Abstract
Purpose
We determined the effect of passive secondhand cigarette smoke on 1) erectile function in vivo, 2) molecular mechanisms involved in penile vascular function, and 3) erectile function and penile molecular signaling in the presence of phosphodiesterase type 5 inhibitor therapy.
Materials and Methods
Four groups of mice were used, including group 1—controls, group 2—mice exposed to 3 weeks of secondhand smoke (5 hours per day for 5 days per week), group 3—control plus sildenafil (100 mg/kg per day) and group 4—smoke exposed plus sildenafil (100 mg/kg per day). Cavernous nerve electrical stimulation and intracavernous injection of acetylcholine were done to assess erectile function. Constitutive and inducible nitric oxide synthase activity, reactive oxygen species generation, nitrotyrosine formation and superoxide anion levels were assessed.
Results
Decreased erectile responses to cavernous nerve electrical stimulation and impaired endothelium dependent erectile responses to ACh in mice exposed to secondhand smoke were observed. Superoxide anion was increased in endothelial and corporeal smooth muscle cells of smoking mouse penises. In mice exposed to secondhand smoke constitutive nitric oxide synthase activity was decreased, and inducible nitric oxide synthase activity, reactive oxygen species generation and nitrotyrosine formation increased. Sildenafil therapy restored constitutive nitric oxide synthase activity in the penis of smoking mice, decreased inducible nitric oxide synthase activity, reactive oxygen species generation and nitrotyrosine formation, and improved erectile responses to cavernous nerve electrical stimulation and acetylcholine.
Conclusions
Short-term exposure to secondhand smoke impairs erectile function through excessive penile reactive oxygen species signaling and inducible nitric oxide synthase activity. Decreased penile constitutive nitric oxide synthase activity may be attributable to the decreased endothelial nitric oxide synthase activity resulting from increased oxidative stress. Sildenafil therapy restored nitric oxide synthase activity and decreased reactive oxygen species signaling, resulting in improved erectile function.
Keywords: penis, erectile dysfunction, smoking, sildenafil, mice
Cigarette smoke is associated with the development of ED and cardiovascular disease.1,2 Underlying the comorbidity of ED and cardiovascular disease is endothelial dysfunction. Endothelial dysfunction is broadly defined as decreased endothelium derived NO bioavailability in the vasculature, resulting in increased smooth muscle tone, inflammatory cytokine levels and platelet activation.3 Enhanced oxidative stress resulting from increased ROS production in the vasculature underscores the development of endothelial dysfunction.
Smoking and ED have been linked in a number of large epidemiological studies.1,4-7 Although cigarette smoking is an established risk factor for ED, the precise molecular mechanisms by which cigarette smoke causes ED remain elusive. Preclinical studies have shown that animals exposed to or treated with cigarette smoke extract have decreased penile NOS activity and neuronal NOS expression, suggesting impaired neuroregulatory control of penile erection with smoking.8-10 Although direct smoke and cigarette smoke extract have been studied for their effects on erectile physiology, little research has been done on secondhand smoke as an inciting factor for the development of penile vascular dysfunction.
In the systemic vasculature secondhand smoke increases oxidative stress through the production of superoxide anion.11,12 Superoxide anion reacts with NO, thus substantially decreasing NO dependent vasodilation. The reaction of superoxide anion and NO forms the toxic molecule peroxynitrite, which is known to cause tissue injury and alterations in vascular tone. Therefore, we determined whether secondhand smoke adversely affects erectile physiology in vivo and identified the molecular factors contributing to the development of ED. Our hypothesis was that secondhand smoke exposure would induce ED by increasing oxidative stress and impairments in penile endothelium dependent vasodilation. Also, we examined the effect of PDE5 inhibition on erectile function in vivo after exposure to secondhand smoke to further delineate the biochemical changes that occur in the penis after exposure to secondhand smoke and we determined the effect of PDE5 inhibition on NO signaling in the penis.
MATERIALS AND METHODS
Cigarette Smoke Exposure
Ten-week-old C57/BL-6 mice were exposed to cigarette smoke 5 hours per day for 5 days per week for 3 weeks.13 Exposure was done by burning 2R4F reference cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, Kentucky) using a TE-10 smoking machine (Teague Enterprises, Davis, California). Each cigarette was puffed for 2 seconds once every minute for a total of 8 puffs at a flow rate of 1.05 l per minute to provide a standard puff of 35 cm3. The smoke machine was adjusted to produce a mixture of sidestream and mainstream smoke (89% and 11%, respectively). The smoke chamber was monitored daily for total suspended particles and carbon monoxide with concentrations of 90 mg/m3 and 350 ppm, respectively. Air exposed control mice were housed in a filtered air environment.
PDE5 Inhibitor Therapy
Oral treatment with the PDE5 inhibitor sildenafil citrate (Viagra®) was provided by mixing drug into semisoft rodent chow (Bioserv, Laurel, Maryland) (4 to 6 gm per day), which also provided full daily nutrition. We treated controls with drug vehicle mixed in food. For in vivo chronic studies we used 100 mg/kg per day sildenafil citrate in semisoft rodent chow for 3 weeks and then performed all biochemical and physiological analyses.14 Mean free plasma concentration was approximately 10 to 20 nm, a concentration of drug that inhibited 50% of PDE5 activity in the presence of substrate. This was comparable to levels in humans at doses of 25 mg orally 3 times daily and reflects the almost 100-fold higher rate of metabolism of sildenafil in mice.14 Treatment with sildenafil citrate was started on the day that cigarette smoke exposure was initiated.
NOS Enzyme and PKG Activity Assays
Penile NOS activity was assayed by radiolabeled L-arginine to L-citrulline conversion.15 Measurements were performed in the presence (constitutive NOS or eNOS activity) or absence (iNOS activity) of calcium. PKG activity was determined by colorimetric analysis (CycLex, Nagano, Japan) according to manufacturer instructions.16
ROS and Nitrotyrosine Analysis
Superoxide anion production in penile tissue homogenates was determined by luminol-enhanced chemiluminescence (EMD Biosciences, San Diego, California).17 Penile lysate was resuspended in assay buffer to a final concentration of 100 μM luminol. In a separate series of experiments penile lysate was incubated with the nonselective NOS inhibitor L-NAME (1 mM) and the antioxidant SOD (10 μM) before determining ROS production. Peroxynitrite was quantitatively assessed by nitrotyrosine measurement using an enzyme-linked immunosorbent assay (Oxis International, Foster City, California). Dihydroethidine (Molecular Probes®), an oxidative fluorescent dye, was used to evaluate superoxide anion levels in situ.17 Dihydroethidine fluorescence images were obtained with an upright fluorescence microscope (Nikon, Tokyo, Japan).
Physiological Erection Studies
Mice were initially anesthetized by being placed in a jar containing isoflurane soaked gauze and then intubated and ventilated while supine with 95% O2/5% CO2 and 2% isoflurane using a custom designed, constant flow mouse ventilator with tidal volume set to 6.7 μl/gm at 140 breaths per minute. Carotid artery and jugular vein cannulation were performed for systemic monitoring. Surgical pelvic dissection was performed for CNS, intracavernous injection of the vasoactive agonists ACh and CGRP, and intracavernous monitoring.15
Statistical Analysis
Data are expressed as the mean ± SEM. Differences between multiple groups were compared by ANOVA, followed by Tukey’s multiple comparisons test. Two-group analysis was performed by the t test, which was paired or unpaired as appropriate. Serial studies were tested by repeated measures ANOVA with p <0.05 considered statistically significant.
RESULTS
NOS and PKG Activity
We assessed penile constitutive NOS (eNOS) and iNOS activities as well as PKG activity in control mice exposed to filtered air and in mice exposed to secondhand smoke for 3 weeks (fig. 1). There was a significant decrease in constitutive NOS (eNOS) and PKG activities in mouse penises exposed to secondhand smoke (p <0.05, fig. 1, A and C). Inducible NOS activity was significantly increased in mouse penises exposed to secondhand smoke (p <0.05, fig. 1, B).
Figure 1.
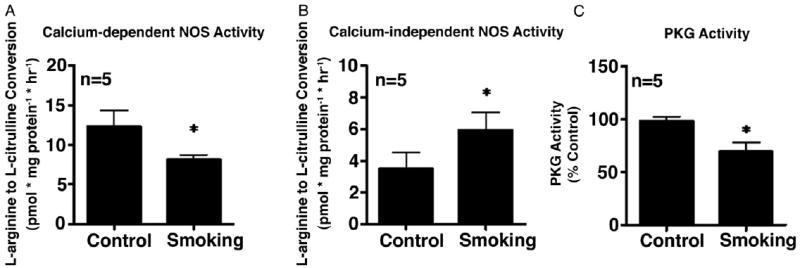
Constitutive NOS (eNOS) (A) and iNOS (B) activity, as measured by calcium dependent and independent conversion of L-arginine to L-citrulline, and PKG activity in control and secondhand smoke exposed mouse penises (C). Asterisk indicates p <0.05 vs control.
ROS Production and Nitrotyrosine Analysis
Tissue sections from the penis of control and secondhand smoke exposed mice were evaluated for the presence of superoxide anion (fig. 2). Smoke exposed mouse penises had qualitatively higher levels of superoxide anion according to EtBr fluorescence compared to those in control mouse penises (fig 2, A to F). Superoxide anion was localized to endothelium and corporeal smooth muscle cells (fig. 2, E and F). To quantify ROS production the superoxide anion concentration was measured using the lucigenin enhanced chemiluminescence assay (fig. 2, G). Secondhand smoke significantly increased penile ROS production compared to that in age matched control (p <0.05, fig. 2, G). ROS production could be significantly inhibited when penile lysate was pre-incubated with L-NAME (1 mM) and it was completely inhibited by SOD (10 μM) (p <0.05, fig. 2, H). ROS production was also partially inhibited by xanthine oxidase and NADPH (reduced nicotinamide adenine dinucleotide phosphate) oxidase inhibitors (data not shown). Nitrotyrosine, which is a specific marker of the toxic radical peroxynitrite, was also significantly increased in the penis of mice exposed to secondhand smoke (p <0.05, fig. 2, I).
Figure 2.
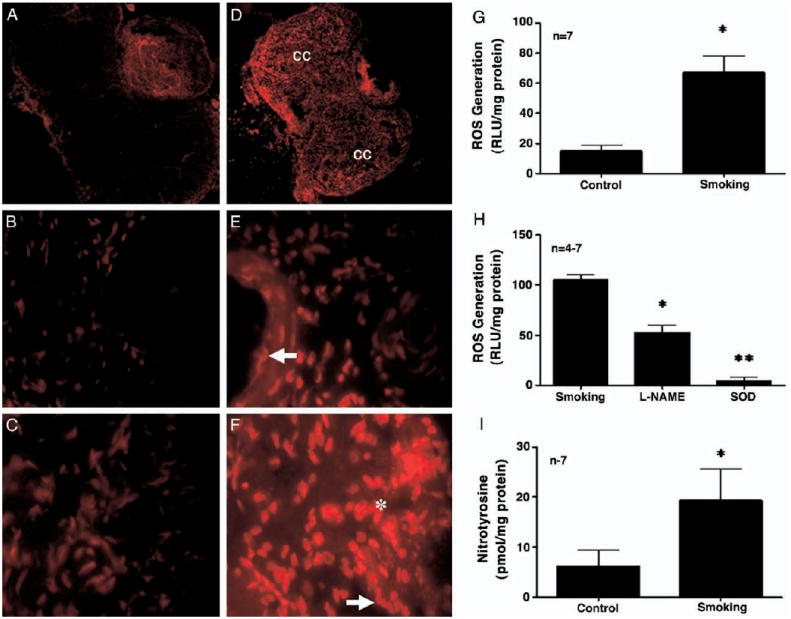
In situ detection of superoxide anion in control (A to C) and secondhand smoke exposed (D to F) mouse penises. Penises of control mice demonstrated minimal fluorescence in endothelium and corpora cavernosa smooth muscle, while smoke exposed mouse penises had marked increase in EtBr fluorescence, reflective of increased superoxide anion levels in endothelium (E, arrow) and corpora cavernosa smooth muscle (F, asterisk). Similar results were obtained in 3 groups of mice. Luminol activity assay to evaluate ROS signaling in control and secondhand smoke exposed mouse penises (G). Asterisk indicates p <0.05 vs control. Luminol activity assay in secondhand smoke exposed penises with co-administration of L-NAME and SOD to determine relative role of uncoupled NOS in ROS signaling associated with smoke exposure (H). SOD served as assay control. Double asterisks indicate p <0.05 vs secondhand smoke. Quantitative nitrotyrosine on enzyme-linked immunosorbent assay in control and secondhand smoke exposed mouse penises (I).
Effect of Secondhand Smoke on Erectile Physiology In Vivo
Three weeks after daily exposure to secondhand smoke or filtered air as the control mice were anesthetized and erectile function was assessed in vivo (see table). Neurogenic mediated erectile responses to CNS were significantly decreased in mice exposed to secondhand smoke compared to those in control mice (p <0.05, see table). Additionally, endothelium dependent erectile responses to intracavernous injection of ACh were significantly decreased in mice exposed to secondhand smoke (p <0.05, see table). There was no change in erectile response to intracavernous administration of the endothelium independent vasodilator CGRP (see table).
In vivo erectile response to CNS, and endothelium dependent and independent agonists in control and secondhand smoke exposed mice
| Mean ± SEM Pear Intracavernous Pressure (mm Hg)
|
|||
|---|---|---|---|
| No. Mice | Control | Smoke | |
| CNS (V): | 6-9 | ||
| Baseline | 10.1 ± 1.3 | 8.9 ± 2.5 | |
| 1* | 21.5 ± 1.8 | 13.4 ± 1.5 | |
| 2* | 29.4 ± 2.4 | 17.8 ± 2.4 | |
| 3* | 41.7 ± 5.1 | 27.3 ± 4.9 | |
| 4* | 53.8 ± 6.0 | 33.2 ± 4.4 | |
| Agonist: | 7-9 | ||
| 10 μg ACh* | 39.2 ± 2.6 | 22.3 ± 2.4 | |
| 0.03 nmol CGRP | 41.7 ± 3.5 | 39.6 ± 2.7 | |
Vs control p <0.05.
PDE5 Inhibition Increased eNOS Activity and Decreased iNOS and ROS Signaling
After concomitant treatment with sildenafil citrate (100 mg/kg per day) and exposure to secondhand smoke for 3 weeks constitutive and inducible NOS activities, and ROS signaling were determined in controls and in mice exposed to secondhand smoke alone or treated with sildenafil (figs. 3 and 4). Secondhand smoke profoundly decreased constitutive NOS (eNOS) activity and increased Ca2+ independent NOS activity (iNOS) (fig. 3), thus preferentially promoting ROS generation and nitrotyrosine production in mouse penises exposed to secondhand smoke (fig. 4). This decrease in eNOS activity was ameliorated by sildenafil co-treatment with subsequently less iNOS activity, ROS generation and nitrotyrosine production in sildenafil treated mouse penises exposed to secondhand smoke for 3 weeks (figs. 3 and 4).
Figure 3.
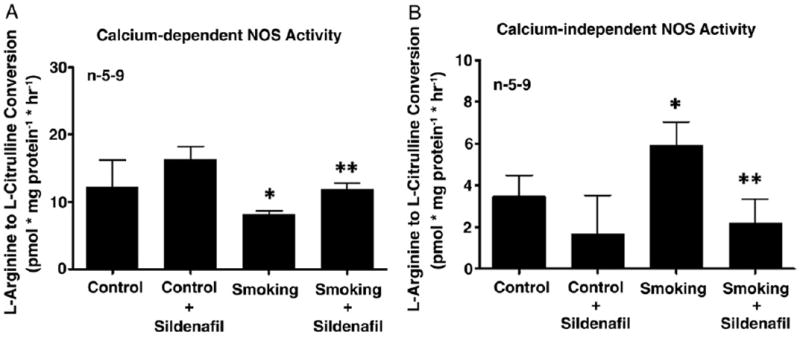
Constitutive NOS (eNOS) (A) and iNOS (B) activity, as measured by calcium dependent and independent conversion of L-arginine to L-citrulline in control and secondhand smoke exposed mouse penises alone or treated with 100 mg/kg sildenafil citrate per day. n indicates number of tissue samples. Single asterisk indicates p <0.05 vs control. Double asterisks indicate p <0.05 vs secondhand smoke.
Figure 4.
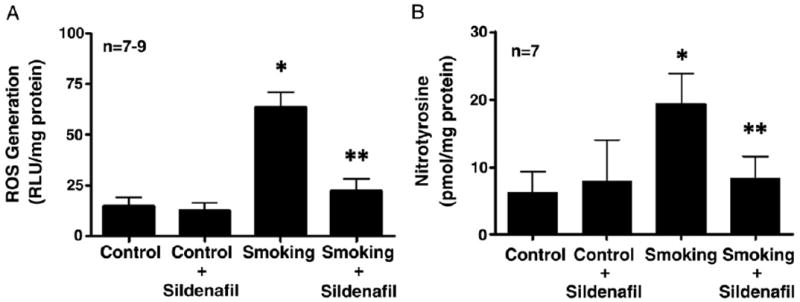
Luminol activity assay to evaluate ROS signaling (A) and nitrotyrosine (B) in control and secondhand smoke exposed mouse penises alone or treated with 100 mg/kg sildenafil citrate per day. Single asterisk indicates p <0.05 vs control. Double asterisks indicate p <0.05 vs secondhand smoke.
PDE5 Inhibition Restored Neurogenic and Endothelium Dependent Erectile Responses
Secondhand smoke resulted in significant decreases in neurogenic mediated (CNS) and endothelium dependent erectile responses to ACh (each p <0.05, fig. 5). The administration of sildenafil (100 mg/kg per day) to secondhand exposed mice resulted in significant improvements in erectile responses to CNS and ACh (p <0.05, fig. 5). Treatment of control (filtered air) mice with sildenafil (100 mg/kg per day) for 3 weeks had no significant hemodynamic effect on erectile responses except at the highest voltage setting of 4 V (fig. 5, A). Endothelium independent erectile responses were unchanged in secondhand smoke exposed mice with or without sildenafil treatment (data not shown).
Figure 5.
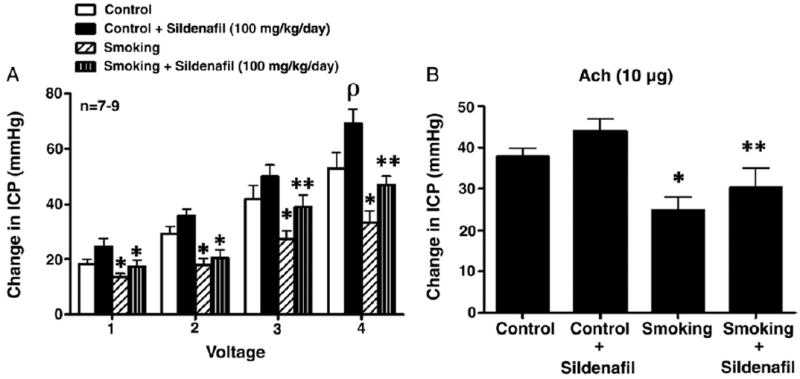
Increased intracavernous pressure (ICP) in response to CNS (A) and intracavernous injection of 10 μg ACh (B) in control and secondhand smoke exposed mice treated with 100 mg/kg sildenafil citrate per day. Single asterisk indicates p <0.05 vs control. Double asterisks indicate p <0.05 vs secondhand smoke.
DISCUSSION
To our knowledge the results of the current study demonstrate for the first time a mouse model of secondhand smoke induced ED that is associated with decreased penile eNOS activity, and increased ROS signaling and iNOS activity. We observed superoxide anion localization to the penile arterial and corporeal vascular endothelium as well as to the smooth muscle cells of the corporeal vascular bed. Moreover, to our knowledge this study is the first to identify a pathophysiological role for acute secondhand smoke in modulating erectile function in vivo by decreasing penile eNOS activity and, thus, decreasing PKG activity. Furthermore, this study demonstrates that the PDE5 inhibitor sildenafil citrate decreases ROS signaling, nitrotyrosine (peroxynitrite) levels and iNOS activity, thus improving endothelium and neurogenic mediated erectile function in vivo as a direct result of increased penile eNOS activity and decreased superoxide oxide anion (fig. 6). These findings imply that secondhand smoke may influence erectile function through decreased endothelium derived NO in the penis.
Figure 6.
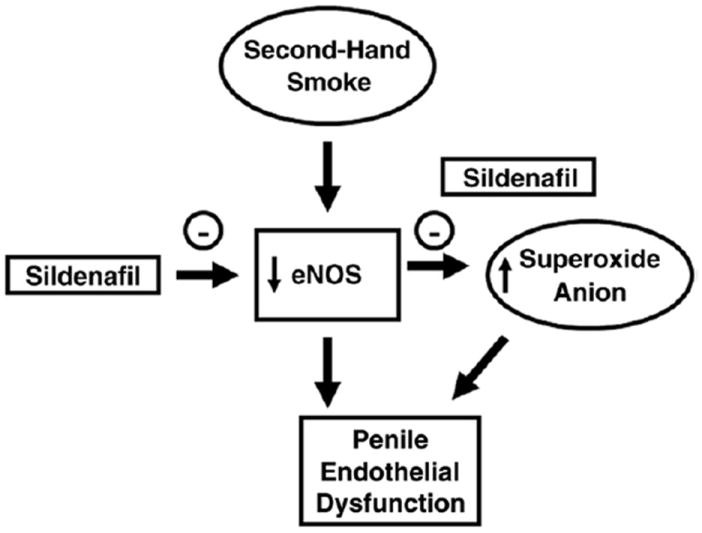
Mechanism of action of beneficial sildenafil effect on penile vascular function in secondhand smoke exposed mice. Secondhand smoke decreased eNOS activity and increased ROS generation (superoxide anion) in penis. Sildenafil therapy prevented decreases in eNOS activity that were likely related to decreased eNOS uncoupling, thus, preventing penile superoxide anion formation. These molecular events prevent penile endothelial dysfunction, restoring erectile function in mice exposed to secondhand smoke.
NO, a vasodilator produced by the endothelium and nitrergic neurons in the penis, regulates penile vascular tone by directing relaxing corporeal smooth muscle.3 NO formation occurs via the regulatory actions of NOS in nitrergic nerves and penile endothelial cells. NO directly binds to soluble guanylate cyclase, thus increasing cGMP and PKG activation, and hyperpolarizing the corporeal myocyte. cGMP activity is terminated by hydrolysis of the 3′5′ bond by PDE5. The constitutive forms of the enzyme, neuronal NOS and eNOS, coupled to Ca2+ and calmodulin, are the principal NOS isoforms involved in the induction of penile erection. iNOS is independent of Ca2+ and calmodulin. It is primarily found in macrophages and is generally believed to be upregulated in inflammatory conditions.
Cigarette smoke is one of the most important preventable risk factors for ED development. Cigarette smoke significantly impairs endothelium dependent flow mediated vasodilation in humans, suggesting that smoking may lead to penile endothelial dysfunction.18 The Massachusetts Male Aging Study showed that cigarette smoking almost doubled the likelihood of moderate or complete ED after controlling for other risk factors.1,5,6 Epidemiological evidence suggests that passive exposure to cigarette smoke also significantly predicts incident ED.5 In our animal model we found significant impairments in endothelium dependent erectile responses and cavernous nerve mediated responses after short-term exposure to secondhand smoke, providing supporting evidence for recent epidemiological studies in men. These data highlight the importance of developing disease specific therapeutic agents to treat smoking induced ED. Previous investigations using smoking animal models to study ED did not indicate impairments in erectile physiology.9 The difference in hemodynamic in vivo outcomes between the study of Xie et al9 and our series may be a result of species differences and the route of exposure to smoke (direct vs secondhand).
Cigarette smoke inhibits the eNOS/NO/cGMP signaling pathway. However, the exact pathophysiological mechanisms of the deleterious effect of cigarette smoke on NO production is not clear. Recently cigarette smoke extract decreased penile eNOS activity and secondhand smoke impaired endothelium dependent and neurogenic mediated corporeal vasoreactivity in vitro.8,19 A potential mechanism by which nicotine impairs endothelium dependent vasoreactivity is related to the superoxide anion mediated degradation of NO. We observed that secondhand smoke leads to decreased penile eNOS activity, and increased superoxide anion signaling and peroxynitrite production. Because this relates to penile vascular function, ROS signaling in the corporeal bodies leads to endothelial dysfunction and impaired corporeal smooth muscle relaxation. The source of superoxide anion is likely related to the up-regulation by nicotine of NADPH oxidase with subsequent eNOS uncoupling.20,21 In such an uncoupled state electrons normally flowing from the reductase domain of the enzyme to the oxygenase domain are diverted to molecular oxygen rather than to L-arginine, resulting in the production of superoxide anion rather than NO.20 Incubation of secondhand smoke exposed penile lysate with L-NAME significantly decreased superoxide anion production, suggesting that iNOS or uncoupled eNOS is a source of superoxide anion in the penis of mice exposed to secondhand smoke.
Nicotine is known to induce a systemic inflammatory response.12 Exposure to cigarette smoke results in oxidant stress with iNOS activation locally in tissues and macrophages.22 In our model short-term exposure of secondhand smoke increased iNOS activity in the penile corporeal bed, which was likely related to cytokine expression, transcriptional activation or a systemic inflammatory response.
The bio-effectiveness of NO/cGMP/PKG signaling in the penis is determined by the inactivation of cGMP by PDE5. The importance of PDE5 in normal penile vascular homeostasis is exemplified by the therapeutic benefit of PDE5 inhibitors for male ED.23 However, PDE5 inhibitors may exert their biological effect beyond the prevention of cGMP hydrolysis. Corporeal smooth muscle cells exposed to nicotine have shown increased superoxide anion production, which was inhibited by sildenafil in vitro.24 PDE5 inhibition with sildenafil successfully decreased iNOS activity, and superoxide anion production and nitrotyrosine (peroxynitrite) levels in the penis of mice exposed to secondhand smoke. Importantly sildenafil therapy increased eNOS activity in the penis with the restoration of endothelial function and neurogenic mediated erectile responses. The mechanism by which eNOS activity was increased by PDE5 inhibitor therapy may be related to the prevention of eNOS uncoupling in the corporeal bed of mice. However, additional studies are warranted to validate this hypothesis and characterize penile NOS isoform protein expression in response to secondhand smoke exposure.
CONCLUSIONS
Acute exposure to secondhand smoke significantly impairs in vivo endothelium and neurogenic mediated erectile responses in mice. The mechanism by which penile vascular dysfunction occurs is related to decreased eNOS activity and increases in superoxide anion, thus, decreasing NO bioavailability. PDE5 inhibitor therapy successfully restored erectile responses in vivo in mice exposed to secondhand smoke by decreasing superoxide anion, increasing penile eNOS activity and restoring endothelial derived NO in the penis.
Acknowledgments
Study received approval from The Johns Hopkins Medical Institutions animal care and use committee.
Supported by an American Urological Association Foundation Astellas USA Foundation MD/PhD Fellowship and National Kidney Foundation of Maryland Research Grant (TJB), The Bernard A. and Rebecca S. Bernard Foundation, an American Heart Association Scientist Development Grant, the WW Smith Foundation, NIH P50 Hl084946, American Heart Association, Pulmonary Vascular Research Institute, an American College of Cardiology Zipes Distinguished Young Investigator Award, the Shin Chun-Wang Young Investigator Award and an American Physiological Society Giles F. Filley Memorial Award (HCC), and a grant from the FAMRI foundation (DEB).
Abbreviations and Acronyms
- ACh
acetylcholine
- cGMP
cyclic guanosine monophosphate
- CGRP
calcitonin gene related peptide
- CNS
cavernous nerve electrical stimulation
- ED
erectile dysfunction
- eNOS
endothelial NOS
- iNOS
inducible NOS
- L-NAME
N(G)-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
NO synthase
- PDE5
phosphodiesterase type 5
- PKG
protein kinase G
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 3.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 4.Millet C, Wen LM, Rissel C, Smith A, Richters J, Grulich A, et al. Smoking and erectile dysfunction: findings from a representative sample of Australian men. Tobacco Control. 2006;15:136. doi: 10.1136/tc.2005.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupelian V, Link CL, McKinlay JB. Association between smoking, passive smoking, and erectile dysfunction: results from the Boston Area Community Health (BACH) survey. Eur Urol. 2007;52:416. doi: 10.1016/j.eururo.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 7.McVary KT, Carrier S, Wessells H. Smoking and erectile dysfunction: evidence based analysis. Subcommittee on Smoking and Erectile Dysfunction Socioeconomic Committee, Sexual Medicine Society of North America. J Urol. 2001;166:1624. [PubMed] [Google Scholar]
- 8.Imamura M, Waseda Y, Marinova GV, Ishibashi T, Obayashi S, Sasaki A, et al. Alterations of NOS, arginase, and DDAH protein expression in rabbit cavernous tissue after administration of cigarette smoke extract. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2081. doi: 10.1152/ajpregu.00406.2007. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Garban H, Ng C, Rajfer J, Gonzalez-Cadavid NF. Effect of long-term passive smoking on erectile function and penile nitric oxide synthase in the rat. J Urol. 1997;157:1121. [PubMed] [Google Scholar]
- 10.Juenemann KP, Lue TF, Luo JA, Benowitz NL, Abozeid M, Tanagho EA. The effects of cigarette smoking on penile erection. J Urol. 1987;138:438. doi: 10.1016/s0022-5347(17)43181-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air. 2005;15:35. doi: 10.1111/j.1600-0668.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Liu Y, Shi J, Larson DF, Watson RR. Side-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med (Maywood) 2002;227:823. doi: 10.1177/153537020222700916. [DOI] [PubMed] [Google Scholar]
- 13.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 15.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, et al. Gene transfer of extracellular SOD to the penis reduces O2-* and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol. 2003;284:H1408. doi: 10.1152/ajpheart.00770.2002. [DOI] [PubMed] [Google Scholar]
- 18.Karatzi K, Papamichael C, Karatzis E, Papaioannou TG, Stamatelopoulos K, Zakopoulos NA, et al. Acute smoke-induced endothelial dysfunction is more prolonged in smokers than in non-smokers. Int J Cardiol. 2007;120:404. doi: 10.1016/j.ijcard.2006.07.200. [DOI] [PubMed] [Google Scholar]
- 19.Göçmez SS, Utkan T, Duman C, Yildiz F, Ulak G, Gacar MN, et al. Secondhand tobacco smoke impairs neurogenic and endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle: improvement with chronic oral administration of L-arginine. Int J Impot Res. 2005;17:437. doi: 10.1038/sj.ijir.3901341. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GC, Newby AC, et al. Acute inhibition of superoxide anion formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analogue, U46619. Prostaglandins Leukot Essent Fatty Acids. 2008;78:247. doi: 10.1016/j.plefa.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang WC, Lee YC, Liu CL, Hsu JD, Wang HC, Chen CC, et al. Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol. 2001;75:28. doi: 10.1007/s002040000168. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 24.Hotston MR, Jeremy JY, Blood J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-α in cavernosal vascular smooth muscle cells: mediation by superoxide. BJU Int. 2006;99:612. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]


