Abstract
Cardiac oxytocin (OT) is structurally identical to that found in the hypothalamus, which thereby indicates that cardiac OT is derived from the same gene and is an active form of OT. The abundance of OT and OT receptors in atrial myocytes shows that, directly and/or via the release of the cardiac hormone atrial natriuretic peptide, OT can regulate the force of cardiac contractions. Previous studies have demonstrated the role of OT in the myocardial inflammatory response. The mechanism by which OT elicits protective myocardial effects in the immediate post-transplantation period is not yet clear, and the role of the early phase inflammatory elements in this mechanism has not yet been studied. As a result, in this study, we have investigated the anti-inflammatory effects of OT on myocardial protection in the immediate post-transplantation period.
Methods:
Adult male Albino rats were grouped into: Sham, Control, and OT-treated groups. The control and treated groups sustained cervical heterotopic heart transplant. Myocardial injury was assessed by measuring: Plasma cardiac troponin I, myocardial proinflammatory cytokines, and histopathological assessment for score of injury, and degree of apoptosis. Myocardial myeloperoxidase, neutrophil infiltration, and neutrophil chemotactant agents, reactive oxygen species, and reactive nitrogen species formation all were measured in the myocardium after 3 hour of reperfusion to assess the neutrophil-dependant myocardial injury and the mechanism involved.
Results:
Oxytocin down-regulates the neutrophil chemotactant agents the KC/CXCL1 and MIP-2/CXCL2 which recruit less neutrophil into myocardium, this decrement in myocardial PMN infiltration is associated with less reactive oxygen species and reactive nitrogen species formation in the myocardium after 3 hours of global ischemia reperfusion. These oxytocin-induced down-regulation inflammatory and oxidative processes will end in less myocardial injury through impedance in the post-myocardial ischemia/reperfusion apoptotic process.
Conclusion:
Oxytocin ameliorates myocardial injury in heart transplant through down-regulation the myocardial inflammatory response, reactive oxygen species, and neutrophil-dependant myocardial apoptosis.
Keywords: Heart transplant, neutrophil-dependant myocardial apoptosis, oxytocin
INTRODUCTION
Oxytocin (OT) is a hormone and neurotransmitter whose primary function is typically associated with being a neuromodulator in the hypothalamus.[1] Although it is best known for its role in female reproduction, it is also significantly involved in social recognition and bonding,[2] as well as empathy and stress reactivity.[3] It is a nonapeptide with the systemic name, cysteine- tyrosine- isoleucine- glutamine - asparagine- cysteine- proline-leucine-glycine-amine.
Research has shown that OT and OT receptors are present in the heart as well.[4] In a specific study, OT synthesis and release were reported in all four chambers of the rat heart. The study demonstrated that cardiac OT is structurally identical to that found in the hypothalamus, which thereby indicates that cardiac OT is derived from the same gene and is an active form of OT.[5]
The OT and OT receptors in atrial myocytes directly and/or via the release of the cardiac hormone atrial natriuretic peptide,[6] can regulate the force of cardiac contractions.[5] Furthermore, a number of studies have shown that the perfusion of isolated rat hearts with OT can lower cardiac flow rate and result in bradycardia.[1] The negative chronotropic inotropic effects of OT in the heart were examined in a particular study of isolated dog right atria in the absence of central mechanisms. The perfusion of the atria through the sinus node artery with 10 (−6) mol/L of OT for over 5 minutes significantly lowered both the beating rate and force of contraction.[1] The results were further confirmed by follow-up studies. In one instance, co-perfusion with 10 (−6) mol/L of OT receptor antagonist completely inhibited the effects of OT on the rate and force of contraction.[1] Hence, these studies demonstrate the negative chronotropic and inotropic effects of OT.
While there is increasing evidence regarding the homeostatic functions of cardiac OT, research is budding on the multi-faceted cardio-protective role of this hormone in the injured heart.[7,8] One specific study examined the effects of OT infusion on inflammation in the acute and sub-acute phases of a myocardial infarction.[9] In this study, OT infusion resulted in the suppression of inflammation through a diminution of neutrophils, macrophages, and T lymphocytes. It also caused an obstruction in the expression of proinflammatory cytokines: Tumor necrosis factor-alpha and interleukin-6.[9,10] Thus, the results show that continuous OT delivery causes a reduction in inflammation, thereby ameliorating the function of the injured heart.
Although still in the initial stages of investigation, research on the effects of OT on inflammation related to heart injury is extending to the field of heart transplantation. The early inflammatory response is addressed by neutrophil infiltration into inflammatory sites including ischemic tissue which is directed by chemoattractants including KC/CXCL1 and MIP-2/CXCL2.[11,12,13,14] In addition to directing neutrophil recruitment, stimulated neutrophils release granules containing proteases, cytokines, chemokines, and other chemoattractants that amplify the intensity and extend the duration of tissue inflammation.[15,16] In reperfusion phase RNS and ROS formations are involved in the early myocardial injury through upregulation of apoptosis.[17,18] The mechanism by which OT elicits protective myocardial effects in the immediate post-transplantation period is not yet clear, and the role of the early phase inflammatory elements in this mechanism has not yet been studied. As a result, in this study, we have investigated the anti-inflammatory, the possible antiapoptotic and antioxidant effects of OT on myocardial protection in the immediate post-transplantation period.
Objective
This study is designed to assess the protective effects of OT in myocardial injury induced by global ischemia reperfusion injury.
MATERIALS AND METHODS
Animal design
Adult male Albino rats weighing 220-280 g and ages ranging from 3 to 4 months were housed in the animal shelter in a temperature-controlled (25°C) room with alternating 12-h light/12-h dark cycles. They were allowed free access to water and a chow diet until the start of the experiments. The Institutional Animal Care and Use Committee of Howard University reviewed and approved the experimental design of all animal experiments. After the first week of acclimatization, the rats were randomized into three groups. There were 12 animals in each group, the groups included.
Sham group
A group in which the animals received anesthetic medications and cervical incisions.
Control group
This is where the animals underwent heterotopic cervical heart transplants.
OT-treated group
The recipient animals in this treatment group received OT (600 ng/kg). intraperitoneally 60 minutes before heart transplantation, different doses of in previous studies were used in myocardial ischemia in rat and in different timing, we used this dose and timing as we found it more effective in protection. The doses were repeated immediately after transplantation.[8]
Anesthesia and surgical procedure
Intraperitoneally injections of a mixture of ketamine and xylazine were given to the rats in a dose of 100 mg/kg and 10 mg/kg, respectively, in order to anesthetize them and render them unconscious within 5-10 minutes.
Surgical procedure
A cervical heterotopic heart transplantation (HHT) was carried out by using the cuff method.[19] Animals were intraperitoneally anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine.[20]
Donor operation
A transverse abdominal incision was performed. The viscera were slightly retracted to the left side, and 100 U of heparin was injected through the infrahepatic inferior vena cava (IVC). Approximately 3 minutes later, the thorax was opened through left and right dorsolateral incisions with a pair of rigid scissors, and the diaphragm was separated from the anterior chest wall at the same time. The right superior vena cava (SVC) was ligated and cut off at the distal side of the ligation with a segment of the suture left. The first branch of the aorta was ligated with a 6-0 silk suture, and the suture was retained to the first branch of the aorta. After cutting the aorta at the site distally close to its first branch, the heart was gently pulled upward by the silk sutures, which were attached to the right SVC and the first branch of the aorta. Retraction was maintained with forceps that were fixed to the corkboard. The left SVC, pulmonary arteries, pulmonary veins, and azygos vein were then ligated together with a 4-0 silk suture. Then, the heart was harvested from the donor and stored in a cold lactated Ringer's solution.
Recipient operation
A right anterolateral incision parallel to the neck was made. The jugular vein was isolated from its incoming branches. An 18-G cuff was sleeved, everted, and secured with a circumferential 6-0 silk suture using the conventional method that was previously reported. An 18-G cuff was always attached in this HHT series. The common carotid artery was ligated as far away from the subclavian artery as possible, and the manipulation was very gentle in order to prevent the injury of the endothelium and the formation of a thrombosis. The cuffing techniques of the artery were the same as those used with the jugular vein. After making a stay suture in the posterior wall of the right SVC of the graft heart, the graft was gently slipped over the cuff that had been fixed to the jugular vein of the recipient, and secured with a circumferential 6-0 silk suture. The anastomosis method between the donor aorta and the common carotid artery of recipient was the same as that used for the vein. A final step was removing all the bulldog clamps and making sure that the beating graft was working well with no congestion.
Myocardial perfusate
After removal, the donor heart was immediately immersed in a Ringer's lactate solution. As the myocardial perfusate cooled to 4°C, OT (120 ng) was added to 10-ml perfusate in the treatment group, to yield concentration of 1200 ng/ml.
Cold myocardial ischemic time
After the donor heart was removed, it was immersed in a myocardial perfusate for 3 hours in both the control and treated groups.
Plasma and myocardial homogenate protein assay
The collected blood from each rat was centrifuged (in 10000 RPM, for 10 minutes at 4°C) and the yielded plasma and myocardial homogenate samples of each animal was subjected to protein assay for different chemokines including: IL-1β, cTn-I, and tumor necrosis factor-α (TNF-α).
Assessing myocardial injury
Myocardial injury during ischemia reperfusion injury was assessed through the following:
Measurement of cardiac troponin-I
Measurement of cardiac troponin-I in rat plasma (cTnI).[21]
Measurement of TNFα
This ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of rat TNF.[22]
Measurement of IL-1β
This ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme measurement tissue of IL-1β.[23]
Myocardial histopathology
After 3 hours of heterotopic heart transplantation, the hearts were excised. The process of the slide preparation for the histopathological examination started with tissue fixation by 10% formalin, paraffin embedding, and staining with hematoxylin and eosin (H and E). Myocardial injury at the histopathological level was assessed based on the morphological criteria[24] and scored as follows: Score 0, zero damage; score 1 (mild), interstitial edema and localized necrosis; score 2 (moderate), widespread myocardial cell swelling and necrosis; score 3 (severe), necrosis with contraction bands, neutrophil infiltration and compressed capillaries; and score 4 (highly severe), diffuse necrosis with contraction bands, neutrophil infiltration, compressed capillaries and hemorrhage.
Assessment of the amount of neutrophil infiltration
As previously stated, the process of slide preparation for the histopathological examination began with tissue fixation with 10% formalin, paraffin embedding, and staining with H and E. Then, with oil immersion ×40, the neutrophils were counted in the studied groups in the examined field.
Assessing myocardial neutrophils infiltration through myeloperoxidase activity assay
Myocardial myeloperoxidase (MPO) activity is a marker of neutrophils infiltration, using method of Curtis et al0.,[25] MPO is assessed in sham, control, OT-treated myocardial tissue samples collected and freezed in −80°C. Homogenization was done as follows: Myocardial tissue was homogenized in 0.5% (w/v) hexadecyltrimethylammonium bromide in 50-mM potassium phosphate buffer (pH 6), in the ratio of 1-ml hexadecyltrimethylammonium bromide to 100 mg of tissue. The homogenized myocardial tissue was centrifuged at 13000 RPM for 20 min at 4°C. Supernatant samples were frozen at −80°C. The measurement myeloperoxidase activity: 50 μl of each sample was added to 50 μl of o-dianisidine dihydrochloride (0.025% v/v in phosphate buffer with 0.5% hexadecyltrimethylammonium bromide) in a 96-well plate. Fifty microliters of 0.01% v/v hydrogen peroxide was added and the increase in optical density at 510 nm was measured over 3 min. Duplicates were run for each sample, and the average value was used to calculate the number of units of MPO present by interpolation with a standard curve established using dilutions of a commercially available preparation of human neutrophils MPO. Protein in the supernatant was then determined according to the bicinchoninic acid method by interpolation with a standard curve established using dilutions of bovine serum albumin. Duplicates were run for each sample, and the average value was used for the calculation of protein content MPO concentration was expressed as units MPO per mg protein.
Measurement of neutrophil myocardial chemotactant agents
In sham group and after 3 hour of global myocardial ischemia reperfusion in the studied groups the CXCL1/CINC-1/KC and MIP-2/CXCL2/Rat CINC-3 concentrations were quantitated by Quantikine_Rat Immunoassay (R and D Systems) in the homogenized myocardium and processed according to kit recommendations.
Assessment of reactive oxygen species in the myocardial rats through oxidative stress measurement
GSH myocardial level was measured using Quantichrom TM glutathione assay Kit (from BioAssay Systems, USA). MDA, the end product of lipid peroxidation, was analyzed according to the method of Buege and Aunt[26] which based on the reaction of MDA with thiobarbituric acid (TBA) to form MDA-TBA complex, a red chromophore, which can be quantitated spectrophotometrically according to this method.
Assessment of reactive nitrogen species levels in rats myocardial tissue NOx (nitrite and nitrate, stable metabolites of NO)
Quantity in supernatants was determined by the Griess reaction and assayed utilizing a NOx concentration assay kit (R and D Systems). Nitrotyrosine is represented the in vivo ONOO¯ The concentration of nitrotyrosine in rat cardiac tissue homogenate was determined via ELISA kit (Cell Sciences Inc., Canton, MA, USA), as previously described (Fan et al. 2005). In the homogenized myocardium and processed according to kit recommendations.
Determine myocardial apoptosis quantitatively
Apoptosis Detection Using Terminal Transferase and Biotin-16-dUTP (TUNEL Enzyme Method).
Description
This protocol is used for detection and quantification of apoptosis (programmed cell death) at single cell level, based on labeling of DNA strand breaks (TUNEL technology). Cleavage of genomic DNA during apoptosis may yield double stranded as well as single-strand breaks (“nicks”), which can be identified by labeling free 3’- OH terminal with modified nucleotides in an enzymatic reaction.
Positive controls
1) Incubate sections with DNase I (3000U/ml in 50 mM Tris-HCl, pH 7.5, 1mg/ml BSA) for 10 minutes at 15-25ºC to induce DNA strand breaks, prior to labeling procedure. 2) Using known positive control is an alternative.
Negative control
Incubate sections with label solution only (without terminal transferase) instead of TUNEL reaction mixture. It is done according to protocol of life science products and Services Company. In this procedure, apoptotic nuclei are stained blue. TUNEL-positive myocytes were determined by randomly counting 15 fields for each tissue section then calculate the average no.
Statistical analysis
Statistical analysis was performed with an ANOVA analysis to explore all possible comparisons of means comprising a factor using the equivalent of multiple t-tests. This procedure was named the Least Significant Difference (LSD), and a variable is considered significant if P < 0.05.
RESULTS
Rats treated with OT have decreased myocardial injury marker after global ischemia reperfusion injury
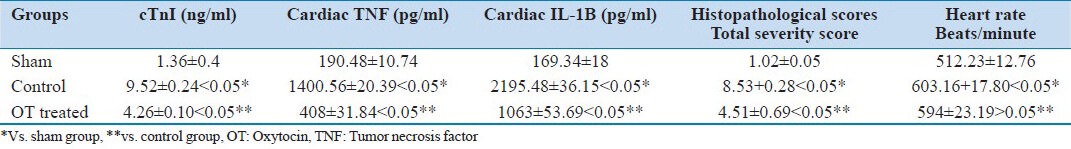
There are consistent differences between cardiac troponin-I levels in rat plasma cTnI (ng/ml). In rats treated with OT, the cTnI (ng/ml) in the rat plasma levels has an average of 4.26 ± 0.10, while the untreated group has an average of 9.52 ± 0.24, and the sham group has an average of 1.36 ± 0.4. This is shown in Table 1.
Table 1.
Cardiac troponin-I in rat plasma cTnI (ng/ml), TNF-α in myocardial tissue, cardiac IL-1B in myocardial tissue, myocardial injury score, heart rate in different groups
OT reduces tNF-α and IL-1β post-global ischemia reperfusion injury
The levels of TNF-α and IL-1β were up-regulated in the control group compared with the OT-treated group. Both these proinflammatory chemokines are sensitive biomarkers of tissue injury in laboratory animals Table 1.
Rats treated with OT have a reduced myocardial injury score after global ischemia reperfusion injury
Again, there are consistent differences between the OT-treated group and the control regarding the myocardial injury score. In rats treated with OT, the score has an average of 4.51 ± 0.69. The untreated group has an average of 8.53 ± 0.28, and the sham group has an average of 1.02 ± 0.05. This is shown in Table 1, Figures 1a (sham), 1b (control), and 1c (OT treated).
Figure 1.
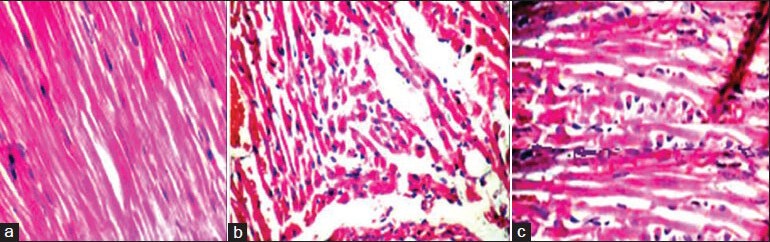
Myocardial injury score in tissue section stained with H and E magnified to ×40, with score ranged from 0 to 4 (a) Sham, (b) Control, (c) Oxytocin group
Rats treated with OT have reduced neutrophil infiltration
Under a high power microscope H/E staining, significant differences are evident in neutrophil infiltration among groups. There is significantly less neutrophil infiltration in the myocardium of OT-treated rats in comparison to the untreated group. This is shown in Figure 2.
Figure 2.
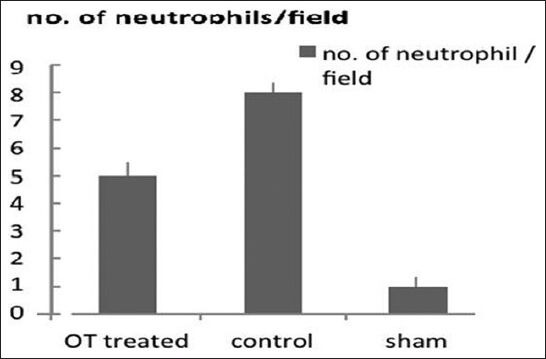
Count of neutrophil infiltration in myocardium in different groups staining with H and E with magnification of ×40. P < 0.05 OT-treated group vs. control
Rats treated with OT have reduced myeloperoxidase activity assay
MO assay has significantly lower activity in OT-treated group than control group. The myocardial tissue in the control set which sustained global ischemia reperfusion injury shows higher activity than the normal sham myocardium which reflected increased neutrophils infiltration in the globally ischemic myocardium. This is shown in Figure 3.
Figure 3.
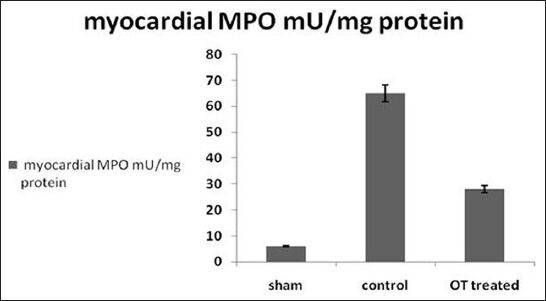
Myeloperoxidase activity assays in homogenized myocardium in different groups. P < 0.05 OT-treated group vs. control
Measurement of neutrophil myocardial chemotactant agents
KC/CXCL1 concentrations as shown in Figure 4a, global myocardial ischemia reperfusion exerts significant elevation in myocardial KC compared with sham group (3.07 pg/mg vs 0.001 pg/mg, P < 0.05), and OT-treated group has significant reduction in KC compared with control group (1.28 pg/mg vs 3.07 pg/mg, P < 0.05).
Figure 4.
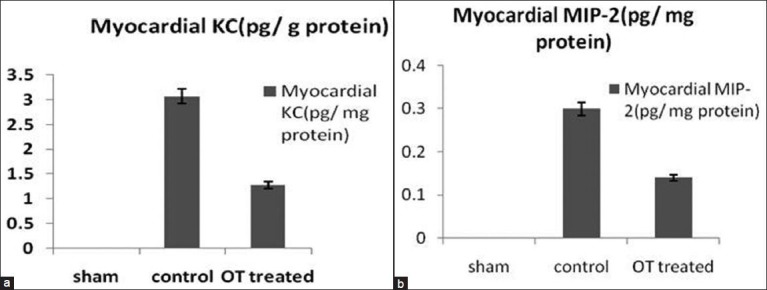
(a and b) Measurements of different chemokines with neutrophil myocardial chemotactant activity in homogenized myocardium by immunoassay A-CXCL1/CINC-1/KC and B-MIP-2/CXCL2/Rat CINC-3. P < 0.05 OT-treated group vs. control
MIP-2/CXCL2/Rat CINC-3 concentrations shown in Figure 4b, global myocardial ischemia reperfusion exerts significant elevation in myocardial KC compared with sham group (0.3 pg/mg vs. 0.001 pg/mg, P < 0.05), and OT-treated group has significant reduction in MIP-2 compared with control group (1.14 pg/mg vs. 0.3 pg/mg, P < 0.05).
Reactive oxygen species in myocardium
After 3 hours of reperfusion, the myocardium shows marked elevation in MDA and reduction in GSH compared to sham group (0.32 μmol/g vs. 0. 0.048 μmol/g, P < 0.05 and 0.94 μmol/g vs. 3.0 μmol/g, P < 0.05, respectively, for MDA and GSH). OT-treated group myocardium shows significantly less increment in MDA, and significantly less decrement in GSH level compared to control group (0.106 μmol/g vs. 0.32 μmol/g, P < 0.05 and 1.63 μmol/g vs. 0.94 μmol/g, P < 0.05, respectively, for MDA and GSH). As shown in Figures 5a and b.
Figure 5.
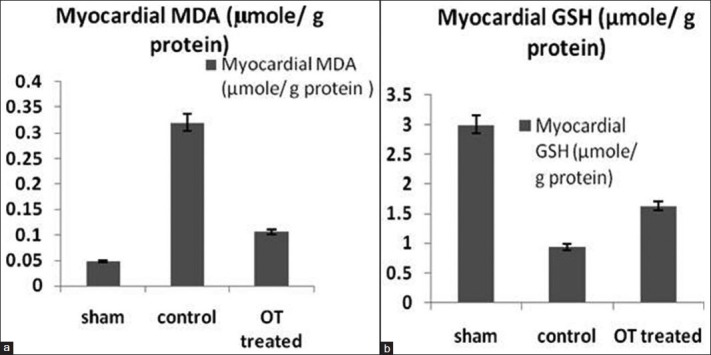
(a and b) Measurement of myocardial (a) MDA and (b) GSH in homogenized myocardium for assessment of oxidative stress. P < 0.05 OT-treated group vs. control
Reactive nitrogen species in myocardium
After 3 hours of reperfusion the rats myocardium in myocardium shows significant elevation in NOx (nitrate/nitrite) and nitrotyrosine levels compared to sham group, (1.35 mmol/g vs. 0.25 mmol/g, P < 0.05 and 2.5 nmol/g vs. 0.20 nmol/g, P < 0.05, respectively, for NOx and nitrotyrosine). OT-treated group myocardium shows significantly reduction in NOx, and nitrotyrosine levels compared to control group (0.57 mmol/g vs 1.35 mmol/g, P < 0.05 and 1.07 μmol/g vs. 2.5 nmol/g, P < 0.05, respectively, for NOx and nitrotyrosine). As shown in Figures 6a and b.
Figure 6.
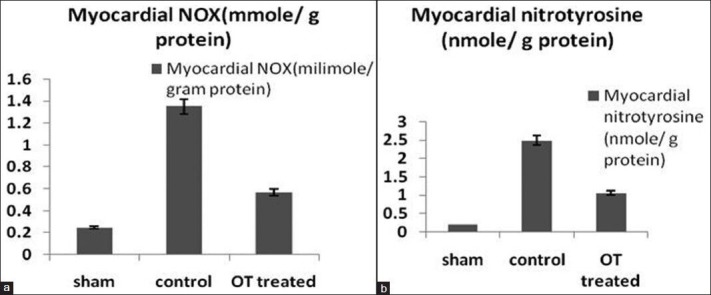
(a and b) Reactive nitrogen species RNS (A- NOx, B-nitrotyrosine) in homogenized myocardium. P < 0.05 OT-treated group vs. control
OT has antiapoptotic action
As shown in Figures 7a and b, tissue section from OT-treated animals processed for TUNEL staining shows significant reduction in apoptotic bodies after 4 hour of global ischemia reperfusion compared to control group. Figures 7a and b show average apoptotic bodies seen in 15 fields for each tissue section in the three studied groups.
Figure 7.
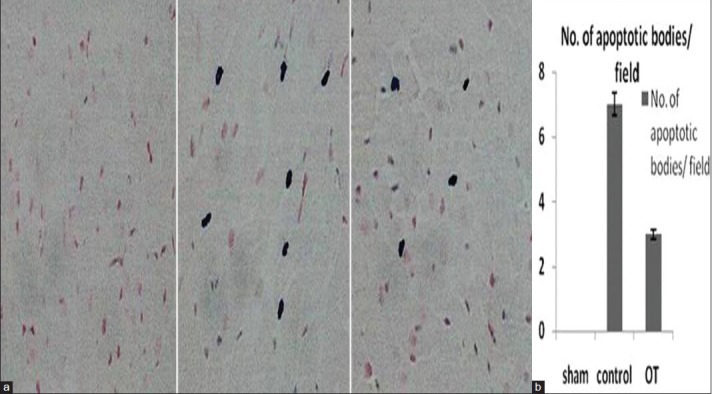
(a and b) Myocardial apoptosis: A- TUNEL stain tissue sections in the studied groups B- average number of myocardial apoptotic bodies per field in the studied groups. P < 0.05 OT-treated group vs. control
OT has no significant effect on heart rate in a transplanted heart
There are no consistent differences between the OT-treated group and the control regarding the heart rate in the transplanted heart. Rats treated with OT have an average of 594 ± 23.19 beats/minute. The control group has an average of 603.16 ± 17.80 beats/minute (P > 0.05), and the sham group (P < 0.05) has an average of 512.23 ± 12.76 beats/minute. This is shown in Table 1.
DISCUSSION
Heterotopic heart transplantation is considered a global myocardial ischemia reperfusion injury and this will induce systemic inflammatory responses in the myocardium. This inflammatory response will augment myocardial injury.[27] This study clearly shows that the control group has a significant elevation in myocardial proinflammatory cytokines and plasma cTnI. Also, there is significant myocardial damage compared to the sham group. The association of this inflammatory change with myocardial tissue damage after heterotopic heart transplantation is already well observed in many previous studies.[28]
Research demonstrates that OT is already well known for its negative chronotropic and inotropic effects.[1] In our research, we observed the chronotropic effects of OT, and the results show no significant difference between the control and OT-treated groups. However, there is a significant difference in the heart rate of animals receiving OT before heart transplantation. This may be explained by the fact that the SA node may be damaged during cold preservation,[29] which is why, during myocardial preservation; the myocardial perfusate (with OT) will not affect the heart rate.
The OT-treated group displays significant myocardial protection against global ischemia reperfusion injury. However, this protection is not through an OT negative chronotropic effect. Nonetheless, this group shows significant myocardial protection and is associated with the expression of less proinflammatory cytokine, less myocardial injury markers, and less neutrophil infiltration. The association of OT and anti-inflammatory potential is also known from previous studies; one of the theories to put mechanism for this effect is that OT may induce elevation in corticosteroid tissue level which will inhibit neutrophil infiltration to the target tissue.[30,31]
Reperfusion of the globally ischemic myocardium will be a signal not just for recruiting more neutrophils but also increases the grade of neutrophils activation which will emit more free radicals and reactive oxygen molecules which in turn augment tissue injury after reperfusion.[32] This will be a vicious circle that is the more injury then the more neutrophil infiltration.
This study clearly shows that OT decreases neutrophil recruitment in the reperfused myocardium but this decrement is not paralleled to the more downregulation of MPO induced by OT, as it causes approximately one-third reductions in neutrophils in target zone compared to two-third reductions in MPO activity. So OT induces less neutrophil recruitment but with more downregulation in its MPO activity resulting in less global myocardial ischemia reperfusion injury. It had been known that RNS not ROS has a critical role in mediating myocardial apoptosis during regional ischemia reperfusion.[17,18] NO, superoxide reaction increases rapidly during myocardial I/R resulting in more harmful species, among those the ONOO¯ which is the most myocardial injurious agent. ONOO¯ is known for its triggering apoptotic activity in myocardium,[17,18] as it reacts with most molecules in tissue including lipids and proteins and through this reaction it causes myocardial tissue injury.[18] This study demonstrated that oxytocin downregulates the neutrophil chemotactant agents the KC/CXCL1 and MIP-2/CXCL2 which recruit less neutrophil into myocardium, this decrement in myocardial PMN infiltration is associated with less ROS and RNS formation in the myocardium after 3 hours of global ischemia reperfusion. These oxytocin-induced downregulation inflammatory and oxidative processes will end in less myocardial injury through impedance in the post-myocardial ischemia/reperfusion apoptotic process.
CONCLUSIONS
Oxytocin ameliorates the immediate myocardial injury in heart transplant through downregulation the myocardial inflammatory response, reactive oxygen species, and neutrophil-dependant myocardial apoptosis.
ACKNOWLEDGMENTS
It is our pleasure to acknowledge Dr. Sandi Rufail who did the histopathological work. The funds of this research are our personal private source.
Footnotes
Source of Support: Private personal support
Conflict of Interest: None declared.
REFERENCES
- 1.Mukaddam-Daher S, Tin YL, Roy J, Gotjowska J, Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–6. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- 2.Pournajafi-Nazarloo H, Perry A, Partoo L, Papademeteriou E, Azizi F, Carter CS, et al. Neonatal oxytocin treatment modulates oxytocin receptor, atrial natriuretic peptide, nitric oxide synthase and estrogen receptor mRNAs expression in rat heart. Peptides. 2007;28:1170–7. doi: 10.1016/j.peptides.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grewen KM, Light KC. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol Psychol. 2011;87:340–9. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, et al. Rat heart: A site of oxytocin production and action. Proc Natl Acad Sci U S A. 1998;95:14558–63. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM, et al. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc Natl Acad Sci U S A. 1997;94:11704–9. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danalache BA, Gutkowska J, Slusarz MJ, Berezowska I, Jankowski M. Oxytocin-Gly-Lys-Arg: A novel cardiomyogenic peptide. PloS One. 2010;5:e13643. doi: 10.1371/journal.pone.0013643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizadeh AM, Faghihi M, Sadeghipour HR, Mohammadghasemi F, Imani A, Houshmand F, et al. Oxytocin protects rat heart against ischemia-reperfusion injury via pathway involving mitochondrial ATP-dependent potassium channel. Peptides. 2010;31:1341–5. doi: 10.1016/j.peptides.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol. 2010;105:205–18. doi: 10.1007/s00395-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 10.Szeto A, Nation DA, Mendez AJ, Dominquez-Bendala J, Brooks LG, Schneiderman N, et al. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295:E1495–501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 12.Leigh LE, Ghebrehiwet B, Perera TS, Bird LN, Strong P, Kishore U, et al. C1q-mediated chemotaxis by human neutrophil involvement of gC1qR and G-protein signaling. Biochem J. 1998;330(Pt 1):247–54. doi: 10.1042/bj3300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 14.Ward PA, Newman LI. A neutrophil chemotactic factor from human C5. J Immunol. 1969;102:93–9. [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–53. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 17.Montecucco F, Lenglet S, Braunersreuther V, Pellia G, Pellieux C, Montessuit C, et al. Single administration of the CXC chemokine-binding protein Evasin-3 during ischemia prevents myocardial reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1371–7. doi: 10.1161/ATVBAHA.110.206011. [DOI] [PubMed] [Google Scholar]
- 18.Fan Q, Chen M, Fang X, Lau WB, Xue L, Zhao L, et al. Aging might augment reactive oxygen species (ROS) formation and affect reactive nitrogen species (RNS) level after myocardial ischemia/reperfusion in both humans and rats. Age (Dordr) 2012 May 12; doi: 10.1007/s11357-012-9421-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Chen D, Chen L. Chen. A modified model of cervical heterotopic cardiac transplantation for chronic rejection research. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi (Chinese journal of reparative and reconstructive surgery) 2008;22:1508–10. [PubMed] [Google Scholar]
- 20.Gomes OM, Magalhaes Mde M, Abrantes RD. Myocardium functional recovery protection by omeprazole after ischemia-reperfusion in isolated rat hearts. Rev Bras Cir Cardiovasc. 2010;25:388–92. doi: 10.1590/s0102-76382010000300016. [DOI] [PubMed] [Google Scholar]
- 21.Ferraro S, Ardoino I, Boracchi P, Santagostino M, Ciardi L, Antonini G, et al. Inside ST-elevation myocardial infarction by monitoring concentrations of cardiovascular risk biomarkers in blood. Clin Chim Acta. 2012;413:888–93. doi: 10.1016/j.cca.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Ock S, Ahn J, Lee SH, Park H, Son JW, Oh JG, et al. Receptor activator of nuclear factor-kB ligand is a novel inducer of myocardial inflammation. Cardiovasc Res. 2012;94:105–14. doi: 10.1093/cvr/cvs078. [DOI] [PubMed] [Google Scholar]
- 23.Radin MJ, Holycross BJ, McCune SA, Altschuld RA. Crosstalk between leptin and interleukin-1beta abrogates negative inotropic effects in a model of chronic hyperleptinemia. Exp Biol Med (Maywood) 2011;236:1263–73. doi: 10.1258/ebm.2011.011144. [DOI] [PubMed] [Google Scholar]
- 24.Meij JT, Sheikh F, Jimenez SK, Nickerson PW, Kardami E, Cattini PA. Exacerbation of myocardial injury in transgenic mice overexpressing FGF-2 is T cell dependent. Am J Physiol Heart Circ Physiol. 2002;282:H547–55. doi: 10.1152/ajpheart.01019.2000. [DOI] [PubMed] [Google Scholar]
- 25.Clements-Jewery H, Hearse DJ, Curtis MJ. Neutrophil ablation with anti-serum does not protect against phase 2 ventricular arrhythmias in anaesthetised rats with myocardial infarction. Cardiovasc Res. 2007;73:761–9. doi: 10.1016/j.cardiores.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Buege JA, Aust SD. Lactoperoxidase-catalyzed lipid peroxidation of microsomal and artificial membranes. Biochim Biophys Acta. 1976;444:192–201. doi: 10.1016/0304-4165(76)90236-1. [DOI] [PubMed] [Google Scholar]
- 27.Timmers L, Pasterkamp G, deHoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–83. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 28.Vuohelainen V, Raitoharju E, Levula M, Lehtimaki T, Pelto-Huikko M, Honkanen T, et al. Myocardial infarction induces early increased remote ADAM8 expression of rat hearts after cardiac arrest. Scand J Clin Lab Invest. 2011;71:553–62. doi: 10.3109/00365513.2011.591424. [DOI] [PubMed] [Google Scholar]
- 29.Ning XH, Chen SH. Mild hypothermic cross adaptation resists hypoxic injury in hearts: A brief review. Chin J Physiol. 2006;49:213–22. [PubMed] [Google Scholar]
- 30.Perretti M. Lipocortin 1 and chemokine modulation of granulocyte and monocyte accumulation in experimental inflammation. Gen Pharmacol. 1998;31:545–52. doi: 10.1016/s0306-3623(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35:487–91. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- 32.Andreoli SP. Reactive oxygen molecules, oxidant injury and renal disease. Pediatr Nephrol. 1991;5:733–42. doi: 10.1007/BF00857888. [DOI] [PubMed] [Google Scholar]


