Abstract
Hereditary hemorrhagic telangiectasia (HHT) is a disease characterized by mucocutaneous telangiectasias and visceral arteriovenous malformations. The genetic mutations that cause this disease result in elevated levels of vascular endothelial growth factor, which is inhibited by bevacizumab. Previous studies have shown bevacizumab treatment to be effective in reducing symptoms, but study protocols have all used oncological dosing parameters, which carry several well-described serious side effects. This study investigates whether drastically lower dosages of bevacizumab than normally used in oncological treatment could control epistaxis in patients with HHT and medically refractory epistaxis. A prospective, open-label, noncomparative study enrolled six patients receiving 0.125-mg/kg infusions of bevacizumab once every 4 weeks for a total of six infusions. Severity of epistaxis was assessed with the epistaxis severity score, and quality-of-life measures were followed with the 20-item Sino-Nasal Outcome Test and 36-item Short Form surveys. A statistically significant improvement was seen in the control of epistaxis severity and frequency, with minimal negative side effects and high patient satisfaction. Very low dose bevacizumab treatment is an effective method of controlling medically refractory epistaxis in patients with HHT and additional investigation to optimize dosing guidelines is warranted.
Keywords: Angiogenesis, Avastin, bevacizumab, epistaxis, hereditary hemorrhagic telangiectasia, Osler–Weber–Rendu disease, VEGF
Hereditary hemorrhagic telangiectasia (HHT; Osler–Weber–Rendu syndrome) is an autosomal dominant inherited disorder characterized by mucocutaneous telangiectasias and visceral arteriovenous malformations (AVMs).1 It is a rare disorder that affects 1 in 5000–8000 patients.2 The visceral AVMs, especially of the pulmonary and hepatic systems, can cause significant arteriovenous shunting and lead to high output heart failure as well as portal and pulmonary hypertension.3,4 However, telangiectasias of the nasal mucosa are extremely common in these patients, and epistaxis is the most common complaint (>90%). For most patients, the epistaxis creates disruptive social embarrassment as well as fatigue from chronic anemia.
HHT is primarily a disorder of inappropriate blood vessel growth. Mutations to genes encoding endoglin, ALK1, and Smad4 have been established in previous genetic studies.5 These mutated gene products are involved in transforming growth factor (TGF) β signaling and lead to the characteristic abnormal vascular structures, ranging from small telangiectasias to large AVMs. Angiogenesis is stimulated by TGF-β as well as vascular endothelial growth factor (VEGF), which itself is also stimulated by TGF-β.6 Recently, it has been shown that patients with HHT have significantly elevated levels of both TGF-β and VEGF.7 In that study, although VEGF levels showed a wide range, the average levels of plasma VEGF in patients with HHT were >10 times greater than healthy controls (330 pg/mL versus 20 pg/mL). Thus, VEGF presents itself as a tantalizing target for medical therapy in the treatment of HHT.
Bevacizumab is a VEGF inhibitor that has been approved for use in the treatment of certain cancers, including metastatic breast, colorectal, and renal cell carcinoma as well as lung cancer and glioblastomas. A number of reports have looked at both intravenous (i.v.) and intranasal administration of bevacizumab for the treatment of HHT.
Intranasal administration has been shown to be effective in reducing the severity and frequency of epistaxis in patients with HHT.8,9 Both intranasal injection and intranasal spray have been used and show similar efficacy.10 Most of these studies have been retrospective chart reviews, not prospective trials. Drawbacks to these administration methods include the lack of systemic delivery and concomitantly eliminating any potential therapeutic efficacy on visceral AVMs. Additionally, questions about dosage delivered to the tissue are important. Local delivery of lower doses may still result in a high tissue load that is similar to or greater than that seen with systemic oncological dosing regimens. This is an important consideration because of the known side effect of poor wound healing when using bevacizumab.11,12
Case studies and prospective trials have shown that inhibiting VEGF with bevacizumab reduces visceral AVM size and blood flow and can eliminate the need for liver transplant, although it has not been shown to decrease pulmonary or cerebral AVM size.13,14 It has also been shown that i.v. bevacizumab can improve iron deficiency anemia and epistaxis in HHT patients.15–17 Notably, however, the studies that investigated the use of i.v. bevacizumab were mainly conducted at oncological dosages of 5–10 mg/kg, with no studies using <1 mg/kg. At these doses there are several well-described side effects that include potential intestinal perforation, ovarian failure, and poor wound healing.11 This dose range has been shown to produce initial blood levels >100 μg/mL.11,18 Complete suppression of free serum VEGF is seen at blood levels of 10–30 μg/mL of bevacizumab; thus, dosing protocols are typically designed to maintain full suppression. However, in HHT patients, oncological cell kill is not a consideration, so complete suppression of VEGF is likely to be unnecessary. Pharmacokinetic studies have shown that dosing of bevacizumab at 0.3 mg/kg results in complete suppression of free serum VEGF.11 It was therefore decided that a low dose of 0.125 mg/kg would be sufficient to reduce free serum VEGF levels as well as its biological activity without fully suppressing it, thus drastically reducing the negative side effects seen at higher doses.
METHODS
Study Design
This was a prospective, open-label, noncomparative study conducted in a single institution. This study involved human subjects and was approved by the Institutional Review Board at St. Vincent's Medical Center. Patient consent was obtained from all participants before treatment.
Enrollment and Exclusion Criteria
Enrollment began in 2012, and nine patients were enrolled between the ages of 46–67 years with clinically diagnosed HHT (at least three of the Curacao Criteria). All patients had moderate-to-severe epistaxis that was refractory to medical management. Three of the patients had received bevacizumab treatment before the initiation of the study; thus, we did not have a baseline for comparison in these patients, and they were excluded. The remaining six patients completed the bevacizumab therapy protocol. The median age was 61 years and the ages ranged from 46 to 67 years. Of the six patients included in this study, three were male and three were female subjects. The study period spanned from September 2012 to October 2013. Premenopausal women were excluded from this study because of the known increased rate of ovarian failure from bevacizumab therapy.12
All of the patients had previously undergone laser treatment for their epistaxis. One patient had a prior septodermoplasty and one patient had undergone a unilateral Young's procedure but continued to have epistaxis on the open nare. Three of the patients were receiving oral iron, two were receiving regular i.v. iron, and one patient was not receiving any iron therapy. The known systemic AVMs included three patients with pulmonary involvement, one patient with brain involvement, and one patient with gastrointestinal involvement.
Treatment
Patients were administered an infusion of bevacizumab i.v. at a dose of 0.125 mg/kg every 4 weeks for a total of six injections. Infusions were given in saline over 90 minutes at the first infusion, over 60 minutes at the second infusion, and over 30 minutes for the remaining infusions. During the trial period, the patients did not undergo any other surgical procedures or receive other medications for their epistaxis.
Evaluation of Treatment
The primary efficacy criterion was a decrease in symptoms of epistaxis by the epistaxis severity score (ESS)19 for patients with HHT, which was measured at every infusion. Additional quality-of-life surveys, the 20-item Sino-Nasal Outcome Test (SNOT-20)20 and the 36-item Short Form (SF-36)21,22 survey, were administered on each infusion day to evaluate for improvement in quality-of-life measures and decreased sinonasal symptoms. Hemoglobin and hematocrit levels were measured at each infusion. Finally, side effects of treatment were monitored by a short survey.
Statistics
All patients who completed the protocol were considered in this study for statistical analysis. Comparisons made between initial and final scores on the ESS, SNOT-20, and SF-36 surveys, as well as effect of treatment on hemoglobin and hematocrit, were made using a paired-sample Student's t-test.
RESULTS
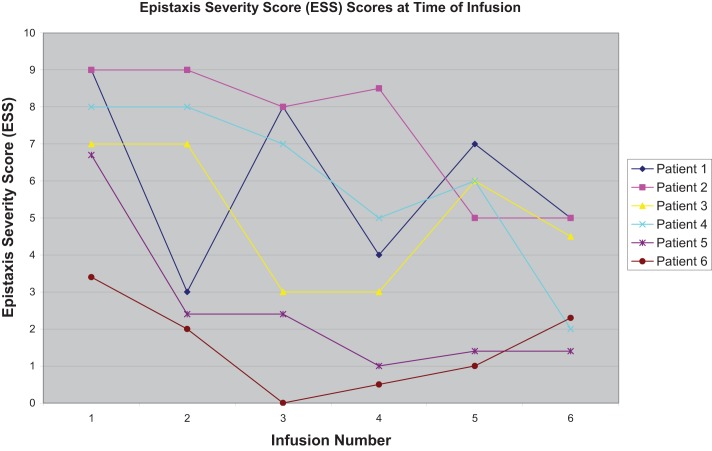
Epistaxis severity and frequency declined for all participants in the study. There was a statistically significant decrease in the ESS value over the course of the treatment protocol. Initial mean ESS value was 7.2, which fell to 3.3 after the last infusion (p < 0.01; Fig. 1). Additionally, the composite scores from the SF-36 indicated a statistically significant improvement in patients' overall mental well-being, which is measured by the mental composite score. Over the course of treatment, the average mental composite score improved from 47.7 to 56.4 (p < 0.05). The mean physical composite score also improved from 37.4 to 42.2, which tracked with the trend toward improvement seen in the SNOT-20 survey results, with the average initial score of 36.5 falling to 19.2 (Table 1).
Figure 1.
Calculated epistaxis severity score (ESS) at each time of Avastin infusion. The decrease in ESS was variable among the patients but decreased by the time of the second infusion in one-half of the patients and by the third infusion in all of the patients.
Table 1.
Effect of low-dose bevacizumab treatment measured by four quality-of-life surveys
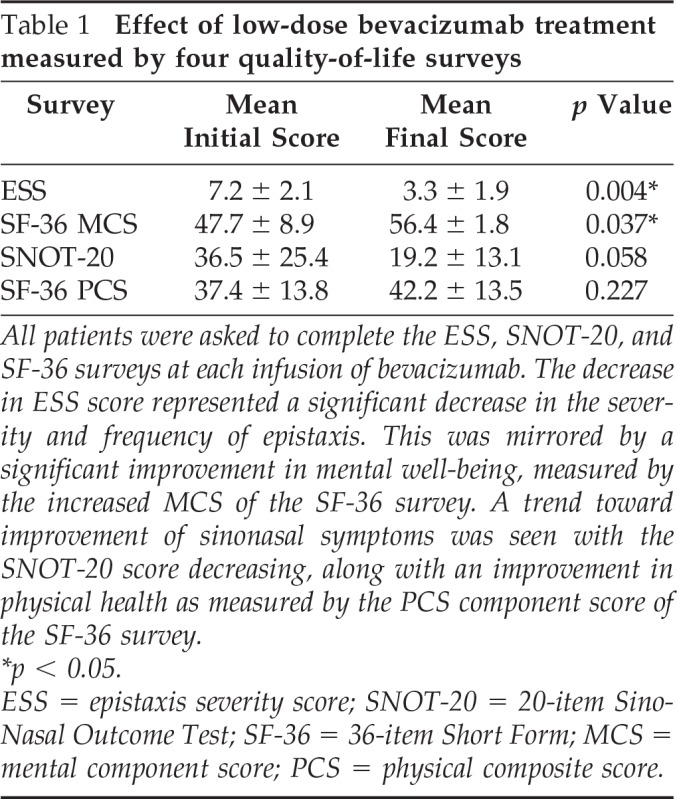
All patients were asked to complete the ESS, SNOT-20, and SF-36 surveys at each infusion of bevacizumab. The decrease in ESS score represented a significant decrease in the severity and frequency of epistaxis. This was mirrored by a significant improvement in mental well-being, measured by the increased MCS of the SF-36 survey. A trend toward improvement of sinonasal symptoms was seen with the SNOT-20 score decreasing, along with an improvement in physical health as measured by the PCS component score of the SF-36 survey.
*p < 0.05.
ESS = epistaxis severity score; SNOT-20 = 20-item Sino-Nasal Outcome Test; SF-36 = 36-item Short Form; MCS = mental component score; PCS = physical composite score.
Side effects of the bevacizumab treatment were also monitored during the trial. The most common adverse effect reported during the trial was headache, followed by change in taste sensation. One patient reported no adverse events at all, and all patients reported a moderate-to-significant decrease in epistaxis (Table 2).
Table 2.
Side effects of treatment with low-dose systemic bevacizumab
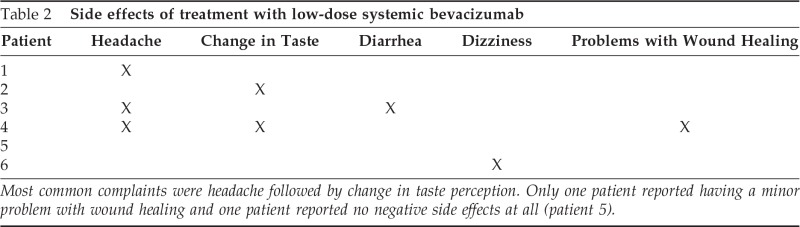
Most common complaints were headache followed by change in taste perception. Only one patient reported having a minor problem with wound healing and one patient reported no negative side effects at all (patient 5).
This dosing protocol had no statistically significant effect on either hemoglobin or hematocrit levels. The mean hemoglobin/hematocrit before and after treatment was 11.0 g/dL and 34.4% and 11.4g/dL and 35.7%, respectively. Four of the six patients had an increase in hemoglobin/hematocrit but the results were not statistically significant.
DISCUSSION
Our study shows that dosages 40–80 times lower than approved oncological protocols will significantly improve epistaxis severity and frequency, as well as overall sinonasal symptoms and mental well-being of patients with HHT. Evidence of benefit in the reduction of symptoms and severity of both epistaxis and visceral involvement has been previously established with both i.v. and intranasal administration of bevacizumab. However, ideal dosing guidelines and administration frequency are currently lacking, with most studies following oncological protocols despite pharmacokinetic evidence that lower dosages are capable of producing significant reduction of free serum VEGF levels. Our study clearly demonstrates that oncological dosing parameters are not necessary for significant improvement in medically refractory epistaxis in patients with HHT.
Additionally, side effects of bevacizumab treatment were minimal to none. Hypertension is a common side effect, but was not observed in this trial. Patients were extremely pleased with the treatment regimen, with the most common side effects noted during treatment being headache and altered taste perception. All patients have elected to continue on bevacizumab therapy after completion of the study.
Additional research is required to optimize the dosing and delivery route for bevacizumab in the treatment of epistaxis caused by HHT. Although intranasal delivery has shown promise, there have also been complications of nasal septal perforation. Additionally, systemic therapy has been shown to improve the more serious visceral AVMs but has suffered from the established side effects of bevacizumab treatment. An optimal approach would be to show that much lower doses of bevacizumab could improve both epistaxis and visceral AVM involvement, with significantly reduced side effects.
It is notable that the patient group as a whole did not show a statistically significant improvement in their hemoglobin or hematocrit parameters over the course of the study. This brings into question whether or not the improvement on the subjective measures of the study were caused by a placebo effect. Given the small number of patients and the open-label nature of the study, placebo at any level can not be ruled out. However, it could also be caused by too infrequent measurements. Any recent acute bleeding before the final hemoglobin and hematocrit being tested could easily eliminate any sustained rise in those values that had been brought about by the treatment protocol. Indeed, three of the six patients had slightly reduced hemoglobin and hematocrit levels at the end of the study compared with at the beginning of the study, and the other three had increased hemoglobin and hematocrit levels. Based on the ESS scores it seems likely that bleeding was actually reduced and a measurable increase in hemoglobin and hematocrit values would be seen with increased measurement frequency and a greater patient pool.
Limitations to this study are primarily its small sample size, which was limited primarily because of the high cost of the drug. We had more patients willing to enroll in the study than we could enroll because of the financial burden of acquiring the bevacizumab.
Our study indicated that very low dose bevacizumab administered once every 4 weeks was sufficient to improve epistaxis in patients with HHT without any serious adverse events. Subjective reporting of satisfaction with the treatment was very high, and study continuation will be pursued with a focus on determining the maximal effective dose spacing that will lead to sustained reduction of epistaxis.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: A clinical and scientific review. Eur J Hum Genet 17:860–871, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: A population based study of prevalence and mortality in Danish patients. J Intern Med 245:31–39, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: Current views on genetics and mechanisms of disease. J Med Genet 43:97–110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: An overview of diagnosis and management in the molecular era for clinicians. Genet Med 6:175–191, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Dupius-Girod S, Bailly S, Plauchu H. Hereditary hemorrhagic telangiectasia: From molecular biology to patient care. J Thromb Haemost 8:1447–1456, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Swerlick RA. Angiogenesis. J Dermatol 22:845–852, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Sadick H, Riedel F, Naim R, et al. Patients with hereditary hemorrhagic telangiectasia have increased plasma levels of vascular endothelial growth factor and transforming growth factor-beta1 as well as high ALK1 tissue expression. Haematologica 90:818–828, 2005 [PubMed] [Google Scholar]
- 8. Karnezis TT, Davidson TM. Efficacy of intranasal bevacizumab (Avastin) treatment in patients with hereditary hemorrhagic telangiectasia-associated epistaxis. Laryngoscope 12:636–638, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Simonds J, Miller F, Mandel J, Davidson TM. The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 119:988–992, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Davidson TM, Olitsky SE, Wei JL. Hereditary hemorrhagic telangiectasia/avastin. Laryngoscope 120:432–435, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850, 2001 [DOI] [PubMed] [Google Scholar]
- 12. FDA Prescribing Information for Bevacizumab. Available online at www.accessdata.fda.gov/drugsatfda_docs/label/2011/125085s225lbl.pdf; accessed January 14, 2014
- 13. Dupuis-Girod S, Ginon I, Saurin JC, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. J Am Med Assoc 307:948–955, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Mitchell A, Adams LA, MacQuillan G, et al. Bevacizumab reverses need for liver transplantation in hereditary hemorrhagic telangiectasia. Liver Transpl 14:210–213, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Suppressa P, Liso A, Sabbà CS. Low dose intravenous bevacizumab for the treatment of anaemia in hereditary hemorrhagic telangiectasia. Brit J Haematol 152:365, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Fleagle JM, Bobba RK, Kardinal CG, Freter CE. Iron deficiency anemia related to hereditary hemorrhagic telangiectasia: Response to treatment with bevacizumab. Am J Med Sci 343:249–251, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Lazaraki G, Akriviadis E, Pilpilidis I, et al. Low dose of bevacizumab is safe and effective in preventing bleeding episodes in hereditary hemorrhagic telangiectasia. Am J Gastroenterol 106:2204–2206, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Wu JY, Wu XN, Ding L, et al. Phase I safety and pharmacokinetic study of bevacizumab in Chinese patients with advanced cancer. Chin Med J 123:901–906, 2010 [PubMed] [Google Scholar]
- 19. Hoag JB, Terry P, Mitchell S, et al. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 120:838–843, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg 126:41–47, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483, 1992 [PubMed] [Google Scholar]
- 22. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 31:247–263, 1993 [DOI] [PubMed] [Google Scholar]