Abstract
Patients with bronchial asthma develop various types of asthmatic response to bronchial challenge with allergen, such as immediate/early asthmatic response (IAR), late asthmatic response (LAR) or delayed asthmatic response (DYAR), because of different immunologic mechanisms. The DYAR, occurring between 24 and 56 hours after the bronchial allergen challenge (p < 0.01), differs from IAR and LAR in clinical as well as immunologic features. This study investigates the expression of CD molecules (markers) on the surface of particular cell populations in the peripheral blood and their changes during the DYAR. In 17 patients developing the DYAR (p < 0.01), the bronchial challenge with allergen was repeated 2–6 weeks later. The repeated DYAR (p < 0.001) was combined with recording of CD molecule expression on various types of blood cells by means of flow cytometry up to 72 hours after the challenge. The results were expressed in percent of the mean relative fluorescence intensity. The DYAR was accompanied by (a) increased expression of CD11b, CD11b/18, CD16,CD32, CD35, CD62E, CD62L, CD64, and CD66b on neutrophils; CD203C on basophils; CD25 and CD62L on eosinophils; CD14, CD16, CD64, and CD86 on monocytes; CD3, CD4, CD8, CD11a, CD18, and CD69 on lymphocytes; CD16, CD56, CD57, and CD94 on natural killer (NK) cells; and CD31, CD41, CD61, CD62P, and CD63 on thrombocytes and (b) decreased expression of CD18 and CD62L on eosinophils, CD15 on neutrophils, and CD40 on lymphocytes. These results suggest involvement of cell-mediated hypersensitivity mechanism, on participation of Th1- lymphocytes, neutrophils, monocytes, NK cells, and thrombocytes in the DYAR.
Keywords: Bronchial provocation tests with allergen, CD molecules, delayed asthmatic response, monocytes, neutrophils, NK cells, Th1 lymphocytes, thrombocytes
Bronchial asthma has classically been attributed to the IgE-mediated hypersensitivity mechanism on participation of mast cells, eosinophils, and Th2-lymphocytes.1,2 However, during the last decades evidence has been found for possible involvement of other hypersensitivity mechanisms, so-called “nonimmediate” or “IgE-independent” mechanisms, in this disorder.3–20 Patients with allergic bronchial asthma, when challenged by allergen, develop various types of asthmatic response, such as immediate asthmatic response (IAR), late asthmatic response (LAR), or dual late asthmatic response (DLAR), because of different immunologic mechanisms, having been already studied from various points of view.3–7,11,14,19,21
In some bronchial asthma patients examined at our department, an asthmatic response appearing 24–48 hours after the bronchial provocation tests (BPT) with allergens has been recorded.22,23 This type, designated by us as “delayed asthmatic response” (DYAR), differs substantially from the IAR and the LAR in clinical and immunologic features and in the hypersensitivity mechanisms underlying the particular asthmatic response types.3,4,7,14,19,21–25 The DYAR became a target of our research interest.22–25
Each of the hypersensitivity mechanisms can be characterized by the typical involvement of particular cell types, generating and/or releasing various factors and expressing typical receptors (CD molecules) on their membrane. The particular cell types, stimulated or inhibited by various intercellular signal mechanisms, interact with other circulating and/or tissue-resident cell types and follow specific traffic patterns in the blood and bronchial tissue leading to a variety of immunologic processes, bronchoconstriction, increased sputum production, and airway remodeling.1–25
The CD molecules, or so-called “surface/membrane-bound receptors,” being dynamic structures expressed on the membranes of various cells, represent important components of the immunologic system and processes by executing manifold intra- as well as extracellular functions, especially the activation of the bearing cells and intercellular communication on various levels.1,2,26–28 The purpose of this study was to investigate expression profiles of membrane-bound CD molecules (markers) on the particular cell types circulating in the peripheral blood during the DYAR and thereby to establish the course of their possible involvement in the mechanism(s) underlying the DYAR.
METHODS
Patients
Seventeen bronchial asthma patients having been referred to our Department of Allergology and Immunology (Institute of Medical Sciences “De Klokkenberg, Breda, The Netherlands) between 1998 and 2000 for diagnostic analysis and developing the DYAR to BPTs with allergens (BPT) during the routine diagnostic procedure, volunteered to participate in this study. These patients (Table 1) suffered from reversible bronchoconstriction (one to three times monthly), alternating with symptom-free periods, without restrictive lung function changes. They did not suffer from chronic infections and used no oral corticosteroids.
Table 1.
Characteristics of the patients and control subjects
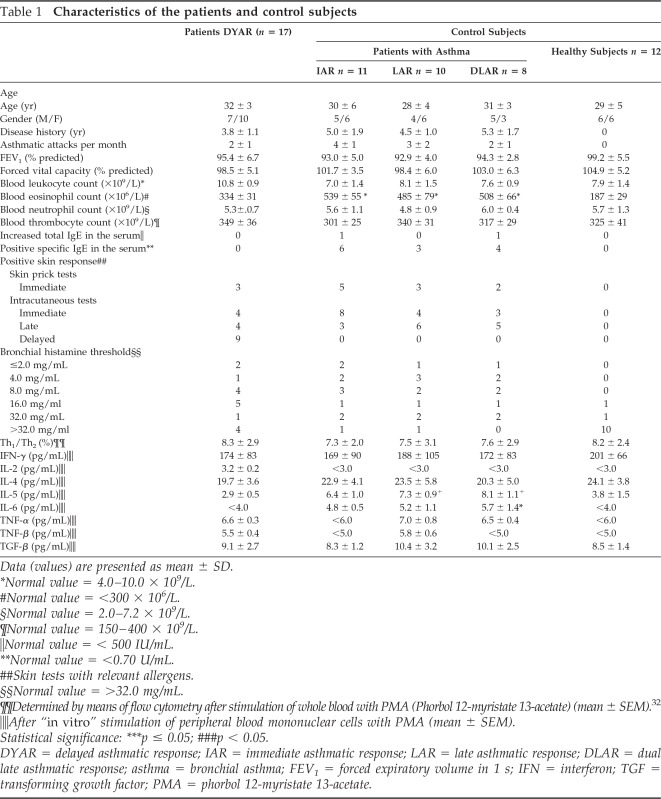
Data (values) are presented as mean ± SD.
*Normal value = 4.0–10.0 × 109/L.
#Normal value = <300 × 106/L.
§Normal value = 2.0–7.2 × 109/L.
¶Normal value = 150–400 × 109/L.
‖Normal value = < 500 IU/mL.
**Normal value = <0.70 U/mL.
##Skin tests with relevant allergens.
§§Normal value = >32.0 mg/mL.
¶¶Determined by means of flow cytometry after stimulation of whole blood with PMA (Phorbol 12-myristate 13-acetate) (mean ± SEM).32
‖‖After “in vitro” stimulation of peripheral blood mononuclear cells with PMA (mean ± SEM).
Statistical significance: ***p ≤ 0.05; ###p < 0.05.
DYAR = delayed asthmatic response; IAR = immediate asthmatic response; LAR = late asthmatic response; DLAR = dual late asthmatic response; asthma = bronchial asthma; FEV1 = forced expiratory volume in 1 s; IFN = interferon; TGF = transforming growth factor; PMA = phorbol 12-myristate 13-acetate.
They were examined by a routine diagnostic procedure serving also as inclusion–exclusion criteria, consisting of (1) general part—disease history, physical examination, basic laboratory tests, x ray of the chest, lung function, blood gases determination, bacteriological examination of the sputum; (2) allergologic part—basic skin tests, bronchial histamine threshold29 and determination of the serum immunoglobulins (Table 1); (3) control BPT with phosphate-buffered saline (PBS); and (4) BPTs with inhalant allergens (n = 38). The allergens for BPT were chosen with respect to the disease history and skin tests (Table 2).
Table 2.
Survey of the allergens causing particular types of asthmatic response
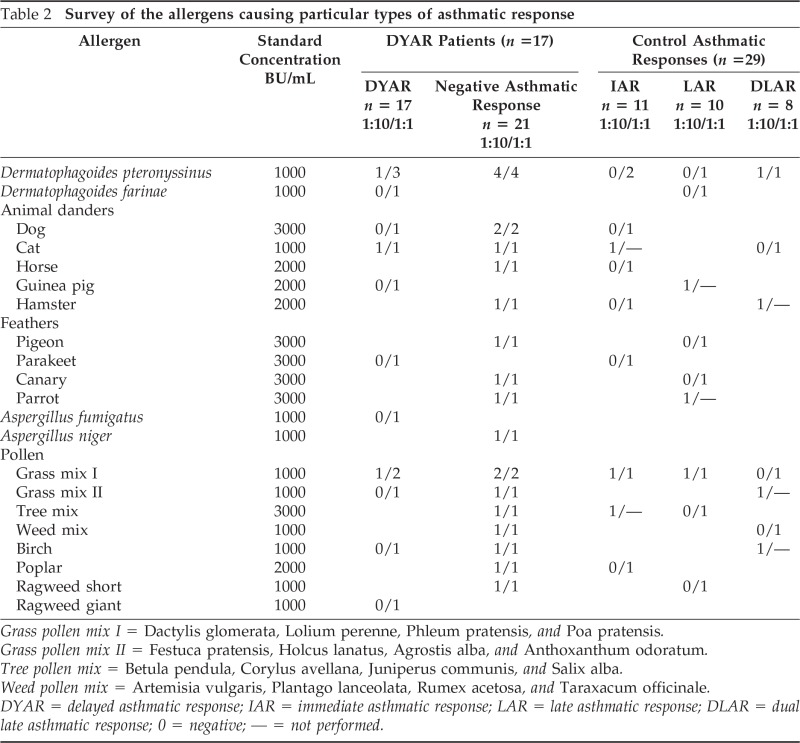
Grass pollen mix I = Dactylis glomerata, Lolium perenne, Phleum pratensis, and Poa pratensis.
Grass pollen mix II = Festuca pratensis, Holcus lanatus, Agrostis alba, and Anthoxanthum odoratum.
Tree pollen mix = Betula pendula, Corylus avellana, Juniperus communis, and Salix alba.
Weed pollen mix = Artemisia vulgaris, Plantago lanceolata, Rumex acetosa, and Taraxacum officinale.
DYAR = delayed asthmatic response; IAR = immediate asthmatic response; LAR = late asthmatic response; DLAR = dual late asthmatic response; 0 = negative; — = not performed.
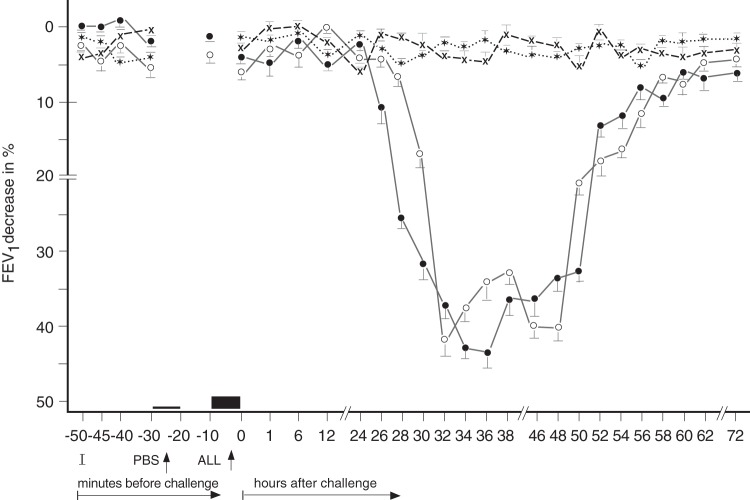
In these 17 patients, developing the DYAR (Fig. 1), the BPTs and the PBS controls were repeated 2–6 weeks later, and combined with recording of various immunologic parameters, including the CD molecules on the blood cells before and at 1, 6, 12, 24, 36, 48, 56 and 72 hours after the allergen challenge (Tables 3 and 4). A 5-day interval was always inserted between the end of the preceding test and the start of the following test, to preclude any carry-over effect. All BPT were performed in a period without manifest bronchial complaints, out of the allergen-related season and during hospitalization of the patients under the standard conditions. Inhaled corticosteroids (n = 5) and long-acting β2-sympathomimetics (n = 4) were withdrawn 4 weeks, disodium cromoglycate (n = 3), nedocromil sodium (n = 5) 2 weeks, while short-acting β2-sympathomimetics and H1 -receptor antagonists 48 hours before every BPT. The decrease in FEV1 of 50% or more, with respect to the predicted values, was treated with a single dose of 200–400 mcg Salbutamol (n = 3).
Figure 1.
Delayed asthmatic response (DYAR) to allergen challenge and phosphate-buffered saline (PBS) control challenge. The mean percentage changes in the forced expiratory volume in 1 secibd (FEV1) values calculated from 17 DYARs and 17 PBS control challenges. ●, the initial DYAR; ○, the repeated DYAR; *, the initial PBS; +, the repeated PBS; I, initial (baseline) values; ALL, allergen challenge; bars, means ± SEM.
Table 3.
Survey of the DYAR-related diagnostic parameters
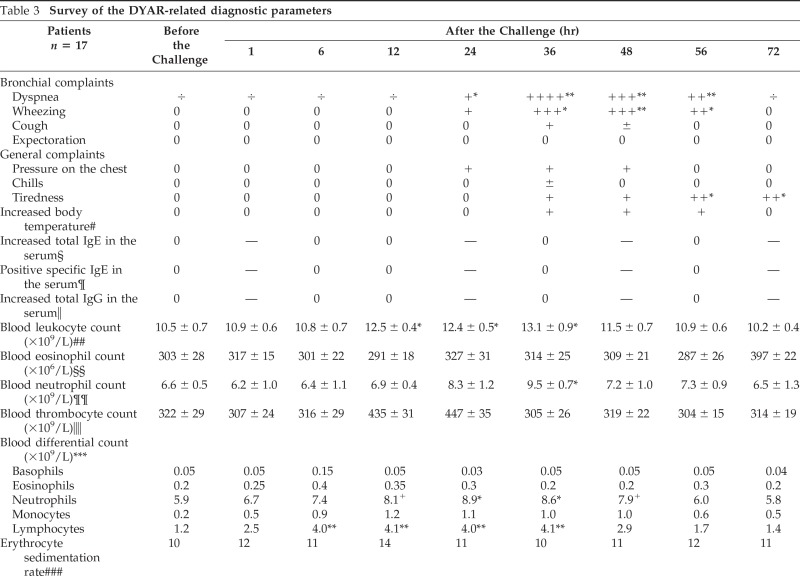
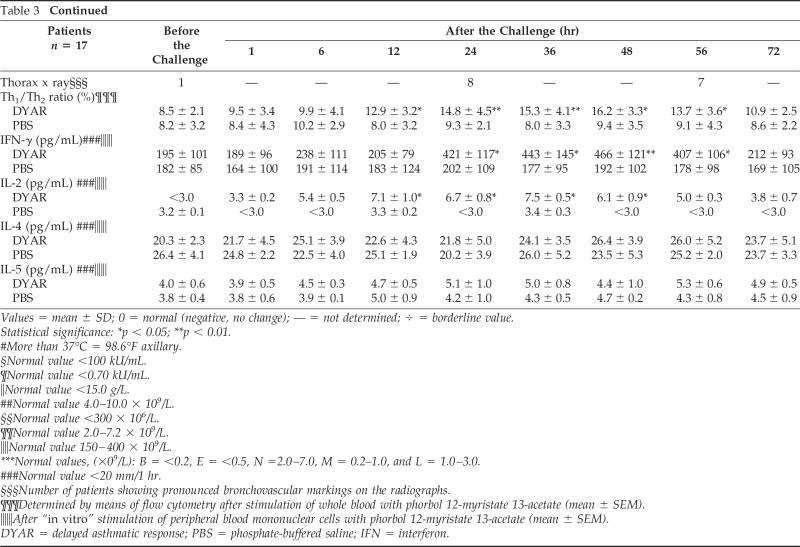
Values = mean ± SD; 0 = normal (negative, no change); — = not determined; ÷ = borderline value.
Statistical significance: *p < 0.05; **p < 0.01.
#More than 37°C = 98.6°F axillary.
§Normal value <100 kU/mL.
¶Normal value <0.70 kU/mL.
‖Normal value <15.0 g/L.
##Normal value 4.0–10.0 × 109/L.
§§Normal value <300 × 106/L.
¶¶Normal value 2.0–7.2 × 109/L.
‖‖Normal value 150–400 × 109/L.
***Normal values, (×09/L): B = <0.2, E = <0.5, N =2.0–7.0, M = 0.2–1.0, and L = 1.0–3.0.
###Normal value <20 mm/1 hr.
§§§Number of patients showing pronounced bronchovascular markings on the radiographs.
¶¶¶Determined by means of flow cytometry after stimulation of whole blood with phorbol 12-myristate 13-acetate (mean ± SEM).
‖‖‖After “in vitro” stimulation of peripheral blood mononuclear cells with phorbol 12-myristate 13-acetate (mean ± SEM).
DYAR = delayed asthmatic response; PBS = phosphate-buffered saline; IFN = interferon.
Table 4.
Significant changes in expression of cellular markers (CD molecules) on granulocytes from peripheral blood before and during DYAR and PBS control tests, in %MFI
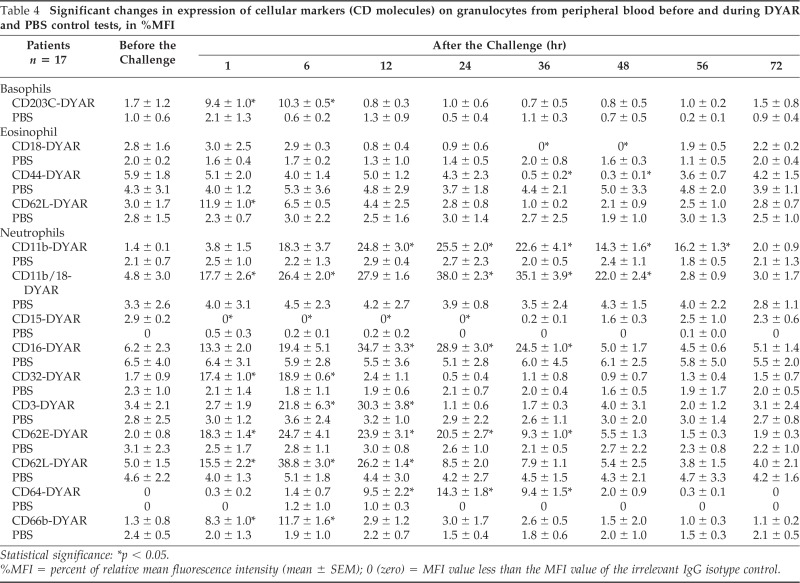
Statistical significance: *p < 0.05.
%MFI = percent of relative mean fluorescence intensity (mean ± SEM); 0 (zero) = MFI value less than the MFI value of the irrelevant IgG isotype control.
Control Groups
The 29 bronchial asthma patients, 11 of them developing an IAR, 10 demonstrating a LAR, and 8 patients developing a DLAR to BPT with various inhalant allergens (Tables 1 and 2), and 12 healthy subjects, being family members of the patients investigated, volunteered to participate as controls in this study (Table 1).
Ethical Committee Approval
The study had been approved by the institutional ethical committee (IRB-MCK) and a written informed consent was obtained from all participants in this study. The study was carried out in accordance with the WMA Declaration of Helsinki concerning the “Principles for medical research involving the human subjects”.
Allergens
Dialyzed and lyophilized allergen extracts (Allergopharma, Reinbek, Germany), diluted in PBS, were used in concentrations of 100–500 BU/mL for skin tests and 1000–3000 BU/mL for BPTs (Table 2). The manufacturer recommended concentrations of 500 BU/mL for the skin tests and up to 5 000 BU/mL for the bronchoprovocation.
Skin Tests
Skin-prick tests with allergens in concentrations of 500 BU/mL were evaluated after 20 minutes. If they were negative, the intracutaneous (i.c.) tests in concentrations of 100–300 BU/mL (our standard concentrations) were then performed. In the case of positive skin-prick tests, the following i.c. tests were performed in a dilution 1:5. The i.c. tests were evaluated 20 minutes and 6, 12, 24, 36, 48, 72, and 96 hours after the injection. A skin wheal (>7.0 mm in diameter) appearing 20 minutes after the injection was considered to be a positive immediate skin response, the skin infiltration observed between 6 and12 hours was considered a late skin response, and the skin induration recorded later than 48 hours was designated a delayed skin response.3,4,22–25
Bronchial Provocation Tests
The BPTs were performed using spirometry (Spirograph D-75; Lode B.V., Groningen, The Netherlands) recording the forced vital capacity and forced expiratory volume in 1 second (FEV1) values.3,4,22–25,29 The aerosols of allergenic extracts and PBS were administered by Wiesbadener Doppel-inhalator at an airflow of 10 L/min. The nebulizer output was 0.12–0.14 mL/min. The aerosol particles had a median mass diameter of 2.8–3.6 μm. The initial BPTs have always been performed with allergenic extracts diluted 1:10 and if they were negative, the BPTs with undiluted extracts (= our standard) followed (Table 2).
The BPTs were performed according to the European standard,29 modified by us, applying the following schedule: (1) baseline values recorded at 0, 5, and 10 minutes; (2) PBS control values recorded at 0, 5, and 10 minutes after a 10-minute inhalation; (3) inhalation of the allergen aerosol for 2 × 5 minutes, with inserted spirometric recording, followed by recording of the spirometric values at 0, 5, 10, 20, 30, 45, 60, 90, and 120 minutes and then every hour up to the 12th hour and every 2nd hour during the 22nd to 38th, the 46th to 56th, and at the 72nd hour.4–6,30–32 The BPTs were evaluated by the following criteria: (1) the FEV1 decrease of <10% with respect to the baseline values as negative, from 10 to 20% as doubtful and of 20% or more as positive; (2) the decrease in FEV1 values recorded at least at three consecutive time intervals was considered to be a positive asthmatic response; (3) the asthmatic response appearing later than 24 hours after the allergen challenge was considered to be a positive DYAR.22,25
Routine Diagnostic Parameters
Various “in vivo” and “in vitro” diagnostic parameters were recorded either singly (Tables 1 and 2) or repeatedly during the DYAR (Table 3). The Th1/Th2 ratio values in peripheral blood were measured by means of flow cytometry and the intracellular cytokines, from the cells stimulated with phorbol 12-myristate 13-acetate, by means of commercial immunoassay (ELISA and EIA) kits, as described previously.22–25
Experimental Parameters
CD Molecules.
The CD molecules were measured by means of fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)-conjugated specific (mouse–anti-human) monoclonal antibodies (MoAb). The antibodies were products either of Becton Dickinson (BD), previously PharMingen (San Diego, CA), Immunotech (IMT, Marseille, France), Ortho Biotech (ORT, Inc., Raritan, NJ), or Caltag Medical System, Ltd. (CAT, Toucester, U.K.). The following MoAbs were used: (1) basophils—FITC-CD32 (BD), FITC-CD45 (BD), FITC-CD62L (BD), PE-CD63 (IMT), FITC-CD69 (IMT), and PE-CD203C (IMT); (2) eosinophils—FITC-CD9 (BD), PE-CD11b (BD), FITC-CD18 (BD), FITC-CD25 (BD), FITC-CD44 (BD), FITC-CD62L (BD), FITC-CD66b {BD), FITC-CD69 (IMT), and FITC-CD86 (CAT); (3) neutrophils—FITC-CD11a (BD), PE-CD11b (BD), FITC-CD11b/18 (BD), FITC-CD15 (BD), FITC-CD16 (BD), FITC- CD18 (BD), FITC-CD32 (BD), FITC-CD35 (BD), biotin-CD62E (BD), FITC-CD62L (BD), FITC-CD64 (BD), and FITC-CD66b (BD); (4) monocytes—FITC-CD14 (BD), FITC-CD16 (BD), FITC-CD64 (BD), FITC-CD80 (BD), and FITC-CD86 (CAT); (5) lymphocytes—PE-CD3 (ORT), FITC-CD4 (BD), PE-CD8 (BD), FITC-CD11a (BD), FITC-CD18 (BD), FITC-CD40 (BD), and FITC-CD69 (IMT); (6) natural killer (NK) cells—FITC-CD16 (BD), PE-CD56 (BD), FITC-CD57 (BD), AND FITC-CD94 (BD); AND (7) thrombocytes—FITC-CD31 (BD), FITC-CD36 (BD), FITC-CD41 (IMT), FITC-CD42b (BD), FITC-CD61 (BD), FITC-CD62P (BD), AND PE-CD63 (IMT).
Separation of Particular Cell Types from Peripheral Blood
Heparinized venous blood (5 mL) was mixed with 0.25 volumes of 6% dextran and erythrocytes were allowed to settle for 30 minutes at room temperature (RT). Aliquots of cell-rich plasma were diluted with Hank's balanced salt solution (HBSS) without Ca++ and Mg++ and particular cell types were isolated by three-step discontinuous centrifugation on Percoll density gradients (Pharmacia, Uppsala, Sweden) and centrifuged at 1000 × g for 20 minutes at 4°C. The cells were separated at the following density degrees: basophils, 1.080 g/mL (1.075–1.081 g/mL); eosinophils, 1.088 g/mL (1.085–1.100 g/mL); neutrophils, 1.090 g/mL (1.080–1.099 g/mL); lymphocytes, 1.077 (1.066–1.077 g/mL); and monocytes, 1.065 g/mL (1.059–1.068 g/mL). The residual erythrocytes were lysed hypotonically. The cell-reach layers were recovered and washed in PBS with 0.5% bovine serum albumin (BSA) and 2 mM of EDTA (pH 7.2).
The cells were additionally purified using immunomagnetic beads, coated with a cocktail of biotin-conjugated MoAb as primary labeling agents (Miltenyi-Biotech isolation kits—human; Miltenyi-Biotech, Bergisch Gladbach, Germany) and anti-biotin MoAb conjugated to MicroBeads as secondary labeling agents (Miltenyi-Biotech) at 4°C for 30 minutes. The positive versus negative selection (depletion) of the cells was performed by magnetic cell sorting using the magnetic cell separator system, including LS column (MACS; Miltenyi-Biotech), according to manufacturers' protocol. The cells were then washed and resuspended in HBSS with 0.2% BSA counted and assessed for purity. The purity of the cell preparations was 96–99% and the viability was 94–97% as measured by trypan blue exclusion. The cells were finally washed in Ca2++- and Mg2++-free HBSS and their count was adjusted at a concentration of 1 × 106 cells/mL. The NK-rich population was additionally separated by four-step discontinuous Percoll gradient centrifugation adjusted to isotonicity by addition of 10%vol/vol 10-fold concentrated HBSS and preparation of four Percoll concentrations (40–50%), varying by 3.5 increments, layered in 15-mL conical tubes (Falcon; BD). After incubation on nylon–wool columns and washing with PBS, resuspension in RPMI-1640 at a concentration of 1 × 106 cells/mL and centrifugation at 400 × g for 30 minutes, the NK cell-rich populations were collected from the second (40:43.5%) the third (43.5:47%), and the fourth (47:54%) interfaces, washed three times in PBS, and again resuspended in RPMI-1640 at a concentration of 1 × 106 cells/mL. The thrombocytes were prepared from separate samples of venous blood collected into EDTA-Vacutainer tubes (BD), fixed with 1% paraformaldehyde in PBS (pH 7.4) at 22°C for 20 minutes and centrifuged at 250 × g for 15 minutes. The platelet-rich plasma was removed and platelets were washed twice in Ca2+- and Mg2+-free PBS, adjusted to 1 × 106/mL, and incubated with saturating concentrations of the FITC-conjugated MoAb. After incubation at 22°C in the dark for 30 minutes, the reactions were stopped by addition of 500 μL of 1% paraformaldehyde in PBS and the samples were analyzed using flow cytometry (BD).
Determination of the CD Molecules by Flow Cytometry
The particular cell preparations, except thrombocytes, were resuspended at a concentration of 1 × 106 cells/mL in 1 mL of RPMI-1640 medium (pH 7.4; Sigma-Aldrich, St. Louis, MO) containing 2 mmol/L of l-glutamine (Gibco, Paisley, U.K.), 100 IU/mLof penicillin, and 100 μg/mL of streptomycin and kept for 10 minutes at 37°C. After addition of 1 μmol/L of ionomycin (Sigma) and 1 μg /mL of Brefeldin A (Sigma), the cell preparations were cultured in the tubes (BD) for 4 hours at 37°C with 5% CO2, centrifuged at 300 × g for 15 minutes at 4°C, washed in PBS containing 0.5% BSA and 0.1% sodium azide, and subsequently resuspended in 500 μL of FACS permeabilizing solution (BD) for 10 minutes at RT in the dark.
The cells were washed twice with PBS and incubated with saturating concentrations (10 μL) of the FITC- or PE- conjugated MoAb directed against the particular CD molecules for 30 minutes in the dark at RT. After the incubation, the cells were washed in PB, resuspended in 500 μL of 1% paraformaldehyde, collected by centrifugation 350 × g for 5 minutes, at 4°C, washed twice with ice-cold PBS, and, finally, centrifuged at 400 × g for 10 minutes at RT. The supernatants were discarded, and the pellets were resuspended in HBSS (pH 7.4) to a final concentration of 1 × 106 cells/mL and stored at 4°C for 12–24 hours until analyzed by flow cytometry. The FITC- and PE-conjugated mouse IgG1 and/or IgG2 MoAb (BD) were used as isotype-specific controls to set the threshold values. The data were generated by the three-color FACS-Calibur flow cytometer (BD), equipped with a 15-mW argon ion laser and appropriate filters for FITC (530 nm; FL-1), PE (585 nm; FL-2), and PerCP (>650 nm; FL-3). The typical forward and side scatter gate for particular cell types together with their logical gates were set and the second gate within this gated area was further selected. The analyses were performed on the cells in the second gate. From each sample, at least 10,000 events have been measured. All determinations, performed in duplicate, were accompanied by parallel incubations with FITC- or PE-conjugated irrelevant antibodies matching the particular antibody isotypes. The results were expressed in percent of the mean relative fluorescence intensity (%MFI), which was actual MFI minus MFI of the irrelevant IgG isotype control.
Statistical Analysis
The initial and repeated DYARs and PBS controls were analyzed by means of fitting polynomials to the mean curves over time, 8 time points within 120 minutes and 20 time points up to 56 hours after the allergen or PBS challenge. The hypotheses were tested by means of the generalized multivariate analysis of variance model.33
The expression changes of CD molecules (ΔMFI%) on particular cell types during the repeated DYARs and PBS controls have also been statistically analyzed by means of the multivariate analysis of variance model method.33 The individual postchallenge expression values of particular CD (in ΔMFI%) recorded at each of the time points during the repeated DYAR and PBS control in each of the patients were compared with their prechallenge (baseline) values and additionally evaluated by Wilcoxon matched-pairs signed-rank test. Moreover, the single postchallenge expression values of particular CD molecules measured at each of the time points during the repeated DYAR in all patients were compared with the corresponding postchallenge PBS values and additionally evaluated by Mann–Whitney U test. A value of p < 0.05 was considered statistically significant for all statistical methods.
RESULTS
Initial and Repeated DYAR
The 17 patients developed 17 positive DYARs (p < 0.01) and 21 negative asthmatic responses (p > 0.1) to 38 BPTs with allergens. The DYAR began within 26–32 hours, reached maximum within 32–48 hours, and resolved within 56 hours (Fig. 1). The DYAR was statistically significant both when compared with the prechallenge FEV1 values (p < 0.01) and with the PBS controls (p < 0.001). No significant differences were found between the initial and the repeated DYAR (p > 0.1; Fig. 1). No significant differences were found in the appearance of the DYAR with respect to the individual allergens (p > 0.2; Table 2).
Association of DYAR with Other Diagnostic Parameters
Patients developing the DYAR showed allergen-relevant positive immediate skin response in 29%, late skin response in 12%, delayed skin response in 59%, and allergen-specific IgE antibodies in the serum in 0% and decreased bronchial histamine threshold in 70% (Table 1). The positive DYAR was accompanied by significantly increased dyspnea, wheezing, and tiredness; increased blood leukocyte, lymphocyte, and neutrophil counts; significant changes in the Th1/Th2 cell ratio in peripheral blood in favor of Th1 cells, and significantly increased intracellular concentrations of interferon (IFN) γ and IL-2 (Table 3).
Expression of CD Molecules (Markers) on the Particular Blood Cell Types during the DYAR
The prechallenge expression of the CD molecules on the particular blood cells in DYAR patients did not differ significantly from their expression on the blood cells in the healthy control subjects (data are not shown).
The repeated DYAR was associated with significant changes (p < 0.05) in the expression of the following CD molecules on the surface of the circulating blood cells (Tables 4 and 5): (a) basophils—increased expression of CD203C at 1 and 6 hours; (b) eosinophils—increased expression of CD25 and CD62L at 1 hour and decreased expression of CD18 and CD44 at 36 and 48 hours; (c) neutrophils—increased expression of CD11b at 6, 12, 24, 36, 48, and 56 hours, CD11b/18 at 1, 6, 12, 24, 36, and 48 hours, CD16 at 6,12, 24, and 36 hours, CD32 at 1 and 6 hours, CD35 at 6 and 12 hours, CD62E at 1, 6, 24, 12, and 36 hours, CD64 at 12, 24, and 36 hours, CD66b at 1 and 6 hours, and decreased expression of CD15 at 1, 6, 12, and 24 hours; (d) monocytes—increased expression of CD14 at 1, 6, 12, 24, and 36 hours, CD16 at 1, 6, 12, 34, and 36 hours, CD64 at 1, 6, and 12 hours, and CD86 at 1 and 6 hours; (e) lymphocytes—increased expression of CD3 at 12, 24, and 36 hours, CD4 at 6, 12, 24, 36, and 48 hours, CD8 at 24, 36, and 48 hours, CD11a at 24 and 36 hours, CD18 at 1 and 6 hours, CD69 at 1, 6, 12, and 24 hours, and decreased expression of CD40 at 1, 6, and 12 hours; (f) NK cells—increased expression of CD16 at 1, 6, and 12 hours, CD56 at 12, 24, and 36 hours, CD57 at 24, 36, and 48 hours, and CD94 at 6 and 12 hours; (g) platelets—increased expression of CD31 at 24 and 36 hours, CD41 at 6 and 12 hours, CD61 at 6 hours, CD62P at 12, 24, and 36 hours, and CD63 at 6 and 12 hours. No significant changes in the expression of the CD molecules were measured on these cells during the PBS control challenges (p > 0.1).
Table 5.
Significant changes in expression of cellular markers (CD molecules) on mononuclear cells and thrombocytes from peripheral blood before and during DYAR and PBS control tests, in %MFI
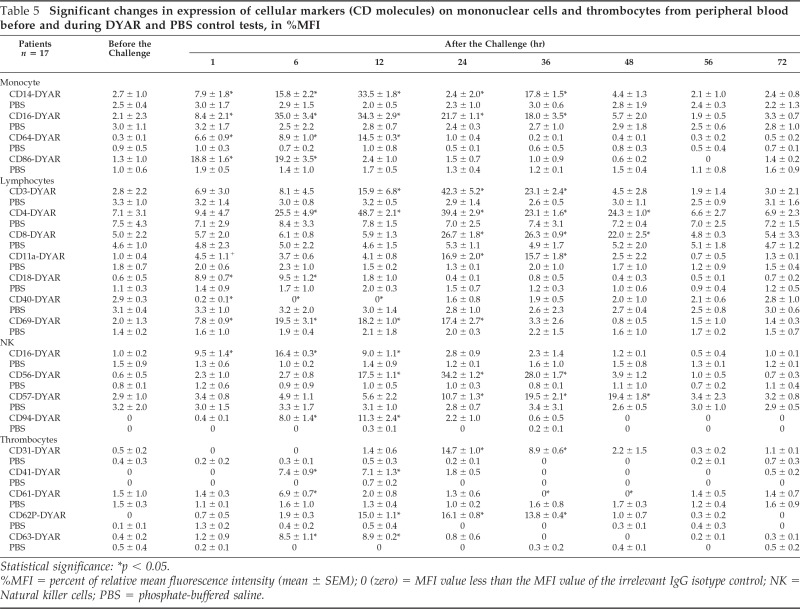
Statistical significance: *p < 0.05.
%MFI = percent of relative mean fluorescence intensity (mean ± SEM); 0 (zero) = MFI value less than the MFI value of the irrelevant IgG isotype control; NK = Natural killer cells; PBS = phosphate-buffered saline.
Control Subjects
No significant differences in the CD molecule expression were recorded on the particular blood cells of the control patients with IAR, LAR, or DAR when compared with the healthy control subjects (p > 0.01, p > 0.01, and p > 0.05, respectively; data are not shown).
DISCUSSION
The DYAR differs from the other asthmatic response types, such as IAR, LAR, and DAR, in the clinical and immunologic features, and presumably, also, in hypersensitivity mechanisms underlying these asthmatic responses.3,4,7,14,19,21–25
The CD molecules are dynamic structures executing manifold regulatory and effector functions and a signal transmission.26,27,30,31,34–36 They act in close relation not only with their ligands, but also with other components of the immunologic system, such as mediators, cytokines, chemokines, and adhesion molecules, with parts of the metabolic system, and with factors released by a variety of cell types.26,27,30,31,34 A relatively high number of various CD molecules can exist on the membrane of one particular cell type, and vice versa a certain type of the CD molecule can be expressed on the surface of various circulating cell types, or cells resident in various types of tissue.31–33 Furthermore, the same CD molecule can execute different functions on the surface of different cell types. Results of this study showed changes in expression of various membrane-bound CD markers on the cells in peripheral blood during the DYAR, such as (a) increased expression on the T-helper (probably Th1) lymphocytes (CD3, CD4, CD8, CD11a, CD18, and CD69), neutrophils (CD11b, CD11b/18, CD16, CD32, CD35, CD62E, CD62L, CD64, and CD66b); monocytes (CD14, CD16, CD64, and CD86), NK cells (CD16, CD56, CD57, and CD94), thrombocytes (CD15, CD41, CD61, CD62P, and CD63), basophils (CD203C), and eosinophils (CD25 and CD62L); (b) decreased expression of the other CD molecules on the eosinophils (CD18 and CD62L), neutrophils (CD15), lymphocytes (CD40), and thrombocytes (CD61). These findings indicate an increased activation of the Th1 and NK cells, neutrophils, monocytes, and thrombocytes during the DYAR.
These results are also consistent with outcomes of our previous studies22–25 concerning the changes of various cells and factors in peripheral blood during the DYAR, such as (1) increased counts of total leukocytes, neutrophils, monocytes, lymphocytes, and Th1 cells22–25; (2) increased plasma/serum concentrations of leukotriene B4 and myeloperoxidase23; (3) increased serum concentration of some soluble adhesion molecules such as soluble intracellular adhesion molecule, soluble vascular adhesion molecule, soluble platelet-endothelial cell adhesion molecule, soluble E-selectin, and soluble L-selectin and decreased serum concentration of soluble E-cadherin24; (4) increased serum concentrations of IL-2, IL-10, IL-12p70, IL-18, granulocyte-colony stimulating factor, IFN-γ, TFN-α, and transforming growth factor β and decreased serum concentration of IL-725; (5) increased concentrations of intracellular IFN-γ, IL-2, IL-10, TNF-α, TNF-β, and transforming growth factor β and changes in the Th1/Th2 ratio in favor of Th1 cells after the “in vitro” stimulation with phorbol 12-myristate 13-acetate.22,23 These data provide evidence for an active involvement of neutrophils, monocytes, thrombocytes, and lymphocytes, especially their Th1 and NK cells in the immunologic mechanism(s) underlying the clinical DYAR.
An increased expression of CD203C on the basophils at 1 and 6 hours and of CD25 and CD62L at 1 hour and decreased expression of CD18 and CD44 at 36 and 48 hours, on the eosinophils, without any significant changes in their counts in the blood, suggest either a limited activation of these cells during the early stages of the DYAR mechanism or their induction by other CD molecules because of a cross-reactivity, or, finally, it may be an artifact. Unfortunately, we have no explanation for this finding. Despite abundant literature, there is a dearth of information concerning the expression of individual CD molecules on the particular cell types and their changes during the individual asthmatic response types. The studies dealing with expression of CD molecules on circulating blood cells in patients with (allergic) bronchial asthma are not numerous.5,12,15,35–40 Moreover, in most of the relevant studies, the expression of a limited number of CD molecules has been measured either on the cellular pool of the whole blood or on the surface of some selected blood cell types, by means of a single determination after a nonspecific “in vitro” stimulation.
The results of this study are not fully comparable with other investigators' data for several reasons. The most important reason is the clinical DYAR itself, studied predominantly by us.22–25 The other reasons can be seen in (a) the existence of various phenotypes of bronchial asthma and various types of asthmatic response to allergen challenge; (b) the difference between a single measurement and the repeated determination of the CD molecules during a particular asthmatic response, generating a dynamic course of such changes; (c) the difference between the “in vivo” exposure with inhaled allergen and the “in vitro” laboratory stimulation of the cells with a nonspecific agent; (d) some technical aspects, such as differences between the fluorescence measured in the whole pooled blood and fluorescence measured on the particular isolated cell types, variations in the methods themselves as well as the commercial antibodies used. Finally, the interpretation of the results may also be an important factor. Is the expression of the particular marker a part of a certain immunologic process, is it a consequence of that process, or is it a combination of both the roles? This crucial question would then lead to a different evaluation of the changes in the CD molecules expression.
Another aspect, which can influence the attained results, is the BPT method.41–44 We have used the inhalational bronchial challenge, whereas a number of other investigators have used the segmental challenge technique.8,9,15,21 Both of the techniques have a number of advantages and disadvantages.3,4,7,9,15,21–25,29,41–44 The advantage of the segmental challenge is the provocation of a limited lung area with a small allergen amount and direct access to the site of the immunologic event. Its disadvantage is an exclusion of the part of the respiratory system (from the mouth to the secondary bronchi) being regularly the site of the initial stage of the immunologic process leading to the asthmatic response. This method is laborious, requires application of anesthetics, and the less natural bronchoalveolar lavage (BAL) technique can be a burden on the patient. The advantage of the inhalational technique is a simulation of the natural allergen exposure on inclusion of all parts of the airways, relatively low strain for the patient, and low risk of postinterventional complications besides the expected bronchoconstriction. Its disadvantage is the need of a rather large effective allergen dose.
These study results are limited to the peripheral blood cells and they can not reflect the topical processes in the bronchial tissue. The CD expression on the surface of Th17 and T-regulatory45 and B lymphocytes, in the peripheral blood as well as cytological and immunologic features in BAL fluid and bronchial mucosa biopsy during the DYAR due to the inhalational challenge with allergens, has not been investigated. These aspects, being a certain shortcoming of this study, should be investigated in the future.
The current results, together with our previous findings,22–25 showed the existence of an asthmatic response phenotype (DYAR), differing essentially from the already established response types, in some patients with allergic bronchial asthma. The DYAR, appearing later than 24 hours after an allergen exposure and lasting up to 48 hours, at least, can not be diagnosed reliably by the skin tests, determination of the IgE antibodies in the serum, eosinophil count in the blood, and/or the bronchial histamine/methacholine threshold. The DYAR can only be confirmed by BPT recorded up to 36–48 hours, at least. According to our preliminary results, the DYAR can only be prevented by inhaled glucocorticosteroid and partly by inhaled nedocromil sodium.46 Nevertheless, further concurrent clinical and immunologic research will be indispensable to clarifying fully the hypersensitivity mechanism(s) underlying the DYAR and to defining its clinical position, significance, and its difference from the other asthmatic response types. In particular, the data indicating and defining the immunologic processes on the topical level in the bronchial tree, gained by means of BAL and by the biopsy of the bronchial mucosa, during the clinical DYAR, will be of a great importance. Another aspect that should also be investigated is the possible development of DYAR after segmental provocation with allergen.
Footnotes
The author has no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Holgate ST, Lemanske RF, O'Byrne PM, et al. Asthma pathogenesis. In Middleton's Allergy, Principles and Practice, 7th ed Adkinson NF, Bochner BS, Busse WW, et al. (Eds). Philadelphia, PA: Mosby- Elsevier, 893–919, 2009 [Google Scholar]
- 2. Christodoulopoulos P, Tulic MK, Kontolemas M, et al. Immunopathology of allergic airway inflammation. In Middletons's Allergy, Principles & Practice, 6th ed Adkinson NF, Yunginger JW, Busse WW, et al. (Eds). St. Louis, MO: Mosby, Inc., 501–514, 2003 [Google Scholar]
- 3. Pelikan Z, Pelikan M, Kruis M, Berger MP. The immediate asthmatic response to allergen challenge. Ann Allergy 56:252–260, 1986 [PubMed] [Google Scholar]
- 4. Pelikan Z, Pelikan-Filipek M. The late asthmatic response to allergen challenge—Part I and II. Ann Allergy 56: 414–420, 421–435, 1986 [PubMed] [Google Scholar]
- 5. Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: Lack of inhibition by prednisolone therapy. Respir Res 7:59–69, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw DE, Berry MA, Hargadon B, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 132:1871–1875, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bentley AM, Kay AB, Durham SR. Human late asthmatic responses. In Allergy and Allergic Diseases. Kay AB. (Ed). Oxford, Berlin, Wien: Blackwell Science Publishing, 1113–1130, 1997 [Google Scholar]
- 8. Liu LY, Mathur SK, Sedgwick JB, et al. Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy 61:589–597, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Becky Kelly EA, Busse WW, Jarjour NN. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol 111:79–86, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: Importance and possible mechanisms. Thorax 57:643–64836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu L, Jarjour NN, Busse WW, Kelly EA. Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma. Am J Respir Crit Care Med 169:1118–1124, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Majori M, Corradi M, Caminati A, et al. Predominant Th1 cytokine pattern in peripheral blood from subjects with chronic obstructive pulmonary disease. J Allergy Clin Immunol 103:458–462, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Tang C, Inman MD, van Rooijen N, et al. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol 166:1471–1481, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Yoshida M, Watson RM, Rerecich T, O'Byrne PM. Different profiles of T-cell IFN-gamma and IL-12 in allergen-induced early and dual responders with asthma. J Allergy Clin Immunol 115:1004–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kelly EA, Rodriguez RR, Busse WW, Jarjour NN. The effect of segmental bronchoprovocation with allergen on airway lymphocyte function. Am J Respir Crit Care Med 156:1421–1428, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Huang TJ, MacAry PA, Eynott P, et al. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol 166:207–217, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Oriss TB, McCarthy SA, Morel BF, et al. Crossregulation between T helper cell (Th)1 and (Th) 2: Inhibition of Th2 proliferation by IFN-gamma involves interference with IL-1. J Immunol 158:3666–3672, 1997 [PubMed] [Google Scholar]
- 18. Borish L, Rosenwasser LJ. Cytokines in allergic inflammation. In Middleton's Allergy, Principles and Practice, 7th ed Adkinson NF, Bochner BS, Busse WW, et al. (Eds). Philadelphia, PA: Mosby-Elsevier, 165–179, 2009 [Google Scholar]
- 19. Matsumoto K, Gauvreau GM, Rerecich T, et al. IL-10 production in circulating T cells differs between allergen-induced isolated early and dual asthmatic responders. J Allergy Clin Immunol 109:281–286, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18, and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10, and IL-13) in patients with allergic asthma. Clin Exp Immunol 125:177–183, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarjour NN, Calhoun WJ, Kelly EA, et al. The immediate and late allergic response to segmental bronchopulmonary provocation in asthma. Am J Respir Crit Care Med 155:1515–1521, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Pelikan Z. Delayed type of asthmatic response to bronchial challenge with allergen. I: Clinical features. Ann Allergy Asthma Immunol 104:394–404, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Pelikan Z. Delayed asthmatic response to bronchial challenge with allergen-mediators, eicosanoids, eosinophil, and neutrophil constituents in the blood and urine. Respiration 82:225–236, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Pelikan Z. Delayed asthmatic response: A new phenotype of bronchial response to allergen challenge and soluble adhesion molecules in the serum. Ann Allergy Asthma Immunol 106:119–130, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Pelikan Z. Delayed type of asthmatic response to allergen challenge and cytokines in the peripheral blood. Respiration 84:385–395, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Chinen J, Fleischer TA, Shearer WT. The immune system: An overview. In Middleton's Allergy, Principles and Practice, 7th ed Adkinson NF, Bochner BS, Busse WW, et al. (Eds). Philadelphia, PA: Mosby-Elsevier, 3–17, 2009 [Google Scholar]
- 27. Zola H, Swart B, Nicholson I, Voss E. In Leukocyte and Stromal Cell Molecules: The CD Markers. Hoboken, NJ: John Wiley & Sons, Inc., 1–581, 2007 [Google Scholar]
- 28. Gomperts BD, Kramer IM, Tatham PER. In Signal Transduction, 2nd ed Burlington, MA: Academic Press/Elsevier, 1–576, 2009 [Google Scholar]
- 29. Melillo G, Bonini S, Cocco G, et al. Provocation tests with allergens. Allergy 52(suppl 35):5–36, 1997 [PubMed] [Google Scholar]
- 30. Delves PJ. In CD Antigens. Hoboken, NJ: John Wiley & Sons, Inc., 2006. (doi: 10.1002/9780470015902.a0000924) [Google Scholar]
- 31. Macey MG. Leukocyte cell surface antigens. In Cytometric Analysis of Cell Phenotype and Function. McCarthy DA, Macey MG. (eds). Cambridge, MA: Cambridge University Press, 315–377, 2000 [Google Scholar]
- 32. Lensmar C, Katchar K, Eklund A, et al. Phenotypic analysis of alveolar macrophages and lymphocytes following allergen inhalation by atopic subjects with mild asthma. Respir Med 100:918–925, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Hand DJ, Taylor CC. In Multivariate Analysis of Variance and Repeated Measures. London: Chapman and Hall, 1–304, 1987 [Google Scholar]
- 34. Barclay AN, Brown MH, Law SKA, et al. The architecture and interaction of leucocyte surface molecules. In The Leucocyte Antigen, 2nd ed Barclay AN, Brown MH, Law SKA, et al. (eds). San Diego, London, Boston, New York, Sydney,Tokyo, Toronto: Academic Press Harcourt Brace and Composition Publishing, 101–129, 1997 [Google Scholar]
- 35. Sklar LA, Tsuji H, Edwards BS, et al. Eosinophil traffic in the circulation following allergen challenge. Allergy 59:596–605, 2004 [DOI] [PubMed] [Google Scholar]
- 36. MacGlashan D., Jr Expression of CD203c and CD63 in human basophil: Relationship to differential regulation of piecemeal and anaphylactic degranulation process. Clin Exp Allergy 40:1365–1377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pacheco KA, Tarkowski M, Klemm J, Rosenwasser LJ. CD49d expression and function on allergen-stimulated T cells from blood and airway. Am J Respir Cell Mol Biol 18:286–293, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Chirumbolo S, Vella A, Ortolani R, et al. Differential response of human basophil activation markers: A multi-parameter flow cytometry approach. Clin Mol Allergy 6:12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sandilands GP, Hauffe B, Loudon E, et al. Detection of cytoplasmic CD antigens within normal human peripheral blood leucocytes. Immunology 108:329–337, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ono E, Taniguchi M, Higashi N, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol 125: 483–489.e3, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Fish JE, Peters SP. Bronchial challenge testing. In Middletons's Allergy, Principles & Practice, 6th ed Adkinson NF, Yunginger JW, Busse WW, et al. (Eds). St. Louis, MO: Mosby, Inc., 657–670, 2003 [Google Scholar]
- 42. Busse WW, Wanner A, Adams K, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med 172:807–816, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Metzger WJ, Zavala D, Richerson HB, et al. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis 135:433–440, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Terashima T, Amakawa K, Matsumaru A, et al. BAL induces an increase in peripheral blood neutrophils and cytokine levels in healthy volunteers and patients with pneumonia. Chest 119:1724–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Kinoshita T, Baatjes A, Smith SG, et al. Natural regulatory T cells in isolated early responders compared with dual responders with allergic asthma. J Allergy Clin Immunol 133:696–703, 2014 [DOI] [PubMed] [Google Scholar]
- 46. Pelikan Z, Pelikan-Filipek M, Oostenbrink JH. Delayed asthmatic response (DYAR), its clinical features and pharmacologic modulation. J Allergy Clin Immunol 99:321, 1997. (Abs 1313) [Google Scholar]