Severe steroid-refractory hepatic graft versus host disease (GVHD) is a highly lethal complication of allogeneic bone marrow transplantation (alloBMT) with very few successful treatment options. Orthotopic liver transplantation (OLT) from a cadaveric or a living donor has been very rarely performed as a last-resort therapeutic option in patients with hematologic malignancies who develop refractory hepatic GVHD following alloBMT. Here we report a patient with acute lymphoblastic leukemia (ALL) who successfully underwent OLT for severe refractory hepatic GVHD post alloBMT and review relevant literature.
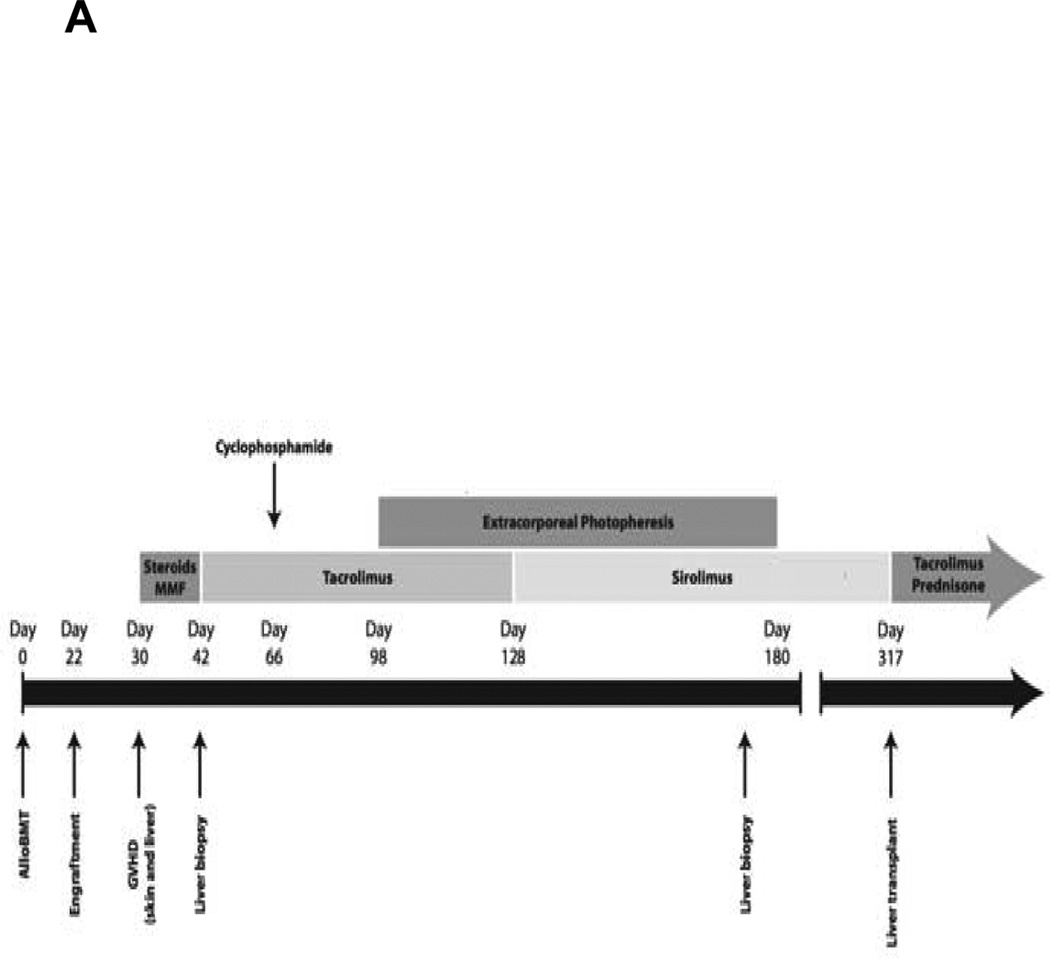
An asymptomatic 33-year old male was diagnosed with T-cell ALL after routine laboratory work showed a white blood cell count (WBC) of 31,900/µL with 28% blasts. The patient received induction chemotherapy with daunorubicin, vincristine, peg-asparaginase, prednisone, methotrexate, cyclophosphamide, cytarabine, and 6-mercaptopurine. A bone marrow (BM) evaluation showed complete remission (CR), but minimal residual disease was detectable by flow cytometry (1%). After additional chemotherapy, he underwent a myeloablative, HLA-matched related donor (MRD) alloBMT from his sister 5 months after diagnosis [Figure 1A]. The conditioning regimen consisted of busulfan and fludarabine, followed by post-transplant high dose cyclophosphamide (PTCy) 50 mg/kg IV on days 3 and 4 as sole graft-versus-host-disease (GVHD) prophylaxis on institutional protocol. Neutrophil engraftment occurred by day 22 and full donor chimerism was observed on the day +30.
Figure 1.
A Timeline of clinical course of the patient.
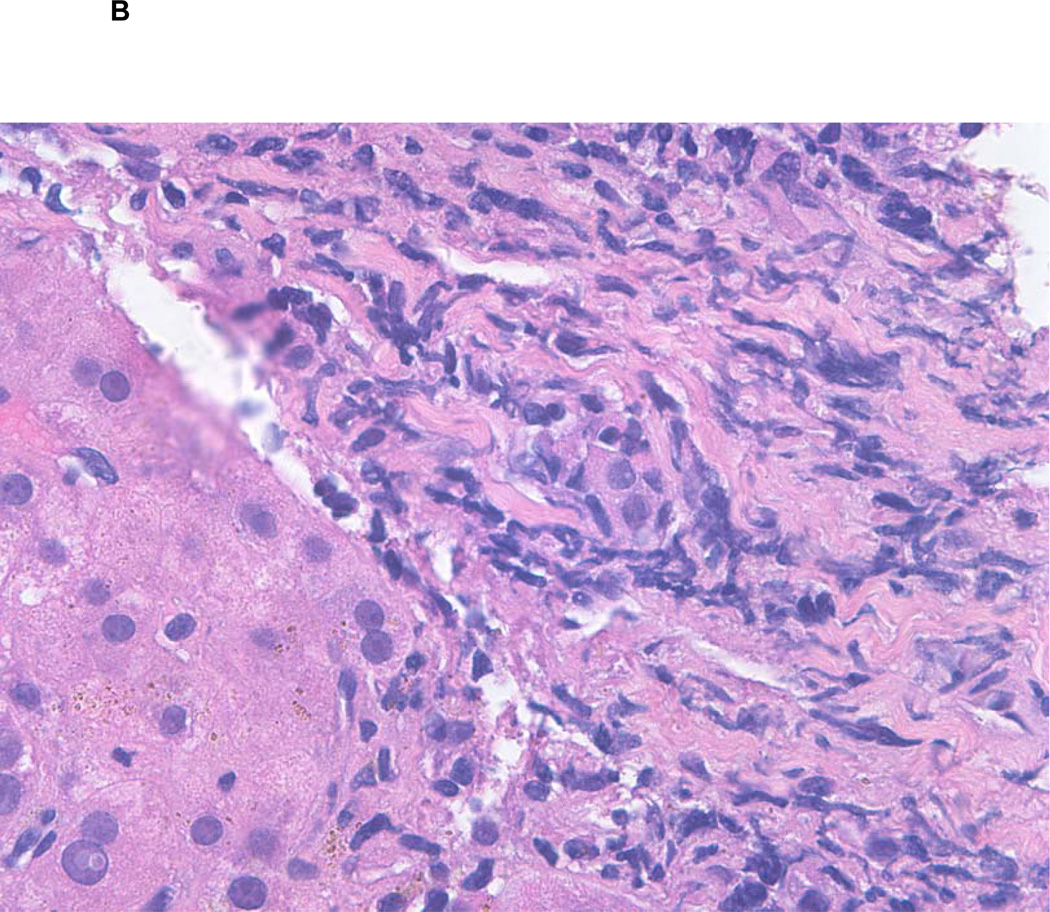
B Liver biopsy showing active graft versus host disease (GVHD). H&E, original magnification 260X. A portal tract shows mild chronic inflammation with bile duct injury.
On day 30, he developed a diffuse erythematous maculopapular rash and marked transaminitis. Imaging of the liver was unremarkable and viral hepatitis B and C, cytomegalovirus, and Epstein-Barr virus studies were all negative. A skin biopsy was consistent with acute GVHD (aGVHD). Treatment with high dose steroids (prednisone 2.5 mg/kg daily) and on a clinical trial mycophenolate mofetil versus placebo was initiated. The skin and hepatic GVHD was refractory to therapy and the liver enzymes continued to rise, so a transjugular liver biopsy was performed on day +42. Pathology showed patchy bile duct injury and apoptosis, consistent with hepatic GVHD, and moderate lobular spotty necrosis. Viral stains were negative, including CMV. He was switched to tacrolimus and steroids without improvement. Based on the diffuse skin involvement and markedly elevated bilirubin, the aGVHD was stage IV by the Keystone Criteria. On day +53 surveillance quantitative CMV polymerase chain reaction became positive and he developed CMV pneumonitis which was treated successfully with ganciclovir and anti-CMV immunoglobulin. There was no response to subsequent therapies for refractory GVHD with high dose cyclophosphamide, extracorporeal phototherapy, or sirolimus [Figure 1A].
Despite multiple infectious complications, he remained in complete remission from ALL with full donor chimerism. Another liver biopsy on day +170 showed a mild patchy portal chronic inflammation and reactive epithelial changes in the bile ducts with patchy inflammation and injury, consistent with active GVHD [Figure 1B]. The bilirubin level peaked at 53.3 mg/dL [Normal range (NR), 0.6-1.2 mg/dL], alanine transaminase (ALT) 2331 IU/L [NR, 12-40 IU/L), and prothrombin time (PT) of 75.7 seconds [NR, 10.7-13.8 seconds]. These abnormalities, in conjunction with the development of ascites, were all consistent with end-stage liver disease. Therefore, he was referred for evaluation for liver transplantation and underwent an OLT from a cadaveric donor on day +317, with prednisone and tacrolimus utilized for post-OLT immune suppression. Although the immediate postoperative course was complicated by multiple infections and persistent hemorrhagic cystitis, he fully recovered with normalization of liver function (albumin 3.9 g/dL, bilirubin 0.2 mg/dL, ALT 94 IU/L, PT of 11.1 seconds). Twenty-seven months after the BMT and 18 months after the OLT, he remains in CR with full donor chimerism, continues on tacrolimus with normal liver function, and returned to work.
AlloBMT is the only known curative therapeutic modality for some high-risk and refractory hematologic malignancies. Despite recent improvements, alloBMT is still associated with a significant incidence of life-threatening complications including severe GVHD. Up to 60% of patients undergoing alloHSCT develop some form of GVHD, and those who suffer from aGVHD are at higher risk of developing chronic (c)GVHD [1–3]. High-dose steroids are the first line of therapy for severe GVHD, but steroids alone result in complete or partial remission of GVHD in less than half of the cases1. Steroid-refractory severe GVHD is frequently refractory to subsequent therapies and is associated with dismal outcomes and very high mortality rates. It has been estimated that up to 10% of hepatic GVHD cases may become severe and can lead to hepatic cirrhosis [4].
Patients with significantly compromised liver function due to hepatic GVHD who fail to respond to steroids or immunosuppressive therapies (IST) have very limited options. Therefore, OLT has been proposed as a last resort for refractory severe liver GVHD with significant hepatic functional derangement. The rationale for this treatment is the removal of the antigenic targets on the native liver that are being targeted by the aberrant immune response [3]. Use of cadaveric donor liver transplantation (CDLT) has been reported very rarely in the literature but never received wide acceptance as a therapeutic modality for severe refractory hepatic GVHD [1, 3, 5–10]. This is most likely due to concerns of relapse of the underlying hematologic malignancy or hepatic GVHD, the scarcity of cadaveric liver grafts, and possibly the multiple medical and infectious complications common to patients with severe GVHD. To address the issue of the scarcity of cadaveric liver grafts, more recently several case reports were published describing successful living donor liver transplantation (LDLT) from the same HLA-identical related BM donor [11], the same HLA-haploidentical related BM donor [12, 13], the same HLA-matched unrelated BM donor [2], and from an HLA-haploidentical mother LDLT donor after a MUD BMT [14].
In our literature search, we found only 13 case reports of OLT performed for severe hepatic GVHD that occurred after alloBMT, in addition to our patient [Table 1]. The indication for alloBMT was a hematologic malignancy in 11 (most common indication was ALL). Eleven patients were 30 years old or younger at the time of alloBMT (age range at BMT, 0.5-34 years). The BM donor was MRD for 7 patients, matched unrelated donor (MUD) for 5 patients, and an HLA-haploidentical donor for 2 patients. Most OLT were performed a year or more after BMT (8), while only 3 were performed within the first 4 months (all 3 after 100 days post BMT). Our patient had CDLT 10 months after his BMT. Nine patients underwent cadaveric donor liver transplants [Table 1A], while the other 5 underwent LDLT [Table 1B]. Several patients, especially those who received the liver and the BM grafts from the same donor, eventually discontinued IST. Among the 14 patients, only one was reported to have developed liver graft rejection, and only 2 deaths were reported. After a follow-up period that varied between 23 days to 128 months post the LDLT (8 patients with follow-up of 2 years or longer), 12 of the 14 patients were still alive, with many having no evidence of GVHD or recurrence of malignancy, including our patient.
Table 1.
Reported cases of orthotopic liver transplantation (OLT) for treatment of refractory severe hepatic graft versus host disease (GVHD) following blood and bone marrow transplantation (BMT).
| A: Reports of cadaveric OLT. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference And Year |
Age (years) at BMT, gender |
Indication for BMT |
Donor | GVHD timing, location |
HSCT-OLT interval |
IST regimen after OLT |
Rejection | Post-OLT outcome |
Follow-up Post OLT |
|
Rhodes et al. 1990 [5] |
19, F | AML | MRD | 23 days: acute: liver and skin; chronic: cirrhosis |
37 months | CyA, S | No | Alive, no recurrence |
2 years |
|
Marks et al. 1992 [6] |
21, M | CML | MRD | 20 days: skin and liver |
104 days | CyA, S | No | Alive, no recurrence |
6 months |
|
Dowlati et al. 1995 [7] |
4, M | ALL | MRD | 10 days: skin and gut, 82 days: liver |
107 days | CyA, S | Yes | Death, DIC and GIB |
23 days |
|
Figuera et al. 1996 [8] |
31, M | MDS | MRD | 25 days: skin and liver |
109 days | CyA, S | No | Death , disseminated aspergillosis |
129 days |
|
Rosen et al. 1996 [9] |
32, M | ALL | Matched donor (not specified) |
120 days: skin and liver |
330 days | CyA, S, Azathio |
No | Alive, no recurrence, rejection or liver GVHD |
2.5 years |
|
Urban et al. 2002 [10] |
0.5, M | Sideroblastic anemia |
MRD | 27 days: skin, gut and liver. Chronic hepatic GVHD |
5 years | CyA, S, Azathio |
No | Alive, no recurrence |
8.5 years |
| Current Case | 33, M | ALL | MRD | 30 days: skin and liver. Chronic hepatic GVHD. |
305 days | S, Tacrolimus |
No | Alive, no recurrence |
18 months |
|
Orlando et al. 2005 [3] |
27, M | CML | MUD | 51 days: skin and liver |
39 months | CyA, S | No | Alive, no liver GVHD |
128 months |
|
Barshes et al. 2005 [1] |
15, M | Idiopathic aplastic anemia |
MUD | "several months": skin and liver |
10 months | S, Tacrolimus |
No | Alive, no recurrence or liver GVHD |
3 years |
|
Summary of 9 cases of cadaveric OLT |
Median age 21 years (range, 0.5– 33 years) M(8) F(1) |
ALL (3) CML (2) AML (1) MDS (1) Aplastic anemia (1) Sideroblastic anemia (1) |
MRD (6) MUD (2) Matched unspecified (1) |
Median time 27 days (range, 10–120 days) |
Median 10 months (range, 3.5–60 months) |
CyA, S (5) CyA, S, Azathio (2) S, tacrolimus (2) |
No (8) Yes (1) |
Alive (7) Death (2) |
Median 24 months (range, 0.8– 102 months) |
| B: Reports of living OLT. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference And Year |
Age (years) at BMT, gender |
Indication for BMT |
Donor | GVHD timing, location |
HSCT- OLT interval |
Donor living OLT |
IST regimen after OLT |
Rejection | Post-OLT outcome |
Follow-up Post OLT |
|
Shimizu et al. 2006 [12] |
4, M | ALL | Haplo (Mother) |
"after engraftment": skin and liver. Chronic hepatic GVHD |
3 years | Mother | S, Tacrolimus* |
No | Alive, no recurrence or liver GVHD |
8 months |
|
Yokoyama et al. 2008 [14] |
10, M | CGD | MUD | "after engraftment": skin, gut and liver. Chronic hepatic GVHD |
3 years | Mother | S, Tacrolimus* |
No | Alive, no recurrence or liver GVHD |
12 months |
|
Mori et al. 2009 [11] |
30, F | ALL | MRD (sibling) |
Day 22: skin, day 41: gut. Day 356: chronic hepatic GVD. |
421 days | Same sibling |
S, Tacrolimus |
No | Alive, no recurrence or liver GVHD |
18 months |
|
Englert et al. 2012 [2] |
2, M | ALL | MUD | Chronic skin, gut and liver GVHD-cirrhosis. |
4 years (from same MUD) |
Same MUD donor |
S, CyA | No | Alive, no recurrence or liver GVHD |
3 years |
|
Granot et al. 2012 [13] |
2.5, M | ALL | Haplo (father) |
Acute severe gut and hepatic GVHD. Chronic hepatic GVHD |
6.5 years | Father | None | No | Alive, no recurrence or liver GVHD |
7 years |
|
Summary of 5 cases of living OLT |
Median age 4 years (range, 2– 30 years) M (4) F (1) |
ALL (4) CGD (1) |
MRD (1) MUD (2) Haplo (2) |
N/A | Median 36 months (range, 14–78 months) |
Parent (3) Sibling (1) MUD(1) |
S, Tacrolimus (3) S, CyA (1) None (1) |
No (5) Yes (0) |
Alive (5) Death (0) |
Median 18 months (range, 8– 84 months) |
IST: immunosuppressive therapy. C: Cadaveric. L: Living donor. AML: Acute myeloid leukemia. ALL: Acute lymphoblastic leukemia. MDS: Myelodysplastic syndrome, CML: Chronic myeloid leukemia. CGD: Chronic granulomatous disease. HLA: Human leukocyte antigen. MRD: HLA-matched related donor. MUD: HLA-matched unrelated donor. Haplo: HLA-Haploidentical donor.
Hepatic artery and portal veins of hepatic graft flushed with the antibody muromonab-CD3. CyA: Cyclosporine, S: steroids, Azathio: Azathioprine.
It is very likely that more cases of OLT for severe hepatic GVHD have been performed and not reported in literature, especially cases with worse outcomes. Therefore, the numbers might overestimate the success rates and underestimate the complications associated with OLT for hepatic GVHD. Barshes et al conducted a search of the UNOS Organ Procurement and Transplant Network database from 1988 to 2003 [1]. The authors reported 73 patients who received OLT for severe liver GVHD after a non-liver organ transplant (65 had alloBMT [89.1%]). The median age for the entire cohort was 35 years. Median waiting time from transplantation registration to OLT was 8.5 days, and most patients received a cadaveric whole liver transplant (85%) while 11% received cadaveric partial liver transplant. At time of reporting with a median follow-up of 357 days after OLT, 25% of patients had died. Interestingly, none of these deaths were attributed to GVHD or graft rejection, and only 2 patients died from cancer (lymphoma-not clear if recurrent or secondary- and metastatic squamous cancer). The 5-year graft and patient survival rates of 57.3% and 69%, respectively, but the authors did not comment on malignancy relapse or GVHD recurrence [1].
A recently published questionnaire surveyed 67, 578 patients who underwent alloBMT in 107 European centers for performance of solid organ transplantation subsequent to alloBMT [15]. The authors documented only 45 solid organ transplants, of which 15 were OLT performed in 14 patients. Of the 14 patients who had OLT, the indication for OLT was refractory hepatic GVHD in six (3 acute and 3 chronic). The authors did not report characteristics and outcomes of the GVHD patients individually, but reported an overall 5-year survival rate of 71%. The authors noted that these patients were very carefully selected [15].
In conclusion, liver transplantation, whether from a cadaveric or living donor, can be an effective therapeutic modality for refractory hepatic GVHD after alloBMT. Acknowledging the retrospective nature of the available literature and the associated selection and publication biases, the literature suggests low rates of recurrent hepatic GVHD, graft rejection, and favorable survival in stringently selected patients. Careful selection of patients based on the underlying malignancy, risk of relapse, and medical co-morbidities are all very important considerations for candidacy for liver transplantation and the choice of the graft source.
Acknowledgment
The authors like to thank Dr. Michael Schweizer for his help in design of Figure 1A.
Footnotes
DISCLAIMER: The ideas and opinions expressed in the journal’s Just Accepted articles do not necessarily reflect those of Informa Healthcare (the Publisher), the Editors or the journal. The Publisher does not assume any responsibility for any injury and/or damage to persons or property arising from or related to any use of the material contained in these articles. The reader is advised to check the appropriate medical literature and the product information currently provided by the manufacturer of each drug to be administered to verify the dosages, the method and duration of administration, and contraindications. It is the responsibility of the treating physician or other health care professional, relying on his or her independent experience and knowledge of the patient, to determine drug dosages and the best treatment for the patient. Just Accepted articles have undergone full scientific review but none of the additional editorial preparation, such as copyediting, typesetting, and proofreading, as have articles published in the traditional manner. There may, therefore, be errors in Just Accepted articles that will be corrected in the final print and final online version of the article. Any use of the Just Accepted articles is subject to the express understanding that the papers have not yet gone through the full quality control process prior to publication.
References
- 1.Barshes NR, Myers GD, Lee D, Karpen SJ, Lee TC, Patel AJ, et al. Liver transplantation for severe hepatic graft-versus-host disease: An analysis of aggregate survival data. Liver Transpl. 2005;11:525–531. doi: 10.1002/lt.20389. [DOI] [PubMed] [Google Scholar]
- 2.Englert C, Ganschow R. Liver transplantation in a child with liver failure due to chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation from the same unrelated living donor. Pediatr Transplant. 2012;16:E325–E327. doi: 10.1111/j.1399-3046.2012.01685.x. [DOI] [PubMed] [Google Scholar]
- 3.Orlando G, Ferrant A, Schots R, Goffette P, Mathijs J, Lemaire J, et al. Liver transplantation for chronic graft-versus-host disease: Case report with 10-year follow-up. Transpl Int. 2005;18:125–129. doi: 10.1111/j.1432-2277.2004.00008.x. [DOI] [PubMed] [Google Scholar]
- 4.Strasser SI, Sullivan KM, Myerson D, Spurgeon CL, Storer B, Schoch HG, et al. Cirrhosis of the liver in long-term marrow transplant survivors. Blood. 1999;93:3259–3266. [PubMed] [Google Scholar]
- 5.Rhodes DF, Lee WM, Wingard JR, Pavy MD, Santos GW, Shaw BW, et al. Orthotopic liver transplantation for graft-versus-host disease following bone marrow transplantation. Gastroenterology. 1990;99:536–538. doi: 10.1016/0016-5085(90)91039-9. [DOI] [PubMed] [Google Scholar]
- 6.Marks DI, Dousset B, Robson A, Imvrios G, Buckels JA, Elias E, et al. Orthotopic liver transplantation for hepatic GVHD following allogeneic BMT for chronic myeloid leukaemia. Bone Marrow Transplant. 1992;10:463–466. [PubMed] [Google Scholar]
- 7.Dowlati A, Honore P, Damas P, Deprez M, Delwaide J, Fillet G, et al. Hepatic rejection after orthotopic liver transplantation for hepatic venoocclusive disease or graft-versus-host disease following bone marrow transplantation. Transplantation. 1995;60:106–109. doi: 10.1097/00007890-199507150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Figuera A, Tomas JF, Otero MJ, Moreno E, Garcia I, Fernandez- Ranada JM. Orthotopic liver transplantation for acute grade IV hepatic graft-versus-host disease following bone marrow transplantation. Am J Hematol. 1996;52:68–69. doi: 10.1002/(SICI)1096-8652(199605)52:1<68::AID-AJH19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Rosen HR, Martin P, Schiller GJ, Territo M, Lewin DN, Shackleton CR, et al. Orthotopic liver transplantation for bone-marrow transplantassociated veno-occlusive disease and graft-versus-host disease of the liver. Liver Transpl Surg. 1996;2:225–232. doi: 10.1002/lt.500020308. [DOI] [PubMed] [Google Scholar]
- 10.Urban CH, Deutschmann A, Kerbl R, Lackner H, Schwinger W, Konigsrainer A, et al. Organ tolerance following cadaveric liver transplantation for chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;30:535–537. doi: 10.1038/sj.bmt.1703688. [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Tabata S, Hashimoto H, Inoue D, Kimura T, Shimoji S, et al. Successful living donor liver transplantation for severe hepatic GVHD histologically resembling autoimmune hepatitis after bone marrow transplantation from the same sibling donor. Transpl Int. 2010;23:e1–e4. doi: 10.1111/j.1432-2277.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Kasahara M, Tanaka K. Living-donor liver transplantation for chronic hepatic graft-versus-host disease. N Engl J Med. 2006;354:1536–1537. doi: 10.1056/NEJMc052628. [DOI] [PubMed] [Google Scholar]
- 13.Granot E, Loewenthal R, Jakobovich E, Gazit E, Sokal E, Reding R. Living related liver transplant following bone marrow transplantation from same donor: Long-term survival without immunosuppression. Pediatr Transplant. 2012;16:E1–E4. doi: 10.1111/j.1399-3046.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama S, Kasahara M, Fukuda A, Sato S, Mori T, Nakagawa A, et al. Successful living-donor liver transplantation for chronic hepatic graftversus- host disease after bone marrow transplantation for chronic granulomatous disease. Transplantation. 2008;86:367–368. doi: 10.1097/TP.0b013e31817c16eb. [DOI] [PubMed] [Google Scholar]
- 15.Koenecke C, Hertenstein B, Schetelig J, van Biezen A, Dammann E, Gratwohl A, et al. Solid organ transplantation after allogeneic hematopoietic stem cell transplantation: A retrospective, multicenter study of the EBMT. Am J Transplant. 2010;10:1897–1906. doi: 10.1111/j.1600-6143.2010.03187.x. [DOI] [PubMed] [Google Scholar]