Abstract
After being a neglected and poorly-understood disorder for many years, there has been a recent explosion of data regarding the complex pathogenesis of myelodysplastic syndromes (MDS). On the therapeutic front, the approval of azacitidine, decitabine, and lenalidomide in the last decade was a major breakthrough. Nonetheless, the responses to these agents are limited and most patients progress within 2 years. Allogeneic stem cell transplantation remains the only potentially curative therapy, but it is associated with significant toxicity and limited efficacy. Lack or loss of response after standard therapies is associated with dismal outcomes. Many unanswered questions remain regarding the optimal use of current therapies including patient selection, response prediction, therapy sequencing and combinations, and management of resistance. It is hoped that the improved understanding of the underpinnings of the complex mechanisms of pathogenesis will be translated into novel therapeutic approaches and better prognostic/predictive tools that would facilitate accurate risk-adaptive therapy.
Keywords: Myelodysplastic syndromes (MDS), Hypomethylating agents, Azacitidine, Decitabine, Lenalidomide, Immunosuppressive therapy, Chemotherapy, Erythropoiesis-stimulating agents, Allogeneic stem cell transplantation
1. Introduction
Myelodysplastic syndromes (MDS) comprise a group of biologically and clinically heterogeneous clonal hematopoietic neoplasms characterized by aberrant myeloid differentiation, dysplastic changes, ineffective hematopoiesis and increasing genomic instability that manifest clinically into peripheral blood (PB) cytopenias and variably increased rates of leukemic progression [1,2]. The age-adjusted incidence of MDS in USA has been estimated at 3.3 to 4.6 per 100,000 persons/year, corresponding to 15,000–20,000 new cases per year and slightly exceeding the incidence of AML [3,4]. As the incidence of MDS increases with age with a median age at diagnosis of 71 to 76 years, the number of patients will continue to increase with the increasing longevity of the population [4,5].
After being a neglected, poorly-understood hematologic disorder for many years, the last two decades have seen a significantly renewed interest in the disease and an explosion of data regarding the prognostication and the complex mechanisms underlying the pathogenesis of MDS [6]. On the therapeutic front, three agents have been specifically approved for MDS indications: the hypomethylating agents (HMAs) azacitidine and decitabine, and lenalidomide. These agents, in addition to supportive care, hematopoietic growth factors, immunosuppressive therapies (IST), and allogeneic stem cell transplantation (alloSCT), constitute the therapeutic interventions commonly used, typically deployed in a risk-adaptive fashion [7]. Decisions on how to apply and sequence these therapies in individual patients are usually based on disease-specific factors (e.g. karyotype, bone marrow [BM] blast percentage, severity of cytopenias and other factors whose collective effects are usually summed in different validated prognostic tools) and patient-specific factors (e.g. age, comorbidities, patient preference, etc.).
Although alloSCT remains the only known potentially curative therapy, a minority of patients undergo the procedure due to advanced age, medical comorbidities, and limited availability of appropriate stem cell donors [8]. Even for those patients who proceed to alloSCT, significant treatment-related mortality (TRM) and morbidity, including acute (a) and chronic (c) graft-versus-host-disease (GVHD), and high relapse rates compromise long-term disease-free survival [9]. The optimal selection of patients to undergo alloSCT, the timing of the procedure, the use of prior cytoreductive therapy or HMAs, the intensity of the conditioning regimen, the optimal use of maintenance strategies, and management of disease relapse are among the intensely debated issues in the field [7]. For the majority of patients who do not undergo alloSCT, the optimal use of available therapies including patient selection, treatment sequencing and combinations, and how to incorporate the newly discovered genetic and epigenetic lesions and the many available prognostic/predictive models in therapeutic decision-making remain largely unknown.
It is hoped that scientific advances in the next few years will shed more light on these questions. New therapeutic strategies will likely emerge based on our improved understanding of the complex pathogenesis of the disease and its underlying genetic, epigenetic, and immune aberrations [6,7]. Our prognostic and predictive tools will hopefully improve to allow for more accurate, evidence-based, risk-adaptive therapeutic approaches for MDS. In the following sections we will discuss the clinical use of the currently available pharmacologic therapies for MDS and briefly overview the future directions in the management of patients with MDS. Due to space limitations, we will not discuss the role and controversies of alloSCT in MDS.
2. Prognostication in MDS
MDS are heterogeneous not only because of the varied mechanisms of pathogenesis and morphologic appearance, but also in the natural history and outcomes of patients. The disease course in individual patients varies significantly from very symptomatic disease with survival limited to few months, to minimally symptomatic disease with survival of a decade or longer [3]. Most patients with MDS die from complications related to severe cytopenias rather than from leukemic progression. These observations were the first basis of the risk-adaptive therapeutic approach used for management of MDS. A vital component of the successful implementation of a risk-adaptive management approach is the ability to accurately predict the outcomes of patients with regard to expected survival and risk of leukemic progression. Accurate prediction of outcomes can be used to estimate the risk/benefit ratio of the various treatment options, especially disease-modifying interventions with incremental toxicities such as HMAs and alloSCT.
The first classification schemes for MDS were based on morphologic features and did not account for clinical parameters [10,11]. The French-American-British (FAB) system [10] uses pathologic criteria for classification of MDS while the World Health Organization (WHO) classification [11] accounts for some characteristic cytogenetic aberrations in addition to pathologic features, but still did not incorporate clinical parameters [6]. To account for the important prognostic clinical parameters, several prognostic tools have been developed and validated, each has certain limitations. An important shortcoming common to all of the clinically-used prognostic and predictive models is that the prognostic estimates generated using these tools generally reflect those of the risk-category to which the patient belongs and do not reflect that patient’s individual risk [6,12]. Additionally, most of these models do not include co-morbid conditions that can significantly limit both the patient’s survival and the extent to which therapies can be tolerated. Therefore, although generally accurate as a risk-category estimate, making therapeutic decisions for individual patients should merely be guided by these estimates and not completely rely on them. In other words, the prognostic estimate should be considered as only one element in the decision-making process and the limitations of its derivation (including the possibly wide variability in individual patient outcomes) should be taken into account.
The most commonly used clinical prognostication tool for patients with MDS is the International Prognostic Scoring System (IPSS) published in 1997 [Table 1] [13]. In this system, points are scored based on 3 criteria: the percentage of BM blasts, the number of PB cytopenias, and the cytogenetic risk-class. Based on the total point score, the patient is assigned to 1 of 4 risk-categories that vary significantly in outcomes: Low-risk (LR), intermediate-1 (INT-1), intermediate-2 (INT-2), and high-risk (HR). Despite its wide use, the IPSS suffers significant limitations. The IPSS was derived from a database of 806 patients with MDS, the majority of whom were treated only with supportive measures, and excluded patients with therapy-related MDS or proliferative chronic myelomonocytic leukemia (CMML) [13]. Moreover, the IPSS was intended for use only at the time of diagnosis, and did not incorporate other clinical variables with significant prognostic importance such red blood cell (RBC) transfusion-dependence and the severity of cytopenias and lineage dysplasia [12]. In order to address some of these limitations, the WHO Prognostic Scoring System (WPSS) was developed. This system accounts for the negative prognostic impact of multi-lineage dysplasia and RBC transfusion-dependence (which was subsequently replaced with severity of anemia) [14,15]. In contrast to the IPSS, the WPSS is a flexible prognostic tool that can be utilized not only at the time of diagnosis, but also in a dynamic fashion at different time points of the disease course [14,15].
Table 1.
The International Prognostic Scoring System(IPSS). This widely used prognostic tool was developed using a database of 806 MDS patients, the majority of whom were treated only with supportive measures. Patients with therapy-related MDS and proliferative chronicmyelomonocytic leukemia were excluded.
| Calculation of the IPSS
| |||||
|---|---|---|---|---|---|
| Score value | |||||
| Prognostic variable | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
| Percentage of BM blasts | <5 | 5–10 | – | 11–20 | 21–30 |
| Cytogenetic risk group | Good | Intermediate | Poor | ||
| Number of PB cytopenias | 0 or 1 | 2 or 3 | |||
| Assignment of the IPSS group and estimation of prognosis of untreated patients | |||||
| IPSS Risk category | Total points | Percentage of the IPSS population | Median survival (years) | Median time to 25% of patients progressing to AML (years) |
|---|---|---|---|---|
| Low | 0 | 33% | 5.7 | 9.4 |
| INT-1 | 0.5–1.0 | 38% | 3.5 | 3.3 |
| INT-2 | 1.5–2.0 | 22% | 1.1 | 1.1 |
| High | >2.5 | 7% | 0.4 | 0.2 |
BM: Bone marrow. PB: Peripheral blood. AML: Acutemyeloid leukemia. INT-1: Intermediate-1. INT-2: Intermediate-2. Note: Patients with 21–30% blasts are considered as AML according to the World Health Organization classification. Cytogenetic risk groups: Good: Normal, isolated Y–, isolated 5q–, or isolated 20q–; Poor: Complex with ≥3 abnormalities or chromosome 7 abnormalities; Intermediate; Other chromosomal aberrations. Cutoffs for PB cytopenias: absolute neutrophil count less than 1800/μl, platelet count less than 100,000/μL, Hemoglobin less than 10 g/dL. Adapted from Greenberg et al. [13].
With increasing recognition that an important subgroup of IPSS LR-MDS patients has significantly worse outcomes than predicted by the IPSS, it became clear that identification of these patients is important as they might be candidates for disease-modifying therapy or clinical trials assessing the benefit of early interventions [12,16,17]. The MD Anderson group developed a prognostic tool specifically for IPSS LR-MDS (LR-PSS) that successfully separated these patients into 3 risk-categories with significantly different survivals, therefore identifying patients with IPSS LR-MDS with worse outcomes than predicted by the IPSS [16]. The incorporation of some the newly discovered molecular aberrations such as the mutations in EZH2, TP53, ETV6, RUNX1, and ASXL1 genes in the IPSS allowed refining its prognostic precision by upstaging patients with any of these mutations to the next-highest IPSS risk-category [18,19]. Other groups found that immunologic variables (e.g. size of the effector memory regulatory T-cell compartment) and flow cytometric parameters can provide prognostic information beyond that of the IPSS [20,21]. The incorporation of molecular mutations, immunologic and possibly epigenetic aberrations in the prognostication schemes require further validation in large cohorts and standardization of assays and technical issues before it can be recommended for routine clinical use [12].
Acknowledging these limitations of the discriminatory power of the IPSS and the need to refine prognosis beyond the IPSS to assist clinical decision-making, a much larger database (n = 7012) of international patients with MDS was created and used to update the IPSS. This has become known as the revised IPSS (IPSS-R) [22]. In this revised model, 5 cytogenetic prognostic classes were applied (instead of 3), the low BM blast percentage value (<5%) was split into 2 categories, and the depth of cytopenias (rather than the mere number of lines affected) was taken into account. This generated 5 prognostic categories, rather than the 4 of the original IPSS. While the IPSS-R has been externally validated by other groups [23], it did not incorporate any of the novel prognostic epigenetic, genetic, and immunologic markers and it still did not gain wide clinical use [6,12].
It is important to keep in mind that while these prognostic tools were developed to inform clinical decision-making, none were designed to predict response to any particular therapeutic modality for MDS; i.e. none of them is a predictive model of response to any specific treatment or alloSCT. A recently published French Prognostic Scoring System (FPSS) was proposed to predict response to HMAs therapy [24]. While this predictive tool has been validated in smaller cohorts of patients from other institutions [25,26], it still requires further evaluation before it can be widely incorporated into routine clinical decision-making. To date, no biomarker or clinical decision rule to reliably predict probability of response or survival for HMAs therapy or alloSCT for MDS patients have gained wide acceptance.
3. Therapy for MDS
The first step on deciding the therapeutic strategy for any individual patient with MDS is determining the goals of therapy. The decision to proceed on a curative versus a non-curative therapeutic approach is usually undertaken after discussion between the treating physician and the patient, taking into consideration the prognostic outlook of the disease, the functional status and medical comorbidities, age, and patient’s preferences and attitudes to risk-taking versus risk-aversion [27]. The IPSS, despite its aforementioned limitations, continues to be the most widely used tool for risk-stratification and guiding therapeutic recommendations. For clinical-decision making, patients with the IPSS risk-classes low or INT-1 are usually classified as lower-risk (LR), while those with IPSS categories of INT-2 and high are classified as higher-risk (HR) [13]. Although recent advances in the alloSCT field allow more elderly and/or patients with comorbidities to undergo the procedure, only a minority of patients with MDS are transplanted today [5,8]. Therefore, the majority of patients with MDS are still managed on a non-curative intent therapeutic paradigm [6].
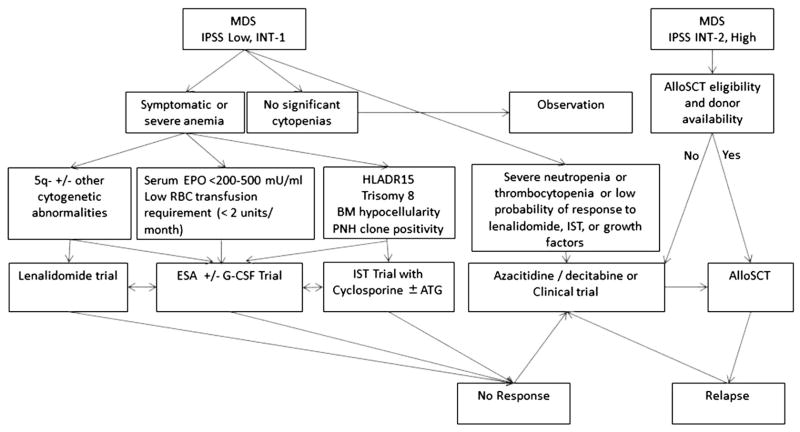
Once the decision is made not to proceed with alloSCT, the next step usually involves deciding whether to use one of the approved disease-modifying agents that can alter the natural history of MDS versus resorting to a supportive care approach. Despite the approval of 3 agents that can alter the disease course, many elderly frail patients with MDS are still treated only with supportive care measures. Supportive measures include RBC and platelet transfusions, use of hematopoietic growth factors, antibiotics, and use of iron chelation therapy as appropriate [7]. In general, therapy with HMAs, in combination with supportive therapies as needed, is usually recommended as frontline treatment for patients with IPSS HR-MDS who do not proceed to immediate alloSCT due its proven survival advantage. For IPSS LR-MDS, although supportive therapies and growth factors are the mainstay of treatment, some patients are treated with lenalidomide or IST. The therapeutic decisions are dynamic and can change during the course of the disease based on changes in the risk-category and the functional status of the patient, response to prior therapies, changes in patient’s preferences, and other factors. Whether the discovery of novel prognostic genetic and epigenetic aberrations and the development of improved prognostic tools such as the IPSS-R will alter this general treatment paradigm is yet to be determined [6]. Fig. 1 provides an overview of the current therapeutic decision-making for patients with MDS.
Fig. 1.
Current decision-making for patients with Myelodysplastic syndromes (MDS) based on the National Comprehensive Cancer Center Network (NCCN) management guidelines (adapted from reference [39]). IPSS: International Prognostic Scoring System; INT-1: Intermediate-1; INT-2: Intermediate 2; alloSCT: Allogeneic stem cell transplantation; EPO: Erythropoietin; G-CSF: Granulocyte colony-stimulating factor; ESAs: Erythropoiesis-stimulating agents; RBC: Red blood cells; BM: Bone marrow, PNH: Paroxysmal nocturnal hemoglobinuria; IST: Immunosuppressive therapy.
4. Treatment of LR-MDS
For patients with IPSS LR-MDS, therapies are directed to improve symptomatology and quality of life. The three most commonly used treatment options, in addition to clinical trials and supportive measures, include: erythropoiesis-stimulating agents (ESAs), IST, and lenalidomide. The choice of therapy depends on the disease phenotype, karyotype, and patient-related factors. The benefits of these treatment options are usually achieved in patients with LR-MDS and anemia, which is the most commonly encountered cytopenia in MDS. Patients with IPSS LR-MDS who fail therapy and those with severe thrombocytopenia or severe neutropenia can be considered for HMA therapy, alloSCT if eligible, or clinical trials [28]. Due to space limitation we will not discuss the controversies of iron chelation therapy in MDS, but the reader is referred to our recent comprehensive review on this issue [29].
4.1. Erythropoiesis-stimulating agents (ESAs) therapy
Anemia is a major contributor to morbidity associated with MDS. Approximately 80 to 90% of MDS patients develop anemia during the course of their disease, of whom 40% become RBC transfusion-dependent [30–32]. Lower Hb levels and RBC transfusion-dependence have been associated with inferior cardiovascular outcomes and increased mortality in patients with MDS, representing a strong rationale for aggressive management of anemia in MDS [31–34]. The suboptimal erythropoietin responses in some patients with MDS constitute one biologic rationale for treating MDS-related anemia with ESAs [13,35–40]. Despite not being approved by the FDA for use in MDS-associated anemia, ESAs are in wide clinical use and are the most commonly used therapy for MDS [41,42]. An analysis of linked SEER-Medicare data between 2001 and 2005 found that 62% of Medicare beneficiaries with MDS received ESAs [43]. Although ESAs have not been shown to prolong survival in MDS patients in randomized prospective trials, large retrospective analyses have suggested a survival benefit with their use [Table 2] [42,44–46].
Table 2.
The impact of erythropoiesis-stimulating agents (ESAs) on overall survival in patients with MDS. Listed below are the 3 large positive retrospective analyses and the negative, but underpowered, prospective randomized study that evaluated impact of ESAs on survival in patients with MDS.
| Study | Number of patients (median age) | Patient characteristics and protocol | Results | Survival |
|---|---|---|---|---|
| Golshayan AR et al. (Reference [45]) Pooled analysis |
Pooled analysis of 162 studies with 2592 patients (68 year for growth factor [GF] group Vs. 66 year for non-growth factor (NGF) group) |
|
|
|
| Park S et al. (Reference [44]) A French Retrospective analysis |
403 (median age 74 years) |
|
|
|
| Jadersten M et al. (Reference [46]) A Nordic Retrospective analysis |
121 EPO + G-CSF-treated patients (71 years) Compared to 237 untreated matched patients (66 years) |
|
|
Multivariate analysis: EPO-G-CSF was associated with better OS (HR 0.61. 95%CI 0.44–0.83; P = 0.002) and decreased risk of non-leukemic death (HR 0.66, 95%CI 0.44–0.99; P = 0.042)
|
| Greenberg PL et al. (Reference [42]) Prospective randomized phase 3 study (designed to provide an 80% power to detect 46% reduction in HR in the ESA group) |
EPO (n = 53) vs. Supportive care (SC) (n = 57) Median age 73 years |
|
At 4 m: Erythroid response rate (ESA: 36%, SC 9.6%) and transfusion dependence (EPO 29%, SC 51%). Response to ESA associated with serum EPO level (45% vs. 5% in patients with serum EPO <200 vs. ≥ 200 mU/mL, respectively). |
|
GF: Growth factor; NGF: Non-growth factor; EPO: Erythropoietin; G-CSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; rEPO: Recombinant human erythropoietin; ORR: Overall response rate; CR: Complete remission; PR: Partial remission; PFS: Progression-free survival; IPSS: International Prognostic Scoring System; INT-1: Intermediate-1; INT-2: Intermediate 2; RA: Refractory anemia; RARS: Refractory anemia with ring sideroblasts; RAEB: Refractory anemia with excess blasts; RBC: Red blood cells; Hb: Hemoglobin; SC: Supportive care; CMML: Chronic myelomonocytic leukemia; IWG: International Working Group criteria.
Taken together, studies suggest treatment with ESAs leads to significant erythroid responses in 20 to 70% of unselected patients with MDS and in approximately 40% of patients with LR-MDS with median response duration in the range of 2 years and without an increase in risk in rates of leukemic progression [30,44,47–49]. The doses of ESAs used for MDS-related anemia, which is associated with relative intrinsic resistance to erythropoietin, are higher than those used for renal disease-related anemia which is usually associated with normal BM responsiveness [37–39]. According to the National Comprehensive Cancer Network (NCCN) management guidelines for MDS, the recommended starting doses are 40,000 to 60,000 units given 1 to 3 times a week for the recombinant human erythropoietin alpha (rEPO) and 150–300 mcg/week for the longer acting form darbepoetin, with both agents administered subcutaneously [39]. Darbepoetin administered every 3 weeks at a dose of 500 mcg appears also effective in correcting anemia associated with LR-MDS [47]. Some preclinical and clinical studies suggested that granulocyte colony-stimulating factor (G-CSF) can have synergistic effects with ESAs and recommended adding small doses of G-CSF to improve erythroid responses in some patients, especially those with RARS, either upfront or in case of lack of response to sole ESA therapy [50]. Additionally, patients with LR-MDS and lower levels of serum erythropoietin (<200–500 mU/mL) and those who had lower RBC transfusion requirements (<2 units/month) had higher probabilities of achieving erythroid responses with ESAs [44,48]. Based on these variables, predictive models of responsiveness to ESA therapy in MDS patients have been developed and validated [44,48]. A minimum duration of ESA therapy of 6 to 8 weeks should be allowed to evaluate for response before therapy is discontinued [39].
A SEER-Medicare claims analysis found significant discrepancies between actual practice and guideline-recommended therapy, especially with regard to rates of ESA use regardless of risk status, low frequency of determination of serum erythropoietin prior to initiation of ESAs (45%), and high prevalence of shorter ESA therapeutic trials than recommended (60.4%) [43]. Still, it is important to note that this analysis evaluated data between 2001 through 2005 and it is likely that these patterns have changed since [43]. Additionally, before starting ESAs (or if suboptimal erythroid responses to ESAs are observed), it is important to rule out any coexisting nutritional deficiencies (e.g. iron or folate deficiency) or other causes (gastrointestinal bleeding) as contributing factors to anemia in MDS patients and to correct these factors if present [39]. The concerns of increased venous thromboembolism rates with ESA therapy in patients with solid tumors have not been substantiated in patients with MDS receiving ESAs [51].
4.2. Lenalidomide therapy
The 5q–syndrome is a subtype of LR-MDS that is characterized by a refractory macrocytic anemia, normal platelet counts or thrombocytosis, low BM blast percentage and small hypolobated dysplastic megakaryocytes, an isolated interstitial deletion in the long arm of chromosome 5 (5q), predilection to elderly females, and a relatively indolent course with lower rates of leukemic progression [52–54]. Although approximately 15% of MDS patients have cytogenetic aberrations in chromosome 5, only a subset of patients with 5q deletions (5q–) have the specific clinicopathologic picture of 5q– syndrome [53,55,56]. Important advances have been achieved in recent years in the understanding of the pathogenesis of this syndrome that was first described in 1974 [52]. Haploinsufficiency of cell-cycle regulatory genes and the ribosomal protein S14 gene (RPS14) located in the commonly deleted region (CDR) of 5q with subsequent ribosomal stress-induced lineage-restricted overexpression of p53 in erythroid precursors leading to increased apoptosis have all been demonstrated to be critical in the pathogenesis of 5q– syndrome [57–59]. Additionally, haploinsufficiency of other genes in the CDR such as the tumor suppressor genes SPARC and genes encoding microRNAs (miR-143, miR-145, and miR-146a) with subsequent upregulation of cytokines such as IL-6 also contribute to the clinicopathologic phenotype [60–62]. There is significant heterogeneity in the clinical outcomes of individual MDS patients with chromosome 5 aberrations that likely reflects biologic heterogeneity [63]. Although patients with IPSS LR-MDS with 5q– were traditionally reported to have lower risk of progression to AML, recent data showed 2-year and 5-year leukemic progression rates of 4.9% and 17.6%, respectively [64]. It has been shown recently that approximately 17% of patients with LR-MDS and 5q– exhibit mutations in TP53 gene, and that these patients have significantly worse OS and increased leukemic progression rates [65,66].
Patients with 5q– syndrome and patients with LR-MDS with 5q deletions were found to have exquisite sensitivity to the oral immunomodulatory agent lenalidomide, a derivative of thalidomide. In prospective clinical trials, lenalidomide resulted in significant responses in this patient population with RBC transfusion-independence rates of 56% to 67% and a median response duration lasting longer than 104 weeks [Table 3] [53,67,68]. Additionally, a significant proportion of these responders achieved cytogenetic responses (50 to 76%), indicating a direct cytotoxic effect of lenalidomide on the neoplastic clones [53,67,68]. Based on these findings, the FDA approved the use of lenalidomide (Revlimid®) for patients with LR-MDS (IPSS low or INT-1) with transfusion-dependent anemia and 5q deletions with or without additional karyotypic aberrations.
Table 3.
The MDS 001, MDS 002, MDS 003, and MDS 004 prospective studies that evaluated lenalidomide for the treatment of lower-risk MDS.
| Study | Number of patients (median age) and protocol | Patient characteristics | Cytogenetics | Clinical and cytogenetic responses |
|---|---|---|---|---|
| List A et al. (Reference [67]) Single center phase 1 MDS 001 Study |
43 (72) Lenalidomide 25 mg daily/10 mg daily/10 mg daily q 21 days on 28 day cycle |
|
|
|
| Raza A et al. (Reference [69]) Multicenter phase 2 MDS 002 Study |
214 (72) Lenalidomide 10 mg po daily for 21 days every 4 weeks or daily |
|
|
|
| List A et al. (Reference [53]) Multicenter international phase 2 MDS 003 Study |
148 (71) Lenalidomide 10 mg po daily for 21 or 28 days every 4 weeks |
|
74% isolated 5q31 deletion, rest with other abnormalities |
|
| Fenaux P et al. (Reference [68]) Phase 3 randomized double blind MDS 004 Study |
205 (69) Lenalidomide 10 mg po daily for 21 days every 4 weeks or 5 mg po daily or placebo |
|
5q31 deletion |
|
EPO: Erythropoietin; IPSS: International Prognostic Scoring System; INT-1: Intermediate-1; INT-2: Intermediate 2; ANC: Absolute neutrophil count; HB: Hemoglobin; CMML: Chronic myelomonocytic leukemia; RBC: Red blood cells; OS: Overall survival; Po: Oral. All studies enrolled patients with 5q– except the MDS 002 which enrolled patients who lack this cytogenetic abnormality.
Major erythroid response: Freedom from the need for transfusion or Hb increase by 2 g/dl;
Hematologic response: Sustained improvement for 8 or more consecutive weeks;
Cytogenetic response: Complete cytogenetic response was defined as the absence of metaphases containing an abnormal clone; partial cytogenetic response was defined as a 50% or greater reduction in proportion of abnormal metaphases after treatment.
The MDS 001 was the first study to show activity of lenalidomide in anemic patients with LR-MDS and suggested selectivity of the drugs to patients with 5q31.1 deletions [67], which was subsequently confirmed in the MDS 003 and MDS 004 trials [Table 3] [53,68]. The lower response frequency and shorter response duration in non-5q– patients in the MDS 002 trial supported the preferential activity of lenalidomide for 5q– MDS [69]. The randomized MDS 004 study suggested a dose–response effect with lenalidomide therapy with significantly higher hematologic and cytogenetic response rates with the 10 mg dose compared to the 5 mg [Table 3] [68]. Despite the excellent responses in 5q– LR-MDS, lenalidomide therapy has not yet been shown prospectively to result in OS benefit in patients with MDS. The cross-over design of this trial probably has significantly reduced the chances of the trial showing an OS benefit [68,70]. Nonetheless, significant improvement in quality of life and statistically significant reductions in relative risk of death and leukemic progression were observed in patients responding to lenalidomide therapy [68].
Concerns have been raised regarding a possibly increased risk of progression to AML in LR-MDS patients with 5q– treated with lenalidomide. Due to these concerns lenalidomide has not yet been approved by the European Medicine Agency for use in Europe for the MDS indication [71]. A recent retrospective analysis compared the outcomes of 295 lenalidomide-treated patients from the MDS 003 and MDS 004 trials to 125 untreated RBC transfusion-dependent patients with LR-MDS and 5q–from a large international registry [72]. Cox proportional hazards models were used to adjust for differences between cohorts. After a median follow-up of 4.3–4.6 years, the 2-year OS rate was significantly higher in the lenalidomide-treated group (89.9%, [95% CI: 84.1–96.0] versus 74.4% [95% CI: 66.1–83.7], HR 0.597, P = 0.012). Leukemic progression rates were not statistically significantly different between the 2 groups (2-year cumulative leukemic progression rate were 6.9% versus 12.1%, HR, 0.969, P = 0.930) [72]. A smaller French retrospective comparative analysis that used a propensity-score approach also failed to show an increased risk of progression to AML in patients with LR-MDS with 5q– treated with lenalidomide compared to historical controls [71]. It has been reported that patients with LR-MDS and 5q– who do not achieve erythroid or cytogenetic remissions after lenalidomide therapy are at a higher risk of clonal evolution and leukemic progression [73]. A randomized prospective comparison is needed to definitively answer this question.
In RBC transfusion-dependent patients with LR-MDS without 5q deletions, lenalidomide results in lower response rates and shorter responses compared to patients with 5q– [Table 3] [69]. An erythroid response rate of 48% and a transfusion-independence rate of 37% have been reported in a retrospective study of 31 consecutive ESA-refractory anemic patients with LR-MDS who lacked 5q–, with a median response duration of 24 months [74]. Therefore lenalidomide can be considered in some transfusion-dependent patients with LR-MDS without 5q– with primary or secondary resistance to ESA therapy, or as upfront therapy in those with high endogenous serum erythropoietin levels who are less likely to respond to ESA therapy [39].
In a multivariate analysis of anemic patients with LR-MDS who have received lenalidomide therapy in the MDS 002 and MDS 003 trials, erythroid responses were associated with younger age, shorter duration of MDS, lower baseline transfusion burden, and development of lenalidomide-related thrombocytopenia [75]. Therefore, the occurrence of lenalidomide-induced cytopenias early in the course of therapy is a surrogate marker of clonal suppression in patients with MDS and 5q– [75]. Using gene expression profiling, an erythroid differentiation molecular signature has been identified to predict response to lenalidomide therapy in patients with LR-MDS who lacked 5q–[76]. A polymorphism in cereblon, an E3 ubiquitin ligase protein that is a direct molecular target for the cytotoxicity of thalidomide and lenalidomide in multiple myeloma, has been recently identified a biomarker of response to therapy with lenalidomide in patients with LR-MDS who lack 5q– [77]. These findings require prospective validation before they can be used to select patients for lenalidomide therapy.
Given the encouraging results of lenalidomide in LR-MDS with 5q–, a phase 2 trial was conducted to assess lenalidomide activity in patients with HR-MDS (IPSS INT-2 and high) with 5q–[78]. Forty-seven patients received a daily lenalidomide dose of 10 mg and achieved a lower hematologic response rate of 27% (15% with cytogenetic responses), including 25.5% who achieved RBC transfusion-independence with a median duration of 26 weeks. There were 7 CR (4 complete and 3 partial cytogenetic responses), 2 marrow CR, and 4 hematologic improvements. Patients with isolated 5q– had higher probabilities of achieving CR compared to those with additional karyotypic aberrations. There was no association between lenalidomide-induced cytopenias and achievement of responses, although this evaluation was difficult due to high rates of baseline cytopenias. Based on these results, Ades et al. [78,79] suggested that the direct cytotoxicity of lenalidomide to the neoplastic 5q– clone in patients with LR-MDS can also been in patients with this clone and excess blasts. A 36% overall response rate (ORR) was reported in a prospective evaluation of 11 patients with IPSS HR-MDS and 5q– using higher doses of lenalidomide (30 mg daily) [80].
Our understanding of the mechanisms of action of lenalidomide has lagged behind the empiric demonstration of the selective activity of the drug in patients with MDS with 5q–. Despite noteworthy recent discoveries in the pathogenesis of 5q– syndrome and the molecular effects of lenalidomide therapy in these patients, the mechanisms of action of lenalidomide in MDS are not yet fully understood. Several pleiotropic karyotype-specific mechanisms have been proposed [56,58,61,81–83]. In LR-MDS with 5q–, lenalidomide seems to have a karyotype-specific mechanism of action in which direct cytotoxic effect is exerted on the neoplastic 5q– clone [75]. Lenalidomide has been shown to inhibit haplodeficient cell-cycle regulatory phosphatases (PP2A and CDC25C) in LR-MDS patients with 5q–, thereby stabilizing the MDM2 protein and accelerating p53 degradation [82,84,85]. In addition, lenalidomide has been shown to cause significant increases in RPS14 expression in 5q– patients [82,86].
In contrast, a number of potential mechanisms of action of lenalidomide have been suggested to explain the effects of lenalidomide in patients with LR-MDS without 5q–. These proposed mechanisms include direct stimulation of erythropoiesis, angiogenesis inhibition, immunomodulatory effects, anti-inflammatory changes, BM microenvironment alterations [56,58,61,81–85]. In a recent study, low levels of RPS14 expression in patients with LR-MDS without 5q– were associated with higher rates of apoptosis erythroid progenitors and predicted better survival and possible response to lenalidomide [87]. It has been suggested that lenalidomide may also exhibit direct cytotoxic effects on the neoplastic clone in patients with HR-MDS and isolated 5q– as well [69,78,79,88].
Given the poor outcomes in patients who exhibit primary or secondary failure of response to lenalidomide therapy and those who exhibit TP53 mutations, more research is required to understand the biologic basis of resistance and develop novel therapeutic approaches [89]. For example, overexpression of PP2A and p53 reaccumulation in erythroid progenitors has been shown to mediate resistance to lenalidomide in some patients [82,85]. Targeting p53 with anti-sense oligonucleotide (e.g. Cenersen) has been suggested as a strategy to overcome lenalidomide resistance [90,91]. The S-enantiomer of lenalidomide, C-21359, is 3 to 4 times more potent than the racemic lenalidomide used clinically currently and will be undergoing testing in patients with LR-MDS [91].
4.3. Immunosuppressive therapy (IST)
The rationale of using IST as a therapeutic option for some patients with MDS rests on several important observations. First, an association was noted between MDS and some autoimmune disorders, connective tissue and rheumatologic disorders, and large granular lymphocytic leukemia (LGL), prompting the question of a possibly shared autoimmune etiology [92]. Second, marked aberrations in the humoral and cellular elements of both arms of the immune system, the innate and the adaptive, have been documented in patients with MDS. In early stage MDS, increased pro-inflammatory cytokines such as TNF-alpha and interferon-gamma, increased frequency of CD4+CD25high + CD127low + regulatory T-cells, and skewing of the T-cell receptor V-beta repertoire due to selective oligoclonal expansion in cytotoxic CD8+ T-cells have been observed [93–96]. In some subsets of MDS, the selective proliferation of both helper CD4+ T-cells (mostly polyclonal) and cytotoxic CD8+ T-cells (mostly oligoclonal) is believed to underlie an autoimmune attack against the dysplastic hematopoietic progenitors [93]. In some forms of MDS, this CD8+ T-cell-mediated insult against the neoplastic MDS clone, and possibly by bystander cytokine-mediated inhibition of normal hematopoietic cells, seems to result in the development of the BM failure state [97,98]. Sloand and colleagues showed that MDS patients with trisomy 8 had clonally expanded CD4+ and CD8+ T-cell populations that attacked the neoplastic clones by recognizing and exhibiting responses against an overexpressed Wilms Tumor 1 (WT1) antigen [99,100].
Multiple prospective studies evaluated different forms of IST as a therapeutic intervention for MDS. The most commonly studied agents included cyclosporine A (CSA) [101,102], horse and rabbit antithymocyte globulin (ATG) [103–107], the anti-CD52 monoclonal antibody alemtuzumab [108], the TNF alpha inhibitor etanacerpt [109], and sirolimus [110]. Comparison of results among these different trials that used different doses and schedules is very difficult due to variable inclusion criteria and studied patient populations. Although reported hematologic responses were largely variable ranging between 0% and 73%, studies that selected patients more likely to respond to IST generally have yielded higher response rates [98]. Hematologic responses to IST are generally slow and may require up to 6 months to fully manifest [103]. Although no reliable clinical or laboratory response-predicting marker has been discovered to date, several factors have been associated with hematologic responses to IST in patients with MDS [Table 4] [97,98,111,112]. The presence of a paroxysmal nocturnal hemoglobinuria (PNH) clone and BM hypocellularity have also been associated with response in some reports, but they could not be reproduced in analysis of large patient numbers [98,105,113].
Table 4.
Factors reported in the literature to be associated with clinical responses to immunosuppressive therapy in patients with myelodysplastic syndromes (MDS).
| Age (<60 years) |
| Early stage MDS (FAB RA and IPSS low and INT-1) |
| HLA-DR15+ Status |
| Trisomy 8 karyoptype |
| Female gender |
| Short duration of RBC transfusion dependency |
| Lower RBC transfusion requirements |
| Presence of a PNH clone |
| BM hypocellularity |
In a large analysis of 139 MDS patients who received IST (ATG, CSA, or in combination)with a median follow-up of 3 years, the hematologic overall response rate was 30% (8% for CSA, 24% for ATG, and 48% for ATG + CSA combination) [113]. The authors identified only 3 factors to be independently predictive of response in multivariate analysis: younger age, HLA-DR15 status, and ATG + CSA combination therapy. In a multivariate analysis of this cohort combined with the patient cohort used to develop the IPSS, the factors significantly associated with improved survival were younger age, IST therapy, and intermediate or low IPSS scores. Based on their findings, the authors developed a predictive score that identified MDS patients who were IST-responders with 88% probability and IST-non-responders with 7% probability of response [113]. This analysis also confirmed long-term safety of IST in patients with MDS as IST-responders had lower rates of leukemic progression compared to the IPSS-matched control untreated group, while the IST-non- responders had leukemic progression at similar rates to the IPSS-matched control untreated group. This observation alleviated the concerns over possible increased risk of progression to AML with IST in MDS patients by inhibiting a protective autoimmune suppression of the neoplastic clones. The authors concluded that IST results in clinically meaningful hematologic responses in a significant proportion of MDS patients and leads to improved OS and PFS, especially in younger patients with LR-MDS [113].
A prospective trial randomized 45 patients receive horse ATG (15 mg/kg of for 5 days) with oral CSA (for 6 months) and 43 patients to best supportive care (BSC) [105]. Most patients had LR-MDS (IPSS low or INT-1, RA or RARS). By month 6, 29% achieved hematologic responses in the ATG + CSA arm compared to 9% in the BSC arm (P = 0.0156). Nonetheless, there were no statistically-significant differences in the 2-year transformation-free survival rates (46% vs. 55%, P = 0.730) or in OS (49% vs. 63%, P = 0.828) between the 2 groups [105]. Another prospective non-randomized phase 1/2 trial evaluated alemtuzumab monotherapy (test dose of 1 mg IV on day 1 followed by 10 mg IV daily for 10 days) in 32 patients with MDS who were preselected based on high likelihood of responding to IST [108]. In this highly selected patient population, the hematologic ORR was an impressive 68% (77% in the INT-1 patients and 57% in INT-2 patients) with a median time to response of 3 months. Four out of 7 evaluable responders with karyotypic aberrations prior to therapy achieved normalization of cytogenetics by 1 year. Additionally, 5 of 9 responding patients (56%) evaluable at 12 months had normal blood counts and 7 out of 9 patients (78%) were transfusion independent. Treatment was well tolerated with no excess toxicity, leukemic progression, or clinically significant cases of Epstein–Bar virus or cytomegalovirus reactivations [108]. These results require further prospective validation. Based on these results, carefully selected patients with symptomatic MDS can be considered for a trial of IST with ATG, CSA, ATG + CSA, or alemtuzumab [Table 4].
5. Therapy for HR-MDS
Patients with HR-MDS have poor outcomes and their survival is significantly limited. If untreated with disease-modifying therapy or alloSCT, these patients have a median survival of less than one year [13]. The goal of therapy in this patient group is to alter the natural course of the disease by prolonging survival of patients who can tolerate HMA or more intensive interventions [6]. In addition to enrollment in clinical trials, the 3 commonly used therapies are HMA therapy with azacitidine or decitabine, alloSCT, or, rarely, intensive chemotherapy.
5.1. Hypomethylating agents (HMAs)
The 2 HMAs approved by the Food and Drug administration (FDA) for treatment of MDS are azacitidine (5-azacytidine, Vidaza®, Celgene), and decitabine (5-aza-2′-deoxycytidine, Dacogen®, Eisai). Both of these azanucleosides are cytidine analogues which inhibit a group of enzymes called DNA methyltransferases (DNMTs) and subsequently lead to demethylation of the cytosine residues in the promoter-associated CpG islands [114]. Although the mechanisms of action of these azanucleosides in MDS are not fully understood, hypomethylation and subsequent reversal of transcriptional inhibition of important tumor-suppressor and DNA repair genes is believed to represent the main mechanism of action [115]. Nonetheless, these drugs exhibit other biologic effects such as immunomodulation and incorporation into DNA and, in case of azacitidine, RNA as well [114–116]. The degree to which mechanisms other than epigenetic modulation contribute to the clinical activity of HMAs in MDS is not yet established [115].
Both HMAs are active across the entire morphologic and prognostic spectrum of MDS and result in objective hematologic responses in 40– 60% of patients including 10–20% CR rates and 10–20% PR rates, delay leukemic progression, improve quality of life in patients with MDS, and, in the case of azacitidine, prolong survival [Table 5] [91,117–120]. The two drugs are yet to be compared head-to-head in a randomized prospective fashion. Therefore, since only azacitidine is the only HMA shown to prolong OS in a randomized phase 3 trial, most experts recommend azacitidine over decitabine as first-line therapy for HR-MDS [39].
Table 5.
The four large randomized phase 3 trials that evaluated the hypomethylating agents (HMAs) azacitidine (AZA) and decitabine (DAC) for the treatment of MDS.
| Study | Number of patients (median age) and protocol | Patient characteristics | Clinical results | Survival |
|---|---|---|---|---|
| Silverman LR et al. (Reference [119]) Phase III randomized study of Azacitidine (AZA) vs. Best supportive care (BSC). Crossover allowed. |
191 (68 years) AZA 75 mg/m2/d SQ for 7 days every 28 days Vs. BSC |
IPSS AZA/SC %: Low 2%/6% INT-1 26%/20% INT-2 11%/16% High 9%/10% |
ORR: AZA arm 60% (CR 7%, PR 16%, and HI 37%) Vs. BSC arm 5%
|
|
| Fenaux P et al. (Reference [118]) Phase III randomized study of Azacitidine (AZA) Vs. Conventional care regimen (CCR). Crossover NOT allowed. |
358 (AZA 69 years; CCR 70 years) AZA 75 mg/m2/d SQ for 7 days every 28 days Vs. CCR (best supportive care [BSC], low dose cytarabine, or intensive chemotherapy) |
IPSS AZA/CCR %: Low excluded INT-1 3%/7% INT-2 43%/39% High 46%/48% |
|
|
| Kantarjian H et al. (Reference [117]) Phase III randomized study of Decitabine (DAC) vs. Best supportive care (BSC) |
170 (70) DAC 15 mg/m2 IV q 8 h for 3 days every 6 weeks Vs. BSC |
IPSS DAC/BSC%: Low excluded INT-1 31%/30% INT-2 43%/44% High 26%/26% |
|
|
| Lubbert M et al. (Reference [120]) Phase III randomized study of decitabine (DAC) Vs. Best Supportive Care (BSC) in elderly patients deemed ineligible for intensive chemotherapy |
233 (70) DAC 15 mg/m2 IV over 4 h 3 times a day for 3 days every 6 weeks or BSC |
IPSS DAC/BSC%: Low excluded INT-1 6.7%/7% INT-2 53.8%/55.3% High 38.7%/36.8%
|
|
|
BSC: Best supportive care; IPSS: International Prognostic Scoring System; INT-1: Intermediate-1; INT-2: Intermediate 2; ORR: Overall response rate; CR: Complete remission; PR: Partial remission; HI: Hematologic improvement; OS: Overall survival; CCR: Conventional care regimen; AML: Acutemyeloid leukemia; PFS: Progression-free survival.
5.1.1. Azacitidine
The first randomized phase 3 trial to study azacitidine was the Cancer and Leukemia Group B (CALGB) 9221 study which failed to show a survival advantage, probably because it allowed crossover from placebo to azacitidine [Table 5] [119]. The first and only randomized phase 3 trial to show an OS advantage in MDS was the AZA 001 study which randomized 358 HR-MDS patients equally to either an azacitidine arm using a similar regimen or to conventional care regimens (CCR) arm that included BSC, low-dose cytarabine, or intensive chemotherapy pre-assigned at physician discretion without allowing cross-over to azacitidine [Table 5] [118]. The median survival advantage was 9.5 months. A survival advantage was maintained for older patients, in patients with 20–30% BM blasts, in patients with adverse karyotypes, and when compared to low-dose cytarabine alone [118,121–123]. A statistically-significant OS difference between patients receiving azacitidine and those receiving intensive chemotherapy could not be detected, probably due to the small number of patients who received chemotherapy. Additionally, azacitidine therapy was also associated with shorter hospitalizations, decreased transfusions and infections, delayed leukemic progression, and improvements in reported quality-of-life [118,121–125].
Objective responses to azacitidine can require 4–6 months to manifest, and response appears to deepen with time in many patients. The first objective response to azacitidine therapy was observed after a median of 2 to 3 cycles (range, 1–16), with approximately 90% of first responses observed after 6 cycles [126,127]. Moreover, continuation of azacitidine therapy beyond first response deepened it in 48% of patients, with the best response observed in 92% of responders by 12 cycles of therapy. The median time from first response to best response (CR or PR) was 3 to 3.5 cycles [127]. Compared to AML therapy where achieving an OS advantage requires attainment of CR or PR, a survival benefit with azacitidine therapy for HR-MDS patients was also observed for patients whose best responses were hematologic improvement (HI) [24,128,129]. Whether patients whose best response to azacitidine therapy is stable disease (SD) achieve an OS benefit it not known at this time [128].
The approved 7-day regimen of azacitidine requires weekend administration, which can be difficult to arrange. Other azacitidine regimens that do not require therapy on weekends were evaluated in a randomized study of 151 MDS patients, of whom two thirds had LR-MDS [130]. The 3 regimens were 5-2-2 (75 mg/m2/day SQ for 5 days, followed by 2 days without therapy, then 75 mg/m2/day for 2 days); 5-2-5 (50 mg/m2/day SQ for 5 days, followed by 2 days without therapy, then 50 mg/m2/day for 5 days); and 5-0-0 (75 mg/m2/day SQ for 5 days). The rates of HI were 44%, 45%, and 56%, respectively, and transfusion-independence rates were 50%, 55%, and 64%, respectively [130]. Since these regimens were not directly compared to the approved regimen, most patients on the study had LR-MDS, and because marrow responses and survival were not reported, these regimens need further evaluation before they can be adopted for wide clinical use for HR-MDS patients. Prolonged administration of lower doses of azacitidine therapy instead of the 7-day course have a biologic rationale as it might result in a lesser degree of cell cycle inhibition with subsequent increased incorporation into DNA and more effective methylation reversal [121].
Despite limited available data, most experts feel that IV administration of azacitidine (same dose/schedule) instead of SQ is a reasonable alternative in cases of significant local reactions to the SQ formulation or limited SQ tissue due to cachexia [124,125]. An oral formulation of azacitidine showed promising results in early phase trials and is currently undergoing evaluation in a large international randomized phase 3 trial [131]. Given that objective responses are seen in approximately only half of HR-MDS patients treated with azacitidine, that responses require 4 to 6 months to manifest, and that therapy can be associated with significant side effects, it is a research priority to discover biomarkers or develop decision tools that can accurately predict the probability of achieving response or survival benefit for any individual MDS patient before or early after initiating azacitidine therapy. Unfortunately, no such biomarker or predictive tool has been widely validated. Ten-eleven-translocation 2 (TET2) mutations were reported to predict response to azacitidine therapy [132]. Recently, a French prognostic scoring system (FPSS) was reported to predict response to azacitidine therapy in HR-MDS patients and was validated in 2 single-institution European cohorts [24–26,129,133,134]. Both TET2 mutations and the FPSS await wide-scale and prospective validation.
5.1.2. Decitabine
Two large randomized studies of decitabine in MDS have been reported, but neither one showed an OS survival [Table 5] [117,120]. The infusional decitabine regimen used in both of these studies requires hospitalization, so alternative easier-to-administer schedules were studied [135]. In a randomized phase 2 trial, 95 patients were assigned to one of three regimens of decitabine: 20 mg/m2/day for 5 days; 20 mg/m2/day SQ for 5 days; or 10 mg/m2/day IV for 10 days [136]. In total, 73% achieved objective responses, including 34% with CR. The 5-day IV regimen resulted in the highest CR rate (39%) and was chosen as the optimal regimen. In a subsequent phase 2 trial, this regimen resulted in a significantly less responses with a CR rate of only 17% [137]. Similar to the case with azacitidine, predictors of response are sought after. A 10-gene methylation signature was reported to be predictive independent of the IPSS, but further validation and technical standardization is required [138].
5.1.3. Side effects of HMAs
The most common side effects of HMAs are hematologic in form of new or worsening cytopenias, especially grade 3 or 4 neutropenia and thrombocytopenia [117,118,136]. These hematologic side effects are usually observed with initial cycles of therapy but generally improve in subsequent cycles [139]. Dose reductions, dose delays, or maintaining the same intensity with supportive therapy are all commonly used approaches to manage hematologic toxicity [124,125]. Some investigators argue that cytopenias could reflect response at the level of malignant clones and therefore delaying or reducing doses is not needed in the absence of life-threatening complications, and can lead to emergence of resistance [124,125]. Other researchers argue that no dose or interval threshold has been established for therapy with HMAs in MDS and that gene expression modulation has been observed with very low doses of HMAs; therefore maintaining same intensity of therapy without dose reduction or delay despite development of significant cytopenias exposes patients to significant risks without proven benefit [131,140]. The non-hematologic side effects of HMAs are mild and usually well tolerated. The most common ones include fatigue, local site reactions, and gastrointestinal disturbances [124].
5.1.4. HMAs use in MDS
Based on available evidence, therapy with HMAs in indicated in patients with IPSS INT-2 and high-risk MDS not proceeding to transplantation immediately, and should be considered in lower risk patients not responding to other therapies, especially those with severe thrombocytopenia or neutropenia. A therapeutic trial of 4 to 6 cycles of HMAs is needed to allow time for objective responses to manifest, unless frank progression or excessive toxicity develop [39,124,125]. For patients who achieve objective responses and are not proceeding to transplantation, therapy should continue until progression or the development of significant toxicity [124,141]. Although no dose or duration threshold has been established, the only regimen shown to prolong survival was azacitidine at 75 mg/m2/day for 7 days every 28 days, and this is the recommended regimen for use especially in patients with HR-MDS in whom modification of the natural history of the disease is the goal of therapy [39,124,125]. Many aspects of the use of HMAs in MDS remain controversial require more evaluation including the optimal schedules and doses, combination therapy, and management of therapy-related cytopenias.
Patients who do not respond to HMAs, lose initial response, or progress to AML after therapy with HMAs have very poor outcomes with median survival of less than 6 months [142–144]. The mechanisms of primary and secondary resistance to HMA therapy are complex and poorly understood. Multiple levels of resistance have been proposed including inter-individual variations in effective drug levels due to genetic and pharmacokinetic differences such as changes in cellular transport proteins and in enzymes responsible for metabolism (e.g. phosphorylation) of HMAs, and changes in the target enzymes of the drugs (DNMTs) [145,146]. Better understanding of the mechanisms of primary and secondary resistance is required in order to rationally develop agents that prevent or overcome resistance. Without understanding the mechanisms of resistance, the choice of subsequent therapy post-HMA failure will remain empiric with high rates of futility. Novel more potent azanucleosides, e.g. SGI-110, and oral formulations of the current HMAs, e.g. CC-486 which is an oral form of azacitidine are being evaluated [91,131].
5.2. Intensive chemotherapy for HR-MDS
Intensive AML-like chemotherapy regimens result in significant toxicity and modest responses in patients with HR-MDS; a largely elderly patient population with high prevalence of medical comorbidities; therefore limiting their use. The CR rate associated with intensive chemotherapy for MDS is lower in than that seen in patients with de novo AML (40–60%) and is typically more limited in duration (median CR duration less than one year) [147–152]. Additionally, more hematologic and non-hematologic toxicity is observed leading to higher rates of induction-related mortality of approximately 20% [147–151]. Poor-risk karyotypes, advanced age, and poor performance status have been associated with adverse outcomes in MDS patients receiving intensive chemotherapy [149–151]. A retrospective study evaluated 510 patients with HR-MDS who were treated with intensive chemotherapy, and reported a CR rate of 53%, induction-related mortality of 17%, and a dismal 5-year overall survival probability of 8% [151]. Those younger than 65 years with normal karyotypes had the best outcomes (5-year survival rate of 27%), while those older than 65 years in age had an induction mortality rate of 29% [151]. No specific intensive induction chemotherapy regimen appears superior to others in HR-MDS [149,151].
The outcomes of patients who receive intensive chemotherapy for AML arising from MDS are similarly dismal [147,153]. Intensive chemotherapy is yet to be compared head-to-head with HMA in a randomized study. Although the landmark AZA 001 study allowed for intensive chemotherapy as part of the CCR arm, only few patients received chemotherapy to allow for informed conclusions [118]. The clinical benefit associated with azacitidine in AZA 001 trial was maintained irrespective of the karyotype [118]. Therefore given the high toxicity associated with chemotherapy and the absence of demonstrated superior outcomes compared to HMA therapy, the use of intensive chemotherapy in MDS is usually restricted to the rare younger patient requiring cytoreduction prior to alloSCT, especially those with normal karyotypes [7,152,154]. Older patients with co-morbidities, especially those with monosomy 7 and other poor-risk cytogenetics, are probably better treated with HMA therapy.
6. Combination strategies
Azacitidine is the only therapy, short of alloHSCT, that was shown prospectively to prolong survival in patients with MDS. Nonetheless, HMA therapy leads to objective responses in only half the patients including a modest CR rate of 10–20%, patients with primary or secondary resistance to HMA have dismal prognosis, and even responsive patients are not cured and most will lose their response in less than 2 years [155]. Therefore novel therapeutic approaches are desperately needed. Given the proven survival advantage of azacitidine, HMA-based platforms represented an appealing combination approach.
Histone deacetylase inhibitors (HDACIs) inhibit a group of enzymes called histone deacetylases (HDACs) that are important in post-translational histone modification and exert epigenetic control over gene expression. Inhibiting HDACs pharmacologically may result in cell cycle arrest and subsequent apoptosis [156]. Additionally, HDACIs can induce reactive oxygen species, directly acetylate chaperone proteins and interfere with nuclear factor kappa-B (NF-kB) and death receptor pathways, and can cause differentiation in the malignant cells [156,157]. Although HDACIs such as vorinostat, panobinostat, entinostat, and belinostat demonstrated very modest single agent activity in MDS and AML clinical trials [158–160], in-vitro experiments showed that they potentiated reversal of promoter methylation of silenced genes [161].
Synergistic antileukemic activity can be achieved in vitro by combining HMA and HDACI [162]. Early phase trials that evaluated the combination of valproic acid or sodium phenylbutyrate, both older HDACIs, with azacitidine or decitabine showed that the combinations were safe and associated with modest clinical activity [163–165]. Several newer HDACIs (e.g. entinostat, belinostat, vorinostat, panobinostat) have been evaluated in early phase trials in combination with HMAs. Pracinostat (SB939), a novel competitive HDACI with >1000-fold selectivity for HDAC Classes 1 and 2 versus Class 3 was combined with azacitidine in a phase II study at MD Anderson Cancer Center. The combination resulted in an impressive CR/CRi rate of 78% (7 out of 9 patients). A randomized phase 2 study of 150 patients reported only in abstract format compared azacitidine monotherapy to azacitidine + vortinostat combination did not find a significant difference in median OS between the 2 groups after a median follow up of 17 months (18 months in the monotherapy arm vs. 13 months in the combination arm B, P = 0.15) [166].
The possibly complementary mechanisms of action of azacitidine, which targets the neoplastic clone, and lenalidomide, which has significant effects on the BM microenvironment, and their single-agent activity in MDS, were the basis of trials combining both agents in HR-MDS [155,167–169]. The goal of this combination is to increase and/or deepen and/or prolong the responses, and ultimately prolong survival compared to single-agent azacitidine. In a phase 1 study, 18 patients with IPSS HR-MDS or BM blasts of 5% or more received azacitidine (75 mg/m2/day for 5 or 10 days) concurrently with lenalidomide (5 or 10 mg daily for 14 or 21 days in 4-week cycles), and the combination was well tolerated [167]. A phase 2 expansion of this trial study enrolled 18 more patients (azacitidine 75 mg/m2/day for 5 days and lenalidomide 10 mg daily in the first 3 weeks of 4-week cycles) [168]. This study confirmed the safety and tolerability of the combination. Overall, 72% of treated patients achieved objective responses with a CR rate of 44% with a median duration of more than 17 months. The median OS for complete responders was more than 37 months. A phase 1 trial that evaluated a sequential combination approach of azacitidine (75 mg/m2/day for 5 days) followed by escalating doses of lenalidomide on day 6 through day 19 in 20 patients with IPSS HR-MDS (65%) or AML (35%) with 5q– has been recently reported [169]. Overall, 26% of patients achieved objective responses, including 44% of previously untreated patients. An ongoing 3-arm randomized trial is comparing 2 combination regimens (azacitidine + lenalidomide and azacitidine + vorinostat) with azacitidine monotherapy (NCT01522976). Other azacitidine-based combinations have been evaluated in HR-MDS such as azacitidine–cytarabine combination and azacitidine-CD33-immunoconjugate gemtuzumab ozogamicin combination [170–172].
In order to improve erythroid responses, combination therapies have been evaluated in patients with LR-MDS as well. For LR-MDS, a recently published trial evaluated the use of a combination regimen of lenalidomide with rEPO in transfusion-dependent patients who failed prior ESA therapy [173]. In the first stage of the trial, patients were treated with lenalidomide monotherapy (10 or 15 mg daily) for 16 weeks. Erythroid non-responders received the combination regimen using rEPO at 40 000 U per week. In the first stage, 6 patients out of 7 with 5q– (86%) and 8 patients out of 32 with non-5q– LR-MDS (25%; 17.7% for the 10 mg dose and 33.3% for the 15 mg dose) achieved HI-E with lenalidomide monotherapy. Twenty-three patients received the combination therapy and 6 of them (26%) achieved HI-E including 4 of 19 non-5q– patients (21.1%). These results led to a randomized phase 3 study that is evaluating the benefits of lenalidomide plus rEPO therapy [173].
7. Allogeneic stem cell transplantation (AlloSCT)
AlloSCT is the only therapeutic approach with a known curative potential for patients with MDS. Nonetheless, most MDS patients do not undergo alloSCT due concerns of excessive procedure-related toxicity related to compromised organ function reserve with advanced age and coexisting medical conditions, or due to difficulties of locating an appropriate stem cell graft source [174,175]. Even for patients who undergo alloSCT, a considerable risk of transplant-related mortality (TRM) and disease relapse results in long-term OS in only 30%–50% of transplanted patients, many of whom suffer from long-term sequelae such as cGVHD [176–179]. As recent advances in the field of alloSCT and the introduction of reduced intensity conditioning (RIC) regimens have led to important reductions in TRM, relapse has become the leading impediment to achieving long-term survival of transplanted MDS patients [180,181]. Therefore, although alloSCT should be considered in all MDS patients, careful selection of patients based on a thoughtful evaluation of risk/benefit ratio is of paramount importance [182–184].
Discussion of the role and controversies of alloSCT in MDS including patient selection, pre-transplantation therapy, the intensity and type of preparatory regimens, the impact of transfusion-dependency and iron overload on transplantation outcomes, the prevention and management of GVHD and relapse is beyond the scope of this paper. The interested reader is referred to an excellent review published recently in this journal [184] and other timely comprehensive reviews [176,181,183,185–188].
The outcomes of the alloSCT for MDS are largely dependent on disease- and patient-related factors. The cytogenetic status, the BM blast percentage, age, medical co-morbidities, transfusion-dependency and iron overload all affect alloSCT outcomes [185,189–193]. The FAB and WHO classifications, the IPSS (at diagnosis and at time of alloSCT), the WPSS, and cytogenetic risk scores (the IPSS 3-group cytogenetic classification and the recent 5-group cytogenetic risk categories) were all retrospectively shown to predict post-alloSCT outcomes [189,194–196]. A Markov decision-analysis published in 2004 found that maximal gain of years of life is achieved for patients with IPSS INT-2 and high risk if they proceed to alloSCT without delay, while patients with low and INT-1 IPSS scores achieve better life expectancy by delaying alloSCT to time of leukemic progression [194]. This analysis was limited to patients younger than 60 years who underwent myeloablative conditioning (MAC) before the wide use of HMA therapy. To address these limitations, another Markov decision-analysis was recently published based on evaluation of 514 MDS patients aged 60 to 70 years [178]. Patients with IPSS low and INT-1 scores were treated with BSC and growth factors, while those with INT-2 and high IPSS scores were treated with RIC alloSCT (n = 132) or HMAs (n = 165). It was concluded that for patients with de novo MDS aged 60 to 70 years who belonged to the IPSS low or INT-1 risk groups, non-transplantation approaches are preferred. In contrast, for those patients in the INT-2 and high IPSS risk categories, RIC alloSCT offered an advantage in terms of OS and quality-adjusted survival [178]. Currently, most experts and practice guidelines recommend that transplant-eligible patients with INT-2 or high risk IPSS scores undergo alloSCT at or soon after diagnosis if an appropriate donor is available [39,188].
In addition to patients with IPSS INT-2 or high, patients with IPSS LR-MDS with certain characteristics should be considered for alloSCT such as those with refractory life-threatening cytopenias [28,181]. Data suggests that some of the newer risk models (e.g. the MD Anderson model) and the recently discovered important prognostic gene mutations (e.g. TP53, EZH2, and ASXL1) can define a subgroup of MDS patients with IPSS low or INT-1 who actually have poor outcomes, but whether the negative prognostic impact of these mutations can be overcome with alloSCT remains to be determined [18,19,189].
8. Future directions and investigational agents
Despite the recent advances in management of MDS, outcomes are far from optimal, especially for high-risk patients. There is a clear unmet need for newer therapies for patients with MDS, especially for patients with primary or secondary resistance to HMA therapy and those who relapse after alloSCT. The increasing knowledge about the complex pathogenesis of MDS, the key genetic alterations that drive progression, and the mechanisms of action and resistance to current therapies will be vital for development of novel and targeted treatments. The lack of adequate animal models that recapitulate the full spectrum of MDS biology has been a major obstacle for these efforts. Still, a large number of agents are undergoing clinical trials for MDS. Detailed discussion of the agents undergoing preclinical and early clinical evaluation for MDS is beyond the scope of this paper.
In addition to HDACIs, novel azanucleosides, agents targeting P53 and lenalidomide resistance mechanisms, and combination regimens mentioned earlier, a large number of agents have and are been evaluated for therapeutic potential in patients with MDS. Some of the recently studied agents for HR-MDS include oral and intravenous formulations of clofarabine [197,198], the multikinase inhibitor rigosertib (previously known as ON-01910) [199], the novel oral deoxycytidine nucleoside sapacitabine [200], farnesyl-transferase inhibitors [201–203], arsenic trioxide [204,205], and the tyrosine kinase inhibitors erlotinib and dasatinib [206]. For LR-MDS, the glutathione S-transferase P1-1 inhibitor ezatiostat (TLK199) is showing promising activity for management of anemia [207,208]. The thrombopoietin-receptior agonists romiplostim and eltrombopag improved thrombocytopenia in patients with LR-MDS but concerns have been raised about the risk of leukemic progression with these agents [209,210].
Some of the promising agents that are entering early phase clinical trials for MDS include INCB024360, a potent oral inhibitor of the key enzyme in breakdown pathways of tryptophan, indoleamine 2,3 dioxygenease 1 [IDO1] [211,212]. This agent modulates the BM microenvironment by metabolically targeting the regulatory T-cells and the myeloid-derived suppressor cells [91,213]. Other agents that target the BM microenvironment include sotatercept, also known as ACE-011, and LY2157299, which target the inhibitory transforming growth factor beta (TGF-β)-SMAD2 pathway whose constitutive activation contributes to ineffective hematopoiesis in LR-MDS [214–216]. Inhibitors of the enzyme aminopeptidase necessary for cancer cell survival, e.g. tosedostat, and inhibitors of the p38 mitogen-activated protein kinase (p38MAPK) pathway, e.g. SCIO-469 and Array614, will be further tested in MDS [217–220]. Lastly, efforts at targeting the MDS stem call by interfering with vital pathways for stem cell survival such as the Wnt/β-catenin and Notch pathways might hold promise for overcoming drug resistance and possibly achieving cure or long-term control of MDS [91,221]. An example of this approach is the use of the smoothened inhibitor PF-04449913, which interferes with the sonic hedgehog self-renewal pathway in stem cells [222]. This agent demonstrated preliminary activity in a phase 1b study of select myeloid malignancies including MDS [222].
9. Practice points
Myelodysplastic syndromes (MDS) are characterized by significant biologic heterogeneity with consequent wide variations in the clinical course and outcomes of individual patients, necessitating an individualized risk-adaptive therapeutic approach.
Several prognostic tools are in clinical use to facilitate prognosis prediction and inform treatment decisions, but each of these prediction tools has its own limitations that have to be considered when contemplating therapeutic options.
Significant advances occurred on the therapeutic front in last decade with the approval of azacitidine, decitabine, and lenalidomide. Nonetheless, outcomes remain suboptimal as all patients eventually progress, most within 2 years of therapy.
Most MDS patients are not eligible for allogeneic hematopoietic stem transplantation (alloSCT), which remains as the only known curative therapeutic intervention.
Patients who do not respond or relapse after hypomethylating agent therapy or alloSCT have dismal outcomes and should be considered for clinical trials.
The improved understanding of the complex pathogenesis of MDS and the underlying driver genetic, epigenetic, and immunologic mechanisms will help develop novel effective rationally-designed therapies.
10. Research agenda
Understanding the biologic consequences and signaling pathways changes of the newly discovered genetic mutations
Discovery of reliable predictive biomarkers and/or accurate clinical rules that can guide choices of therapy
Development of better prognostic tools that can more accurately identify patients with worse outcomes, especially among patients in the IPSS low and INT-1 risk-categories, to select such patients for more aggressive risk-adaptive therapies or clinical trials.
Understanding mechanisms of action and resistance of azacitidine, decitabine, and lenalidomide and development of strategies to prevent and overcome resistance.
Development of new strategies to improves outcomes of alloSCT for MDS, including selection of patients, pre-transplantation therapy, conditioning regimens and intensity, GVHD prophylaxis, and relapse prevention and treatment.
Footnotes
Conflict of interest: Steve Gore received funding from Celgene and owned Celgene stocks till August 2011. The other authors report no relevant conflicts of interest. All authors contributed significantly to the manuscript and approved the final version.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 2.Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12:849–59. doi: 10.1038/nrc3321. [DOI] [PubMed] [Google Scholar]
- 3.Sekeres MA. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9:57–63. doi: 10.6004/jnccn.2011.0006. [DOI] [PubMed] [Google Scholar]
- 4.Ma X. Epidemiology of myelodysplastic syndromes. Am J Med. 2012;125:S2–5. doi: 10.1016/j.amjmed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee S, Sekeres MA. What’s all the fuss about? Facts and figures about bone marrow failure and conditions. Curr Hematol Malig Rep. 2012;7:300–9. doi: 10.1007/s11899-012-0134-1. [DOI] [PubMed] [Google Scholar]
- 6.Zeidan A, Faltas B, Douglas BS, Gore S. Myelodysplastic syndromes: what do hospitalists need to know? J Hosp Med. 2013;8(6):351–7. doi: 10.1002/jhm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faltas B, Zeidan A, Gergis U. Myelodysplastic syndromes: towards a risk-adaptive treatment approach. Expert Rev Hematol. 2013 doi: 10.1586/17474086.2013.840997. [in press] [DOI] [PubMed] [Google Scholar]
- 8.Mufti GJ, Potter V. Myelodysplastic syndromes: who and when in the course of disease to transplant. Hematology Am Soc Hematol Educ Program. 2012:49–55. doi: 10.1182/asheducation-2012.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–11. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–99. [PubMed] [Google Scholar]
- 11.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 12.Zeidan AM, Smith BD, Komrokji RS, Gore SD. Prognostication in myelodysplastic syndromes: beyond the International Prognostic Scoring System (IPSS) Am J Med. 2013;126:e25. doi: 10.1016/j.amjmed.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 14.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution inmyelodysplastic syndromes. J Clin Oncol. 2007;25:3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 15.Malcovati L, Della Porta MG, Strupp C, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS) Haematologica. 2011;96:1433–40. doi: 10.3324/haematol.2011.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–43. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bejar R, Stevenson KE, Caughey BA, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30:3376–82. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mailloux AW, Sugimori C, Komrokji RS, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol. 2012;189:3198–208. doi: 10.4049/jimmunol.1200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Loosdrecht AA, Ireland R, Kern W, et al. Rationale for the clinical application of flow cytometry in patients with myelodysplastic syndromes: position paper of an International Consortium and the European LeukemiaNet Working Group. Leuk Lymphoma. 2013;54:472–5. doi: 10.3109/10428194.2012.718341. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breccia M, Salaroli A, Loglisci G, Alimena G. Revised IPSS (IPSS-R) stratification and outcome of MDS patients treated with azacitidine. Ann Hematol. 2013;92:411–2. doi: 10.1007/s00277-012-1581-4. [DOI] [PubMed] [Google Scholar]
- 24.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–11. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 25.Zeidan A, Sun Z, Thomas P, Greenberg P, Juckett M, Smith MR, et al. Application of the French prognostic score (FPS) to assess overall survival (OS) in a U.S.-based cohort of patients (pts) treated with azacitidine (Aza) J Clin Oncol. 2013;(suppl) abstr 7126. [Google Scholar]
- 26.van der Helm LH, Alhan C, Wijermans PW, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br J Haematol. 2011;155:599–606. doi: 10.1111/j.1365-2141.2011.08893.x. [DOI] [PubMed] [Google Scholar]
- 27.Lyons RM. Myelodysplastic syndromes: therapy and outlook. Am J Med. 2012;125:S18–23. doi: 10.1016/j.amjmed.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Fenaux P, Ades L. How we treat lower risk myelodysplastic syndromes. Blood. 2013;121:4280–6. doi: 10.1182/blood-2013-02-453068. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell M, Gore S, Zeidan A. Iron chelation therapy for myelodysplastic syndromes-associated iron overload: where do we stand? Expert Rev Hematol. 2013 doi: 10.1586/17474086.2013.814456. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santini V. Clinical use of erythropoietic stimulating agents in myelodysplastic syndromes. Oncologist. 2011;16:35–42. doi: 10.1634/theoncologist.2011-S3-35. [DOI] [PubMed] [Google Scholar]
- 31.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 32.Leitch HA. Controversies surrounding iron chelation therapy for MDS. Blood Rev. 2011;25:17–31. doi: 10.1016/j.blre.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28:2847–52. doi: 10.1200/JCO.2009.25.2395. [DOI] [PubMed] [Google Scholar]
- 34.Oliva EN, Schey C, Hutchings AS. A review of anemia as a cardiovascular risk factor in patients with myelodysplastic syndromes. Am J Blood Res. 2011;1:160–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Parker JE, Fishlock KL, Mijovic A, Czepulkowski B, Pagliuca A, Mufti GJ. ‘Low-risk’ myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro- versus anti-apoptotic bcl-2-related proteins. Br J Haematol. 1998;103:1075–82. doi: 10.1046/j.1365-2141.1998.01114.x. [DOI] [PubMed] [Google Scholar]
- 36.Parker JE, Mufti GJ. Ineffective haemopoiesis and apoptosis inmyelodysplastic syndromes. Br J Haematol. 1998;101:220–30. doi: 10.1046/j.1365-2141.1998.00708.x. [DOI] [PubMed] [Google Scholar]
- 37.Kelaidi C, Beyne-Rauzy O, Braun T, Sapena R, Cougoul P, Adès L, et al. High response rate and improved exercise capacity and quality of life with a new regimen of darbepoetin alfa with or without filgrastim in lower-risk myelodysplastic syndromes: a phase II study by the GFM. Ann Hematol. 2013;92:621–31. doi: 10.1007/s00277-013-1686-4. [DOI] [PubMed] [Google Scholar]
- 38.Santini V. Treatment of low-risk myelodysplastic syndrome: hematopoietic growth factors erythropoietins and thrombopoietins. Semin Hematol. 2012;49:295–303. doi: 10.1053/j.seminhematol.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg PL, Attar E, Bennett JM, et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs A, Janowska-Wieczorek A, Caro J, Bowen DT, Lewis T. Circulating erythropoietin in patients with myelodysplastic syndromes. Br J Haematol. 1989;73:36–9. doi: 10.1111/j.1365-2141.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 41.Casadevall N, Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colony-stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood. 2004;104:321–7. doi: 10.1182/blood-2003-07-2252. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg PL, Sun Z, Miller KB, et al. Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996) Blood. 2009;114:2393–400. doi: 10.1182/blood-2009-03-211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidoff AJ, Weiss SR, Baer MR, et al. Patterns of erythropoiesis-stimulating agent use among Medicare beneficiaries with myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res. 2013;37:675–80. doi: 10.1016/j.leukres.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S, Grabar S, Kelaidi C, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111:574–82. doi: 10.1182/blood-2007-06-096370. [DOI] [PubMed] [Google Scholar]
- 45.Golshayan AR, Jin T, Maciejewski J, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol. 2007;137:125–32. doi: 10.1111/j.1365-2141.2007.06546.x. [DOI] [PubMed] [Google Scholar]
- 46.Jadersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26:3607–13. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 47.Gabrilove J, Paquette R, Lyons RM, et al. Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes. Br J Haematol. 2008;142:379–93. doi: 10.1111/j.1365-2141.2008.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120:1037–46. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 49.Davidoff AJ, Weiss Smith S, Baer MR, et al. Patient and physician characteristics associated with erythropoiesis-stimulating agent use in patients with myelodysplastic syndromes. Haematologica. 2012;97:128–32. doi: 10.3324/haematol.2011.049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negrin RS, Stein R, Doherty K, et al. Maintenance treatment of the anemia of myelodysplastic syndromes with recombinant human granulocyte colony-stimulating factor and erythropoietin: evidence for in vivo synergy. Blood. 1996;87:4076–81. [PubMed] [Google Scholar]
- 51.Smith SW, Sato M, Gore SD, et al. Erythropoiesis-stimulating agents are not associated with increased risk of thrombosis in patients with myelodysplastic syndromes. Haematologica. 2012;97:15–20. doi: 10.3324/haematol.2011.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux JL, Sokal G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251:437–8. doi: 10.1038/251437a0. [DOI] [PubMed] [Google Scholar]
- 53.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 54.Steensma DP. Historical perspectives on myelodysplastic syndromes. Leuk Res. 2012;36:1441–52. doi: 10.1016/j.leukres.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Kantarjian H, O’Brien S, Ravandi F, et al. The heterogeneous prognosis of patients with myelodysplastic syndrome and chromosome 5 abnormalities: how does it relate to the original lenalidomide experience in MDS? Cancer. 2009;115:5202–9. doi: 10.1002/cncr.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padron E, Komrokji R, List AF. Biology and treatment of the 5q– syndrome. Expert Rev Hematol. 2011;4:61–9. doi: 10.1586/ehm.11.2. [DOI] [PubMed] [Google Scholar]
- 57.Ebert BL, Pretz J, Bosco J, et al. Identification of RPS14 as a 5q– syndrome gene by RNA interference screen. Nature. 2008;451:335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bejar R, Tiu R, Sekeres M, Komrokji R. Myelodysplastic syndromes. Am Soc Clin Oncol Educ Book. 2013:256–70. doi: 10.14694/EdBook_AM.2013.33.e256. [DOI] [PubMed] [Google Scholar]
- 59.Dutt S, Narla A, Lin K, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–76. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuchs O. Important genes in the pathogenesis of 5q– syndrome and their connection with ribosomal stress and the innate immune system pathway. Leuk Res Treat. 2012 doi: 10.1155/2012/179402. http://dx.doi.org/10.1155/2012/179402 [2012;2012:179402, Electronic publication ahead of print 2012 Feb 13.] [DOI] [PMC free article] [PubMed]
- 61.Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del(5q)myelodysplastic syndromes. Expert Rev Anticancer Ther. 2010;10:1663–72. doi: 10.1586/era.10.135. [DOI] [PubMed] [Google Scholar]
- 62.Kumar MS, Narla A, Nonami A, et al. Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q– syndrome. Blood. 2011;118:4666–73. doi: 10.1182/blood-2010-12-324715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komrokji RS, List AF. Lenalidomide for treatment of myelodysplastic syndromes. Curr Pharm Des. 2012;18:3198–203. doi: 10.2174/1381612811209023198. [DOI] [PubMed] [Google Scholar]
- 64.Germing U, Lauseker M, Hildebrandt B, et al. Survival, prognostic factors and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia. 2012;26:1286–92. doi: 10.1038/leu.2011.391. [DOI] [PubMed] [Google Scholar]
- 65.Jadersten M, Saft L, Smith A, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29:1971–9. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]
- 66.Sebaa A, Ades L, Baran-Marzack F, et al. Incidence of 17p deletions and TP53 mutation in myelodysplastic syndrome and acute myeloid leukemia with 5q deletion. Genes Chromosomes Cancer. 2012;51:1086–92. doi: 10.1002/gcc.21993. [DOI] [PubMed] [Google Scholar]
- 67.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 68.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–76. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 69.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 70.Sekeres MA. Lenalidomide in MDS: 4th time’s a charm. Blood. 2011;118:3757–8. doi: 10.1182/blood-2011-07-369009. [DOI] [PubMed] [Google Scholar]
- 71.Ades L, Le Bras F, Sebert M, et al. Treatment with lenalidomide does not appear to increase the risk of progression in lower risk myelodysplastic syndromes with 5q deletion. A comparative analysis by the Groupe Francophone des Myelodysplasies. Haematologica. 2012;97:213–8. doi: 10.3324/haematol.2011.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuendgen A, Lauseker M, List AF, et al. Lenalidomide does not increase AML progression risk in RBC transfusion-dependent patients with Low- or Intermediate-1-risk MDS with del(5q): a comparative analysis. Leukemia. 2013;27:1072–9. doi: 10.1038/leu.2012.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gohring G, Giagounidis A, Busche G, et al. Patients with del(5q) MDS who fail to achieve sustained erythroid or cytogenetic remission after treatment with lenalidomide have an increased risk for clonal evolution and AML progression. Ann Hematol. 2010;89:365–74. doi: 10.1007/s00277-009-0846-z. [DOI] [PubMed] [Google Scholar]
- 74.Sibon D, Cannas G, Baracco F, et al. Lenalidomide in lower-risk myelodysplastic syndromes with karyotypes other than deletion 5q and refractory to erythropoiesis-stimulating agents. Br J Haematol. 2012;156:619–25. doi: 10.1111/j.1365-2141.2011.08979.x. [DOI] [PubMed] [Google Scholar]
- 75.Sekeres MA, Maciejewski JP, Giagounidis AA, et al. Relationship of treatment-related cytopenias and response to lenalidomide in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2008;26:5943–9. doi: 10.1200/JCO.2007.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ebert BL, Galili N, Tamayo P, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Med. 2008;5:e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sardnal V, Rouquette A, Kaltenbach S, et al. A G polymorphism in the CRBN gene acts as a biomarker of response to treatment with lenalidomide in low/int-1 risk MDS without del(5q) Leukemia. 2013;27(7):1610–3. doi: 10.1038/leu.2013.59. [DOI] [PubMed] [Google Scholar]
- 78.Ades L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–52. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 79.Ades L, Fenaux P. Immunomodulating drugs in myelodysplastic syndromes. Hematol Am Soc Hematol Educ Program. 2011:556–60. doi: 10.1182/asheducation-2011.1.556. [DOI] [PubMed] [Google Scholar]
- 80.Mollgard L, Saft L, Treppendahl MB, et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica. 2011;96:963–71. doi: 10.3324/haematol.2010.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Padron E, Komrokji R, List AF. The 5q– syndrome: biology and treatment. Curr Treat Options Oncol. 2011;12:354–68. doi: 10.1007/s11864-011-0165-1. [DOI] [PubMed] [Google Scholar]
- 82.Wei S, Chen X, Rocha K, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci U S A. 2009;106:12974–9. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliva EN, Cuzzola M, Nobile F, et al. Changes in RPS14 expression levels during lenalidomide treatment in Low- and Intermediate-1-risk myelodysplastic syndromes with chromosome 5q deletion. Eur J Haematol. 2010;85:231–5. doi: 10.1111/j.1600-0609.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 84.Komrokji RS, List AF. Lenalidomide for treatment of myelodysplastic syndromes: current status and future directions. Hematol Oncol Clin North Am. 2010;24:377–88. doi: 10.1016/j.hoc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Wei S, Chen X, McGraw K, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2013;32:1110–20. doi: 10.1038/onc.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zandberg DP, Hendrick F, Vannorsdall E, et al. Tertiary center referral patterns for myelodysplastic syndrome patients are indicative of age and race disparities: a single-institution experience. Leuk Lymphoma. 2012;54:304–9. doi: 10.3109/10428194.2012.710904. [DOI] [PubMed] [Google Scholar]
- 87.Wu L, Li X, Xu F, Zhang Z, Chang C, He Q. Low RPS14 expression in MDS without 5q —aberration confers higher apoptosis rate of nucleated erythrocytes and predicts prolonged survival and possible response to lenalidomide in lower risk non-5q– patients. Eur J Haematol. 2013;90:486–93. doi: 10.1111/ejh.12105. [DOI] [PubMed] [Google Scholar]
- 88.Sekeres MA, Gundacker H, Lancet J, et al. A phase 2 study of lenalidomide monotherapy in patients with deletion 5q acutemyeloid leukemia: Southwest Oncology Group Study S0605. Blood. 2011;118:523–8. doi: 10.1182/blood-2011-02-337303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voutsadakis IA, Cairoli A. A critical review of the molecular pathophysiology of lenalidomide sensitivity in 5q—myelodysplastic syndromes. Leuk Lymphoma. 2012;53:779–88. doi: 10.3109/10428194.2011.623255. [DOI] [PubMed] [Google Scholar]
- 90.Caceres G, Johnson J, McGraw K, et al. Targeted repression of TP53 promotes erythropoiesis in Del(5q) MDS and overcomes clinical resistance to lenalidomide. ASH Ann Meet Abstr. 2012;120:920. [Google Scholar]
- 91.List AF. New therapeutics for myelodysplastic syndromes. Leuk Res. 2012;36:1470–4. doi: 10.1016/j.leukres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Giannouli S, Kanellopoulou T, Voulgarelis M. Myelodysplasia and autoimmunity. Curr Opin Rheumatol. 2012;24:97–102. doi: 10.1097/BOR.0b013e32834db4ee. [DOI] [PubMed] [Google Scholar]
- 93.Fozza C, Contini S, Galleu A, et al. Patients with myelodysplastic syndromes display several T-cell expansions, which are mostly polyclonal in the CD4(+) subset and oligoclonal in the CD8(+) subset. Exp Hematol. 2009;37:947–55. doi: 10.1016/j.exphem.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Kordasti SY, Afzali B, Lim Z, et al. IL-17-producing CD4(+)T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145:64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- 95.Kordasti SY, Ingram W, Hayden J, et al. CD4+CD25high Foxp3 + regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 96.Fozza C, Longu F, Contini S, et al. Patients with early-stage myelodysplastic syndromes show increased frequency of CD4+CD25+CD127(low) regulatory T cells. Acta Haematol. 2012;128:178–82. doi: 10.1159/000339498. [DOI] [PubMed] [Google Scholar]
- 97.Barrett AJ, Sloand E. Autoimmune mechanisms in the pathophysiology of myelodysplastic syndromes and their clinical relevance. Haematologica. 2009;94:449–51. doi: 10.3324/haematol.2009.006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parikh AR, Olnes MJ, Barrett AJ. Immunomodulatory treatment of myelodysplastic syndromes: antithymocyte globulin, cyclosporine, and alemtuzumab. Semin Hematol. 2012;49:304–11. doi: 10.1053/j.seminhematol.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sloand EM, Melenhorst JJ, Tucker ZC, et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood. 2011;117:2691–9. doi: 10.1182/blood-2010-04-277921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sloand EM, Mainwaring L, Fuhrer M, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–51. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dixit A, Chatterjee T, Mishra P, et al. Cyclosporin A in myelodysplastic syndrome: a preliminary report. Ann Hematol. 2005;84:565–8. doi: 10.1007/s00277-005-1016-6. [DOI] [PubMed] [Google Scholar]
- 102.Chen S, Jiang B, Da W, Gong M, Guan M. Treatment of myelodysplastic syndrome with cyclosporin A. Int J Hematol. 2007;85:11–7. doi: 10.1532/IJH97.A10513. [DOI] [PubMed] [Google Scholar]
- 103.Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, Barrett AJ. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99:699–705. doi: 10.1046/j.1365-2141.1997.4423249.x. [DOI] [PubMed] [Google Scholar]
- 104.Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120:679–84. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 105.Passweg JR, Giagounidis AA, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/9. J Clin Oncol. 2011;29:303–9. doi: 10.1200/JCO.2010.31.2686. [DOI] [PubMed] [Google Scholar]
- 106.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17:2101–6. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- 107.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18:460–5. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 108.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28:5166–73. doi: 10.1200/JCO.2010.29.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scott BL, Ramakrishnan A, Fosdal M, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149:706–10. doi: 10.1111/j.1365-2141.2010.08145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Platzbecker U, Haase M, Herbst R, et al. Activity of sirolimus in patients with myelodysplastic syndrome—results of a pilot study. Br J Haematol. 2005;128:625–30. doi: 10.1111/j.1365-2141.2005.05360.x. [DOI] [PubMed] [Google Scholar]
- 111.Saunthararajah Y, Nakamura R, Nam JM, et al. HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2002;100:1570–4. [PubMed] [Google Scholar]
- 112.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102:3025–7. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 113.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–11. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vigna E, Recchia AG, Madeo A, et al. Epigenetic regulation in myelodysplastic syndromes: implications for therapy. Expert Opin Investig Drugs. 2011;20:465–93. doi: 10.1517/13543784.2011.559164. [DOI] [PubMed] [Google Scholar]
- 115.Aimiuwu J, Wang H, Chen P, et al. RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood. 2012;119:5229–38. doi: 10.1182/blood-2011-11-382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griffiths EA, Gore SD. DNA methyltransferase inhibitors: class effect or unique agents? Leuk Lymphoma. 2008;49:650–1. doi: 10.1080/10428190801947575. [DOI] [PubMed] [Google Scholar]
- 117.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 118.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 120.Lubbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–96. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 121.Gore SD. New ways to use DNA methyltransferase inhibitors for the treatment of myelodysplastic syndrome. Hematol Am Soc Hematol Educ Program. 2011:550–5. doi: 10.1182/asheducation-2011.1.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 123.Fenaux P, Gattermann N, Seymour JF, et al. Prolonged survival with improved tolerability in higher-risk myelodysplastic syndromes: azacitidine compared with low dose ara-C. Br J Haematol. 2010;149:244–9. doi: 10.1111/j.1365-2141.2010.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steensma DP, Stone RM. Practical recommendations for hypomethylating agent therapy of patients with myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:389–406. doi: 10.1016/j.hoc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 125.Fenaux P, Bowen D, Gattermann N, et al. Practical use of azacitidine in higher-risk myelodysplastic syndromes: an expert panel opinion. Leuk Res. 2010;34:1410–6. doi: 10.1016/j.leukres.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 126.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 127.Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. 2011;117:2697–702. doi: 10.1002/cncr.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gore SD, Fenaux P, Santini V, et al. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013;98(7):1067–72. doi: 10.3324/haematol.2012.074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeidan A, Gore S. Management of high-risk myelodysplastic syndrome. In: Deeg HJ, editor. Myelodysplastic syndromes. 2. Springer; 2013. [in press] [Google Scholar]
- 130.Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27:1850–6. doi: 10.1200/JCO.2008.17.1058. [DOI] [PubMed] [Google Scholar]
- 131.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronicmyelomonocytic leukemia, and acutemyeloid leukemia. J Clin Oncol. 2011;29:2521–7. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147–52. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 133.Breccia M, Loglisci G, Cannella L, et al. Application of French prognostic score to patients with International Prognostic Scoring System intermediate-2 or high risk myelodysplastic syndromes treated with 5-azacitidine is able to predict overall survival and rate of response. Leuk Lymphoma. 2012;53:985–6. doi: 10.3109/10428194.2011.643408. [DOI] [PubMed] [Google Scholar]
- 134.Itzykson R, Thepot S, Quesnel B, et al. Long-term outcome of higher-risk MDS patients treated with azacitidine: an update of the GFM compassionate program cohort. Blood. 2012;119:6172–3. doi: 10.1182/blood-2012-04-422204. [DOI] [PubMed] [Google Scholar]
- 135.Estey EH. Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia. Leukemia. 2013 doi: 10.1038/leu.2013.173. [Electronic publication ahead of print] [DOI] [PubMed] [Google Scholar]
- 136.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 137.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–8. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shen L, Kantarjian H, Guo Y, et al. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol. 2010;28:605–13. doi: 10.1200/JCO.2009.23.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Santini V, Fenaux P, Mufti GJ, et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol. 2010;85:130–8. doi: 10.1111/j.1600-0609.2010.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saunthararajah Y, Hillery CA, Lavelle D, et al. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102:3865–70. doi: 10.1182/blood-2003-05-1738. [DOI] [PubMed] [Google Scholar]
- 141.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol. 2011;29:516–23. doi: 10.1200/JCO.2010.31.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–7. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prebet T, Gore SD, Thepot S, et al. Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure. Br J Haematol. 2012;157(6):764–6. doi: 10.1111/j.1365-2141.2012.09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–4. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kadia TM, Jabbour E, Kantarjian H. Failure of hypomethylating agent-based therapy in myelodysplastic syndromes. Semin Oncol. 2011;38:682–92. doi: 10.1053/j.seminoncol.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–67. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oosterveld M, Muus P, Suciu S, et al. Chemotherapy only compared to chemotherapy followed by transplantation in high risk myelodysplastic syndrome and secondary acute myeloid leukemia; two parallel studies adjusted for various prognostic factors. Leukemia. 2002;16:1615–21. doi: 10.1038/sj.leu.2402591. [DOI] [PubMed] [Google Scholar]
- 148.Wattel E, De Botton S, Luc Lai J, et al. Long-term follow-up of de novo myelodysplastic syndromes treated with intensive chemotherapy: incidence of long-term survivors and outcome of partial responders. Br J Haematol. 1997;98:983–91. doi: 10.1046/j.1365-2141.1997.2973114.x. [DOI] [PubMed] [Google Scholar]
- 149.Beran M, Shen Y, Kantarjian H, et al. High-dose chemotherapy in high-risk myelodysplastic syndrome: covariate-adjusted comparison of five regimens. Cancer. 2001;92:1999–2015. doi: 10.1002/1097-0142(20011015)92:8<1999::aid-cncr1538>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 150.Garcia-Manero G. Myelodysplastic syndromes: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:490–8. doi: 10.1002/ajh.22047. [DOI] [PubMed] [Google Scholar]
- 151.Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer. 2006;106:1099–109. doi: 10.1002/cncr.21699. [DOI] [PubMed] [Google Scholar]
- 152.Knipp S, Hildebrand B, Kundgen A, et al. Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer. 2007;110:345–52. doi: 10.1002/cncr.22779. [DOI] [PubMed] [Google Scholar]
- 153.Bello C, Yu D, Komrokji RS, et al. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. 2011;117:1463–9. doi: 10.1002/cncr.25598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Platzbecker U. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndromes. Semin Hematol. 2012;49:342–9. doi: 10.1053/j.seminhematol.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 155.Zeidan A, Gore S, Komrokji R. Higher-risk myelodysplastic syndromes with del5q: Is sequential azacitidine–lenalidomide combination the way to go? Expert Rev Hematol. 2013;6(3):251–4. doi: 10.1586/ehm.13.30. [DOI] [PubMed] [Google Scholar]
- 156.Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–8. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 158.Prebet T, Vey N. Vorinostat in acute myeloid leukemia and myelodysplastic syndromes. Expert Opin Investig Drugs. 2011;20:287–95. doi: 10.1517/13543784.2011.542750. [DOI] [PubMed] [Google Scholar]
- 159.Cashen A, Juckett M, Jumonville A, et al. Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS) Ann Hematol. 2012;91:33–8. doi: 10.1007/s00277-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dimicoli S, Jabbour E, Borthakur G, et al. Phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in patients with low or intermediate-1 risk myelodysplastic syndrome. Am J Hematol. 2012;87:127–9. doi: 10.1002/ajh.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 162.Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res. 2005;29:739–48. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–9. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–8. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 165.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–9. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 166.Prebet T, Gore S, Sun Z, et al. Prolonged administration of azacitidine with or without entinostat increases rate of hematologic normalization for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup Trial E1905. ASH Annu Meet Abstr. 2010;116:601. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sekeres MA, List AF, Cuthbertson D, et al. Phase I combination trial of lenalidomide and azacitidine in patients with higher-risk myelodysplastic syndromes. J Clin Oncol. 2010;28:2253–8. doi: 10.1200/JCO.2009.26.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–51. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Platzbecker U, Braulke F, Kundgen A, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27(6):1403–7. doi: 10.1038/leu.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Borthakur G, Huang X, Kantarjian H, et al. Report of a phase 1/2 study of a combination of azacitidine and cytarabine in acute myelogenous leukemia and high-risk myelodysplastic syndromes. Leuk Lymphoma. 2010;51:73–8. doi: 10.3109/10428190903318329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bayraktar UD, Domingo GC, Schmit J, Pereira D. Azacitidine combined with gemtuzumab ozogamicin in patients with relapsed/refractory acute myeloid leukemia. Leuk Lymphoma. 2011;52:913–5. doi: 10.3109/10428194.2010.551570. [DOI] [PubMed] [Google Scholar]
- 172.Nand S, Godwin J, Smith S, et al. Hydroxyurea, azacitidine and gemtuzumab ozogamicin therapy in patients with previously untreated non-M3 acute myeloid leukemia and high-risk myelodysplastic syndromes in the elderly: results from a pilot trial. Leuk Lymphoma. 2008;49:2141–7. doi: 10.1080/10428190802451254. [DOI] [PubMed] [Google Scholar]
- 173.Komrokji RS, Lancet JE, Swern AS, et al. Combined treatment with lenalidomide and epoetin alfa in lower-risk patients with myelodysplastic syndrome. Blood. 2012;120:3419–24. doi: 10.1182/blood-2012-03-415661. [DOI] [PubMed] [Google Scholar]
- 174.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–11. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 175.Giralt SA, Horowitz M, Weisdorf D, Cutler C. Review of stem-cell transplantation for myelodysplastic syndromes in older patients in the context of the Decision Memo for Allogeneic Hematopoietic Stem Cell Transplantation for Myelodysplastic Syndrome emanating from the Centers for Medicare and Medicaid Services. J Clin Oncol. 2011;29:566–72. doi: 10.1200/JCO.2010.32.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu F, Deeg HJ. Current status of allogeneic hematopoietic cell transplantation for MDS. Curr Pharm Des. 2012;18:3215–21. doi: 10.2174/1381612811209023215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Giralt S. Bone marrow transplant inmyelodysplastic syndromes: new technologies, same questions. Curr Hematol Rep. 2005;4:200–7. [PubMed] [Google Scholar]
- 178.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31(21):2662–70. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Platzbecker U, Schetelig J, Finke J, et al. Allogeneic hematopoietic cell transplantation in patients age 60–70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: comparison with patients lacking donors who received azacitidine. Biol Blood Marrow Transplant. 2012;18:1415–21. doi: 10.1016/j.bbmt.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Appelbaum FR. The role of hematopoietic cell transplantation as therapy for myelodysplasia. Best Pract Res Clin Haematol. 2011;24:541–7. doi: 10.1016/j.beha.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 182.Cutler C. Allogeneic hematopoietic stem-cell transplantation for myelodysplastic syndrome. Hematol Am Soc Hematol Educ Program. 2010:325–9. doi: 10.1182/asheducation-2010.1.325. [DOI] [PubMed] [Google Scholar]
- 183.Cutler C. Patient selection for transplantation in the myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:469–76. doi: 10.1016/j.hoc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 184.Gyurkocza B, Deeg HJ. Allogeneic hematopoietic cell transplantation for MDS: for whom, when and how? Blood Rev. 2012;26:247–54. doi: 10.1016/j.blre.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Armand P, Kim HT, Rhodes J, et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:852–60. doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cutler C. Transplantation for MDS in the elderly: more evidence, or more bias? Biol Blood Marrow Transplant. 2012;18:1320–1. doi: 10.1016/j.bbmt.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 187.Deeg HJ, Bartenstein M. Allogeneic hematopoietic cell transplantation for myelodysplastic syndrome: current status. Arch Immunol Ther Exp (Warsz) 2012;60:31–41. doi: 10.1007/s00005-011-0152-z. [DOI] [PubMed] [Google Scholar]
- 188.Oliansky DM, Antin JH, Bennett JM, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant. 2009;15:137–72. doi: 10.1016/j.bbmt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 189.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120:1398–408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96:441–9. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–54. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 192.Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica. 2009;94:729–32. doi: 10.3324/haematol.2008.002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–84. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 195.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112:895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 196.Lee JH, Lee JH, Lim SN, et al. Allogeneic hematopoietic cell transplantation for myelodysplastic syndrome: prognostic significance of pre-transplant IPSS score and comorbidity. Bone Marrow Transplant. 2010;45:450–7. doi: 10.1038/bmt.2009.190. [DOI] [PubMed] [Google Scholar]
- 197.Faderl S, Garcia-Manero G, Estrov Z, et al. Oral clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. J Clin Oncol. 2010;28:2755–60. doi: 10.1200/JCO.2009.26.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Faderl S, Garcia-Manero G, Jabbour E, et al. A randomized study of 2 dose levels of intravenous clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. Cancer. 2012;118:722–8. doi: 10.1002/cncr.26327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reddy MV, Venkatapuram P, Mallireddigari MR, et al. Discovery of a clinical stage multi-kinase inhibitor sodium (E)-2-{2-methoxy-5-[(2′,4′,6′-trimethoxystyrylsulfonyl) methyl] phenylamino} acetate (ON 01910. Na): synthesis, structure-activity relationship, and biological activity. J Med Chem. 2011;54:6254–76. doi: 10.1021/jm200570p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kantarjian H, Garcia-Manero G, O’Brien S, et al. Phase I clinical and pharmacokinetic study of oral sapacitabine in patients with acute leukemia and myelodysplastic syndrome. J Clin Oncol. 2010;28:285–91. doi: 10.1200/JCO.2009.25.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fenaux P, Raza A, Mufti GJ, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109:4158–63. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 202.Itzykson R, Fenaux P. Optimal sequencing of treatments for patients with myelodysplastic syndromes. Curr Opin Hematol. 2009;16:77–83. doi: 10.1097/MOH.0b013e3283257a74. [DOI] [PubMed] [Google Scholar]
- 203.Jabbour E, Kantarjian H, Ravandi F, et al. A phase 1–2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer. 2011;117:1236–44. doi: 10.1002/cncr.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schiller GJ, Slack J, Hainsworth JD, et al. Phase II multicenter study of arsenic trioxide in patients with myelodysplastic syndromes. J Clin Oncol. 2006;24:2456–64. doi: 10.1200/JCO.2005.03.7903. [DOI] [PubMed] [Google Scholar]
- 205.Vey N, Bosly A, Guerci A, et al. Arsenic trioxide in patients with myelodysplastic syndromes: a phase II multicenter study. J Clin Oncol. 2006;24:2465–71. doi: 10.1200/JCO.2005.03.9503. [DOI] [PubMed] [Google Scholar]
- 206.Duong VH, Jaglal MV, Zhang L, et al. Phase II pilot study of oral dasatinib in patients with higher-risk myelodysplastic syndrome (MDS) who failed conventional therapy. Leuk Res. 2013;37:300–4. doi: 10.1016/j.leukres.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 207.Raza A, Galili N, Smith S, et al. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009;113:6533–40. doi: 10.1182/blood-2009-01-176032. [DOI] [PubMed] [Google Scholar]
- 208.Raza A, Galili N, Mulford D, et al. Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS) J Hematol Oncol. 2012;5 doi: 10.1186/1756-8722-5-18. [18-8722-5-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sekeres MA, Kantarjian H, Fenaux P, et al. Subcutaneous or intravenous administration of romiplostim in thrombocytopenic patients with lower risk myelodysplastic syndromes. Cancer. 2011;117:992–1000. doi: 10.1002/cncr.25545. [DOI] [PubMed] [Google Scholar]
- 210.Oliva EN, Santini V, Zini G, et al. Efficacy and safety of eltrombopag for the treatment of thrombocytopenia of low and intermediate-1 IPSS risk myelodysplastic syndromes: interim analysis of a prospective, randomized, single-blind, placebo-controlled trial (EQoL-MDS) ASH Ann Meet Abstr. 2012;120:923. [Google Scholar]
- 211.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 212.Muller AJ, Malachowski WP, Prendergast GC. Indoleamine 2,3-dioxygenase in cancer: targeting pathological immune tolerance with small-molecule inhibitors. Expert Opin Ther Targets. 2005;9:831–49. doi: 10.1517/14728222.9.4.831. [DOI] [PubMed] [Google Scholar]
- 213.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084–95. doi: 10.1182/blood-2011-07-365817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou L, McMahon C, Bhagat T, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71:955–63. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhou L, Nguyen AN, Sohal D, et al. Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS. Blood. 2008;112:3434–43. doi: 10.1182/blood-2008-02-139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Raje N, Vallet S. Sotatercept, a soluble activin receptor type 2A IgG-Fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther. 2010;12:586–97. [PubMed] [Google Scholar]
- 217.Lowenberg B, Morgan G, Ossenkoppele GJ, et al. Phase I/II clinical study of Tosedostat, an inhibitor of aminopeptidases, in patients with acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2010;28:4333–8. doi: 10.1200/JCO.2009.27.6295. [DOI] [PubMed] [Google Scholar]
- 218.Navas T, Zhou L, Estes M, et al. Inhibition of p38alpha MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenvironment. Leuk Lymphoma. 2008;49:1963–75. doi: 10.1080/10428190802322919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Navas TA, Mohindru M, Estes M, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–7. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sokol L, Cripe L, Kantarjian H, et al. Randomized, dose-escalation study of the p38alpha MAPK inhibitor SCIO-469 in patients with myelodysplastic syndrome. Leukemia. 2013;27:977–80. doi: 10.1038/leu.2012.264. [DOI] [PubMed] [Google Scholar]
- 221.Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740–50. doi: 10.1172/JCI31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jamieson C, Cortes J, Oehler V, et al. Phase 1 dose-escalation study of PF-04449913, an oral hedgehog (Hh) inhibitor, in patients with select hematologic malignancies. ASH Ann Meet Abstr. 2011;118:424. [Google Scholar]