with suitable climatic conditions corresponds to high prevalence of fungi during these periods. The existence of two peaks of high fungal density was also reported from Delhi.34–39
Fungal spore calendars are important because they provide important information on the seasonal dynamics of airborne fungi. These calendars are of immense help to the clinicians in management of respiratory ailments. In the present investigation, fungal calendars were constructed for 15 dominant viable fungal spore types. Similarly, fungal calendars from different parts of India are also available.8,18,39,40
Cladosporium spp. was the major contributor to the air spora followed by Alternaria spp., A. niger, and Curvularia spp. The dominance of Cladosporium spp. has also been reported in the air spora of different geographical regions of the world including India.30,34,39,41–46 Thus, Cladosporium can be regarded as a “universal dominant” fungus.47 In compliance with our observation, Alternaria spp. has been reported as the second dominant fungi from parts of India such as Pune, Dehradun, and Nagpur.48–50
Poor incidence of fungal spores during monsoon period, i.e., July and August could be attributed to their washing off by rains, thus minimizing their presence in the atmosphere. Moreover, high relative humidity leads to absorption of water by the spores, making them heavier and less transportable by air.51 Similar observations have also been made from Delhi, Bikaner, Modinagar, and Allahabad.23,30,52–53
In our study, SPT was performed on 150 patients suffering from nasobronchial allergy to assess the allergenicity of 17 fungal types because these only were available commercially. Highest positivity (17.3%) was shown to the antigenic extract of P. oxalicum followed by R. nigricans (15.3%). However, low positivity to P oxalicum has been reported from Delhi and Bangalore.18,22 In compliance to our study, Singh reported P. oxalicum to show high positivity in susceptible individuals from Delhi.23 R. nigricans is also reported to be an important allergen from Calcutta.54 Donthi et al. from Hyderabad also reported high sensitivity (36.75%) to Rhizopus among patients of their region.55 However, from Andhra Pradesh, only 5.7% of patients showed 2+ and above reaction to the antigenic extract of R. nigricans.56 With respect to other antigenic extracts, 10% of the patients exhibited markedly positive skin reactions to the antigenic extract of E. purpurascens, F. solani, C. lunata, N. sitophila, and A. flavus. From Andhra Pradesh also these fungi are reported to be important allergens showing markedly positive skin reaction in 6–11% of the patients tested.56 Among all 17 antigenic extracts tested, the least allergenicity (4.7%) was shown against antigenic extract of Alternaria tenuis.
The correlation between skin positivity to individual fungal extract and presence of specific IgE to the respective fungi in the sera of patients varied from 16.7 (T. viride) to 69.2% (C. lunata) with an average of 48.4%. More than 50% concordance was observed for antigenic extract of R. oryzae, N. sitophila, E. purpurascens, F. solani, A. fumigatus, and A. flavus. Variability in the concordance between the results of bioassay and immunoassay has been reported from different parts of the world. From a study in Delhi, a correlation between skin positivity to different fungal extracts and specific IgE to the respective fungi in the sera was recorded in the range of 33–100%.57 However, in a study performed in the united Kingdom, the concordance rates for individual fungi varied from 14 to 56% with an average of only 40%. Highest concordance (56%) was reported for Alternaria; and only 29% was observed for Penicillium, which is in compliance with our study. O'Driscoll et al.58 reported 54% concordance for Aspergillus and a weak concordance (35%) for Cladosporium.
Our results show that the most predominant fungi may not necessarily be the most potent allergens. For example, Cladosporium was the major contributor to the total fungal load (25%) but it showed skin reactivity in only 8% of cases and concordance obtained with ELISA was 38.5%. On the other hand, P. oxalicum contributed <2% to the total catch but was found to be the most important allergen from the Rohtak city. Similarly, Rhizopus contributed only 2.6% to the aerial catch but showed skin positivity in 15.5% of the patients studied. Similar observations have also been made from Netherlands.59,60
STRENGHTS AND LIMITATIONS OF PRESENT STUDY
The present study aimed at identifying the fungal allergens predominant in the atmosphere of Rohtak city. The findings are significant because identification of dominant fungal allergens along with their seasonal variations presented in the form of fungal calendar will help the clinicians in effective and efficient management of allergic ailments of local inhabitants. For the first time, routine SPTs for fungal allergens have been initiated in Post Graduate Institute of Medical Sciences, University of Health Sciences, Rohtak; therefore, the study is of a great benefit to the local patients who had to go to far places for the diagnosis and treatment of their respiratory disorders. The information obtained will also help the allergy sufferers to manage their routine activities so that they can minimize the exposure to various allergens. Moreover, there is paramount scope of using the findings by public authorities in predicting potential fungal types as a source of respiratory allergy in susceptible individuals. This will also prevent unnecessary exposure of patients to the fungal allergens that are not prevalent in the atmosphere of their city. The study is a pioneer and ground-breaking effort in the city, especially in the field of aerobiology and allergy.
Although efforts have been made to understand the aeromycoflora of Rohtak city and identify dominant fungal allergens for the city, this work has a few limitations such as sampling could not be performed at different sites of the city because of limitation of funds and practical inconvenience. Apart from this, the allergenicity of all of the fungal types identified from the atmosphere of study area could not be studied because all of the fungal antigens are not commercially available for skin testing. Based on our observations, we recommend that a comprehensive study over a greater number of years involving more sampling sites is required to understand the relationship between symptoms of respiratory allergies and prevalence of airborne fungi.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Shivpuri DN. Comparative evaluation of the sensitivity of common methods of diagnostic antigen tests in patients of allergy. Indian J Chest Dis 102–107, 1962 [Google Scholar]
- 2. Kang B, Velody D, Hombuager H, Yuninger JW. Analysis of indoor environment and atopic allergy in urban population with bronchial asthma. J Allergy Clin Immunol 63:80, 1979 [PubMed] [Google Scholar]
- 3. Burge H. Bioaerosols: Prevalence and health effects in the indoor environment. J Allergy Clin Immunol 86:687–701, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Singh AB, Singh A. Pollen allergy—A global scenario. In Recent Trends Aerobiology, Allergy and Immunology. Agashe SN. (Ed). New Delhi: Oxford 1BH, 143–170, 1994 [Google Scholar]
- 5. Green BJ, Tovey ER, Sercombe JK, et al. Airborne fungal fragments and allergenicity. Med Mycol 44(suppl 1):S245–S255, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Hamilos DL. Allergic fungal rhinitis and rhinosinusitis. Proc Am Thorac Soc 7:245–252, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Sabokbar A, Ashtiani SSM, Bayat M, et al. Determining allergenic bands for Penicillium species isolated from outdoor air in iran using patients' sera with asthma. Adv Biol Res 5:215–220, 2011 [Google Scholar]
- 8. Chakrabarti HS, Das S, Gupta-Bhattacharya S. Outdoor airborne fungal spora load in a suburb of Kolkata, India: Its variation, meteorological determinants and health impact. Int J Environ Health Res 22:37–50, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Schafer T, Ring J. Epidemiology of allergic diseases. Allergy 52(suppl):14–22, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Burge HA, Rogers CA. Outdoor allergens. Environ Health Perspect 108(suppl 4):653–659, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurup VP, Shen HD, Banerjee B. Respiratory fungal allergy. Microbes Infect 2:1101–1110, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Shivpuri DN. Clinically important pollen, fungal and insect allergens for nasobronchial allergy patients in India. Asp Allergy Appl Immunol 13:19–23, 1980 [Google Scholar]
- 13. Holgate ST. The epidemic of allergy and asthma. Nature 402:B2–B4, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hussan MR, Kabir ARML, Mahmud AM, et al. Self-reported asthma symptoms in children and adults of Bangladesh: Findings of the National Asthma Prevalence Study. Int J Epidemiol 31:483–488, 2002 [PubMed] [Google Scholar]
- 15. Singh AB, Dahiya P. Aerobiological researches on pollen and fungi in India during last fifty years: An overview. J. Allergy Asthma Immunol 22:27–38, 2008 [Google Scholar]
- 16. Bhat MM, Rajasab Incidence of airborne fungal spores at two different sites in Gulbarga. Ind J Aerobiol 4:1–6, 1988 [Google Scholar]
- 17. Shivpuri DN, Singh K. Studies on yet unknown allergenic pollen of Delhi state metropolitan: Botanical aspects. Indian J Med Res 59:1392–1410, 1971 [PubMed] [Google Scholar]
- 18. Agarwal MK, Shivpuri DN. Fungal spores—Their role in respiratory allergy. Adv Pollen Spore Res 1:78–128, 1974 [PubMed] [Google Scholar]
- 19. Shivpuri DN, Singh AB, Babu CR. New allergenic pollens of Delhi state, India and their clinical significance. Ann Allergy 42:49–52, 1979 [PubMed] [Google Scholar]
- 20. Babu CR, Singh AB, Shivpuri DM. Allergenic factors and symptomatology of respiratory allergy patients. J Asthma Res 16:97–101, 1979 [DOI] [PubMed] [Google Scholar]
- 21. Shivpuri DN. Studies in allergy to fungi in India. Asp Allergy Appl Immunol 15:19–30, 1982 [Google Scholar]
- 22. Agashe SN, Anand P. Immediate type hypersensitivity to common pollen and molds in Bangalore city. Asp Allergy Appl Immunol XV:49–52, 1982 [Google Scholar]
- 23. Singh A. Aerobiological and immuochemical studies on fungal spores of different occupational sites. PhD thesis University of Delhi, New Delhi, India: pg 250, 1993 [Google Scholar]
- 24. Gaur SN, Jain SK, Singh AB. Prevalence of asthma, allergic rhinitis and sensitization with pollen and fungal allergens in the Residents of Narora atomic power plant Township, Narora, Uttar Pradesh, India. Indian J Allergy Asthma Immunol 21:1–7, 2007 [Google Scholar]
- 25. Prasad R, Verma SK, Dua R, et al. A study of skin sensitivity to various allergens by skin prick test in patients of nasobronchial allergy. Lung India 26:70–73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair PKK, Joshi AP, Gangal SV. Airborne pollen, spores and other plant materials of India—A survey. Published jointly CSIR Centre for Biochemicals and National Botanical Research Institute, Lucknow: 1–224, 1986 [Google Scholar]
- 27. Udaya Prakash NK. In Indoor Molds—Isolation and Identification. Chennai, India: Color Wings (M) Pvt. Ltd., 1–200, 2003 [Google Scholar]
- 28. Arora A, Jain VK. In A Colour Atlas of Aeroallergens (Pollen and Fungal spores). Madhu Publications, pg 128, 2008 [Google Scholar]
- 29. Sepulveda R, Longbottom J, Pepys J. Enzyme-linked immuno sorbent assay (ELISA) for IgG and IgE antibodies to protein and polysaccharide antigens of Aspergillus fumigatus. Clin Allergy 9:359–371, 1979 [DOI] [PubMed] [Google Scholar]
- 30. Nayak BK, Behra N. Seasonal and diurnal prevalence of airborne fungal spores over Berhampur University, Campus, Orissa. J Palynol 32:29–39, 1996 [Google Scholar]
- 31. Kumari S, Gond DK, Samuel CO, Abbasi P. A comparative study of aeromycospora in different localities of Gorakhpur, U.P. Ind J Sci Res 2:51–55, 2011 [Google Scholar]
- 32. Singh AB, Gangal SV, Subramaniam TAV, Singh BP. Study of fungal airspora in extramural and intramural environments in Delhi with reference to allergic disorders—Final report. The Ministry of Environment and Forests Government of India, New Delhi, 127, 1994 [Google Scholar]
- 33. Adhikari A, Sen MM, Gupta-Bhattacharya S, Chanda S. Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: A 2-year study at five outdoor sampling stations. Sci Total Environ 326:123–141, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Agarwal MK, Shivpuri DN, Mukergi KG. Studies on the allergenic fungal spores of the Delhi, India, metropolitan area: Botanical aspects (aeromycology). J Allergy 44:193–203, 1969 [DOI] [PubMed] [Google Scholar]
- 35. Singh AB, Babu CR. Airborne fungal spore of Delhi. Indian J Chest Dis Allied Sci 25:31–35, 1983 [PubMed] [Google Scholar]
- 36. Sandhu DK, Shivpuri DN, Sandhu RS. Studies on the airborne fungal spores in Delhi. Their role in respiratory allergy. Ann Allergy 22:374–384, 1964 [PubMed] [Google Scholar]
- 37. Kasliwal RM, Sanghvi LM, Gupta KD. Respiratory allergen in Rajasthan. J Ass Phy Ind 3:184, 1965 [Google Scholar]
- 38. Jothish PS, Mohan TK, Nayar TS. Year round fungal airspora of Kovalum-The international beach resort of India. Ind J Aerobiol 17:53–62, 2004 [Google Scholar]
- 39. Das S, Bhattacharya SG. Airborne culturable fungal flora of an agricultural farm in West Bengal and its relationship with meteorological factors. Ind J Aerobiol 20:1–8, 2007 [Google Scholar]
- 40. Agashe SN, Sudha P. Studies on circadian periodicity of total spores in Bangalore city. Ind J Aerobiol 8:17–23, 1995 [Google Scholar]
- 41. Larsen L, Gravesen S. Seasonal variation of outdoor airborne viable microfungi in Copenhagen, Denmark. Grana 30:467–471, 1991 [Google Scholar]
- 42. Hjelmroos M. Relationship between airborne fungal spores and weather variables—Cladosporium and Alternaria. Grana 32:40–47, 1993 [Google Scholar]
- 43. De-Wei Li, Bryce Kendrick. A year-round comparison of fungal spores in indoor and outdoor air. Mycologia 87:190–195, 1995 [Google Scholar]
- 44. Raha S, Bhattacharya K. Aeromycoflora of residential area at two distinct biozones of West Bengal, India. Ind J Aerobiol 10:38–42, 1997 [Google Scholar]
- 45. El-Morsy ESM. Preliminary survey of indoor and outdoor airborne microfungi at coastal building in Egypt. Aerobiologia 22:197–210, 2006 [Google Scholar]
- 46. Oliveira M, Ribeiro H, Delgado L, et al. Outdoor allergenic fungal spores: Comparison between an Urban and a rural area in Northern Portugal. J Investig Allergol Clin Immunol 20:117–128, 2010 [PubMed] [Google Scholar]
- 47. Verma KS, George AM. Fungi of allergenic significance in the air of Jabalpur. Ind Allergy Appl Immunol 11:13–15, 1997 [Google Scholar]
- 48. Chaubal PD, Kotmire SY. Survey of aeromycoflora in Kolhapur. Acta Bot Indica 11:248–251, 1983 [Google Scholar]
- 49. Singh BP, Singh AB, Nair PKK, Gangal SV. Survey of airborne pollen and fungal spores at Dehradun, India. Ann Allergy 59:229–234, 1987 [PubMed] [Google Scholar]
- 50. Kalkar SA, Mothure VM. Concentration of airborne Alternaria and Cladosporium spores in relation to meteorological conditions at Nagpur. Ind J Aerobiol 24:12–18, 2011 [Google Scholar]
- 51. Gonzalez Minero FJ, Canadau P, Cepeda JM. Presencia de sporesde Alternaria en el aire (SO de Espana) y su relacion con factores meterologicos. Rev Iberoam Micol 11:92–95, 1994 [Google Scholar]
- 52. Bhat MS, Gaur RD. Studies on aerobiology—Atmospheric fungal spores. New Phytol 82:519–527, 1979 [Google Scholar]
- 53. Sahney M, Purwar A. A study on airborne fungi at Allahabad. Ind J Aerobiol 20:17–25, 2007 [Google Scholar]
- 54. Batabyal SK, Saha SK, Kundu S, Chanda S. Clinico-immonological studies of some aeroallergens of Calcutta. Ind J Allergy Appl Immunol 4:17–22, 1990 [Google Scholar]
- 55. Donthi S, SivaSai KSR, Lakshmi VV, et al. Prevalence of inhalant allergens in nasobronchial allergy in Hyderabad region: India. Int Sci Res J 3:192–199, 2011 [Google Scholar]
- 56. Acharya PJ. Skin test response to some inhalant allergens in patients of naso-bronchial allergy from Andhra Pradesh. Asp Allergy Appl Immunol XIII:14–18, 1980 [Google Scholar]
- 57. Sharma R, Gaur SN, Singh VP, Singh AB. Association between indoor fungi in Delhi homes and sensitization in children with respiratory allergy. Med Mycol 50:281–290, 2012 [DOI] [PubMed] [Google Scholar]
- 58. O'Driscoll BR, Powell G, Chew F, et al. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clin Exp Allergy 39:1677–1683, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Beaumont F, Kauffman HF, de-Monchy JG, et al. Volumetric aerobiological survey of conidial fungi in the North-East Netherlands. II. Comparison of aerobiological data and skin tests with mould extracts in an asthmatic population. Allergy 40:181–186, 1985 [DOI] [PubMed] [Google Scholar]
- 60. Govind K. Botanical, clinical and immunological studies of intramural fungal spore aeroallergens of Kerala. PhD thesis Mahatma Gandhi University, Kerela, India: 34–55, 2004 [Google Scholar]