Abstract
Strict regulation of gene expression is critical for the development of the malaria parasite within multiple host cell types. However, much remains unexplored regarding gene regulation in Plasmodium falciparum with only a few components of the gene regulation machinery identified thus far. Better characterization of transcript structures with precise mapping of transcript ends will greatly aid in the search of conserved regulatory sequences in the genome. Transcript analysis of maebl, a member of the ebl gene family, in P. falciparum intra-erythrocytic stages has revealed a unique transcript structure for maebl. The 5′ untranslated region of maebl transcript is exceptionally long (>2 kb) with a small multi-exon open reading frame, annotated as a putative mitochondrial ATP synthase (PF11_0485) in the Plasmodium database. Northern blot hybridizations and RT-PCR analysis confirmed a bicistronic message for maebl along with PF11_0485. We further identified the minimal maebl promoter to be upstream of PF11_0485 by using transient chloramphenicol acetyl transferase (CAT) reporter assays. The occurrence of a bicistronic mRNA in Plasmodium is both novel and unusual for a lower eukaryote and adds on to the complexity of gene regulation in malaria parasites.
Index descriptors: Plasmodium falciparum, malaria, maebl, gene regulation, promoter, bicistronic mRNA
1. Introduction
Malaria parasites must invade and multiply within different cell types to sustain their life cycle. Through the course of development in both the vertebrate and the invertebrate hosts, malaria parasites exhibit a strictly regulated pattern of gene expression. Whole-genome transcription profiles have been extremely valuable in providing insights into levels and timing of gene expression in Plasmodium (Bozdech, et al., 2003, Le Roch, et al., 2003). The unavailability of transcript structure for most genes however complicates the precise identification of regulatory regions in the Plasmodium genome.
Identifying conserved regulatory motifs is further hindered in Plasmodium by its AT-rich genome and the presence of extensive repeat sequences. Some recent bioinformatic studies have hence used special algorithms that take into account the unique genetic composition of Plasmodium and identified conserved regulatory motifs in different categories of genes (Young, et al., 2008) (Wu, et al., 2008). Most Plasmodium promoters also contain an over-representation of homopolymeric tracts (dA:dT) (Horrocks, et al., 1998), which could contribute to transcription factor binding and transcriptional regulation as in other organisms (Hori and Firtel, 1994, Struhl, 1985, Winter and Varshavsky, 1989). Although Plasmodium lacks many components of the conserved eukaryotic transcription machinery, the recent characterization of ApiAp2-mediated regulation of gene expression identifies a conserved mechanism of gene regulation in apicomplexan parasites and plants (De Silva, et al., 2008).
maebl is a paralogue of the ebl gene family in P. falciparum that encodes erythrocyte-binding ligands EBA175 and BAEBL (EBA140). maebl transcripts are found in mid-trophozoite stages during intra-erythrocytic development and are expressed at maximum levels in the mosquito midgut sporozoite stages (Blair, et al., 2002, Le Roch, et al., 2003). maebl stands out as a classic example of complex gene structure in Plasmodium, with alternative splicing creating distinct MAEBL isoforms (Saenz, et al., 2008, Singh, et al., 2004). In this study, we further analyzed maebl in P. falciparum erythrocytic stages by characterizing its transcript structure and identifying its promoter sequences.
2. Materials and Methods
2.1. Parasite culture and maintenance
Clones of Plasmodium falciparum 3D7 were obtained from the Naval Medical Research Center and maintained in culture according to standard methods at 37 °C and gassing (5% O2, 5% CO2, Nitrogen balanced) with 5% hematocrit in RPMI 1640 (Invitrogen) supplemented with 0.5% Albumax I (Invitrogen), 0.25% sodium bicarbonate and 0.01mg/ml gentamicin. Human red blood cells were obtained from Indiana Blood bank and washed three times with RPMI 1640 (Invitrogen), resuspended to 50% hematocrit and stored at 4°C.
2.2. Genomic DNA and RNA extraction
Plasmodium falciparum genomic DNA was purified from blood-stage parasites using a standard phenol/chloroform method as described before (Balu, et al., 2005). Total RNA was isolated using TRI REAGENT (Molecular Research Center, Inc) RNA isolation protocol and mRNA was then isolated by purification through an oligo(dT) cellulose column.
2.3. Northern blot hybridizations and RT-PCR
Two μg of P. falciparum 3D7 total RNA was separated by formaldehyde gel electrophoresis along with a 0.5 kb-10 kb RNA ladder (Invitrogen). The gel was then blotted by standard methods on to a nylon membrane and hybridized overnight at 65°C with respective 32P-labeled probes for maebl (PCR amplified from genomic DNA with JA-503 and JA-504) and PF11_0485 (PCR amplified from genomic DNA with JA-739 and JA-741) in 10 ml of 0.1 M disodium hydrogen phosphate with 1% SDS. The blot was washed three times at 65°C with washing solution (0.3 M disodium hydrogen phosphate; 1 mM EDTA; 1% SDS), dried and exposed to an X-Ray film for 2 days at −80°C. RT-PCR for the entire 5′ region of maebl was performed using the one-step RT-PCR kit (Invitrogen) with primers JA-740 and JA-712 (Table 1).
Table 1.
List of primers used in RACE and cloning experiments.
| Primer | Sequence |
|---|---|
| JA-503 | 5′-AATAACATGAAAGGAAATAATAATG-3′ |
| JA-504 | 5′-TGAAAATTCCTTTTTTTTAAAAC-3′ |
| JA-635 | 5′-ATAGGTACCGGATTTATATAATATATTTATG-3′ |
| JA-636 | 5′-ATAAAGCTTATTAAGGAAACAAAATGAAAG-3′ |
| JA-650 | 5′-AAGTGAGTTAAAATGTAAAAGAT-3′ |
| JA-651 | 5′-CTCCATTTATCATCCGTAGCATC-3′ |
| JA-676 | 5′-GCAATCCTTTAAGTTATTCGGAAG-3′ |
| JA-712 | 5′-CATATTTGAATTTCCCCTTTCCATGTATCCCAGAGTTTG-3′ |
| JA-739 | 5′-GAACAAGAAGAATATTTTTTAGTACTGC-3′ |
| JA-741 | 5′-GGCATGCATGGAGAACTACC-3′ |
| JA-817 | 5′-CCAACATGTGTACTGAAAAAGG-3′ |
| JA-818 | 5′-GGGGTCATACTCCTTCATGG-3′ |
| JA-837 | 5′-ATACTCGAGCACATACTACTAAAAACGTTCACAC-3′ |
| JA-838 | 5′-ATAGGATCCCATCTTTCCTCTTGATTTTGCTG-3′ |
| JA-1220 | 5′-ATAGGATCCCAGGTTCAATACAGCTCTTAAG-3′ |
| JA-1221 | 5′-ATAGGTACCCTTATAATGTCTGCTCGAAGC-3′ |
| JA-1433 | 5′-CGGGATCCATTTATTACAATAAAATAAAAGAATATG-3′ |
2.4. Random amplification of cDNA ends (RACE)
The SMART™ RACE cDNA amplification kit (Clontech) was used to identify the transcript ends of maebl by performing 5′ RACE and 3′ RACE. The gene-specific primers used for 5′ RACE of maebl were JA-650 and JA-651 (Table 1). The primer used for 3′ RACE of maebl was JA-676 (Table 1). The conditions for the PCR were 94°C for 1 min followed by 35 cycles of 15 s at 94°C; 30 s at 49°C; 1 min at 65°C.
2.5. PCR amplification, cloning and sequencing
The primer sequences used in all the cloning experiments are summarized in Table 1. Two different regions in the 5′ sequences of maebl were amplified from P. falciparum 3D7 genomic DNA as follows: mp: JA-837 and JA-838; 35 cycles of 15 s at 94°C; 30 s at 45°C; 1 min at 65°C; wm: JA-837 and JA-1433; 35 cycles of 15 s at 94°C; 30 s at 45°C; 2 min at 65°C. The coding sequence of chloramphenicol acetyl transferase (cat) was amplified from the vector pCAT (Promega) by using primers JA-1220 and JA-1221. The PCR conditions were 35 cycles of 15 s at 94°C; 30 s at 48°C; 1 min at 65°C. The PCR products and the RACE PCR-amplified products were cloned into the pCR-2.1 vector by using the TOPO-TA cloning kit (Invitrogen) and sequenced using M13 forward and reverse primers.
2.6. Plasmid constructs
p11zCH: 3′ hsp 86 was amplified as a KpnI/Hind III from P. falciparum 3D7 genome using primers JA- 635 and JA- 636 and T-cloned into pCR2.1 (Invitrogen). Chloramphenicol acetyl transferase coding sequence was amplified as a BamHI/KpnI fragment from the plasmid vector pCAT (Promega) and cloned into pCR2.1 upstream to 3′ hsp 86. The cat coding sequence along with 3′ hsp 86 was excised as a BamHI/HindIII fragment from pCR2.1 and cloned into the vector pGEM-11zf(+) (Promega). p11zCH-mp and p11z-CH-wm: These reporter constructs for maebl were created by cloning the two different regions of maebl 5′ sequences into the vector p11zCH using XhoI and BamHI.
2.7. Exonuclease digestions
Unidirectional exonuclease digestions were performed on the maebl reporter plasmids by using the Erase a Base System (Promega). In brief, plasmid DNA was digested first with AatII and then with Eco01019 and exonuclease digestions were performed according to the manufacturer’s protocol at 22°C and the plasmids containing deletions were then sequenced to identify the deleted sequences.
2.8. Transient transfection of Plasmodium falciparum and Chloramphenicol acetyl transferase (CAT) reporter assays
Plasmodium falciparum 3D7 parasites were transfected as described previously (Wu, et al., 1995). Briefly, Plasmodium falciparum ring stage parasites were synchronized with 5% sorbitol in RPMI 1640 and after one generation of growth, the parasite culture was split into four equal volumes for transfection such that equal number of parasites were transfected with the four different plasmids pHC1CAT, p11zch-mp, p11zch-Δmp1 and p11zch-Δmp2. Parasites were electroporated at 0.31 KV and 950 μF capacitance in 0.2 cm cuvette using a Bio-Rad gene pulser III and reintroduced into a 5 ml culture at 5 % hematocrit. CAT assays were performed 48 hrs later at young trophozoite-stages as described previously (Balu and Adams, 2003) using a FAST CAT green substrate reagent (Invitrogen).
3. Results
3.1. Transcript analyses of maebl
Northern hybridization with a probe in the 3′ coding region of maebl identified a transcript slightly greater than 8 kb for maebl (Figure 1A). The coding sequence for maebl is approximately 6.2 kb in length, indicating that maebl untranslated regions (UTRs) were around 2 kb long. In order to characterize the individual lengths of 5′ and 3′ UTRs of maebl, random amplification of cDNA ends (RACE) was performed on maebl transcripts. Multiple transcription start sites, close to each other, were identified for maebl at 2163 bp, 2139 bp, 2073 and 1937 bp upstream of the translational start codon. (Figure 1B). No RACE products were obtained while using different sets of primers near the start of maebl coding sequence (data not shown) probably due to the inefficiency of the method to amplify greater than 2kb sequences. Two transcription stop sites were identified for maebl at 191 bp and 267 bp downstream of the translational stop codon by using 3′ RACE (Figure 1B).
Fig. 1. Transcript analysis of P. falciparum maebl.
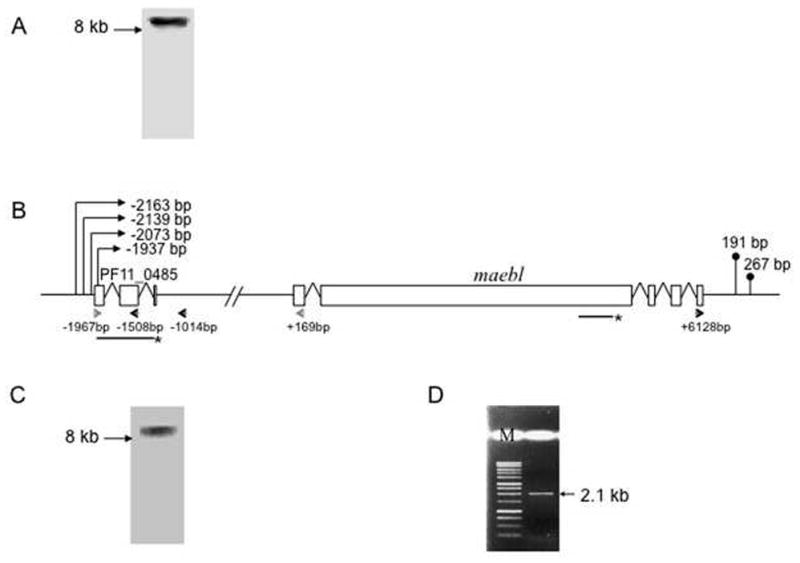
A. Northern hybridization with a probe in the 3′ region of maebl identified a slightly >8kb transcript for maebl. B. A schematic representation of maebl gene structure with the identified transcription start and stop sites and the location of primers and probes (marked with *). Four transcription start sites and two stop sites were mapped for maebl using RACE. Gene specific primers used in RACE are shown as single black arrows. A conserved, multi-exon open reading frame (ORF), annotated as PF11_0485, is present in the 5′ untranslated region of maebl. C. Northern hybridization performed on P. falciparum 3D7 total RNA with PF11_0485 coding sequence as a probe identified a >8 kb transcript similar to that seen with the maebl probe, thereby suggesting a bicistronic transcript for maebl and PF11_0485. D. RT-PCR was performed on P. falciparum 3D7 total RNA with a sense primer in the first exon of PF11_0485 and an antisense primer in the maebl coding sequence (shown as single grey arrows). The 2.1 kb product was confirmed by sequencing to contain maebl along with the intervening 1.3 kb region and the appropriately spliced PF11_0485 coding sequence (M-marker).
3.2. A possible bicistronic transcript for maebl
Sequence analysis of maebl 5′ UTR revealed a multi-exon open reading frame, designated as a mitochondrial ATP-synthase subunit (PF11_0485) in the Plasmodium database (PlasmoDB, ver. 5.4) (Kissinger, et al., 2002) that is well conserved in different Plasmodium species (Figure 1B). To investigate whether this open reading frame is a part of maebl transcript, we repeated the northern hybridization for maebl by using PF11_0485 as a probe and a similar >8 kb transcript was seen suggesting PF11_0485 to be a part of maebl transcript (Figure 1C). As further confirmation, RT-PCR was performed with a sense primer (JA-739) at the start of PF11_0485 coding sequence and an antisense primer in the maebl coding sequence (JA-712). A 2.1 kb fragment was amplified that was confirmed by sequencing to contain the spliced PF11_0485 transcript, the intervening 1.3 kb region and the beginning of maebl transcript (Figure 1D).
3.3. Identification and characterization of maebl promoter
To identify maebl promoter, two different 5′ regions of maebl were cloned into a chloramphenicol acetyl transferase (CAT) reporter construct, p11z-CH that was created for transient expression in P. falciparum. maebl putative promoter (mp)- contained a 329 bp region in 5′ maebl from 2303 bp to 1975 bp upstream of maebl start codon. Whole 5′ maebl (wm)- contained a 2.3 kb 5′ region of maebl from 2303 bp to 1 bp upstream of maebl start codon (Figure 2A). Forty eight hours post transfection with the maebl reporter constructs, parasite CAT activity was assayed and compared to the positive control plasmid pHC1-CAT (Crabb and Cowman, 1996). Parasites transfected with the 5′ region ‘mp’ showed significant CAT expression confirming its promoter activity whereas those transfected with the whole 5′ region of maebl showed very little promoter activity (Figure 2B). To identify the minimal promoter region within ‘mp’, unidirectional exonuclease digestions were performed on the plasmid p11z-CH-mp resulting in two 5′ deletions. The first deletion removed −2303 bp to −2112 bp from mp and the second deletion removed −2303 to −2053 bp giving raise to p11z-CH-Δp 1 and p11z-CH-Δmp 2, respectively. Both these deletions significantly reduced the promoter activity of ‘mp’, suggesting that the 5′ region from -2302 bp to -2112 bp contains maximum promoter activity (Figure 2B). A closer look at the sequences in this promoter region showed a poly (dA) repeat and an (ATT)10 tri nucleotide repeat (Figure 2C) that have been previously described in promoter regions of other organisms.
Fig. 2. Reporter assay analysis identifies possible promoter region of P. falciparum maebl.
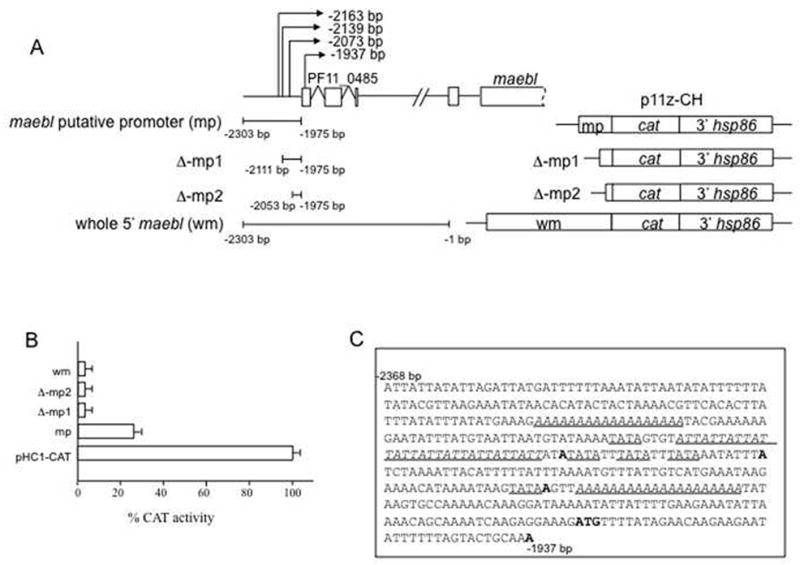
A. Two different regions from 5′ maebl were first tested for promoter activity. maebl putative promoter (mp) consisted of a 329 bp region upstream of PF11_0485. Whole 5′ maebl (wm) contained mp along with the rest of the 5′ UTR of maebl. The two maebl 5′ regions were cloned individually into a chloramphenicol acetyl transferase (CAT) reporter plasmid, p11z-CH, created for transient assays in P. falciparum. Unidirectional exonuclease deletions on mp resulted in Δ-mp1 and Δ-mp2. B. Forty eight hours post-transfection, the parasite cell lysate was assayed for CAT expression. CAT activity was compared to the control plasmid pHC1-CAT that contained a strong, constitutive calmodulin promoter. The 5′ region ‘mp’ around the transcription start sites showed significant promoter activity that was greatly reduced in the deletions Δ-mp1, Δ-mp2. The 5′ region ‘wm’ also displayed only slightly detectable promoter activity. C. Sequences around the transcription start sites show the possible cis regulatory motifs in 5′ maebl (italicized and underlined). Homopolymeric (dA) tracts are seen along with (ATT)10 trinucleotide repeats, which are known to cause structural changes in the DNA. Some TATA boxes are also seen around the start sites. Shown in bold are the four transcription start sites mapped for maebl and the start codon of PF11_0485.
4. Discussion
A member of the P. falciparum ebl gene family of ligands, maebl, serves as a classic example of complex gene regulation mechanisms in this malaria parasite. A cDNA clone from the rodent parasite P. yoelii previously identified the 5′ UTR to be approximately 1.8 kb long (Kappe, et al., 1998). Although uncommon, such long 5′ UTRs have been reported in Plasmodium (Militello, et al., 2004, Myrick, et al., 2003, Porter, 2001). maebl is also alternatively spliced in different Plasmodium species that results in different possible isoforms of the protein (Singh, et al., 2004). In this study we intended to further characterize the transcript structure of maebl in P. falciparum blood stages and identify its promoter sequences.
By using RACE, multiple transcription start sites were mapped for maebl with the farthest start site located approximately 2.1 kb upstream of the translational start codon. Even though RACE is known to yield some false-positive results, the close location of the identified transcription sites strongly suggests the actual start site for maebl to be in this region. Two transcription stop sites were mapped for maebl using 3′ RACE at around 0.2 kb and a consensus eukaryotic polyadenylation signal ATTAAA was found 15 bp before the second stop site.
Sequence analysis of 5′ UTR of maebl identified a 0.7 kb region in the 5′ UTR that is conserved in P. falciparum and the rodent parasites, P. yoelii and P. berghei and contains a small predicted open reading frame (PF11_0485) of 465 bp that spans two introns. Northern blot hybridizations using a probe corresponding to PF11_0485 and RT-PCR analysis confirmed a bicistronic message for PF11_0485 and maebl, which has not been reported in Plasmodium thus far. In bicistronic mRNAs of other organims, ribosomes re-initiate translation at the downstream start codon and the re-initiation efficiency increases with an increase in the length of the inter-cistronic region and a decrease in the length of the upstream ORF (Kozak, 1987). Both these scenario correspond well to PF11_0485 and maebl, where the inter-cistronic region is approximately 1.3 kb and the upstream gene, PF11_0485, codes for a short open reading frame. The presence of this bicistronic message for maebl in other parasite life cycle stages as well as the functional importance of its product remain to be investigated.
Two different 5′ regions of maebl: a small 329 bp region just upstream to PF11_0485 (mp) and this region along with the rest of the 5′ UTR (wm), were tested for promoter activity by transient CAT assays in P. falciparum. The 5′ region ‘mp’ displayed significant promoter activity in the parasite blood stages and loss of CAT expression following exonuclease digestions on this region, further deduced the promoter activity to the first 192 bp of this region. The striking features in this promoter region were the presence of long homopolymeric (dA:dT) tracts that have been shown to be present in Plasmodium promoters previously (Horrocks, et al., 1998) and (ATT)10 tri nucleotide repeats that have been previously reported in other organisms to cause DNA instability and are known to play a role in gene regulation by allowing interactions with gene regulatory proteins (Stallings, 1994, Trotta, et al., 2000). However, an exhaustive analysis of the 5′ regions in the genome will be required to confirm the prevalence of these structures in Plasmodium promoters. Significant reduction in promoter activity with the entire 5′ region of maebl (wm) suggests the presence of possible silencing elements. However, several regions of 5′ maebl would need to be analyzed individually to identify such regions with possible enhancer/silencer activity.
In summary, maebl is transcribed along with the upstream gene, PF11_0485, as a bicistronic transcript and a minimal 5′ region with promoter activity was mapped upstream to PF11_0485 and the mapped transcription start sites. Our study on the transcript structure of P. falciparum maebl has thus revealed another novel and intriguing aspect of Plasmodium gene regulation that further adds to the uniqueness of this parasitic protozoan.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, R01 AI033656. We thank Kirk Deitsch for his helpful advice on the original design of the reporter assay.
Abbreviations
- CAT
chloramphenicol acetyl transferase
- EBA175
erythrocyte binding antigen-175
- EBA140
erythrocyte binding antigen-175
- RACE
random amplification of cDNA ends
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balu B, Adams JH. Fluorescent chloramphenicol as a substitute for radioactive [14C]-chloramphenicol for CAT reporter assays in Plasmodium falciparum. Molecular and Biochemical Parasitology. 2003;126:285–286. doi: 10.1016/s0166-6851(02)00285-2. [DOI] [PubMed] [Google Scholar]
- Balu B, Shoue DA, Fraser MJ, Jr, Adams JH. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16391–16396. doi: 10.1073/pnas.0504679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PL, Witney A, Haynes JD, Moch JK, Carucci DJ, Adams JH. Transcripts of developmentally regulated Plasmodium falciparum genes quantified by real-time RT-PCR. Nucleic Acids Research. 2002;30:2224–2231. doi: 10.1093/nar/30.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The Transcriptome of the Intraerythrocytic Developmental Cycle of Plasmodium falciparum. PLoS Biology. 2003;1(1):e5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML, Llinas M. Specific DNA-binding by apicomplexan AP2 transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8393–8398. doi: 10.1073/pnas.0801993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori R, Firtel RA. Identification and characterization of multiple A/T-rich cis-acting elements that control expression from Dictyostelium actin promoters: the Dictyostelium actin upstream activating sequence confers growth phase expression and has enhancer-like properties. Nucleic Acids Research. 1994;22:5099–5111. doi: 10.1093/nar/22.23.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks P, Dechering K, Lanzer M. Control of gene expression in Plasmodium falciparum. Molecular and Biochemical Parasitology. 1998;95:171–181. doi: 10.1016/s0166-6851(98)00110-8. [DOI] [PubMed] [Google Scholar]
- Kappe SH, Noe AR, Fraser TS, Blair PL, Adams JH. A family of chimeric erythrocyte binding proteins of malaria parasites. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1230–1235. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger JC, Brunk BP, Crabtree J, Fraunholz MJ, Gajria B, Milgram AJ, Pearson DS, Schug J, Bahl A, Diskin SJ, Ginsburg H, Grant GR, Gupta D, Labo P, Li L, Mailman MD, McWeeney SK, Whetzel P, Stoeckert CJ, Roos DS. The Plasmodium genome database. Nature. 2002;419:490–492. doi: 10.1038/419490a. [DOI] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Molecular and Cellular Biology. 1987;7:3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Militello KT, Dodge M, Bethke L, Wirth DF. Identification of regulatory elements in the Plasmodium falciparum genome. Molecular and Biochemical Parasitology. 2004;134:75–88. doi: 10.1016/j.molbiopara.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Myrick A, Munasinghe A, Patankar S, Wirth DF. Mapping of the Plasmodium falciparum multidrug resistance gene 5′-upstream region, and evidence of induction of transcript levels by antimalarial drugs in chloroquine sensitive parasites. Molecular Microbiology. 2003;49:671–683. doi: 10.1046/j.1365-2958.2003.03597.x. [DOI] [PubMed] [Google Scholar]
- Porter ME. The DNA polymerase delta promoter from Plasmodium falciparum contains an unusually long 5′ untranslated region and intrinsic DNA curvature. Molecular and Biochemical Parasitology. 2001;114:249–255. doi: 10.1016/s0166-6851(01)00263-8. [DOI] [PubMed] [Google Scholar]
- Saenz FE, Balu B, Smith J, Mendonca SR, Adams JH. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS ONE. 2008;3:e2287. doi: 10.1371/journal.pone.0002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Preiser P, Renia L, Balu B, Barnwell J, Blair P, Jarra W, Voza T, Landau I, Adams JH. Conservation and Developmental Control of Alternative Splicing in maebl Among Malaria Parasites. Journal of Molecular Biology. 2004;343:589–599. doi: 10.1016/j.jmb.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Stallings RL. Distribution of trinucleotide microsatellites in different categories of mammalian genomic sequence: implications for human genetic diseases. Genomics. 1994;21:116–121. doi: 10.1006/geno.1994.1232. [DOI] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta E, Del Grosso N, Erba M, Paci M. The ATT strand of AAT.ATT trinucleotide repeats adopts stable hairpin structures induced by minor groove binding ligands. Biochemistry. 2000;39:6799–6808. doi: 10.1021/bi0001473. [DOI] [PubMed] [Google Scholar]
- Winter E, Varshavsky A. A DNA binding protein that recognizes oligo(dA).oligo(dT) tracts. EMBO Journal. 1989;8:1867–1877. doi: 10.1002/j.1460-2075.1989.tb03583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Sieglaff DH, Gervin J, Xie XS. Discovering regulatory motifs in the Plasmodium genome using comparative genomics. Bioinformatics. 2008 doi: 10.1093/bioinformatics/btn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sifri CD, Lei HH, Su XZ, Wellems TE. Transfection of Plasmodium falciparum within human red blood cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, Johnson JR, Benner C, Yan SF, Chen K, Le Roch KG, Zhou Y, Winzeler EA. In silico discovery of transcription regulatory elements in Plasmodium falciparum. BMC Genomics. 2008;9:70. doi: 10.1186/1471-2164-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]


