Abstract
The Center for Disease Control guidelines recommend desensitization to metronidazole in patients with trichomoniasis and hypersensitivity to metronidazole. There is only one published oral metronidazole desensitization protocol. The purpose of this study was to design a new, more gradual oral desensitization protocol to decrease systemic reactions that may occur when using the previously published protocol. We present two patients with presumed IgE-mediated allergy to metronidazole who underwent oral desensitization using our modified protocol. Case 1 was a 65-year-old woman with trichomoniasis who presented for metronidazole desensitization with a history of intraoperative anaphylaxis and positive skin tests to metronidazole. The patient tolerated six doses of the modified desensitization but developed systemic symptoms of nasal congestion and diffuse pruritus after the 25- and 100-mg doses. Both reactions were treated with intravenous (i.v.) antihistamines. Because of gastrointestinal irritation, the desensitization was completed at a dose of 250 mg orally every 6 hours. Case 2 was a 42-year-old woman with trichomoniasis and a history of hives immediately after administration of i.v. metronidazole who presented for desensitization. The patient had negative skin-prick and intradermal testing to metronidazole. She developed lip tingling and pruritus on her arms 15 minutes after the 10-mg dose. Fexofenadine at 180 mg was given orally and symptoms resolved. She tolerated the rest of the protocol without reaction and received a total dose of 2 g of metronidazole. Our oral metronidazole desensitization for presumed IgE-mediated reactions offers a second option for physicians wishing to use a more gradual escalation in dose.
Keywords: Anaphylaxis, desensitization, drug allergy, immediate-type allergy, intradermal skin test, metronidazole, nitroimidazoles, skin-prick testing, tinidazole, trichomonas
The nitroimidazoles comprise the only class of drugs useful for oral or parenteral therapy of Trichomonas vaginalis. Of these drugs, metronidazole and tinidazole are available in the United States and are approved by the United States Food and Drug Administration for the treatment of trichomoniasis.
Trichomoniasis is considered the most common curable sexually transmitted infection. An estimated 37 million people in the United States are infected with T. vaginalis, but only 30% will develop symptoms.1 Symptoms may develop 5 days to 4 weeks after the initial infection or much later. Symptoms can range from mild itching to severe inflammation with pain. Without treatment, the infection can last for months or years and can increase the risk of the patient getting or spreading other sexually transmitted diseases.1
Trichomoniasis can be treated with a single dose of metronidazole or tinidazole. There are no equally efficacious alternatives for the treatment of T. vaginalis. Therefore, if a patient has an immediate, presumed IgE-mediated reaction to a nitroimidazole, the Center of Disease Control recommends that the patient be managed by metronidazole desensitization in consultation with a specialist. Topical therapy with drugs other than nitroimidazoles can be attempted, but cure rates are low (<50%).1 The recommended adult dosing is a single dose of metronidazole at 2 g orally or 500 mg orally twice a day for 7 days.
Types I, II, and IV hypersensitivity reactions to metronidazole have been described in the literature. Some case reports classified the type of reaction by history alone; other cases described positive skin-prick testing or patch testing correlating with patient history.2–6 The validity of skin-prick testing to metronidazole in determining the cause of the reaction is not validated. In both of our patients, however, we hypothesized that the mechanism was IgE-mediated because of the rapid onset of symptoms, response to antihistamine therapy, and the characterization (e.g., hives) of the rash. There are only a few reports in the literature detailing metronidazole desensitization protocols7–9 and success in treating trichomoniasis. This is likely because of the fact that metronidazole is generally well tolerated. We performed a literature search on PubMed using the terms “metronidazole hypersensitivity,” “metronidazole hypersensitivity and desensitization,” and “metronidazole allergy and desensitization,” looking for published metronidazole desensitization protocols. The only published protocol for oral metronidazole desensitization by Kurohara et al.9 was initially used in the first patient in this case series. Our rationale for designing a new oral desensitization protocol was because of the first patient having a systemic reaction with this protocol. We believed that there should be an additional published protocol with a more gradual dose escalation for cases where there is a concern for a severe systemic reaction.
RESULTS
Case 1
The patient was a 65-year-old woman with recently diagnosed trichomoniasis and a history of intraoperative anaphylaxis 4 years ago, who had undergone extensive drug allergy evaluation at that time in our Allergy/Immunology section, which is located at a tertiary care multispecialty academic center. Skin testing was performed by prick methods to midazolam at 5 mg/mL, alfentanil at 0.05 mg/mL, propofol at 10 mg/mL, ondansetron at 2 mg/mL, droperidol at 2.5 mg/mL, metronidazole at 2 mg/mL, ceftizoxime at 100 mg/mL, and penicillin G 10,000U/mL and then by intradermal methods if prick testing results were negative, at concentrations of 1:10,000, 1:1000, and 1:100 to the medications. The results of testing showed positive intradermal skin tests to metronidazole, ondansetron, and droperidol and a positive penicillin radioallergosorbent test. Skin and radioallergosorbent testing was negative for latex. The patient avoided use of metronidazole after skin testing because of our findings and recommendations.
When the patient returned with trichomoniasis, outpatient desensitization to oral metronidazole was planned, using the previously published protocol9 (Table 1). The risks and benefits were explained to the patient and informed consent was obtained. The first four doses of the protocol were tolerated. Within 10 minutes after receiving the 25-mg dose of metronidazole, the patient developed a diffuse erythematous, blotchy, pruritic rash on her inner thighs, abdomen, chest, and back. Because of the timing of this reaction, we believe this reaction was a mast cell–mediated hypersensitivity reaction. The patient was treated with intravenous (i.v.) diphenhydramine at 25 mg and the rash resolved in 2.5 hours.
Table 1.
Protocols for oral desensitization to metronidazole
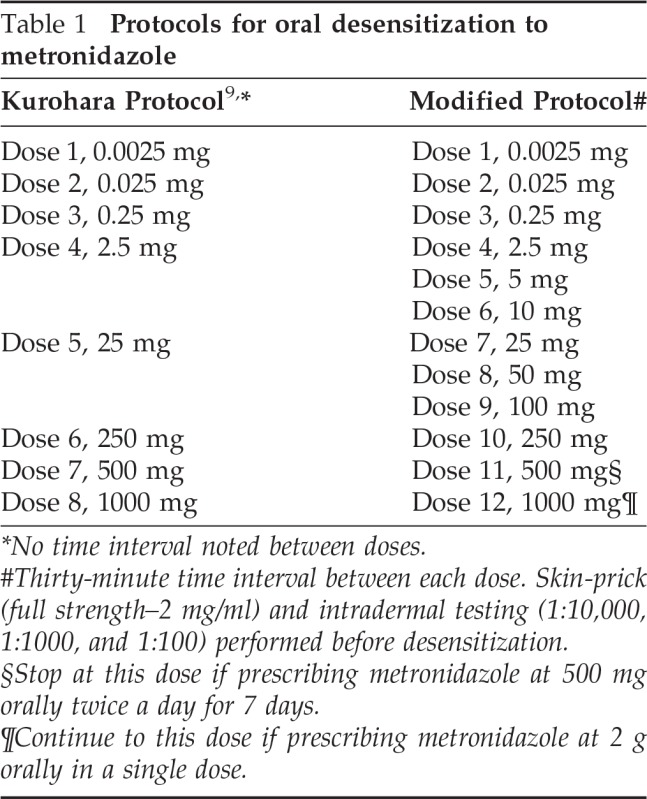
*No time interval noted between doses.
#Thirty-minute time interval between each dose. Skin-prick (full strength–2 mg/ml) and intradermal testing (1:10,000, 1:1000, and 1:100) performed before desensitization.
§Stop at this dose if prescribing metronidazole at 500 mg orally twice a day for 7 days.
¶Continue to this dose if prescribing metronidazole at 2 g orally in a single dose.
Because of concerns that the patient could experience a more severe systemic reaction, desensitization in the intensive care unit (ICU) with a modified desensitization protocol was performed (Table 1). The patient was admitted to the ICU 2 days later after clearance of the initial pruritic rash.
The patient tolerated the first six doses of metronidazole, but developed throat itching, nasal congestion, and mild diffuse pruritus 10 minutes after the 25-mg dose of oral metronidazole; she was treated with 25 mg of oral diphenhydramine. Symptoms resolved and the 10-and 25-mg doses of metronidazole were readministered per the protocol without adverse reactions. The patient also tolerated the 50-mg dose.
A reaction occurred after the 100-mg dose, consisting of nasal congestion and generalized pruritus, and the patient received 50 mg of i.v. diphenhydramine and 20 mg of i.v. famotidine. Resolution of symptoms took 3 hours.
Desensitization continued and the patient tolerated the repeat 50- and 100-mg dose. Twenty-five minutes after the 250-mg dose, she developed a feeling of warmth and then vomited. She did not have any of the previous symptoms (pruritus, nasal congestion, or throat itching); therefore, it was decided that these new symptoms were related to gastrointestinal irritation. Metronidazole therapy can cause nausea in ∼12% of patients.
The patient remained in the ICU and tolerated the next two doses of oral metronidazole (250 mg), which were administered every 6 hours. She was discharged home the next morning to continue oral metronidazole at 250 mg orally every 6 hours for the next 7 days and completed the course without further adverse effect.
Case 2
The patient was a 42-year-old woman with a history of recently diagnosed Trichomonas who presented to our Allergy/Immunology clinic for evaluation of metronidazole allergy. The reaction consisted of hives many years ago after administration of i.v. metronidazole. It was elected by the patient and staff that the patient would return for outpatient skin testing and oral desensitization; this decision was based on the patient's values and preferences and the suspected limited risks of desensitization for this patient. Informed consent was obtained. The patient's gynecologist recommended that she be treated with 2 g of oral metronidazole in a single dose. Skin test results at full-strength prick (2 mg/mL) and intradermal testing (1:10,000, 1:1000, and 1:100) were negative. Because the negative predictive value of skin testing is unknown, it was decided that oral desensitization as planned should be performed.
The patient underwent desensitization using our modified protocol (Table 1). She developed lip tingling and pruritus on her arms 15 minutes after the 10-mg dose and was treated with 180 mg of oral fexofenadine. Symptoms resolved over the next 20 minutes. The patient tolerated the rest of the desensitization, receiving a total dose of 2 g of metronidazole without adverse reactions.
DISCUSSION
In a suspected IgE-mediated hypersensitivity to a medication, a joint decision by the patient and physician must be made to determine the best option for the patient. The choices are avoidance of the offending agent, premedication with readministration of medication, graded-dose challenge, or desensitization to the medication. An IgE-mediated reaction should be suspected in a patient with pruritus, hives, angioedema, difficulty breathing, or change in mental status within 30 minutes of the last dose of a medication or other offending agent.10
Trichomoniasis is unique in that the only effective treatment is metronidazole or tinidazole. No other treatment options have been found to be equally efficacious. Therefore, in a patient with a likely IgE-mediated hypersensitivity to metronidazole, the only therapeutic option is desensitization. Because of the similar chemical structure of nitroimidazoles, patients with hypersensitivity to metronidazole may also have a hypersensitivity to tinidazole.
The patient should be educated on the risks and benefits of desensitization.11 Risks include having a severe systemic allergic reaction, which could require treatment with epinephrine and possibly other treatments or procedures for anaphylaxis, such as oxygen, albuterol, i.v. fluids, and intubation. The patient should be in a monitored setting where treatment for life-threatening reactions can be promptly administered. It is strongly recommended that informed consent be obtained when performing medication desensitization that may lead to an anaphylactic reaction.11
There are limitations to our metronidazole protocol. We have presented a small number of patients and, as in all case reports, there was selection bias. However, we have been able to describe specific details of the desensitization and have shown that despite reactions during the desensitization, the modified protocol used was effective in achieving recommended treatment doses. We were unable to delineate the specific immunologic mechanisms to explain the patients' past reactions to metronidazole and reactions that occurred during the desensitization. There can be consideration of obtaining serum tryptase levels if reactions suggest mast cell activation, as in case 1, when there was concomitant rash, itching, and nasal congestion. One concern regarding our protocol was that case 1 did have reactions at the 25- and 100-mg doses. Because these symptoms suggested a mild systemic reaction, a more gradual increase in medication dosing for patients with severe or recurrent reactions should be considered. No control patients were used for our protocol, making it difficult to ascertain if the metronidazole skin test concentrations were not irritating dilutions in the general population. Although we recognize this as a limitation, it was not feasible to obtain case controls during the desensitization procedures. With future use of our protocol, we plan to test appropriate control patients to avoid false positive and/or irritating reactions.
Metronidazole can be administered i.v. or orally depending on its indication for use and severity of infection. Our oral metronidazole desensitization protocol offers a second option for physicians who wish to use a more gradual escalation in dose when there is no suitable alternative antibiotic. We have presented two patients with trichomoniasis who had a history of a suspected IgE-mediated systemic reaction to metronidazole, with one patient having a positive reaction on skin testing. Both patients underwent successful oral metronidazole desensitization using our protocol and were able to complete treatment despite past adverse immediate reactions to metronidazole. This protocol may be useful and applicable to other patients showing immediate hypersensitivity reactions that physicians need to treat in similar clinical settings.
Footnotes
Presented at the meeting of the American College of Allergy, Asthma, and Immunology, November 5–6, 2011, Boston, Massachusetts
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 59:1–110, 2010 [PubMed] [Google Scholar]
- 2. Garcia-Rubio I, Martínez-Cócera C, Santos Magadán S, et al. Hypersensitivity reactions to metronidazole. Allergol Immunopathol (Madr) 34:70–72, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Lammintausta K, Kortekangas-Savolainen O. The usefulness of skin tests to prove drug hypersensitivity. Br J Dermatol 152:968–974, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 Cases. Contact Dermatitis 56:364–366, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Girardi M, Duncan KO, Tigelaar RE, et al. Cross-comparison of patch test and lymphocyte proliferation responses in patients with a history of acute generalized exanthematous pustulosis. Am J Dermatopathol 27:343–346, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Lomax NJ, Estcourt C, Mirakian R. Symptomatic trichomoniasis, metronidazole allergic and pregnant—A management dilemma. Int J STD AIDS 15:275–276, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Helms DJ, Mosure DJ, Secor WE, Workowski KA. Management of trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol 198:370.e1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Pearlman MD, Yashar C, Ernst S, Solomon W. An incremental dosing protocol for women with severe vaginal trichomoniasis and adverse reaction to metronidazole. Am J Obstet Gynecol 174:934–936, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Kurohara ML, Kwong FK, Lebherz TB, Klaustermeyer WB. Metronidazole hypersensitivity and oral desensitization. J Allergy Clin Immunol 88:279–280, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 Update. J Allergy Clin Immunol 126:477–480.e1–42, 2010 [DOI] [PubMed] [Google Scholar]
- 11. ABIM Foundation. American Board of Internal Medicine; ACP-ASIM Foundation; American College of Physicians-American Society of Internal Medicine; European Federation of Internal Medicine. Medical professionalism in the new millennium: A physician charter. Ann Intern Med 136:243–246, 2002 [DOI] [PubMed] [Google Scholar]


