Abstract
Schizophrenia patients with the deficit syndrome (DS) may represent a homogeneous subgroup. To increase the practicability of diagnosing the DS, Kirkpatrick et al. (Kirkpatrick, B., Buchanan, RW., Breier, A. Carpenter, WT., 1993. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Research 47, 47–56) proposed the use of a ‘proxy’ case identification tool using standardized symptom ratings instead of the Schedule for the Deficit Syndrome (SDS) which requires an independent clinical assessment. The Proxy for the Deficit Syndrome (PDS) is based on the extraction of symptoms that are essentially equivalent or overlap substantially with the restricted affect and diminished emotional range on the SDS. Kirkpatrick et al. (1993) reported good sensitivity and specificity in a comparison of SDS and PDS assessments among 100 chronic schizophrenia outpatients. The present investigation involves the comparison of the deficit syndrome as assessed by the “gold standard” Schedule for the Deficit Syndrome with the ratings of the same symptoms embodied in the “proxy instrument” the PANSS, within the same group of 156 inpatients.
Forty-four patients were assessed by the SDS to have the deficit syndrome. Patients with and without the DS, as defined by the SDS, did not differ for age, education, age at illness onset and duration of illness. The two main ‘proxy’ measures PDS1 and PDS2 discriminated across the SDS groups. The direct dichotomous comparison of the actual SDS and the ‘proxy’ derived PDS groups demonstrated good specificity (78.6% and 79.5%) and moderate to very good sensitivity (61.4% and 86.4%) and there was a moderately low rate of false positive cases (21.4% and 20.5%). For the two main ‘proxy’ measures (PDS1 & PDS2) kappas were .38 and .59, representing poor to good agreement.
In our sample of rigorously diagnosed schizophrenia inpatients, the use of a ‘proxy’ case identification tool for the deficit syndrome would appear to be a viable alternative in identifying a subgroup of schizophrenia patients with the deficit syndrome when the use of the actual SDS is not feasible. Further study is indicated before the PDS as extracted from the PANSS can be used in lieu of the SDS for identifying patients with this syndrome.
Keywords: schizophrenia, deficit syndrome, proxy for the deficit syndrome
Introduction
Schizophrenia is a complex and multi-dimensional disorder that is characterized by heterogeneity of symptoms, course of illness and clinical profiles. In an attempt to reduce this heterogeneity, researchers have utilized a subtyping strategy to identify homogeneous subtypes to facilitate the development of targeted treatment and investigations of the pathophysiology and etiology of schizophrenia. The Deficit Syndrome (DS) in schizophrenia is one subtype that has received much attention. Carpenter et al. (1988) described the DS as a schizophrenia subtype characterized by prominent negative symptoms (persistent/ enduring and primary) that are “trait like” in nature.
The DS is assessed by the Schedule for the Deficit Syndrome (SDS; Kirkpatrick et al. 1989), and is defined by the presence of at least two negative symptoms (restricted affect, diminished emotional range, poverty of speech, diminished social drive, diminished sense of purpose, curbing of interests) that are both primary (i.e., not caused by neuroleptic akinesia, depression, anxiety, paranoia or other psychotic symptoms) and enduring (present during the preceding 12 months as well as during periods of clinical stability). The DS, as assessed by the SDS, has been shown to be stable over time - Fenton et al. (1994) reported that 10 of 12 patients (83%) retained the DS diagnosis from first admission to an index admission, on average 7.5 years later. Similarly, Amador et al. (1999) reassessed a group of 43 patients, 18 with and 25 without the DS, on average some 3.8 years later. Fifteen of 18 (83.3%) retained their DS diagnosis, and 22 of 25 (88.0%) continued to not fulfill criteria for the DS (kappa = 0.71).
To increase the practicability of diagnosing the DS, Kirkpatrick et al. (1993) proposed the use of a proxy case identification tool (Proxy for the Deficit Syndrome, PDS) to identify DS in the absence of an actual SDS assessment. As initially reported the PDS is based on the assessment of symptoms using the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962) that are essentially equivalent or overlap substantially with the restricted affect and diminished emotional range on the SDS (blunted affect, anxiety, guilt feelings, depressive mood and hostility). Kirkpatrick et al. (1993) in their comparison of SDS and PDS assessments among 100 chronic schizophrenia outpatients, reported sensitivity and specificity rates at 79% (identifying 19 of 24 patients with the deficit syndrome) and 89% (67 of 75 non-deficit patients). They concluded that the PDS performed well as a case identification tool for the DS, and may serve as a useful tool to identify DS in the absence of SDS assessments.
Following the Kirkpatrick et al. (1993) report, a number of investigators used the PDS to assess DS (Kirkpatrick et al., 1996, 1998, 2000, 2002; Messias et al., 2001; Subotnik et al. 1998, 2000; Tek et al., 2001). Directly relevant here, Subotnik et al. (1998) evaluated the PDS (using the BPRS) cross-sectionally and longitudinally and reported that the initial presence of deficit syndrome features was not a good predictor of the syndrome longitudinally. Specificity was good in re-identifying 50 of 52 (96%) nondeficit patients, however, sensitivity was disappointing in re-identifying only 10 of 25 (40%) deficit patients. The authors suggest that a large proportion of “false positives” were identified at the initial cross-sectional assessment. However, the original findings by Kirkpatrick et al. (1993), which directly compared the SDS and PDS, have not been replicated by others. Another concern is that the use of the PDS (a proxy measure) in identifying patients with the Deficit Syndrome may dilute the homogeneity of the Deficit Syndrome as a distinct and important schizophrenia subgroup. Thus, our goal is to directly compare the PDS, as derived from the Positive and Negative Symptom Scale ([PANSS], Kay et al. 1987; Kay et al. 1989; Kay et al. 1989; Kay et al. 1992; White et al. 1997), and SDS in order to assess the validity and utility of a newly derived PDS. Our strategy follows closely the Kirkpatrick et al. (1993) article, which examined what measures differ across DS and Nondeficit patient groups, and dichotomize the derived measures to assess sensitivity and specificity compared to the gold standard of the SDS.
Experimental/ Materials and Methods
1.1 Participants
One hundred and fifty-six inpatients (113 males and 43 females) from the Schizophrenia Research Unit (SRU) at New York State Psychiatric Institute participated in this Institutional Review Board approved investigation. Patients met strict diagnostic criteria for Schizophrenia. Diagnoses were made using the Diagnostic Interview for Genetic Studies (DIGS, Nurnberger et al. 1994) and DSM-IV criteria, clinical data, past psychiatric records and symptom ratings; and represented a consensus between clinical and research staff. Patients were assessed as having the capacity to consent and signed informed consent documents. Patients were determined to be medically healthy by recent physical examination and laboratory evaluations. Demographic data included age, gender, education, ethnicity, age at onset of psychotic symptoms, and duration of illness.
1.2 Assessments
The present investigation involves the comparison of the deficit syndrome as assessed by the “gold standard” Schedule for the Deficit Syndrome with the ratings of similar symptoms embodied in the “proxy instrument” the Positive and Negative Symptom Scale (PANSS), within the same group of patients. Patients were assessed for the deficit syndrome using the Schedule for the Deficit Syndrome (SDS) [2/3 by actual face to face interview and 1/3 by review of clinical chart and all available information], which uses historical data, rather than cross-sectional data to assess the severity, prominence and stability of negative symptoms. Symptom information about the patients was obtained from patient and family interviews, chart review and discussions with the clinical staff regarding restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose and diminished social drive. The Deficit Syndrome was rated as present if at least two of these negative symptoms were rated as present, primary and stable illness features. The raters of the deficit syndrome were trained at the Maryland Psychiatric Research Center, where inter-rater reliability was established under the supervision of Brian Kirkpatrick, a developer of the SDS. Briefly, the training consisted of reading the Schedule for the Deficit Syndrome manual, attending a lecture, viewing videotaped SDS interviews of three Maryland Psychiatric Research Center subjects, and co-rating (Brian Kirkpatrick) seven other patients during live interviews. The three raters had 100% agreement on the deficit and nondeficit status of these 10 patients; five were classified as having the deficit syndrome.
In the absence of an actual SDS to define the deficit syndrome, Kirkpatrick et al. 1992 advocated the use of a “proxy” case identification tool that utilizes symptom items from the Brief Psychiatric Rating Scale (BPRS, [blunted affect, emotional withdrawal, motor retardation, anxiety, guilt feelings, depressed mood, hostility, conceptual disorganization, suspiciousness, hallucinatory behavior and unusual thought content]). We use the Positive and Negative Symptom Scale (PANSS, Kay et al. 1989; White et al. 1997) to assess patient symptoms, and these same symptom items are embodied in the PANSS. PANSS interviews were performed by masters level psychologists. Raters achieved high reliability with each other (i.e. Kappa > .80 for individual symptom ratings and 95% agreement on diagnosis) before evaluating subjects.
The “proxy” case identification tool and other related variables as per Kirkpatrick et al. (1993) were calculated from the PANSS items as described below. During the patients in-hospital stay the 30-item PANSS is generally administered several times corresponding to clinical phases (admission/baseline, treatment, discharge). For our purposes here, we used the PANSS clinical ratings near discharge or during the antipsychotic treatment phase, which ever was available. Both the SDS and the PANSS were assessed when the clinical state of the patient was considered stable and after 4 weeks of fixed dose antipsychotic treatment. The “proxy” case identification tool for the deficit syndrome is referred to as the PDS (here as PDS1) and is defined as the sum of the Anxiety, Guilt Feelings, Depressive Mood and Hostility items (the AFFSCALE as per Kirkpatrick) subtracted from the score for Blunted Affect item. We also calculated PDS2 which was defined by Kirkpatrick et al. (1992) as Blunted affect item minus the Depression item score. Similar to Kirkpatrick et al., we also calculated two additional scales: the Psychosis Scale = the summing of the Conceptual Disorganization, Suspiciousness, Hallucinatory Behavior and Unusual Thought Content items; and Factor2 (considered a “negative symptom” measure) = the summing of the Emotional Withdrawal, Motor Retardation and Blunted Affect items. The Blunted Affect, Emotional Withdrawal and the Depressive Mood items will also be examined individually. The PANSS has its own set of factors that we decided to examine in comparison to the “gold standard” deficit syndrome, and to consider their utility as possible Deficit Syndrome proxy measures. We opted to use the new set of recently factor analyzed PANSS factors as per White et al. (1997) These factors included the following: positive, negative, dysthymia, activation and preoccupation with autism symptom factors.
1.3 Data Analysis
The distributional properties of all variables were examined for normality, and preliminary analyses (t-tests, Oneway ANOVA and chi-square) examined the demographic measures across the Deficit Syndrome (DS) and gender and no demographic differences were revealed nor were there any significant interactions. Categorical distributions (i.e. DS by gender) were examined using the Chi-square statistic.
Preliminary t-tests revealed differences within DS group/across gender for the “proxy” and PANSS measures, thus subsequent analysis was performed using two separate 2 by 2 (DS: positive/negative by gender: male/female) multivariate ANOVAs. We performed Receiver-Operator-Characteristics (ROC) analyses predicting the actual Deficit Syndrome rating with the various proxy measures and the PANSS factors. We also calculated two additional dichotomous variables PROXYDS and DSCAT and using severity levels for the specific symptoms (similar to the actual SDS algorithm). The PANSS symptoms are all rated on a 1 to 7 scale with a rating of 3 representing mild and a ratings of 4 and greater representing moderate through extreme. PROXYDS was calculated using the following algorithm: (anxiety, guilt, depression and hostility all less than or equal to 3) and (blunted affect ≥ 4). Patients fitting the above algorithm were considered positive for the PROXYDS variable with all others as negative. DSCAT was calculated using the blunted affect and depression items from the PANSS. Patients with blunted affect ≥ 4 [moderate and higher] and depression ≤ 3 [mild and lower] were coded as positive for DS and all others as negative.
We examined the distributions of the “proxy” measures and PANSS factors and chose cut points to dichotomize each measure so as to 1.) over-estimate (> 44 cases [44 cases were identified by the SDS to have the Deficit Syndrome]) the number of DS cases and then 2.) under-estimate (<44 cases) the number of DS cases. Each of these would then be examined in a two by two cross tabulation to assess agreement, sensitivity, specificity and kappas with the “gold Standard” measure.
As a final integrative analysis we performed a logistic regression, predicting to the deficit syndrome as assessed by the Schedule for the Deficit Syndrome; entering gender, age at onset of illness and education level attained at the first step, followed by the forward stepping of the proxy measures and PANSS factors into the regression. We also performed an exploratory logistic regression examining the actual SDS symptom items and their association to the dichotomous SDS and PDS outcomes.
Results
2.1 Demographics and Multivariate Analysis
Forty-four patients (28.2% of 156) were determined to have the DS as per the Schedule for the Deficit Syndrome. The Deficit syndrome was more frequently diagnosed in males, 38 of 113 (33.6%) males versus 6 of 43 (14.0%) females were diagnosed with DS (Chi-square = 5.95, df=1, p<.015). The distribution of DS among ethnic groups did not differ significantly (Chi-square = 1.55, df=4, p = .818).
The multivariate analysis examining the ‘proxy’ measures (see Table 2) exhibited a significant overall Wilks’ Lambda for DS (F(6/147) = 9.99, p< .001) and the tests of between subjects effects showed that PDS1 (blunted affect minus the affective scale), PDS2 (blunted affect minus depression), blunted affect, emotional withdrawal, and FACTOR2 (sum of emotional withdrawal, motor retardation and blunted affect) all differed significantly with the DS positive patients always exhibiting higher values. There were no significant gender differences revealed, however, there were significant interactions of DS and gender for the PDS1 [F(1/152) = 5.75, p< .018], PDS2 [F(1/152) = 6.49, p< .012] and depression [F(1/152) = 6.19, p< .014] (these statistics are not reported on Table 2). For PDS1 and PDS2, the non-deficit females exhibited lower values and the deficit females exhibited higher values than their male counterparts, and this was obviously due to higher depression values among the non-deficit females and lower depression among the deficit females compared to the males. For depression, the males did not differ across the deficit syndrome however, the deficit females did exhibit lower depression values than the non-deficit females. In spite of the significant interaction between the DS and gender it should be noted that the significant findings for DS are not confounded by gender, i.e. both males and females with the DS differed from the males and females without DS.
Table 2.
Means, standard deviations and MANCOVA results for the Deficit Syndrome “proxy” (PDS) measures and PANSS factors.
| Non-Deficit | Deficit | DS | Gender F | |||
|---|---|---|---|---|---|---|
| Male (N=75) | Females (N=37) | Males (N=38) | Females (N=6) | F(df) / p | F(df) / p | |
| Proxy measures | Multivariate Wilk’s Lambda → | F(6/147) = 9.99, .001 | F(6/147) = 0.87, .52 | |||
| PDS 1* | −4.6 +/− 3.4 | −6.8 +/− 3.6 | −2.4 +/− 2.9 | −.83 +/− 1.3 | F(1/152) = 26.8, .001 | F(1/152) = 0.21, .65 |
| PDS 2* | 0.51 +/− 1.5 | −.65 +/− 1.8 | 2.4 +/− 1.6 | 3.2 +/− 1.7 | F(1/152) = 54.8, .001 | F(1/152) = 0.22, .64 |
| Blunted Affect | 2.3 +/− 1.2 | 2.2 +/− 1.5 | 4.2 +/− 1.4 | 4.5 +/− 1.0 | F(1/152) = 43.7, .001 | F(1/152) = 0.15, .70 |
| Emotional With. | 2.4 +/− 1.4 | 2.2 +/− 1.5 | 3.8 +/− 1.2 | 2.8 +/− 1.5 | F(1/152) = 9.59, .002 | F(1/152) = 2.73, .10 |
| Depression* | 1.8 +/− 1.1 | 2.9 +/− 1.6 | 1.8 +/− 1.2 | 1.3 +/− .82 | F(1/152) = 6.58, .011 | F(1/152) = 0.14, .71 |
| Psychosis | 9.2 +/− 4.0 | 10.8 +/− 4.5 | 10.0 +/− 4.7 | 9.2 +/− 4.5 | F(1/152) = 0.20, .654 | F(1/152) = 0.37, .54 |
| Factor 2 | 6.0 +/− 2.6 | 6.1 +/− 3.2 | 10.4 +/− 3.2 | 9.5 +/− 2.2 | F(1/152) = 31.3, .001 | F(1/152) = 0.97, .33 |
| PANSS Factors | Multivariate Wilk’s Lambda → | F(5/147) = 6.59, .001 | F(5/147) = 1.38, .23 | |||
| Positive | 11.2 +/− 5.4 | 12.5 +/− 5.2 | 10.7 +/− 5.3 | 9.2 +/− 4.6 | F(1/151) = 2.25, .14 | F(1/147) = 0.00, .948 |
| Negative | 17.3 +/− 6.3 | 19.0 +/− 8.7 | 26.8 +/− 7.1 | 23.3 +/− 5.4 | F(1/151) = 16.4, .001 | F(1/147) = 0.29, .593 |
| Dysthymia* | 8.3 +/− 3.8 | 10.6 +/− 4.2 | 8.2 +/− 3.4 | 6.2 +/− 1.2 | F(1/151) = 6.43, .012 | F(1/147) = 0.03, .867 |
| Activation | 9.0 +/− 4.4 | 11.8 +/− 6.0 | 10.2 +/− 3.8 | 10.8 +/− 6.6 | F(1/151) = 0.01, .929 | F(1/147) = 2.31, .131 |
| Autism* | 11.5 +/− 3.8 | 13.4 +/− 4.8 | 14.7 +/− 6.0 | 11.8 +/− 4.8 | F(1/151) = 0.48, .488 | F(1/147) = 0.18, .67 |
significant interaction of DS and Gender described in the results section.
The multivariate analysis examining the PANSS factors also exhibited a significant overall Wilks’ Lambda for DS (F(5/147) = 6.59, p< .001). The tests of between subjects effects for DS revealed that the negative factor was significantly higher among the deficit patients. The dysthymia factor also exhibited a significant DS effect, however, the main finding was a significant interaction of DS and gender (F(1/151) = 5.53, p<.020), with the males exhibiting equivalent values and the deficit females exhibiting lower dysthymia values than their non-deficit counterparts. The preoccupation with autism factor also exhibited a significant interaction of DS and gender, with the deficit males exhibiting higher values than the non-deficit males (14.7 +/− 6.0 vs. 11.5 +/− 3.8) and the deficit females showing lower values than the non-deficit females (11.8 +/− 4.8 vs. 13.4 +/− 4.8).
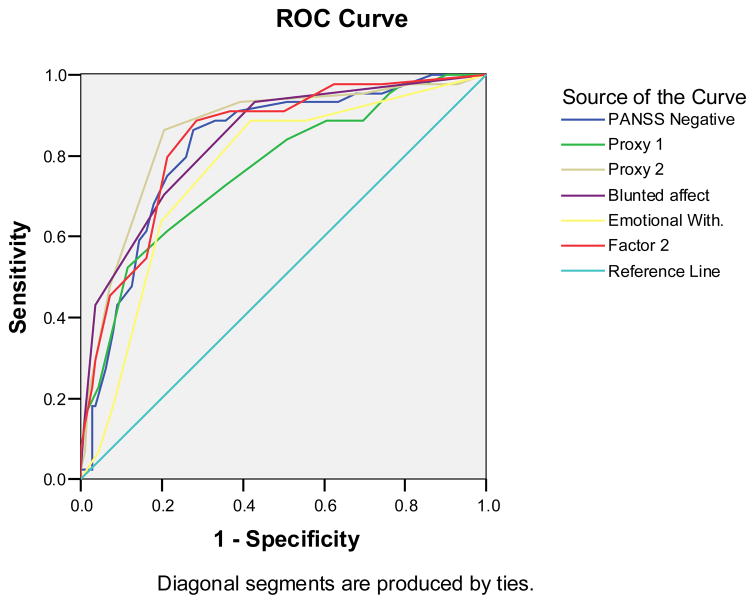
2.2 Receiver-Operator Characteristic (ROC) Analyses
We performed Receiver-Operator Characteristic (ROC) curve analyses to examine the statistically promising ‘proxy’ measures and PANSS Factors - to determine which measures best predicted to the gold standard Deficit Syndrome rating (see ROC Figure1 and Table). Among the ‘proxy’ measures, PDS2 (blunted affect – depression) yielded the greatest area under the curve (auc), followed by FACTOR2 (negative symptom factor) and blunted affect with closely matching aucs. PDS1 (blunted affect – affective scale) and emotional withdrawal (N2) also yielded significant aucs. Among the PANSS factors, only the negative factor yielded a significant auc.
Figure 1.
Receiver Operator Characteristic (ROC) curve analysis results for the ‘Proxy’ measures and the PANSS scales and new factors, predicting to actual Deficit Syndrome status.
2.3 Agreement Characteristics
We examined more closely the distributions of the most prominent (as per ROC results) “proxy” measures and the PANSS negative factor. As a point of clarification, the Schedule for the Deficit Syndrome identified 44 cases as positive for the Deficit Syndrome and 112 cases as negative. We chose cut points to dichotomize each measure so as to 1.) over-estimate the number of DS cases (> 44 cases) and then 2.) under-estimate the number of DS cases (<44 cases). Each of these would then be examined in a two by two cross tabulation to assess total agreement, sensitivity, specificity and kappa with the “gold Standard” measure. These results appear on Table 4.
Table 4.
Agreement characteristics between the ‘proxy’ measures and the actual Deficit Syndrome status. Characteristics include percent agreement, sensitivity, specificity, false positive rate and kappa.
| UNDER ESTIMATE | OVER ESTIMATE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Cases | Agree. | Sen. | Spec. | False Positives | kappa | Cases | Agree. | SEN. | SPEC. | False Positives | Kappa |
| PDS1 | 36 | 78.2% | 52.3% | 88.4% | 11.6% | .43 | 51 | 73.7% | 61.4% | 78.6% | 21.4% | .38 |
| PDS2 | 29 | 80.2% | 47.7% | 92.9% | 7.1% | .45 | 61 | 81.5% | 86.4% | 79.5% | 20.5% | .59 |
| Blunted Affect | 23 | 81.4% | 43.2% | 96.4% | 3.6% | .46 | 54 | 77.0% | 70.5% | 79.5% | 20.5% | .47 |
| Emotional With. | 17 | 71.1% | 18.2% | 92.0% | 8.0% | .13 | 50 | 75.6% | 63.6% | 80.4% | 19.6% | .42 |
| Factor 2 | 42 | 75.7% | 54.5% | 83.9% | 16.1% | .39 | 59 | 78.8% | 79.5% | 78.6% | 21.4% | .53 |
| DSCAT | 42 | 79.5% | 61.4% | 86.6% | 13.4% | .49 | ||||||
| PROXYDS | 44 | 78.2% | 52.3% | 88.4% | 11.6% | .43 | ||||||
| PANSS Negative | 45 | 77.6% | 61.4% | 83.9% | 16.1% | .45 | ||||||
NB: Factor 2, DSCAT, PROXYDS, and the PANSS Negative cutpoints predicted estimates close to the actual N of 44 cases with Deficit Syndrome, therefore only one estimate was used.
For the under estimate of cases the sensitivities were generally lower while specificities were higher. The under-estimate sensitivities reflect the lower number of cases identified by the ‘proxy’ and PANSS measures as DS positive. The sensitivities ranged from a high of 61.4% for the PANSS negative factor and the DSCAT and lows of 18.2% and 43.2% for emotional withdrawal and blunted affect. The sensitivities for the PDS1, PDS2 and Factor2 were 52.3%, 47.7% and 54.5%, respectively. False positive rates were lower as would be expected, ranging from 3.6% for blunted affect to 16.1% for DSCAT and the PANSS negative. The false positive rates for PDS1 and PDS2 were 11.6% and 7.1%, respectively. Kappa’s ranged from .13 for the emotional withdrawal item to .49 for DSCAT. PDS1 and PDS2 kappa’s were .43 and .45, respectively.
The PDS2 ‘proxy‘ measure (blunted affect minus depression) exhibited the highest sensitivity (86.4%) for the over estimate of cases. The next highest sensitivity was exhibited by the Factor2 (79.5%), and the PDS1 ‘proxy’ measure exhibited a low sensitivity of 61.4%. Specificity levels ranged from a low of 78.6% for the PDS1 ‘proxy’ measure to a high of 80.4% for the emotional withdrawal item. The false positive rates ranged from 19.6% for the emotional withdrawal item to 21.4 for both PDS1 and Factor2. PDS2 exhibited the highest kappa statistic at .59, which is indicative of moderate/good agreement. All other kappa’s ranged from .38 for PDS1 to .53 for Factor2 (see Table 4.).
2.4 Logistic regression Analysis
For the logistic regression, gender and education proved to be significant predictors of the deficit syndrome at the first step. The forward stepping procedure subsequently entered blunted affect, PDS1, the PANSS negative factor and the PANSS dysthymia factor as significant predictors to the Deficit Syndrome. With the entry of the PANSS dysthymia factor, the blunted affect symptom was removed from the model (see Table 5). The Deficit Syndrome was significantly more prevalent among males, and was associated with a lower education level. Higher PDS1 scores, and higher PANSS negative and dysthymia factors were significant predictors of the Deficit Syndrome.
Table 5.
Results of the Logistic Regression Analysis predicting to Deficit Syndrome status (coded as 0 = DS negative, 1 = DS positive). Gender and education were entered at the first step as control covariates, and all other Proxy and PANSS measures were forward stepped into the Logistic Regression.
| Variables in the Equation at the final step | ||||||
|---|---|---|---|---|---|---|
| Variable | B | S.E. | Wald | Sig. | Exp (B) | 95% CI |
| Gender | −.799 | .484 | 2.73 | .099 | .450 | .174–1.161 |
| Education | −.169 | .079 | 4.56 | .033 | .845 | .724–.986 |
| PDS1 | .626 | .139 | 20.19 | .000 | 1.870 | 1.42–2.45 |
| PANSS Negative | .105 | .030 | 12.40 | .000 | 1.111 | 1.04–1.17 |
| PANSS Dysthymia | .299 | .106 | 7.93 | .005 | 1.349 | 1.09–1.66 |
Discussion
These results demonstrate that ‘proxy’ measures derived from the PANSS (a clinical state rating) to identify the Deficit Syndrome, may be a viable alternative in identifying a subgroup of rigorously diagnosed schizophrenia patients with the deficit syndrome when the use of the actual SDS is not feasible. Similar to Kirkpatrick et al. (1993; using the BPRS), our DS group as defined by the ‘proxy’ tool derived from the PANSS, exhibited higher PDS1 and PDS2 scores, higher blunted affect and emotional withdrawal and higher Factor2 scores. Depression was marginally lower within the DS group. They reported lower Psychosis scores among their DS subjects however, our two groups had similar Psychosis scores. The ROC results were impressive demonstrating significant findings for five ‘proxy’ measures (PDS1, PDS2, blunted affect and emotional withdrawal and Factor2) as well as for the PANSS negative factor, and corroborating the MANCOVA results.
The results of the Logistic Regression showed PDS1 to be the most salient association with the deficit syndrome, along with the PANSS negative and dysthymia factors. The above suggest strong associations between the various proxy measures and the DS, however, due to the dichotomous nature of the Deficit Syndrome criteria, the true empirical assessment of the ‘proxy’ identification tool should be the cross tabular comparison of it to the actual SDS assessments. For this the specificity numbers were high and the false positive rates were low for both the over and under estimate conditions. The sensitivity numbers ranged from poor to good (18.2% – 61.4%) for the under-estimate comparisons, and good to very good (61.4% – 86.4%) for the over-estimate comparisons. The kappa statistics all fell short of the .60 or higher level indicative of good agreement. The most notable finding here is for the PDS2 ‘proxy’ measure (blunted affect item – depression item). For the over estimation of cases it exhibited a sensitivity of 86.4%, a specificity of 78.6%, a false positive rate of 20.5% and a kappa statistic of .59.
Compared to Subotnik et al. (1998) the PANSS derived ‘proxy’ tools exhibited a slightly lower specificity, paired with a somewhat better sensitivity level. It should also be noted that our false positive rate was consistent across ‘proxy’ tools, averaging 21% for the over-estimate and about 10% for the under-estimate conditions. This was substantially lower then the 35% false positive rate reported by Subotnik et al. Due to this high false positive rate, Subotnik reported that the initial presence of deficit syndrome features was not a good predictor of the syndrome longitudinally. The PANSS like the BPRS used by Subotnik et al. is also a cross-sectional instrument, and as such, would be thwart with the same problem of limited longitudinal application. However, our patients were uniformly assessed at or near discharge in a clinically stable state, or during treatment after several weeks at a fixed dose of anti-psychotic medication in a clinically stable state. The trait-like symptoms that are characteristic of the deficit syndrome should still be present during a clinically stable state. From the results it would seem that the ‘proxy’ measures are very good at identifying those that do not have the deficit syndrome, and moderately good at identifying those that do have the deficit syndrome.
In a meta-analysis examining gender and the deficit syndrome Roy et al. (2001) reported strong associations between male gender and the deficit syndrome for studies using the SDS and detailed review methods, but not for studies using the PDS. They suggest that this lack of association between male gender and the deficit syndrome may be due to the PDS instrument being less accurate than the SDS. Our results regarding gender clearly showed a predominance of males with the actual deficit syndrome, however, similar to Roy et al. (2001), the main proxy measure for the under estimate of cases showed only a weak trend for male preponderance (21.9% versus 12.5%, chisquare = 2.79, df=1, p=.095) and nothing for the over estimate of cases.
Recent work from our group (Kimhy et al. 2005) has shown that the 6 symptoms assessed by the SDS factor analyze into two distinct factors, expressive prosody and volition, also seen in the work of Salem & Kring (1999). We performed several logistic regressions entering these two distinct factors as independent measures predicting to the actual SDS and PDS1 (using over and under estimates of the cases), while controlling for gender, age, education and duration of illness. Both factors showed significant association to the actual SDS, indicating their independence from each other. However, the expressive prosody factor exhibited a stronger association. Using the PDS1 as the dependent measure, only the expressive prosody factor was significantly associated in the case of the over and under cases estimates. This indicates that the PDS1 is only getting at one aspect/factor, the expressive prosody factor as assessed by the blunted affect and emotional withdrawal items of the PANSS. It would seem that the volition factor is not accounted for by the PDS1 measure.
A major concern regarding the use of the PDS in identifying patients with the Deficit Syndrome is that it may dilute the homogeneity of the Deficit Syndrome as a distinct and important schizophrenia subgroup. The notion of the deficit syndrome as a homogeneous schizophrenia subgroup has been supported by empirical reports from many research and clinical domains that include poor premorbid adjustment (Galderisi et al. 2003), increased negative symptoms, neurological impairment (Galderisi et al. 2003), cognitive impairment (Buchanan et al. 1994, Buchanan et al. 1997, Ludewig et al. 2003), poor functioning (Tiryaki et al. 2003), clinical outcome (Tek et al. 2001), regional neuronal densities, regional Cerebral bloodflow (Gonul et al. 2003, Vaiva et al. 2002, Yurekli et al. 2003). The majority of these studies have relied upon the SDS to identify patients with the Deficit Syndrome. Although utilizing a PDS has the potential to open up archived data sets to investigations of the DS (i.e., by reanalyzing symptom data to derive PDS) and making the categorization more feasible in future work, further study is needed to determine if such schemes are reliable.
In our sample of rigorously diagnosed schizophrenia inpatient, using symptoms from the Positive and Negative Symptom Scale, we found the ‘proxy’ identification tool PDS to be a reliable, accurate viable alternative for identification of the Deficit Syndrome. While our sensitivity and specificity levels were somewhat lower than Kirkpatrick et al. (1993), they are at a level that suggests a good degree of utility might be gained from there use in situations where the use of the SDS is not feasible.
Table 1.
ANOVA results for the Demographic measures by Deficit Syndrome and Gender.
| Non-Deficit | Deficit | DS | Gender F | |||
|---|---|---|---|---|---|---|
| Male (N=75) | Females (N=37) | Males (N=38) | Females (N=6) | F(df) / p | F(df) / p | |
| Age | 33.7 +/− 10.5 | 33.1 +/− 9.0 | 33.0 +/− 10.8 | 31.2 +/− 6.2 | F(1/152)=0.31, .58 | F(1/152)=0.24, .62 |
| Age at Onset Of Psychotic Symptoms | 20.5 +/− 4.9 | 21.0 +/− 7.3 | 20.5 +/− 7.0 | 22.7 +/− 6.1 | F(1/148)=0.33, .57 | F(1/152)=0.79, .38 |
| Education | 12.7 +/− 2.6 | 13.1 +/− 2.5 | 12.1 +/− 3.1 | 12.3 +/− 2.1 | F(1/147)=1.01, .32 | F(1/147)=0.17, .68 |
| Duration | 13.4 +/− 9.8 | 11.8 +/− 9.1 | 12.8 +/− 9.8 | 8.5 +/− 10.8 | F(1/148)=0.63, .41 | F(1/148)=1.54, .22 |
NB: there were no significant interactions of DS and Gender.
Table 3.
Receiver – Operator Characteristics (ROC) Analysis results. Area under the ROC curves and the 95% Confidence Intervals for each of the Proxy measures included for analysis.
| Area Under the Curve | |||||
|---|---|---|---|---|---|
| Test Result Variable(s) | Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval
|
|
| Lower Bound | Upper Bound | ||||
|
| |||||
| PANSS Negative | .825 | .036 | .000 | .754 | .895 |
| PDS1 | .765 | .043 | .000 | .680 | .849 |
| PDS2 | .861 | .034 | .000 | .793 | .928 |
| Blunted affect | .840 | .035 | .000 | .771 | .909 |
| Emotional withdrawal | .763 | .042 | .000 | .681 | .846 |
| Factor 2 | .842 | .034 | .000 | .775 | .909 |
The test result variable(s): PANSS Negative, Proxy 1, Proxy 2, Blunted affect, Emotional withdrawal, Factor 2 have at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
Under the nonparametric assumption
Null hypothesis: true area = 0.5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA. Stability of the diagnosis of deficit syndrome schizophrenia. Amer J Psychiatry. 1999;156:637–39. doi: 10.1176/ajp.156.4.637. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT., Jr Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry. 1994;51:804–811. doi: 10.1001/archpsyc.1994.03950100052005. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WT., Jr Attentional impairments in deficit and nondeficit forms of schizophrenia. Amer J Psychiatry. 1997;154:363–70. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: The concept. Amer J of Psychiatry. 1988;145:578–83. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Amer J Psychiatry. 1994;151:351–56. doi: 10.1176/ajp.151.3.351. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Maj M, Mucci A, Cassano GB, Invernizzi G, Rossi A, Vita A, Dell’Osso L, Daneluzzo E, Pini S. Historical, psychopathological, neurological, and neuropsychological aspects of deficit syndrome schizophrenia: A Multicenter Study. Amer J Psychiatry. 2002;159:983–990. doi: 10.1176/appi.ajp.159.6.983. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Kula M, Esel E, Tutus A, Sofuoglu S. A Tc-99m HMPAO SPECT study of regional cerebral blood flow in drug-free schizophrenic patients with deficit and non- deficit syndrome. Psychiatry Research. 2003;123:199–205. doi: 10.1016/s0925-4927(03)00067-2. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13 (2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. Br J Psychiatry Suppl. 1989;7:59–67. [PubMed] [Google Scholar]
- Kay SR, Opler LA. Deficit or negative syndrome? Am J Psychiatry. 1989;146(2):282–283. doi: 10.1176/ajp.146.2.282. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. Positive and Negative Syndrome Scale (PANSS) Manual. Multi-Health Systems; Toronto: 1992. [Google Scholar]
- Kimhy D, Yale S, Goetz R, Marcinko L, Malaspina D. Factorial Structure of the Schedule for the Deficit Syndrome. Schizophrenia Bulletin. 2005 Sep 21; doi: 10.1093/schbul/sbi064. EPUB ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenny PD, Alphs LD, Carpenter WT. The Schedule for the deficit syndrome: An instrument for research in schizophrenia. Psychiatry Research. 1989;30:119–23. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Research. 1993;47:47–56. doi: 10.1016/0165-1781(93)90054-k. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Ram R, Bromet E. The deficit syndrome in the Suffolk County Mental Health Project. Schizophrenia Research. 1996;22:119–126. doi: 10.1016/s0920-9964(96)00057-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Ram R, Amador XF, Buchanan RW, McGlashan T, Tohen M, Bromet E. Summer birth and the deficit syndrome of schizophrenia. Amer J Psychiatry. 1998;155:1221–1226. doi: 10.1176/ajp.155.9.1221. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Castle D, Murray RM, Carpenter WT., Jr Risk factors for the deficit syndrome of schizophrenia. Schizophrenia Bulletin. 2000;26:233–242. doi: 10.1093/oxfordjournals.schbul.a033443. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Paulus MP, Vollenweider FX. Behavioral dysregulation of decision- making in deficit but not nondeficit schizophrenia patients. Psychiatry Research. 2003;119:293–306. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Messias E, Kirkpatrick B. Summer birth and the deficit syndrome of schizophrenia in The Epidemiological Catchment Area study. J Nervous and Mental Dis. 2001;189:608–612. doi: 10.1097/00005053-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T from the NIMH Genetics Initiative collaborators. Diagnostic Interview for Genetic Studies: Rationale, unique features, and training. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Roy MA, Maziade M, Labbe A, Merette C. Male gender is associated with deficit schizophrenia: a meta-analysis. Schizophrenia Research. 1999;47:141–47. doi: 10.1016/s0920-9964(99)00231-5. [DOI] [PubMed] [Google Scholar]
- Salem JE, Kring AM. Flat affect and social skills in schizophrenia: Evidence for their independence. Psychiatry Res. 1999;87:159–167. doi: 10.1016/s0165-1781(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Subotnik KL, Nuechterlein KH, Ventura J, Green MF, Hwang SS. Prediction of the deficit syndrome from initial deficit symptoms in the early course of schizophrenia. Psychiatry Research. 1998;80:53–59. doi: 10.1016/s0165-1781(98)00052-3. [DOI] [PubMed] [Google Scholar]
- Subotnik KL, Nuechterlein KH, Ventura J. MMPI discriminators of deficit vs. nondeficit recent-onset schizophrenia patients. Psychiatry Research. 2000;93:111–123. doi: 10.1016/s0165-1781(00)00102-5. [DOI] [PubMed] [Google Scholar]
- Tek C, Kirkpatrick B, Kelly C, McCreadie RG. Summer birth and deficit schizophrenia in Nithsdale, Scotland. J Nerv Ment Dis. 2001;189:613–617. doi: 10.1097/00005053-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Tek C, Kirkpatrick B, Buchanan RW. A five-year followup study of deficit and nondeficit schizophrenia. Schizophrenia Research. 2001;30:253–60. doi: 10.1016/s0920-9964(00)00146-8. [DOI] [PubMed] [Google Scholar]
- Tiryaki A, Yazici MK, Anil AE, Kabakci E, Karaagaoglu E, Gogus A. Reexamination of the characteristics of the deficit schizophrenia patients. European Arch Psychiatry and Clinical Neuroscience. 2003;253:221–227. doi: 10.1007/s00406-003-0434-5. [DOI] [PubMed] [Google Scholar]
- Vaiva G, Cottencin O, Llorea PM, Devos P, Dupont S, Mazas O, Rascle, Thomas P, Steinling M, Goudemand M. Regional cerebral blood flow in deficit/nondeficit types of schizophrenia according to SDS criteria. Prog Neuropsychopharmacology Biol Psychiatry. 2003;26:481–5. doi: 10.1016/s0278-5846(01)00292-5. [DOI] [PubMed] [Google Scholar]
- White L, Harvey PD, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale The PANSS Study Group. Psychopathology. 1997;30:263–74. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- Yurekli Y, Bodur Z, Gulseren L, Mete L. Comparison of regional cerebral blood flow in deficit and nondeficit schizophrenic patients. Turk Psikiyatri Derg. 2003;14:255–62. [PubMed] [Google Scholar]