Abstract
No treatment is currently available for mucopolysaccharidosis (MPS) IIIB, a neuropathic lysosomal storage disease caused by autosomal recessive defect in α-N-acetylglucosaminidase (NAGLU). In anticipation of a clinical gene therapy treatment for MPS IIIB in humans, we tested the rAAV9-CMV-hNAGLU vector administration to cynomolgus monkeys (n=8) at 1E13 vg/kg or 2E13 vg/kg via intravenous injection. No adverse events or detectable toxicity occurred over a 6-month period. Gene delivery resulted in persistent global central nervous system and broad somatic transduction, with NAGLU activity detected at 2.9–12-fold above endogenous levels in somatic tissues and 1.3–3-fold above endogenous levels in the brain. Secreted rNAGLU was detected in serum. Low levels of preexisting anti-AAV9 antibodies (Abs) did not diminish vector transduction. Importantly, high-level preexisting anti-AAV9 Abs lead to reduced transduction in liver and other somatic tissues, but had no detectable impact on transgene expression in the brain. Enzyme-linked immunoabsorbent assay showed Ab responses to both AAV9 and rNAGLU in treated animals. Serum anti-hNAGLU Abs, but not anti-AAV9 Abs, correlated with the loss of circulating rNAGLU enzyme. However, serum Abs did not affect tissue rNAGLU activity levels. Weekly or monthly peripheral blood interferon-γ enzyme-linked immunospot assays detected a CD4+ T-cell (Th-1) response to rNAGLU only at 4 weeks postinjection in one treated subject, without observable correlation to tissue transduction levels. The treatment did not result in detectable CTL responses to either AAV9 or rNAGLU. Our data demonstrate an effective and safe profile for systemic rAAV9-hNAGLU vector delivery in nonhuman primates, supporting its clinical potential in humans.
Introduction
Mucopolysaccharidosis (MPS) IIIB is a devastating lysosomal storage disease (LSD) caused by mutations in the gene coding for a lysosomal enzyme, α-N-acetylglucosaminidase (NAGLU) (Neufeld and Muenzer, 2001). The lack of NAGLU activity disrupts the stepwise metabolic process for degradation of heparin sulfates, a class of biologically important glycosaminoglycan. This metabolic impairment leads to the accumulation of nondegraded or partially degraded heparan sulfate oligosaccharides in lysosomes in cells of virtually all organs. Cells throughout the central nervous system (CNS) are particularly affected, including both neuronal and nonneuronal cells, resulting in complex secondary neuropathology (Li et al., 1999, 2002; Ohmi et al., 2003, 2009; McGlynn et al., 2004; Ryazantsev et al., 2007; Villani et al., 2007, 2009; DiRosario et al., 2009). Infants with MPS IIIB appear normal at birth, but develop progressive neurological manifestations that lead to premature death (Weber et al., 1999; Yogalingam et al., 2000; Neufeld and Muenzer, 2001). Somatic manifestations of MPS IIIB occur in all patients, and involve virtually all organs, although mild relative to other forms of MPS, such as MPS I, II, and VII.
No definite treatment is currently available for MPS IIIB, and therapies have been limited to palliative care (Rohrbach and Clarke, 2007). The biggest challenge in therapeutic development has been the effect of the blood–brain barrier (BBB) that precludes effective CNS access to the global neuropathology of the disease (Pardridge, 2002).
Gene therapy shows promise for LSDs because of the by-stander effect of secreted lysosomal enzymes, including NAGLU, which reduces the demands for efficient gene transfer (Neufeld and Muenzer, 2001; Sands and Haskins, 2008). The adeno-associated virus (AAV) vector system offers important advantages as a gene delivery tool for treating a great variety of diseases, including a broad tissue tropism and absence of known pathogenesis in humans (Berns and Linden, 1995). Recombinant AAV (rAAV) vectors based on AAV serotype 2 (AAV2) have been shown to transduce both neuronal and nonneuronal cells in the CNS in numerous gene therapy studies, with demonstrated therapeutic benefits in treating neurological diseases (Daya and Berns, 2008), including MPS and other LSDs, in animal models (Fu et al., 2002, 2003, 2007, 2011; Heuer et al., 2002; Desmaris et al., 2004; Liu et al., 2005; Fraldi et al., 2007; Sands and Haskins, 2008; McCarty et al., 2009; Baek et al., 2010; Heldermon et al., 2010; Ruzo et al., 2012). Multiple phase I clinical trials have been completed using rAAV gene delivery approaches in patients with neurological disorders (McPhee et al., 2006; Kaplitt et al., 2007; Worgall et al., 2008; Marks et al., 2010; Mittermeyer et al., 2012).
The characterization of novel AAV serotypes, including the recently recognized trans-BBB neurotropic AAV serotype 9 (AAV9) (Duque et al., 2009; Foust et al., 2009; Zincarelli et al., 2008), has provided invaluable tools for efficient CNS gene delivery to treat neurological diseases, especially diseases such as MPS IIIB with global CNS manifestations and somatic involvement (Foust et al., 2010; Fu et al., 2011). Previously, we treated adult MPS IIIB mice with a single systemic rAAV9 vector injection and successfully achieved permanent restoration of NAGLU activity in the CNS, peripheral nervous system (PNS), and broad somatic tissues, leading to functional correction of neurological disorders and normalized survival (Fu et al., 2011). These results strongly support the translation of this approach to the treatment MPS IIIB in patients.
In this study, we tested our therapeutic vector product, rAAV9-CMV-hNAGLU, in sexually immature and adult nonhuman primates (NHP) by intravenous (IV) injection to assess the feasibility and safety of the approach for clinical application in humans. The treatment reproduced global CNS and widespread somatic transduction, as observed in MPS IIIB mice, with no detectable toxicity in NHP, supporting the clinical potential of this gene therapy approach.
Materials and Methods
rAAV viral vectors
A previously described rAAV vector plasmid (Fu et al., 2011) was used to produce the conventional single-strand rAAV9-CMV-hNAGLU viral vector. The vector genomes contained minimal elements required for transgene expression, including AAV2 terminal repeats, a human cytomegalovirus (CMV) immediate-early promoter, SV40 splice donor/acceptor signal, a human NAGLU (hNAGLU) coding sequence, and polyadenylation signal. The rAAV9 viral vector was produced in 293 cells by transient transfection and purified by banding twice on CsCl density gradients under non-GLP conditions. The vector was dialyzed twice against phosphate-buffered saline, pH 7.4 containing 0.001% pluronic F68 (Gibco). The purified viral vectors were titrated using quantitative real-time PCR (qPCR) and confirmed by dot blotting. The vector was tested for potency by IV injection into MPS mice and assay of vector genome copies in liver by qPCR.
Animals
Animal procedures were approved by the Institutional Animal Care and Use Committee at the Research Institute at Nationwide Children's Hospital (NCH-RI). All animals were housed and cared for at Nationwide Children's Hospital, in accordance with the Guide for the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 85-23]. Ten Macaca cynomolgus were used in this study (Table 1). Before the experiments, the animals were screened for preexisting antibodies (Abs) to AAV9 capsid in the serum, using enzyme-linked immunoabsorbent assay (ELISA). The experiments were not conducted in full compliance with Good Laboratory Practice.
Table 1.
Systemic Delivery of rAAV9-CMV-hNAGLU in Nonhuman Primates
| NHP | Age (years) | AAV9 Abs | hNAGLU Abs | IS | Vector dose (vg/kg) | Blood test | CSF | Termination (pi) |
|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||
| NT1 | 10.3 | 1:64 | 1:100 | − | Saline | Weekly/monthly | + | 6 weeks |
| S1-V | 10.5 | 1:32 | 1:50 | − | 1E13 | Weekly/monthly | + | 6 weeks |
| S2-V | 11.0 | − | − | − | 1E13 | Weekly/monthly | + | 3 months |
| Group 2 | ||||||||
| NT-2 | 2.3 | − | 1:50 | − | Saline | Monthly | + | 3 months |
| M1-V | 2.3 | − | − | − | 2E13 | Monthly | + | 3 months |
| M2-V | 2.1 | − | 1:50 | − | 2E13 | Monthly | + | 3 months |
| M3-V+P | 2.0 | 1:16 | 1:50 | + | 2E13 | Monthly | + | 3 months |
| M4-V | 1.9 | 1:4 | 1:100 | − | 2E13 | Monthly | − | 6 months |
| M5-V+P | 1.8 | 1:32 | 1:100 | − | 2E13 | Monthly | + | 6 months |
| M6-V | 2.3 | 1:1000 | − | + | 2E13 | Monthly | + | 6 months |
AAV, adeno-associated virus; Abs, preexisting antibodies; CSF, cerebrospinal fluid; IS, immunosuppression with prednisolone; NHP, nonhuman primates; NT, nontreated controls; pi, postinjection; V, treated with rAAV9 vector only; V+P, treated with rAAV9 vector and prednisolone.
IV vector delivery
For vector delivery, veterinary staff anesthetized the subjects by an intramuscular injection of Telazol (6 mg/kg). The subjects were then treated by an IV injection of either 1E13 or 2E13 vg/kg of rAAV9-CMV-hNAGLU vector (in saline, 5 ml) or 5 ml saline alone (nontreated controls) via cephalic vein. Upon recovery, the subjects were returned to their housing and were cared for the duration of the experiments.
Two of the subjects treated with 2E13 vg/kg vector also received immunosuppression treatment. These subjects were given daily oral administration of 0.5 mg/kg prednisolone (NDC60432-212-08; Morton Grove Pharmaceutical) for 2 weeks, beginning 1 week before the vector injection, and an IV injection of 4 mg/kg methylprednisolone (NDC0009-0039-30; Pfizer) at 24 and 4 hr before, as well as 24 hr after, the vector injection.
Posttreatment monitoring
After injection, the subjects were observed daily for their well-being and behavior throughout the duration of the experiments.
Blood and tissue analyses
Blood draws were performed before vector injection, and weekly and/or monthly postinjection (pi). The subjects were terminated at 6 weeks, 3 months, or 6 months pi. The veterinary staff euthanized the subjects by an IV injection of pentobarbital (50 mg/kg). Cerebrospinal fluid (CSF) was collected by lumbar puncture. Brain, spinal cord, dorsal root ganglion (DRG), and multiple somatic tissues (liver, kidney, spleen, heart, lung, intestines, stomach, pancreas, skeletal muscles, testis, lymph nodes) were harvested either on dry ice and stored at −80°C, or in 4% paraformaldehyde at 4°C. Each brain was divided into two hemispheres along the midline and then into multiple coronal slabs. Each slab from one sphere was further divided into matrices with 12–14 sections and each section was harvested on dry ice and stored at −80°C. Brain slabs from another sphere were stored in 4% paraformaldehyde for immunofluorescence (IF) assays and hematoxylin and eosin staining.
Blood chemistry and hematology
Blood samples were processed for blood chemistry and hematology by the Child Laboratory at Nationwide Children's Hospital.
ELISA for Ab responses to rAAV9 vector and rNAGLU
Serum samples were assayed by ELISA for Abs to AAV9 or rNAGLU, using the purified rAAV9 vector or full-length hNAGLU protein as antigens (ag). Briefly, 1E10 vg/ml of the rAAV9 vector or 20 μg/ml of the full-length hNAGLU protein in carbonate coating buffer was applied to 96-well plates and incubated over night at 4°C. The plate was then washed and blocked for 1 hr with blocking buffer (5% milk in PBS containing 0.1% Tween-20 [PBS-T]). Serially diluted serum samples in blocking buffer were added to the plates and incubated at room temperature for 1 hr. The plates were washed with PBS-T and then incubated with horseradish peroxidase-conjugated antimonkey IgG (Sigma-Aldrich) for 1 hr at room temperature. After being washed with PBS-T, the plates were then developed with 3,3′,5,5′-tetramethylbenzidine (TMB) at room temperature for 5 min. The reaction was stopped by adding 1 N sulfuric acid. The absorbance was read at 650 nm on a plate reader. Data were analyzed as follows: (OD450-ag+ − OD450-ag−)/OD450-ag−. Values ≥2 were considered to be Ab positive.
Interferon-γ enzyme-linked immunospot assay
Peripheral blood white cells (PBWC) and liver T cell responses to AAV9 and hNAGLU were quantified by interferon-γ enzyme-linked immunospot (IFN-γ ELISPOT) assay, following previously published procedures (Mendell et al., 2009). Briefly, 106 PBMC or monocytes from the liver isolated on Ficoll-Hypaque gradients were cultured with synthetic overlapping peptides (18 amino acids each in length, overlapping by 11 residues) covering the full-length AAV9 capsid protein or human NAGLU protein. Each peptide was dissolved in dimethyl sulfoxide (DMSO). Peptides of each protein were organized into three pools. After incubation at 37°C for 36 hr, the plates were developed according to the manufacture's instruction, and the IFN-γ spot-forming cells (SFC) were counted. DMSO was used as negative control and concanavalin A was used as positive control. Fewer than 10 SFC/well were observed from negative control (DMSO). Responses were considered positive when SFC exceeded 50 per 106 PBMC in duplicate wells. To identify the target peptide, peptide pools that exhibited IFN-γ activity were subdivided for further ELISPOT assay with each peptide present in two of the intersecting mapping subpools.
Flow cytometry
IFN-γ-ELISPOT-positive cells were further analyzed by flow cytometry for CD4, CD8, and intracellular IFN- γ to determine the phenotype of activated T cells.
NAGLU activity assay
Tissue, serum, and CSF samples were assayed for NAGLU enzyme activity following a published procedure with minor modifications (Thompson and Nowakowski, 1991; Li et al., 1999). The assay measures 4-methylumbelliferone (4MU), a fluorescent product formed by hydrolysis of the substrate 4-methylumbellireyl-N-acetyl-α-D-glucosaminide. Tissue NAGLU activity is expressed as unit/mg protein. Serum or CSF NAGLU activity is expressed as units/ml, and 1 unit is equal to 1 nmol of 4MU released/hr at 37°C.
Immunofluorescence
Tissues were processed to obtain paraffin sections (4 μm). The tissue sections were then assayed by IF to identify cells expressing rNaGlu, using Abs against hNAGLU (a kind gift from Dr. E.F. Neufeld, UCLA), and a secondary Ab conjugated with AlexaFluor568 (Millipore), following procedures recommended by the manufacturers. The sections were visualized under a fluorescence microscope.
Quantitative real-time PCR
Total DNA was isolated from tissue samples of treated and nontreated NHP subjects using Qiagen DNeasy columns. Brain DNA was isolated from cerebral cortex, hippocampus, and hypothalamus. The DNA samples were analyzed by qPCR, using Absolute Blue QPCR Mix (Thermo Scientific) and Applied Biosystems 7000 Real-Time PCR System, following the procedures recommended by the manufacturer. TaqMan primers specific for the CMV promoter were used to detect rAAV vector genomes: forward, GGCAGTACATC AAGTGTATC; reverse, ACCAATGGTAATAGCGATG AC; probe, [6∼FAM]AATGACGGT AAATGGCCCGC[TAMRA∼6∼FAM]. Genomic DNA was quantified in parallel samples using β-actin-specific primers: forward, CCTTCCGTCCTCAGAT CATT; reverse, CTTGCTGATCCACATCTGCT; probe, [6∼FAM] CATCCTGGCCTCGCTG CCA[Tamra∼Q]. Genomic DNA from nontreated NHP tissues was used as controls for background levels and absence of contamination.
Histopathology
All collected tissues were processed to produce paraffin sections (4 μm) and then stained with hematoxylin and eosin by the Morphology Core at NCH-RI. The sections were examined in an uncoded (unblinded) fashion, by a board-certified veterinary pathologist from the Comparative Pathology and Mouse Phenotyping Shared Resource at The Ohio State University. Microscopic findings were scored semiquantitatively using a tiered scale: within normal limits, or minimal, mild, moderate, or marked changes.
Statistical analyses
Means, standard deviation (SD), and unpaired Student's t-test were used to analyze brain NAGLU activity data from different brain areas. The significance level was set at p≤0.05.
Results
In anticipation of clinical applications for systemic rAAV9-hNAGLU gene therapy, we tested our therapeutic rAAV9-CMV-hNAGLU vector in two groups of male cynomolgus monkeys (M. cynomolgus) (Table 1) to further assess safety and feasibility. The animals were screened by ELISA for preexisting Abs (total IgG) against AAV9. In general, an AAV Ab titer <1:50 was considered negative. Group 1 (Table 1) included three adult (10–11-year-old) animals. Two animals had either no (S2) or low (S1, 1:32) titer of preexisting AAV9 Ab, and were treated with an IV infusion of 1E13 vg/kg rAAV9-hNAGLU vector. Group 2 (Table 1) included 7 sexually immature (1.8–2.3-year-old) subjects. Four subjects (M1, M2, M4, M6) were treated with 2E13 vg/kg rAAV9-hNAGLU vector alone. Two animals were treated with 2E13 vg/kg vector and also immunosuppression with prednisone, to assess whether the immunosuppression regimen can either enhance transduction or affect safety. The immunosuppression animals were administered daily oral prednisolone (0.75 g/kg/day) for 2 weeks, starting 1 week before vector injection, and acute suppression with an IV infusion of methylprednisolone (4 mg/kg) at 24 and 4 hr before, and 24 hr after, vector injection. To assess whether preexisting anti-AAV9 Abs exacerbate toxicity, an animal with high-level AAV9 Ab (M6) was included in this study. Two subjects [NT-1 (adult) and NT-2 (immature)] were used as nontreated controls (Table 1).
The animals were observed daily for well-being and behavior after the vector treatments. Blood samples from all subjects were assayed weekly and/or monthly for blood chemistry, hematology, secreted rNAGLU, and T-cell and Ab responses. The subjects were terminated at different time points (Table 1) for tissue, blood, and CSF analyses for transgene expression, secretion, vector biodistribution, and immune responses against the vector and transgene product.
Systemic rAAV9-CMV-hNAGLU vector delivery did not lead to observable adverse effects in NHP
Daily observation for the entire 6 months of the experiments demonstrated no abnormalities in either the rAAV9-treated subjects or the nontreated controls, suggesting that administration of the NAGLU-containing vector posed essentially no risk of severe adverse effects for general health or neural function.
Systemic infusion of the rAAV9-CMV-hNAGLU vector did not induce detectable evidence of toxicity in NHP
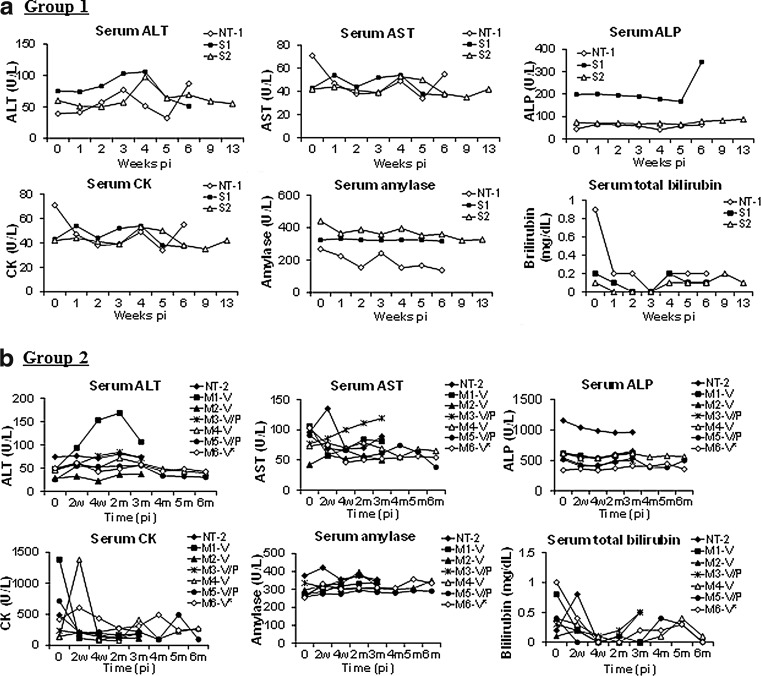
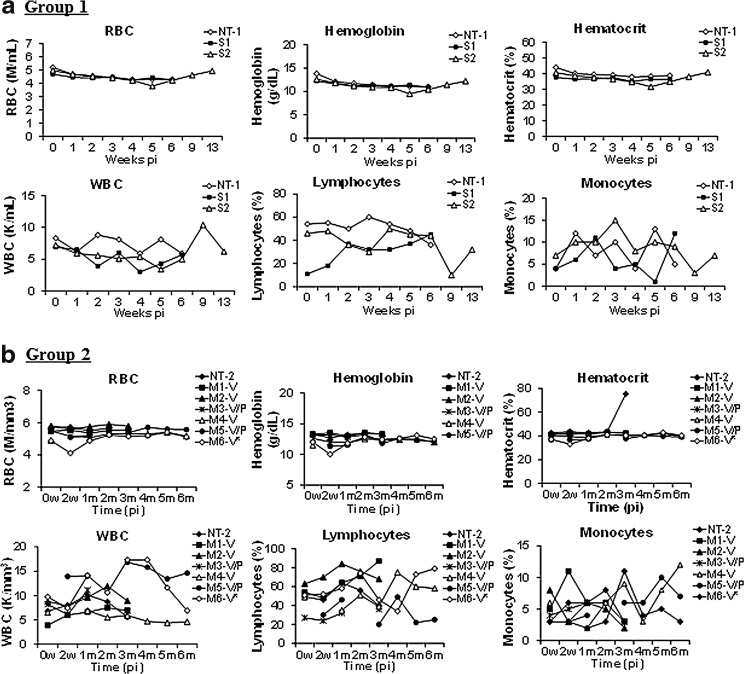
Peripheral blood samples from the rAAV9-treated and nontreated animals were assayed for alterations in blood chemistry and hematology weekly for 6 weeks, and then at 2 and 3 months pi, to assess the potential toxicity of the gene replacement approach. The results showed no specific changes in either blood chemistry (Fig. 1) or hematology (Fig. 2) analytes at the times of testing. Histopathologic examination demonstrated no treatment-related lesions in organs of major systems—cardiovascular, digestive (including liver), lymphoid, nervous (CNS [brain and spinal cord] and PNS [DRG, nerve] and sensory organs [eye]), pulmonary, reproductive, and urinary—and selected organs (skeletal muscle, thyroid gland), indicating that the vector treatment induced no systemic toxicity. These data support the safety of IV injection of the rAAV9-CMV-hNAGLU vector as a potential strategy for gene therapy in human patients.
FIG. 1.
No abnormal changes in blood chemistry in NHP after a systemic rAAV9-hNAGLU vector delivery. NHP were treated with an IV injection of 1E13 vg/kg (a, Group 1, adult) or 2E13 vg/kg (b, Group 2, sexually immature) rAAV9-CMV-hNAGLU vector. Blood samples were assayed for blood chemistry panel before and after the vector delivery. AAV, adeno-associated virus; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate Aminotransferase; CK, creatine kinase; IV, intravenous; NHP, nonhuman primates; NT, nontreated controls; V, treated with vector only; V+P, treated with vector and immunosuppression.
FIG. 2.
No abnormal changes in hematology in NHP receiving a systemic rAAV9-hNAGLU vector delivery. (a) Group 1: 1E13 vg/kg group. (b) Group 2: 2E13 vg/kg group. Blood samples were assayed for hematology before and after the vector delivery. *Subject with high preexisting anti-AAV9 antibodies. RBC, red blood cell count; WBC, white blood cell count.
Global CNS and widespread differential somatic rAAV9-mediated expression of rNAGLU
Brain, spinal cord, and multiple somatic tissues were analyzed at 6 weeks (NT-1, S1), 3 months (S2, NT-2, M1, M2, M3), or 6 months pi (M4, M5, M6) for NAGLU enzymatic activity and/or IF for hNAGLU to determine the level and distribution of rAAV9-mediated transgene expression.
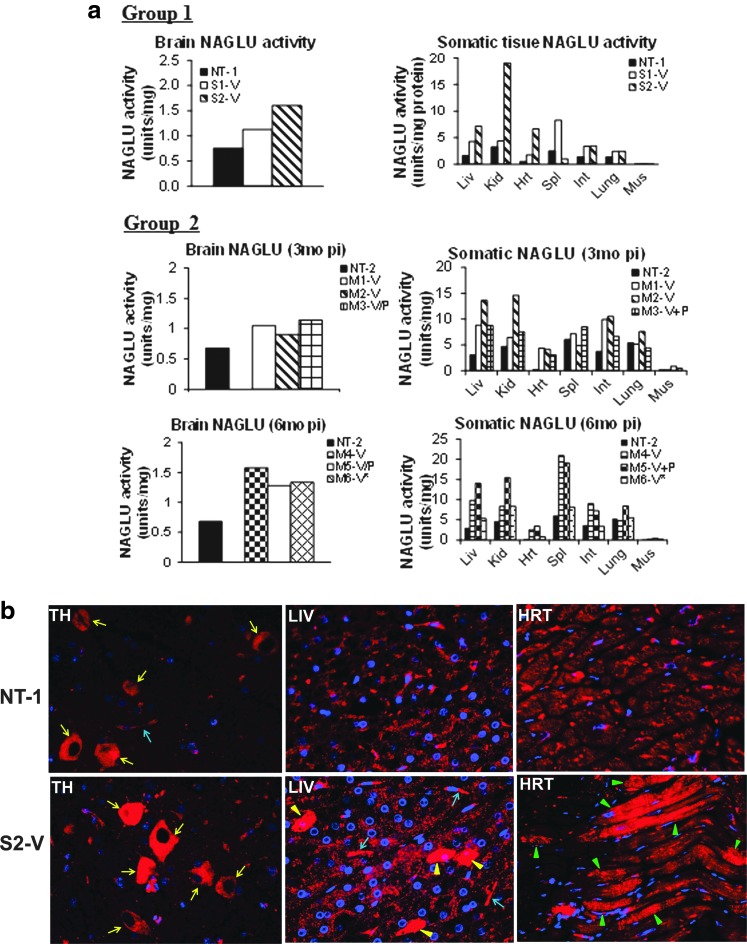
We observed evidence of increased NAGLU enzymatic activity above normal levels in the brain and multiple somatic tissues in rAAV9-treated subjects compared with nontreated controls (Fig. 3). This suggested transduction in both the CNS and somatic system mediated by the systemically delivered rAAV9 vector. While we observed individual variations in tissue NAGLU activity among rAAV9-treated animals (Fig. 3), there was no evident correlation between tissue NAGLU activity levels and low levels of preexisting anti-AAV9 Ab or immunosuppression treatment (Fig. 3). Surprisingly, in subject M6, with high serum anti-AAV9 Ab before vector treatment (Fig. 3a), we detected a two-fold higher than normal level of NAGLU activity in the brain, which was second highest among the rAAV9-treated animals in Group 2. However, NAGLU activities in somatic tissues from this animal were the lowest in the group (Fig. 3a). These results suggest that rAAV9-CNS entry may be somewhat resistant to Ab neutralization. In addition, unlike our previous observations in MPS IIIB mice, we detected high NAGLU activity in kidneys and relatively less NAGLU activity in heart and skeletal muscle in rAAV9-treated subjects compared with controls (Fig. 3a), suggesting possible species-specific differences in tissue tropism of the AAV9 vector.
FIG. 3.
Effective CNS and widespread somatic transduction in NHP after a systemic rAAV9-hNAGLU gene delivery. (a) Tissue NAGLU activity. NAGLU activity is expressed as units/mg protein, 1 unit=release of 1 nmol 4MU/hr. Group 1: treated with 1E13 vg/kg; Group 2: treated with 2E13 vg/kg. (b) Immunofluorescence detection for rNAGLU using a polyclonal Ab against hNAGLU. Red fluorescence indicates NAGLU-positive cells/signals. Yellow arrows indicate neurons; blue arrows, blood vessels; yellow arrowheads, NAGLU-overexpressing hepatocytes; green arrowheads, NAGLU-overexpressing cardiomyocytes. *Subject with high preexisting anti-AAV9 antibodies. CNS, central nervous system; HRT, heart; LIV, liver; TH, thalamus (brain).
Overexpression of rNAGLU was detected by IF staining in individual neurons and endothelial cells throughout the brains and spinal cord of rAAV9-treated subjects compared with nontreated control animal NT-1 (Fig. 3b and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/humc). These data indicated that the systemically delivered rAAV9 vector was able to cross the BBB in normal NHP. In general, the rNAGLU overexpression appeared to be localized in cytoplasmic granules, suggesting lysosomal targeting of the gene product. Because of the cross-reactivity of the NAGLU Ab with endogenous enzyme, we were unable to clearly demonstrate rNAGLU expression in other cell types in the CNS.
IF staining also indicated the overexpression of NAGLU in satellite cells (glia) in DRG from rAAV9-treated subjects (data not shown), suggesting rAAV9-mediated DRG transduction. However, we were unable to clearly distinguish rNAGLU from endogenous enzyme expression in DRG neurons, again, because of NAGLU Ab cross-reactivity. In addition, we observed an increase in NAGLU staining in neurons in myenteric plexus (Supplementary Fig. S2a) and submucosal plexus (data not shown) in the digestive tract, including stomach, duodenum, jejunum, ileum, and colon in treated animals. These data support widespread PNS targeting by AAV9 in NHP, as we had previously observed in MPS IIIB mice.
NAGLU overexpression was also detected by IF in multiple somatic tissues (Fig. 3b). We observed increased NAGLU signals in hepatocytes, cardiomyocytes (Fig. 3b), cuboidal epithelial cells of distal convoluted tubules in the kidney, retinal pigment epithelium in the eye (Supplementary Fig. S2b), and follicular cells in the thyroid gland (data not shown). The NAGLU-positive signals are in fine granules in a majority of hepatocytes in the liver of treated subjects (Fig. 3b), though extensive NAGLU signals were only observed in <10% of hepatocytes. The systemic rAAV9 delivery also resulted in observable transduction in endothelial cells in the liver (Fig. 3b), heart, intestine, and pancreas (data not shown). Again, the NAGLU overexpression appeared to be localized in granules, supporting lysosomal targeting of rNAGLU.
Secretion of rNAGLU: NAGLU activity in the serum and CSF
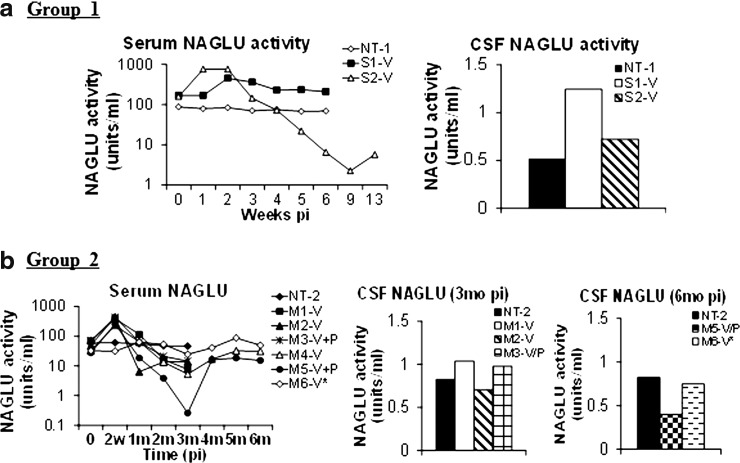
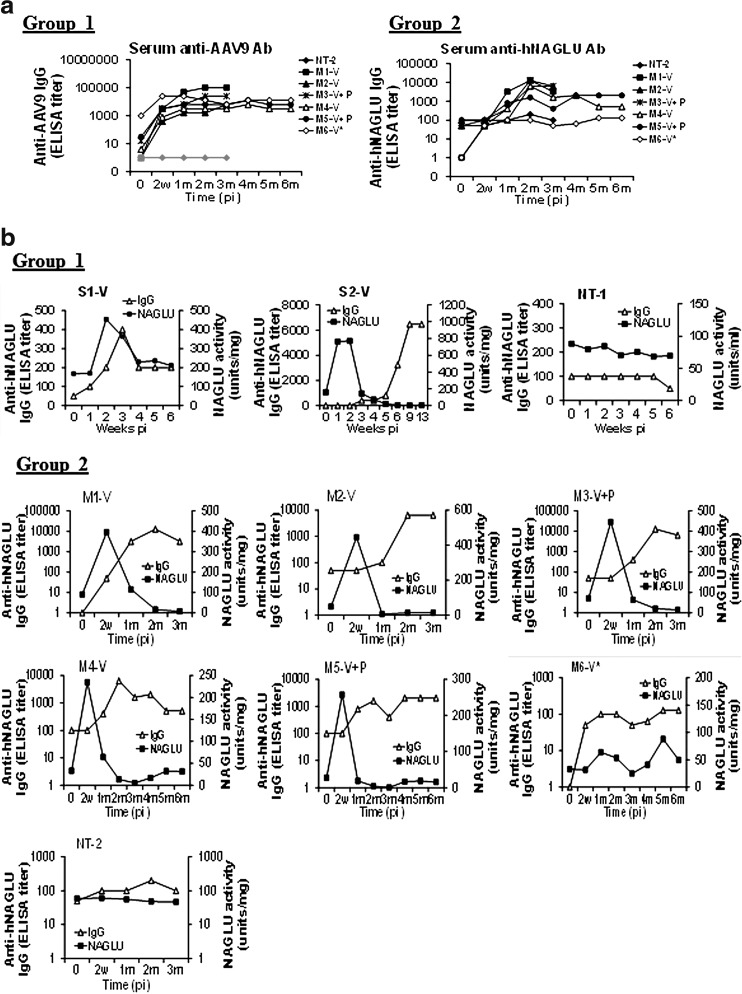
Serum and CSF samples were assayed for NAGLU activity to determine whether secreted recombinant enzyme could be detected. Results showed a rapid 3–5-fold (Group 1) or 6–9-fold (Group 2) increase in serum NAGLU activity that peaked within 2 weeks pi in the majority of treated subjects, indicating that the rNAGLU was secreted (Fig. 4). However, in the majority of rAAV-treated subjects, serum NAGLU activity then declined rapidly to baseline levels by 2 months pi, and further to only 1–28% of baseline levels at 3 months pi (Fig. 4). The NAGLU activity began to rebound at 1–3 months pi to baseline or near baseline levels (Fig. 4). The transient decline of serum NAGLU activity is likely because of the induction of Ab against the rNAGLU (Fig. 5), which then cross-reacts with the endogenous enzyme because of the high homology across species. The stabilization or rebound of serum NAGLU activity in the rAAV9-treated subjects may be because of the emergence of B-cell tolerance to the rNAGLU. Importantly, there was no correlation between the serum and tissue NAGLU activity levels suggesting that tissue NAGLU was unaffected by circulating Ab (Fig. 5). Of equal significance was the absence of correlation between serum NAGLU activity and preexisting anti-AAV9 Ab or immunosuppression, with the exception of subject M6 that had high preexisting anti-AAV9 Ab. In subject M6, serum NAGLU activity stayed within 1.3–2.7-fold of baseline levels, without the sharp increase or decline as observed in the rest of AAV9-treated animals (Fig. 4).
FIG. 4.
Serum and CSF NAGLU activity in NHP after rAAV9 vector delivery (a) Group 1: 1E13 vg/kg group. (b) Group 2: 2E13 vg/kg group. Serum and CSF samples were assayed for NAGLU activity at end point. NAGLU activity is expressed as units/ml. 1 unit NAGLU activity=formation of 4MU/hr. *Subject with high preexisting anti-AAV9 antibodies.
FIG. 5.
Systemic rAAV9 gene delivery-mediated robust antibody response against the vector. Serum samples were assayed weekly and/or monthly for antibodies against AAV9 vector by ELISA. (a) Group 1: 1E13 vg/kg group. (b) Group 2: 2E13 vg/kg group. *Subject with high preexisting anti-AAV9 antibodies. ELISA, enzyme-linked immunoabsorbent assay.
We also detected a 1.3–2.5-fold increase in NAGLU activity in the CSF at or before 3 months pi in the majority of rAAV9-treated subjects compared with nontreated controls, suggesting the presence of secreted rNAGLU in the CSF (Fig. 4). However, at 6 months pi, CSF NAGLU activity was detected in the 2 tested rAAV9-treated animals at 50–91% of that nontreated control (Fig. 4). There was no observable association between NAGLU activity levels in CSF and preexisting anti-AAV9 Abs or immunosuppression treatment. There was no clear association between CSF and tissue NAGLU activity levels.
rAAV9 gene delivery induced robust anti-AAV9 capsid Ab responses that had no detectable impact on tissue, serum, or CSF NAGLU activity
To determine the potential impact of Ab to the AAV9 capsid protein on transgene expression, serum samples were assayed by ELISA in plates coated with the rAAV9 vector. The results showed robust anti-AAV9 Ab responses in all vector-treated subjects (Fig. 5). The anti-AAV9 Ab peaked at 1–3 months pi and persisted at relatively high levels till the endpoint (3–6 months pi) (Fig. 5) with individual variation. The induced AAV9 Ab responses had no detectable impact on tissue or serum NAGLU activity levels (Figs. 3 and 4).
Anti-rNAGLU Abs were responsible for the loss of circulating rNAGLU activity, but had no detectable effect on transgene expression in tissues
Serum samples were also assayed by ELISA for total IgG against the full-length hNAGLU protein, to assess the impact of anti-rNAGLU Ab responses on transgene expression and the function of the recombinant enzyme.
We detected significant increases in IgG against hNAGLU in the serum in all rAAV9-hNAGLU-treated subjects, indicating that the systemic vector delivery induced an Ab response to the transgene product (Fig. 6a). To our surprise, relatively low levels of preexisting hNAGLU-reactive Ab were detected in the majority of animals used in this study (Fig. 6 and Table 1). The NAGLU proteins are highly homologous across species, with approximately 15 amino acid differences between the human and rhesus monkey proteins, which likely account for the observed induction of Ab responses. While the antigenic specificity of the preexisting cross-reactive Ab to hNAGLU is not clear, there was a clear correlation between the induced serum NAGLU Ab levels and serum NAGLU activity levels (Fig. 6b), suggesting that the induction of NAGLU Ab was responsible for the rapid loss of circulating NAGLU in the blood. The reduction in serum NAGLU activity below baseline in the majority of AAV9-treated subjects suggested the loss of not only the rNAGLU but also the endogenous primate enzyme, likely because of cross-reactivity to NAGLU between humans and primates (Fig. 6b). The persistence of serum NAGLU activity above the endogenous level in S1 and M6 and the rebound of serum enzyme activity after 3 months pi in the other individuals (Figs. 4 and 6b) raised the possibility of induction of immune tolerance to rNAGLU in these animals. Importantly, we did not observe a correlation between serum anti-hNAGLU Ab levels and tissue rNAGLU activity (Supplementary Table S1), suggesting that serum anti-hNAGLU Abs have little impact on either the transgene product or rAAV9-transduced cells in tissues.
FIG. 6.
rAAV9-hNAGLU gene delivery-mediated antibody responses against rNAGLU and serum NAGLU activity levels. Group 1: 1E13 vg/kg group. Group 2: 2E13 vg/kg group. Serum samples were assayed weekly and/or monthly for antibodies against the full-length hNAGLU protein by ELISA. (a) Anti-hNAGLU IgG levels. (b) Correlation of anti-hNAGLU IgG levels to serum NAGLU activity. *Subject with high preexisting anti-AAV9 antibodies.
Systemic rAAV9-hNAGLU gene delivery induced the activation of helper T cells
We assayed PBWC and monocytes isolated from liver by IFN-γ-ELISPOT, in the presence of overlapping synthetic peptide libraries covering the full-length AAV9 capsid protein and full-length hNAGLU to detect T-cell responses against the vector or transgene in the treated subjects. Positive IFN-γ-forming spots were detected only in PBWC from one treated animal (1E13 vg/kg), subject S2, at 8 weeks pi against hNAGLU peptideaa134–151. Further, using flow cytometry for T cell markers and intracellular IFN-γ, we showed that only CD4+ T cells in PBWC, not CD8+ T cells, responded to the target hNAGLU peptideaa134–151. These data indicated that the systemic rAAV9-hNAGLU gene delivery induced a Th-1 activation of helper T cells, but not cytotoxic T cells, in this subject. It is likely that these activated helper T cells contributed to the Ab response that resulted in the loss of rNAGLU activity in the blood circulation (Fig. 4). We did not detect positive IFN-γ forming spots in the PBWC from any of the other subjects at any time point. In addition, no positive ELISpot response was observed in monocytes from the livers in either rAAV9-treated or control animals when tested at the endpoints (6 weeks, 3 months, or 6 months pi). These data suggested a limited role for the T-cell response against either the transgene product or the vector in these subjects after a systemic rAAV9 gene delivery.
Differential biodistribution of the rAAV9 vector genomes in tissues
Quantitative real-time PCR was performed to determine the amount of the rAAV9-CMV-hNAGLU vector entering the CNS versus somatic tissues. Table 2 shows the differential biodistribution of vector genomes in different tissues/organs in NHP subjects receiving an IV vector injection versus nontreated animals. The number of vector genome copies per cell showed clear dose dependency. Immunosuppression did not have an observable impact on the biodistribution of the vector. In general, the highest vector concentration was detected in the liver, followed by heart, spleen, muscle, and brain (Table 2). It is worth noting that vector genome copy numbers were higher in the kidneys in rAAV9-treated NHP subjects than had been observed in MPS IIIB mice, consistent with measured NAGLU activity, and suggesting the potential for species-specific tissue affinity toward the rAAV9 vector.
Table 2.
Biodistribution of rAAV9-CMV-hNAGLU Vector Genome in Nonhuman Primates by Systemic Delivery
| Vector genome/cell | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NHP | Time (pi) | Liver | Spleen | Muscle | Heart | Lung | Kidney | Intestine | Brain |
| Group 1 | |||||||||
| NT-1 | 6 weeks | 0.000 | 0.004 | 0.005 | 0.000 | 0.001 | 0.002 | 0.002 | 0.005 |
| S1-V | 6 weeks | 2.060 | 0.849 | 0.008 | 0.003 | 0.010 | 0.011 | 0.006 | 0.008 |
| S2-V | 3 months | 10.310 | 0.660 | 0.407 | 0.212 | 0.020 | 0.045 | 0.008 | 0.014 |
| Group 2 | |||||||||
| NT-2 | 3 months | 0.001 | 0.000 | 0.002 | 0.001 | 0.001 | 0.001 | 0.000 | 0.002 |
| M1-V | 3 months | 75.115 | 0.751 | 0.858 | 8.932 | 0.270 | 0.068 | 0.092 | 0.238 |
| M2-V | 3 months | 17.380 | 0.704 | 0.729 | 3.623 | 0.117 | 0.156 | 0.088 | 0.145 |
| M3-V+P | 3 months | 104.502 | 2.368 | 0.285 | 6.491 | 0.279 | 0.075 | 0.075 | 0.206 |
| M4-V | 6 months | 104.889 | 0.105 | 0.185 | 6.534 | 0.039 | 0.193 | 6.534 | 0.097 |
| M5-V+P | 6 months | 31.042 | 0.078 | 0.076 | 1.144 | 0.051 | 0.061 | 0.044 | 0.120 |
| M6-Va | 6 months | 0.564 | 7.919 | 0.517 | 0.033 | 0.010 | 0.048 | 0.215 | 0.041 |
Total DNA samples were assayed in duplicates by quantitative real-time PCR for vector genome.
Subject with high level of preexisting anti-AAV9 antibodies.
Interestingly, in subject M6 that had high preexisting anti-AAV9 Abs, the vector genome copies were much lower in the liver, heart, lung, and kidney, but not changed in the muscle and only moderately reduced in the brain compared with the other two rAAV9-treated animals (M4, M5) at the same end point (Table 2). In subject M6, while the liver vg/cell was 0.5–1.8% of the values in M4 and M5, the brain vg/cell was 34.2–42.3% of that in M4 and M5. These data correlate to liver and brain NAGLU activity levels, suggesting that the high preexisting AAV9 Ab may have less impact on the vector CNS entry than on somatic transduction.
While the vector copy number per cell in the spleen from M6 was far higher (75.4–101.5-fold) than in M4 and M5 (Table 2), spleen NAGLU activity in M6 was lower (<50%) compared with M4 and M5 (Fig. 3). This suggests that the anti-AAV9 Ab may have driven higher levels of phagocytosis that did not lead to productive infection in the splenocytes.
Discussion
The recently demonstrated capability of AAV9 to efficiently cross the BBB has proven a valuable tool for therapeutic gene delivery to the CNS in a broad range of animal species (Duque et al., 2009; Foust et al., 2009, 2010; Bevan et al., 2011; Fu et al., 2011; Gray et al., 2011). Using the AAV9 vector to deliver the survival motor neuron (SMN) gene, Foust et al. (2010) rescued the spinal muscular atrophy phenotype in a mouse model. In our previous work with LSD, we achieved long-term global CNS and widespread somatic restoration of NAGLU activity, correction of lysosomal storage pathology, and functional neurological benefits in adult MPS IIIB mice by a single IV injection of the rAAV9-CMV-hNAGLU vector (Fu et al., 2011). These results demonstrate the promising clinical potential of this minimally invasive approach to treat both the neurological and somatic disorders of MPS IIIB. To extend the relevance of this approach toward clinical applications in humans, we tested systemic gene delivery in adult NHP in the present study. We demonstrated that the rAAV9-mediated global CNS and broad somatic transduction observed in MPS IIIB mice is largely reproducible in NHP. While there was evident vector transduction throughout the brain, it is difficult to assess the overall levels of vector-expressed NAGLU activity in the context of the endogenous enzyme. Treatment of MPS IIIB mice at the equivalent dose resulted in expression exceeding wild-type levels (Fu et al., 2011), in contrast to the NHP, where increases were fractional in most animals. While the vector genome copy number in the NHP brains exceeded that of mice at the same dose, the expression levels did not correlate well with these values, possibly because of variability in endogenous enzyme levels.
One of the major purposes of this study was to assess the safety profile of systemic rAAV9-hNAGLU gene delivery. The single IV infusion of rAAV9-CMV-hNAGLU did not lead to either observable adverse effects or detectable toxicity in NHP during 6 months of observation. Although we did observe brisk Ab responses to both the rAAV9 vector and the transgene product in the treated animals, there was no evidence of immune-mediated tissue damage. This adds to the already substantial evidence for the safety of systemic rAAV gene delivery, further supporting its potential for clinical application.
A major challenge for rAAV9-mediated gene therapy is the prevalence of preexisting immunity to human serotypes of AAV, including AAV9. Previous studies reported the detection of anti-AAV9 Ab in 47% of the human population, with 33.5% being positive for AAV9 neutralizing Ab (Boutin et al., 2010), which may lead to the clearance of the vector as shown in NHP experiments (Gray et al., 2011). However, it is important to note that low levels (<1:50) of preexisting AAV9 Ab did not greatly impact tissue transgene expression in NHP in this study, suggesting that systemic rAAV9-hNAGLU gene delivery may also be efficacious in treating patients with similarly low levels of preexisting anti-AAV9 Abs. This is consistent with gene delivery in one patient in a recent hemophilia gene therapy clinical trial who had a low-level (1:37) Ab to the AAV8 vector (Nathwani et al., 2011).
Our inclusion of a subject animal (M6) with high preexisting anti-AAV9 Ab (1:1,000) allowed us to assess the potential impact on therapeutic efficacy and safety. In this subject, the anti-AAV9 Ab had less impact on transduction in the CNS than in liver. While the reason for this difference in vector sensitivity to Ab is unclear, it may be related to the kinetics of rAAV9-CNS entry, which may be fast process at the BBB, allowing adsorption of the vector before Ab interaction. Alternatively, the critical ligand for receptor binding at the BBB may be different from that for entry in the liver, such that it is not blocked by the Ab. Whatever the mechanism, the possibility that CNS entry is less sensitive to preexisting Ab would potentially expand the patient population that could benefit from this treatment. This ability is likely to offer significant benefit in the clinical setting considering that almost half of the general human population is AAV9-Ab positive at some level. It also supports the possibility of multiple vector treatments by systemic injection through the use of sophisticated immunomodulation regimens, as demonstrated in a recent study by Mingozi et al. (2012), which allowed a successful rAAV vector re-administration for factor IX (FIX) expression in primates. While the immunosuppression regimen used in the current study did not consistently affect transduction efficiency, further investigations are needed to determine what would be the limiting level of preexisting AAV9 Ab for efficacious NAGLU gene delivery, and what immunomodulation strategies could be used to ameliorate the effect of preexisting immunity. Furthermore, recent studies demonstrate that additional novel AAV serotypes share the trans-BBB neurotropism of AAV9, and some of these are nonhuman serotypes, which may exhibit a lower prevalence of neutralizing Ab in the human population (Zhang et al., 2011).
We also noted that the animal with a high-level preexisting AAV9 Ab also had very high vector genome copy numbers in the spleen compared with the other subjects, yet did not have concordantly high NAGLU enzyme activity in the spleen. We hypothesize that this is a consequence of phagocytosis promoted by Ab binding to the circulating vector as part of the initial innate immune response to vector delivery. However, the vector appears to persist in the splenocytes without further processing to achieve transgene expression. A similar observation was made by Wang et al. (2011) when treating NHPs with an IV injection of an rAAV8 vector.
The present study also addresses another critical issue in rAAV gene therapy, the vector-induced immune responses to the viral capsid and the transgene product. A previous gene therapy clinical trial treating hemophilia B by intravascular (hepatic artery) AAV2-FIX gene delivery showed loss of vector expression because of a cytotoxic immune response to the capsid that was detected during weeks 5–8 pi (Manno et al., 2006). This suggested an immune-mediated loss of transduced cells in the liver. A more recent clinical trial for hemophilia B using IV rAAV8 gene delivery showed that the loss of FIX and concurrent increase in serum ALT were correlated with a T cell response to AAV8 capsid in two patients receiving a high dose of the vector at 8 and 9 weeks pi, suggesting that hepatic damage by cytotoxic T cells was the cause of FIX loss (Nathwani et al., 2011). Importantly, transient immunosuppression using prednisolone led to rapid normalization of ALT levels and persistence of FIX levels in the same study. In our current NHP study, we demonstrate that systemic rAAV9-CMV-hNAGLU gene delivery induced Ab responses to both the vector capsid and rNAGLU. The anticapsid Ab response did not have a major impact on either tissue transgene expression or the levels of secreted rNAGLU circulating in the blood. However, while the induced anti-hNAGLU Ab response does not affect tissue rNAGLU activity, it does clear secreted rNAGLU activity from the serum. Whether a similar response to rNAGLU would be induced in MPS IIIB patients will likely depend on the specific mutation, and whether there is residual protein expression. It is worth noting that in the MPS IIIB mouse model treated with the same vector, cross-correction by secreted enzyme in the CNS and somatic tissues persisted with normalized survival (Fu et al., 2011), though these animals also exhibited anti-hNAGLU Ab response (unpublished data) similar to that observed in the NHP in this study. This suggests that the Ab response to NAGLU, whether ultimately transient or controllable by immune suppression, does not eliminate enzyme activity in organs and tissues where it is most needed. It is not clear why we observed low levels of preexisting hNAGLU-reactive Ab in the majority of NHP in this study. These animals had not previously been used in MPS experiments, or exposed to animals that had been. While we hypothesize that these are nonspecific cross-reactive Abs to hNAGLU, their primary antigenic specificity remains unclear.
The systemic rAAV9-hNAGLU delivery in the current study resulted in only a minimal cellular immune response, with hNAGLU-reactive CD4+ helper T cells detectable in only one subject at 4 weeks pi. As with the Ab response to the transgene, this did not affect tissue transgene expression levels. Notably, the specificity of the reactive CD4+ T cells was directed toward hNAGLU peptideaa134–151, which is 100% homologous in all primate sequences currently available, including cynomolgus macaques. It is not clear how this response was initiated in this animal.
The systemic gene delivery did not induce a detectable IFNγ-positive response against AAV9 capsid, which is consistent with the absence of detectable toxicity or tissue damage in the treated subjects. Although it is unclear whether the absence of T cell response to AAV9 in this study is serotype-specific, our data suggest a minimal impact from T cells on the transduction efficiency in primates.
In summary, we demonstrate that a single systemic delivery of the rAAV9-CMV-hNAGLU vector induces the expression of the transgene in multiple tissues, including the CNS, in NHP without eliciting detectable toxicity or adverse effects. This capability supports the gene delivery strategy as a safe approach for treating patients with MPS IIIB, possibly including those with low levels of preexisting anti-AAV9 Abs. Furthermore, rAAV9-mediated systemic gene delivery may also offer potential for treating many other neurogenetic diseases with somatic manifestations.
Supplementary Material
Acknowledgments
We would like to thank Ben's Dream–The Sanfilippo Research Foundation and Cure Kirby–The Children's Medical Research Foundation for sponsoring this study. H.F., D.M.M., B.J.N., A.S.M., B.B., C.M.W., K.M.F., and K.L.M. are also supported by a grant from NIH (U01 NS069626). We also want to thank Adrienne Morrison, Ashley Artrip, and Natalie Snyder for taking care of the experimental subjects. The Comparative Pathology and Mouse Phenotyping Shared Resource, Department of Veterinary Biosciences and the Comprehensive Cancer Center, The Ohio State University, is supported in part by a grant from NIH/NCI (P30 CA016058).
Author Disclosure Statement
H.F. and D.M.M. are affiliated with a start-up company (Abeona Therapeutics) in gene therapy approaches for LSDs. Other authors declare no conflict of interest.
References
- 1.Baek R.C., Broekman M.L., Leroy S.G., et al. (2010). AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS One 5, e13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns K.I., and Linden R.M. (1995). The cryptic life style of adeno-associated virus. Bioessays 17, 237–245 [DOI] [PubMed] [Google Scholar]
- 3.Bevan A.K., Duque S., Foust K.D., et al. (2011). Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 19, 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutin S., Monteilhet V., Veron P., et al. (2010). Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 [DOI] [PubMed] [Google Scholar]
- 5.Daya S., and Berns K.I. (2008). Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmaris N., Verot L., Puech J.P., et al. (2004). Prevention of neuropathology in the mouse model of Hurler syndrome. Ann. Neurol. 56, 68–76 [DOI] [PubMed] [Google Scholar]
- 7.DiRosario J., Divers E., Wang C., et al. (2009). Innate and adaptive immune activation in the brain of MPS IIIB mouse model. J. Neurosci. Res 87, 978–990 [DOI] [PubMed] [Google Scholar]
- 8.Duque S., Joussemet B., Riviere C., et al. (2009). Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foust K.D., Nurre E., Montgomery C.L., et al. (2009). Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foust K.D., Wang X., Mcgovern V.L., et al. (2010). Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 28, 271–274 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Fraldi A., Hemsley K., Crawley A., et al. (2007). Functional correction of CNS lesions in an MPS-IIIA mouse model by intracerebral AAV-mediated delivery of sulfamidase and SUMF1 genes. Hum. Mol. Genet. 16, 2693–2702 [DOI] [PubMed] [Google Scholar]
- 12.Fu H., Samulski R.J., Mccown T.J., et al. (2002). Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery. Mol. Ther. 5, 42–49 [DOI] [PubMed] [Google Scholar]
- 13.Fu H., Muenzer J., Samulski R.J., et al. (2003). Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol. Ther. 8, 911–917 [DOI] [PubMed] [Google Scholar]
- 14.Fu H., Kang L., Jennings J.S., et al. (2007). Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 14, 1065–1077 [DOI] [PubMed] [Google Scholar]
- 15.Fu H., DiRosario J., Killedar S., et al. (2011). Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol. Ther. 19, 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray S.J., Matagne V., Bachaboina L., et al. (2011). Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 19, 1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heldermon C.D., Ohlemiller K.K., Herzog E.D., et al. (2010). Therapeutic efficacy of bone marrow transplant, intracranial AAV-mediated gene therapy, or both in the mouse model of MPS IIIB. Mol. Ther. 18, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer G.G., Passini M.A., Jiang K., et al. (2002). Selective neurodegeneration in murine mucopolysaccharidosis VII is progressive and reversible. Ann. Neurol. 52, 762–770 [DOI] [PubMed] [Google Scholar]
- 19.Kaplitt M.G., Feigin A., Tang C., et al. (2007). Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369, 2097–2105 [DOI] [PubMed] [Google Scholar]
- 20.Li H.H., Yu W.H., Rozengurt N., et al. (1999). Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase. Proc. Natl. Acad. Sci. USA 96, 14505–14510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H.H., Zhao H.Z., Neufeld E.F., et al. (2002). Attenuated plasticity in neurons and astrocytes in the mouse model of Sanfilippo syndrome type B. J. Neurosci. Res. 69, 30–38 [DOI] [PubMed] [Google Scholar]
- 22.Liu G., Martins I., Wemmie J.A., et al. (2005). Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J. Neurosci. 25, 9321–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manno C.S., Pierce G.F., Arruda V.R., et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 [DOI] [PubMed] [Google Scholar]
- 24.Marks W.J., Jr., Bartus R.T., Siffert J., et al. (2010). Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 [DOI] [PubMed] [Google Scholar]
- 25.McCarty D.M., DiRosario J., Gulaid K., et al. (2009). Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther. 16, 1340–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGlynn R., Dobrenis K., and Walkley S.U. (2004). Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J. Comp. Neurol. 480, 415–426 [DOI] [PubMed] [Google Scholar]
- 27.McPhee S.W., Janson C.G., Li C., et al. (2006). Immune responses to AAV in a phase I study for Canavan disease. J. Gene Med. 8, 577–588 [DOI] [PubMed] [Google Scholar]
- 28.Mendell J.R., Rodino-Klapac L.R., Rosales-Quintero X., et al. (2009). Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann. Neurol. 66, 290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mingozzi F., Chen Y., Murphy S.L., et al. (2012). Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol. Ther. 20, 1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittermeyer G., Christine C.W., Rosenbluth K.H., et al. (2012). Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum. Gene Ther. 23, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathwani A.C., Tuddenham E.G., Rangarajan S., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neufeld E.F., and Muenzer J. (2001). The mucopolysaccharidoses. In The Metabolic & Molecular Basis of Inherited Disease. Scriver C.R., Beaudet A.L., Sly W.S., and Valle D., eds. (McGraw-Hill, New York, NY: ) pp3421–3452 [Google Scholar]
- 33.Ohmi K., Greenberg D.S., Rajavel K.S., et al. (2003). Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. USA 100, 1902–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmi K., Kudo L.C., Ryazantsev S., et al. (2009). Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc. Natl. Acad. Sci. USA 106, 8332–8337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardridge W.M. (2002). Drug and gene delivery to the brain: the vascular route. Neuron 36, 555–558 [DOI] [PubMed] [Google Scholar]
- 36.Rohrbach M., and Clarke J.T. (2007). Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs 67, 2697–2716 [DOI] [PubMed] [Google Scholar]
- 37.Ruzo A., Garcia M., Ribera A., et al. (2012). Liver production of sulfamidase reverses peripheral and ameliorates CNS pathology in mucopolysaccharidosis IIIA mice. Mol. Ther. 20, 254–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryazantsev S., Yu W.H., Zhao H.Z., et al. (2007). Lysosomal accumulation of SCMAS (subunit c of mitochondrial ATP synthase) in neurons of the mouse model of mucopolysaccharidosis III B. Mol. Genet. Metab. 90, 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sands M.S., and Haskins M.E. (2008). CNS-directed gene therapy for lysosomal storage diseases. Acta Paediatr. Suppl. 97, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson J.N., and Nowakowski R.W. (1991). Enzymatic diagnosis of selected mucopolysaccharidoses: Hunter, Morquio type A, and Sanfilippo types A, B, C, and D, and procedures for measurement of 35SO4-glycosaminoglycans. In Techniques in Diagnostic Human Biochemical Genetics—A Laboratory Manual. Hommes F.A., ed. (Wiley-Liss, New York, NY: ) pp567–586 [Google Scholar]
- 41.Villani G.R., Gargiulo N., Faraonio R., et al. (2007). Cytokines, neurotrophins, and oxidative stress in brain disease from mucopolysaccharidosis IIIB. J. Neurosci. Res. 85, 612–622 [DOI] [PubMed] [Google Scholar]
- 42.Villani G.R., Di Domenico C., Musella A., et al. (2009). Mucopolysaccharidosis IIIB: oxidative damage and cytotoxic cell involvement in the neuronal pathogenesis. Brain Res. 1279, 99–108 [DOI] [PubMed] [Google Scholar]
- 43.Wang L., Bell P., Lin J., et al. (2011). AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta). Mol. Ther. 19, 2012–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber B., Guo X.H., Kleijer W.J., et al. (1999). Sanfilippo type B syndrome (mucopolysaccharidosis III B): allelic heterogeneity corresponds to the wide spectrum of clinical phenotypes. Eur. J. Hum. Genet. 7, 34–44 [DOI] [PubMed] [Google Scholar]
- 45.Worgall S., Sondhi D., Hackett N.R., et al. (2008). Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 19, 463–474 [DOI] [PubMed] [Google Scholar]
- 46.Yogalingam G., Weber B., Meehan J., et al. (2000). Mucopolysaccharidosis type IIIB: characterisation and expression of wild-type and mutant recombinant alpha-N-acetylglucosaminidase and relationship with sanfilippo phenotype in an attenuated patient. Biochim. Biophys. Acta 1502, 415–425 [DOI] [PubMed] [Google Scholar]
- 47.Zhang H., Yang B., Mu X., et al. (2011). Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol. Ther. 19, 1440–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zincarelli C., Soltys S., Rengo G., and Rabinowitz J.E. (2008). Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 16, 1073–1080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.