Abstract
Objectives
We sought to examine the prognostic value of subclinical left ventricular (LV) regional myocardial dysfunction (RMD) measured by magnetic resonance imaging (MRI) among asymptomatic individuals.
Background
LV RMD, defined as segmental impairment in systolic wall thickening, predicts adverse events in patients with established cardiovascular disease. MRI is highly accurate for detecting subtle RMD, of which the prognostic significance in a large multiethnic asymptomatic population is not known.
Methods
We used MRI to evaluate baseline regional LV myocardial function and prospectively followed a multiethnic (African American, Caucasian, Chinese, and Hispanic) population-based sample of 4,510 men and women without cardiovascular disease for a mean of 4.6 years. Regional myocardial dysfunction was defined as the presence of impaired systolic wall thickening (<10th percentile of segment-specific population distribution) in ≥2 contiguous LV segments within any given coronary artery territory.
Results
Baseline prevalence of RMD was 25.6%. Heart failure developed in 34 (1.0%) and 30 (2.6%) participants without and with RMD, respectively (p < 0.001). After adjustment for demographics and traditional risk factors, RMD remained independently associated with incident heart failure (hazard ratio [HR]: 2.62; 95% confidence interval [CI]: 1.56 to 4.39; p < 0.001). The relationship persisted after further adjustment for biomarkers of reported association with cardiovascular disease and indexes of global LV systolic dysfunction and hypertrophy (HR: 1.80; 95% CI: 1.02 to 3.20; p = 0.044). Similarly, RMD independently conferred an increased risk for hard coronary events (myocardial infarction or death from coronary heart disease; HR: 1.75; 95% CI: 1.06 to 2.89; p = 0.029), the composite of hard coronary events and stroke (HR: 1.72; 95% CI: 1.16 to 2.56; p = 0.005), and all atherosclerotic cardiovascular events (HR: 1.50; 95% CI: 1.09 to 2.07; p = 0.012).
Conclusions
Among an asymptomatic multiethnic American cohort, RMD is an independent predictor beyond traditional risk factors and global LV assessment for incident heart failure and atherosclerotic cardiovascular events. The clinical utility of early recognition of this subclinical phenotype deserves further investigation.
Keywords: epidemiology, heart failure, magnetic resonance imaging, myocardial dysfunction, prognosis
Epidemiologic studies have reported that left ventricular (LV) hypertrophy and depressed ejection fraction predict development of heart failure in asymptomatic individuals (1–4). These global alterations in LV structure and function have since been recognized as important subclinical therapeutic targets in the effort to delay progression to symptomatic heart failure (5,6). However, the unfavorable progressive nature of heart failure underscores the importance of better defining its earlier subclinical manifestations. Because coronary artery disease is the major cause of LV dysfunction (7,8), it is conceivable that as with coronary atherosclerosis, incipient myocardial dysfunction would commence as a regional process antedating global LV dysfunction.
The assessment of systolic wall thickening (SWT) is a validated technique for evaluating regional LV myocardial function (9). Magnetic resonance imaging (MRI) is the reference standard for assessing regional LV structure and function (10,11). In this study, we used the data collected from the MESA (Multi-Ethnic Study of Atherosclerosis) cohort to evaluate the relationship between subclinical regional myocardial dysfunction (RMD), detected by MRI as reduced SWT, and incident cardiovascular events in a large, multiethnic, asymptomatic population without baseline clinical cardiovascular disease.
Methods
Study design and participants
MESA was a multicenter, prospective cohort study designed to examine the prevalence, correlates, and progression of subclinical cardiovascular disease. Details of its rationale and methodology have been published (12). Briefly, the MESA cohort comprised 6,814 men and women and was a population-based sample from 6 communities (Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota) recruited between 2000 and 2002. Eligible participants were between 45 and 84 years of age from 4 self-identified ethnicities (African American, Caucasian, Chinese, and Hispanic) without known clinical cardiovascular disease at enrollment. The study was approved by the institutional review board of each center, and all participants provided written informed consent.
Cardiac MRI
Consenting eligible participants underwent cardiac MRI at enrollment. The complete MRI protocol was detailed elsewhere (13,14). Briefly, MRI was performed using commercially available 1.5-T scanners. After acquisition of scout images, 2- and 4-chamber cine images were obtained. Short-axis cine images covering the entire LV were then acquired from above the mitral valve plane to LV apex using segmented k-space, electrocardiogram (ECG)-triggered flow-compensated fast gradient echo sequence (time to repetition/echo time: 8 to 10 ms/3 to 5 ms, flip angle: 20°, slice thickness/gap: 6 mm/4 mm, in-plane resolution: 1.4 to 1.6 mm × 2.2 to 2.5 mm, temporal resolution: 46 ± 8 ms).
Using Q-MASS software (version 4.2, Medis, the Netherlands), the endocardial and epicardial borders of the LV were traced semiautomatically at end-systole and end-diastole on short-axis cine images. Regional wall thickness was determined by Q-MASS, which uses the validated modified centerline method incorporating a 3-dimensional analytic approach (15). Systolic wall thickening was calculated as the percentage change in wall thickness from end-diastole to end-systole: SWT (in %) = (ESWT–EDWT)/EDWT × 100%, where ESWT indicates end-systolic wall thickness and EDWT indicates end-diastolic wall thickness, and was measured separately for 6 equally partitioned segments on each of the 3 short-axis planes (apex, mid-cavity, and base) of the LV. The 6 apical segments as partitioned and quantified were condensed into 4 segments by combining, respectively, the 2 adjacent septal and lateral wall segments for consistency with published segmentation definition (16). Because there were segmental variations in SWT measured by MRI among MESA participants without traditional risk factors, abnormal values of SWT on MRI were expected to be segment specific. Accordingly, abnormal SWT in a specific segment was defined a priori as below the 10th percentile of its segment-specific distribution among a healthy reference MESA population without obesity, hypertension, or diabetes (n = 1,778; age 59 ± 10 years; female 49.9%; African American 16.3%, Caucasian 45.9%, Chinese 17.0%, Hispanic 20.8%). LV RMD was defined as the presence of abnormal SWT in ≥2 contiguous segments within the same coronary arterial territory. The assignment of LV segments to coronary territories (left anterior descending [LAD], left circumflex [LCx], and right coronary artery [RCA]) followed published recommendations (16). The means ± SD of the segment-specific abnormal segmental SWT threshold of the LAD, RCA, and LCx segments were 22 ± 9%, 24 ± 14%, and 28 ± 10%, respectively. A participant was considered as having RMD if it was present in at least 1 coronary territory. Details on image analysis, data quality control, calculations for LV ejection fraction and mass, and reproducibility of these global LV measurements have been published (14). For regional LV myocardial function in accordance with the 16-segment model (16), reliability for single segmental measurement of SWT (intraobserver and interobserver intraclass correlation coefficients of 0.73, 95% confidence interval [CI]: 0.69 to 0.76; and 0.68, 95% CI: 0.63 to 0.72, respectively) and its overall variability (intraobserver and interobserver differences of −0.3 ± 15.3% and −0.9 ± 17.3%, respectively) were determined among the same random subset of 75 MESA participants as previously reported (14).
Risk factors and clinical covariates
At baseline, designated research personnel collected clinical information on cardiovascular risk factors, including family history of coronary artery disease, smoking status (never, former, or current) and amount (pack-years), hypertension, diabetes, medication use, and physical activity using the standard semiquantitative MESA Typical Week Physical Activity Survey for derivation of intentional exercise and moderate/vigorous physical activity measures (metabolic equivalent min/week) (17). Physical examination, including measurements of seated brachial blood pressure, resting pulse rate, and anthropometric indexes, was conducted in accordance with a standardized protocol. Hypertension was defined by the recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (18). Diabetes was diagnosed per the American Diabetes Association criteria or use of antihyperglycemic therapy. Laboratory measurements of 12-h fasting glucose and lipoprotein cholesterol concentrations, serum creatinine, and novel biomarkers were collected and analyzed at a central core laboratory.
Follow-up and definition of clinical events
In addition to 6 follow-up MESA examinations, participants were followed at intervals of every 9 to 12 months for interim cardiovascular events via telephone interviews, which were complete in 92% of living participants. Next-of-kin interviews were conducted for participants with out-of-hospital cardiovascular deaths. Medical records of 98% and 95% of all self-reported incident cardiovascular events associated with hospitalizations and outpatient cardiovascular diagnostic encounters, respectively, were reviewed. Two physicians of the MESA mortality and morbidity committee blinded to cardiac MRI findings independently classified events and assigned incidence dates. Disagreement was resolved through adjudication by the full committee.
In this study, the primary outcome measure was incident symptomatic heart failure, which was pre-defined in MESA and required documentation of symptoms and/or signs, as well as physician diagnosis and treatment, with or without additional objective evidence of pulmonary edema/congestion by chest radiograph and/or LV dysfunction by clinical imaging. Ejection fraction by clinical imaging at index event, if available, was used in this investigation for descriptive subclassification into systolic (ejection fraction <50%) or diastolic (ejection fraction ≥50% or qualitatively normal) heart failure. Moreover, we studied 4 secondary outcome measures defined a priori in MESA, based on pre-specified clinical event definitions: 1) myocardial infarction, resuscitated cardiac arrest, and death from coronary disease were classified as hard coronary events; 2) composite of all coronary events additionally included definite angina and probable angina followed by revascularization; 3) hard cardiovascular events encompassed hard coronary events plus fatal and nonfatal stroke; and 4) the composite endpoint of all cardiovascular events was defined by deaths related to atherosclerotic diseases and any of the coronary and hard cardiovascular events. A detailed description of the methodology for follow-up, definitions of the individual clinical endpoints, and adjudication of clinical events is available (19,20).
Statistical analysis
Continuous variables with normal distribution are presented as mean ± SD and were compared by 1-way analysis of variance. All non-normally distributed variables were found to exhibit skewed distribution and are reported as median (interquartile range), with comparisons conducted by Mann-Whitney test. Discrete variables are described as counts and percentages with differences examined by chi-square tests. Differences in baseline characteristics between subgroups with and without RMD were first examined. To contrast intergroup differences in incidence of clinical events since enrollment, Kaplan-Meier cumulative-event curves were constructed and compared using the log-rank test. The unadjusted hazard ratio (HR) for each clinical endpoint with individuals, having no RMD as the reference group, was reported. The independent association between RMD and incident clinical events was examined using Cox proportional hazards models with forced entry of the presence of RMD as a dichotomous predictor variable and adjustment for covariates in a hierarchic manner (models 1 to 4). Continuous variable covariates with skewed distributions were logarithmically transformed before entry into models. Proportionality assumptions were verified by examining time-dependent covariates. In the base models (model 1), adjustment was made for demographics, traditional risk factors, and physical activity. Additional models (model 2) were constructed with further adjustment for biomarkers of reported association with myocardial dysfunction and/or cardiovascular events in asymptomatic populations. Finally, depressed LV ejection fraction and increased LV mass index (LV mass [g] normalized to body surface area [m2]) as respective indicators of global LV systolic dysfunction and LV hypertrophy were entered into a set of more fully adjusted models (models 3 and 4). Because myocardial infarction is a well-recognized cause of heart failure, myocardial infarction during follow-up (interim myocardial infarction) was additionally included and modeled as a time-dependent covariate in each hierarchic model for heart failure. Furthermore, we performed exploratory analyses to examine the prognostic significance of RMD among participants with confirmed normal baseline LV ejection fraction (>50%) only—a conceivably lower-risk subgroup without contemporary regard of harboring subclinical global LV systolic dysfunction. First, we restricted our comparative analyses to within this selected subgroup. Second, we tested for interactions between RMD and ejection fraction to evaluate any heterogeneity in the prognostic significance of RMD across subgroups with normal versus abnormal ejection fraction. All analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, Illinois) and Stata version 10.0 (StataCorp, College Station, Texas). Statistical significance was inferred at 2-sided p < 0.05.
Results
Clinical characteristics of study cohort
Among the 6,814 participants of the MESA cohort, 5,004 (73%) had a baseline cardiac MRI examination. Technically adequate data for complete quantitative measurements of regional SWT of all LV segments was available for 4,514 participants; of these participants, 4,510 (66% of the total cohort) had complete follow-up information and constituted the study population. Of the 4,510 study participants, the prevalence of RMD in the LAD, LCx, and RCA territories was 13.2%, 10.3%, and 11.3%, respectively. Overall, 1,154 participants (25.6%) had RMD, with 815 (18.1%), 263 (5.8%), and 76 (1.7%) of them exhibiting it in 1, 2, and all 3 coronary territories, respectively. The baseline characteristics of the study population according to RMD status are depicted in Table 1. Compared with participants without RMD, those with this abnormality were less likely to be female and had higher diastolic blood pressure and waist-to-hip ratio, lower high-density lipoprotein cholesterol level, higher serum creatinine and homocysteine levels, relatively higher LV mass index and lower ejection fraction (although both were still well within normal limits), and lower average segmental SWT for each of the 3 coronary territories (Table 1).
Table 1.
Clinical Characteristics of MESA Participants Without and With Baseline RMD
| Participants Without RMD (n = 3,356) | Participants With RMD (n = 1,154) | p Value* | |
|---|---|---|---|
| Age, yrs† | 61.5 ± 10 | 61.2 ± 10 | 0.34 |
|
| |||
| Women | 1,788 (53.3) | 530 (45.9) | <0.001 |
|
| |||
| Ethnicity | 0.005 | ||
| African American | 854 (25.4) | 341 (29.5) | |
| Chinese | 442 (13.2) | 115 (10.0) | |
| Hispanic | 739 (22.0) | 250 (21.7) | |
| Caucasian | 1,321 (39.4) | 448 (38.8) | |
|
| |||
| Hypertension | 1,413 (42.1) | 495 (42.8) | 0.68 |
|
| |||
| Systolic blood pressure, mm Hg† | 126 ± 21 | 125 ± 22 | 0.15 |
|
| |||
| Diastolic blood pressure, mm Hg† | 72 ± 10 | 73 ± 11 | 0.001 |
|
| |||
| Pulse rate, beats/min† | 63 ± 9 | 63 ± 10 | 0.12 |
|
| |||
| Body mass index, kg/m2† | 27.7 ± 4.9 | 27.8 ± 5.0 | 0.79 |
|
| |||
| Waist-to-hip ratio† | 0.92 ± 0.08 | 0.93 ± 0.08 | 0.026 |
|
| |||
| Smoking status | 0.13 | ||
| Never | 1,726 (51.6) | 574 (49.9) | |
| Former smoker | 1,208 (36.1) | 409 (35.5) | |
| Current smoker | 412 (12.3) | 168 (14.6) | |
|
| |||
| Cumulative smoking, pack-yrs† | 10.7 ± 23.2 | 11.4 ± 20.0 | 0.34 |
|
| |||
| Total cholesterol, mg/dl† | 194 ± 35 | 194 ± 36 | 0.74 |
|
| |||
| LDL cholesterol, mg/dl† | 117 ± 31 | 118 ± 32 | 0.26 |
|
| |||
| HDL cholesterol, mg/dl† | 52 ± 15 | 50 ± 15 | 0.004 |
|
| |||
| Treatment for hyperlipidemia | 537 (16.0) | 168 (14.6) | 0.24 |
|
| |||
| Diabetes | 382 (11.4) | 136 (11.8) | 0.73 |
|
| |||
| Physical activity, MET-min/week‡ | |||
| Intentional exercise | 938 (210–2,100) | 840 (125–2,100) | 0.12 |
| Moderate and vigorous physical activity | 4,200 (2,091–7,665) | 4,117 (2,100–7,860) | 0.92 |
|
| |||
| Fasting glucose, mg/dl† | 96 ± 28 | 97 ± 31 | 0.29 |
|
| |||
| Serum creatinine, mg/dl† | 0.95 ± 0.24 | 0.99 ± 0.43 | <0.001 |
|
| |||
| Microalbuminuria | 247 (7.4) | 14 (1.2) | 0.98 |
|
| |||
| Macroalbuminuria | 42 (1.3) | 14 (1.2) | 0.98 |
|
| |||
| C-reactive protein, mg/l‡ | 1.77 (0.77–4.05) | 1.80 (0.78–4.04) | 0.52 |
|
| |||
| Fibrinogen, mg/dl† | 342 ± 71 | 345 ± 72 | 0.11 |
|
| |||
| Interleukin-6, pg/ml† | 1.5 ± 1.2 | 1.5 ± 1.1 | 0.84 |
|
| |||
| Homocysteine, μmol/l† | 9.1 ± 3.3 | 9.4 ± 3.4 | 0.008 |
|
| |||
| Q-wave on electrocardiogram | 34 (1.0) | 15 (1.3) | 0.42 |
|
| |||
| LV hypertrophy on electrocardiogram | 32 (1.0) | 17 (1.5) | 0.15 |
|
| |||
| LV ejection fraction, %†§ | 70 ± 6 | 65 ± 9 | <0.001 |
|
| |||
| LV mass index, g/m2†§ | |||
| Women (n = 2,318) | 71 ± 12 | 73 ± 14 | <0.001 |
| Men (n = 2,192) | 84 ± 15 | 89 ± 19 | <0.001 |
|
| |||
| Regional myocardial function, SWT (%)†§ | |||
| Average SWT of LAD segments | 68 ± 21 | 48 ± 20 | <0.001 |
| Average SWT of RCA segments | 66 ± 19 | 47 ± 18 | <0.001 |
| Average SWT of LCx segments | 81 ± 24 | 59 ± 23 | <0.001 |
All data are presented as mean ± SD, n (%), or median (interquartile range).
For categoric variables, p value was generated from the chi-square test.
Intergroup comparison by 1-way analysis of variance.
Intergroup comparison by Mann-Whitney test.
Determined by Q-MASS on magnetic resonance imaging examination.
HDL = high-density lipoprotein; LAD = left anterior descending artery; LCx = left circumflex artery; LDL = low-density lipoprotein; LV = left ventricular; MET = metabolic equivalent; RCA = right coronary artery; RMD = regional myocardial dysfunction; SWT = systolic wall thickening.
RMD, heart failure, and atherosclerotic clinical events
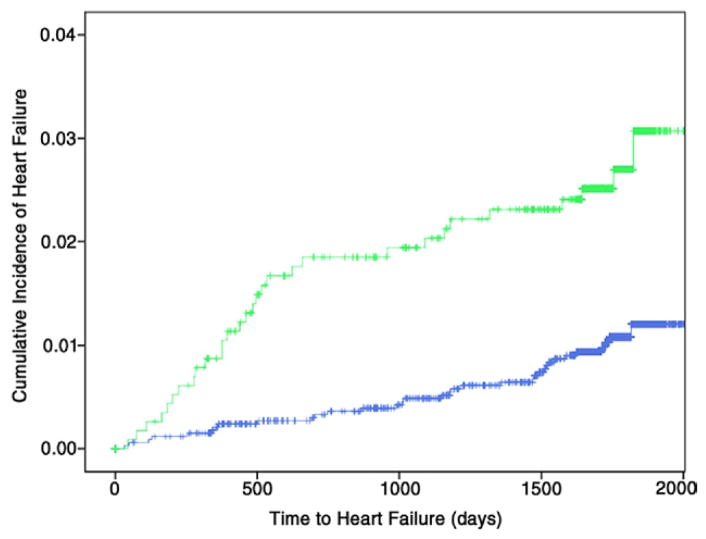
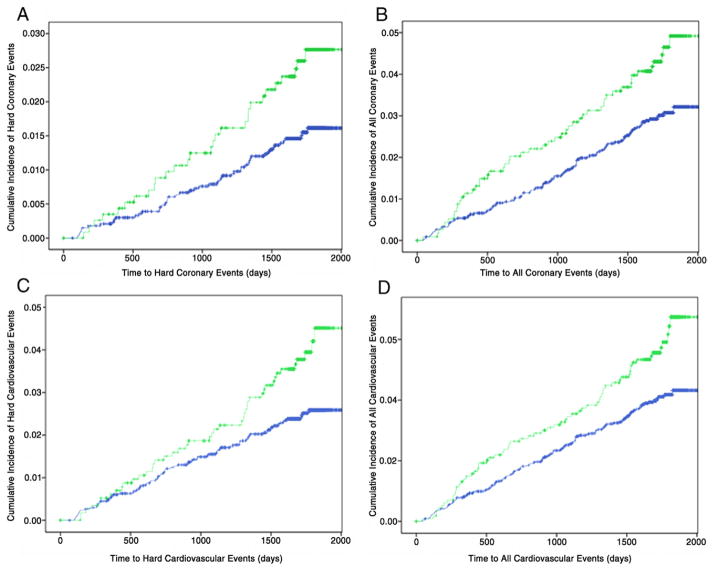
Over a mean follow-up of 4.6 ± 0.8 years, heart failure developed in 64 participants (1.4%), and 16 of them had an interim myocardial infarction (mean elapsed time 48 days). Of these 64 participants with incident heart failure, 35 (54.7%) and 21 (32.8%) had systolic and diastolic heart failure, respectively, whereas the remaining 8 (12.5%) participants had no ejection fraction data recorded at the index event, precluding such mechanistic distinction. There were 148 (3.3%) and 200 (4.4%) members in the study cohort sustaining the composite endpoints of all coronary and all cardiovascular events, respectively. The incidences of heart failure and secondary composite endpoints of hard and all coronary as well as cardiovascular events among subgroups of participants without and with baseline RMD are summarized in Table 2. Intergroup differences in cumulative event rates over time are shown in Figures 1 and 2. Of note, participants with RMD were at significantly higher risk for incident heart failure (unadjusted HR: 2.62; 95% CI: 1.60 to 4.28; p < 0.001) and at elevated risk for the composite of hard and all coronary events as well as cardiovascular endpoints (unadjusted HR: 1.46 to 1.72; p ≤ 0.02) (Table 2, Figs. 1 and 2).
Table 2.
Incidence and Unadjusted HRs of Adverse Clinical Outcome by the Presence/Absence of RMD
| Incident Clinical Events | Participants Without RMD (n = 3,356) | Participants With RMD (n = 1,154) | Unadjusted HR (95% CI) (Referent: No RMD) | p Value |
|---|---|---|---|---|
| Heart failure | 34 (1.0) | 30 (2.6) | 2.62 (1.60–4.28) | <0.001 |
| Hard coronary events* | 50 (1.5) | 29 (2.5) | 1.72 (1.09–2.72) | 0.020 |
| All coronary events† | 98 (2.9) | 50 (4.3) | 1.51 (1.08–2.13) | 0.017 |
| Hard cardiovascular events‡ | 82 (2.4) | 44 (3.8) | 1.59 (1.10–2.29) | 0.013 |
| All cardiovascular events§ | 134 (4.0) | 66 (5.7) | 1.46 (1.09–1.97) | 0.012 |
Values are n (%) unless otherwise indicated.
Myocardial infarction, resuscitated cardiac arrest, or death from coronary artery diseases.
Hard coronary events, definite angina, and probable angina followed by revascularization.
Hard coronary events, nonfatal and fatal stroke.
All coronary events, nonfatal and fatal stroke, and other death from atherosclerotic diseases.
CI = confidence interval; HR = hazard ratio; RMD = regional myocardial dysfunction.
Figure 1. Kaplan-Meier Analysis for the Primary Endpoint of Incident Heart Failure.
Kaplan-Meier curve illustrating cumulative incidence of heart failure among subgroups of participants without (blue) and with (green) baseline regional myocardial dysfunction (log-rank test for difference p < 0.001).
Figure 2. Kaplan-Meier Analyses for the Secondary Atherosclerotic Cardiovascular Endpoints.
Kaplan-Meier curves illustrating cumulative incidences by absence (blue) and presence (green) of baseline regional myocardial dysfunction for (A) hard coronary events (log-rank for difference, p = 0.019), (B) all coronary events (log-rank for difference, p = 0.016); (C) hard cardiovascular events (log-rank for difference, p = 0.012); and (D) all cardiovascular events (log-rank for difference, p = 0.011).
Independent association between RMD and heart failure
The independent association between RMD and clinical events is summarized in Table 3. After adjustment for demographics, traditional risk factors, physical activity, and interim myocardial infarction (model 1), RMD was independently associated with incident heart failure (HR: 2.62; 95% CI: 1.56 to 4.39; p < 0.001). Adjustment for biomarkers reportedly related to impaired regional myocardial function and/or incident heart failure in asymptomatic populations (serum creatinine, C-reactive protein, interleukin 6, fibrinogen, and homocysteine) did not significantly alter the relationship (model 2). After additional adjustment for depressed LV ejection fraction (model 3) and further for LV hypertrophy (model 4), the association with heart failure was attenuated but remained significant (HR: 1.80; 95% CI: 1.02 to 3.20; p = 0.044).
Table 3.
Cox Proportional Hazards Models for Incident Cardiovascular Events as a Function of RMD
| Clinical Endpoint | RMD Adjusted HR (95% CI) | |||
|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | Model 4§ | |
| Heart failure | 2.62 (1.56–4.39); p < 0.001 | 2.52 (1.49–4.26); p = 0.001 | 1.87 (1.06–3.30); p = 0.030 | 1.80 (1.02–3.20); p = 0.044 |
| Hard coronary events (MI, resuscitated cardiac arrest, and death from CAD) | 1.76 (1.10–2.81); p = 0.018 | 1.76 (1.09–2.85); p = 0.021 | 1.82 (1.10–2.99); p = 0.019 | 1.75 (1.06–2.89); p = 0.029 |
| All coronary events (hard coronary events, definite angina, probable angina followed by revascularization) | 1.56 (1.10–2.21); p = 0.012 | 1.56 (1.09–2.22); p = 0.014 | 1.55 (1.07–2.24); p = 0.020 | 1.52 (1.05–2.21); p = 0.026 |
| Hard cardiovascular events (hard coronary events, nonfatal and fatal stroke) | 1.70 (1.17–2.48); p = 0.006 | 1.69 (1.16–2.48); p = 0.007 | 1.78 (1.20–2.64); p = 0.004 | 1.72 (1.16–2.56); p = 0.005 |
| All cardiovascular events (all coronary events, nonfatal and fatal stroke, death from other atherosclerotic diseases) | 1.55 (1.15–2.10); p = 0.004 | 1.54 (1.14–2.10); p = 0.005 | 1.54 (1.12–2.11); p = 0.008 | 1.50 (1.09–2.07); p = 0.012 |
Model 1: adjustment for age, sex, ethnicity, hypertension/antihypertensive medications, systolic and diastolic blood pressure, pulse rate, smoking status and amount in pack-years, physical activity (intentional exercise), diabetes/antihyperglycemic treatment, total cholesterol, HDL, use of lipid-modifying medications, and waist-to-hip ratio. For heart failure, interim MI was additionally included and modeled as a time-dependent covariate.
Model 2: adjustment for covariates in model 1 and biomarkers of reported association with myocardial dysfunction and/or cardiovascular events in asymptomatic populations (fasting glucose, serum creatinine, C-reactive protein, fibrinogen, interleukin 6, and homocysteine).
Model 3: adjustment for covariates in model 2 and global LV systolic dysfunction (LV ejection fraction <61%, 10th percentile of reference MESA population).
Model 4: adjustment for covariates in model 3 and LV hypertrophy (LV mass index >90th sex-specific percentile of reference MESA population).
Independent association between RMD and incident atherosclerotic cardiovascular events
The occurrence of the first hard and the composite of all coronary and cardiovascular events was consistently more frequent in individuals with baseline RMD compared with their counterparts without regional dysfunction (Table 2). In hierarchic multivariable Cox regression analyses, RMD was found to be a significant independent predictor of incident hard and the composite of all coronary and cardiovascular events, albeit with relatively lower HRs (HR estimates: 1.50 to 1.82; p < 0.05) than that in the corresponding model for symptomatic heart failure. Moreover, relationships between RMD at study entry and cardiovascular events persisted after adjustment for global LV systolic function and hypertrophy (Table 3).
RMD despite normal ejection fraction and cardiovascular events
Among participants with normal LV ejection fraction (n = 4,433), RMD conferred an increased risk for heart failure (n = 49) beyond traditional risk factors (model 1 HR: 1.83; 95% CI: 1.00 to 3.32; p = 0.049) but not after controlling for biomarkers and global LV evaluation (model 4 HR: 1.55; 95% CI: 0.82 to 2.93; p = 0.18). Moreover, for atherosclerotic endpoints, RMD portended significantly increased hazards for the composite of hard (n = 118; HR: 1.58; 95% CI: 1.04 to 2.38; p = 0.030) and all cardiovascular events (n = 188; HR: 1.40; 95% CI: 1.01 to 1.96; p = 0.046) independent of risk factors, biomarkers, and global LV assessment in the fully-adjusted models.
As demonstrated for the entire study cohort, the respective associations between RMD and incident heart failure as well as each secondary atherosclerotic endpoint were homogeneous across participants with normal versus abnormal ejection fraction (p for interaction-heart failure = 0.13; atherosclerotic endpoints >0.45).
Discussion
This present study is the first to our knowledge to demonstrate that subclinical RMD quantitatively measured by MRI among asymptomatic adults without known cardiovascular diseases is independently associated with subsequent development of heart failure and adverse cardiovascular events. Specifically, the presence of RMD confers close to a doubling of risk for heart failure and a 1.50- to 1.75-fold increased risk for combined adverse atherosclerotic events over a mean follow-up of 4.6 years among asymptomatic adults of 4 ethnic groups, independent of indexes of global LV assessment. These findings indicate that RMD is an important phenotypic manifestation of early subclinical disease and that its accurate quantitative delineation can potentially refine risk stratification of asymptomatic individuals for incident heart failure and clinically overt cardiovascular diseases.
Earlier clinical and epidemiologic studies have largely employed echocardiography and focused on indexes of global LV structure and function to examine the transition from subclinical to symptomatic disease (2,3). These important studies have firmly established that diffuse alterations of LV architecture and function manifesting as global LV dilation and impaired ejection fraction are largely irreversible, and once they are present, progression to symptomatic disease can be inexorable (2,3,21). This unfavorable evolution highlights the need to characterize and detect earlier stages of asymptomatic myocardial dysfunction upstream to the development of global ventricular dysfunction.
The traditional paradigm linking global LV alterations and clinical events in ischemic heart disease asserts that the genesis and progression of myocardial dysfunction result from repeated myocardial damage secondary to multiple episodes of silent or clinically apparent myocardial infarction, with ensuing compensatory ventricular remodeling. Furthermore, the infliction of myocardial damage by various other etiologic factors leading to myocardial dysfunction was traditionally perceived as homogeneous and global in nature. However, emerging data from other investigators and our myocardial tagging ancillary studies in MESA have suggested a more heterogeneous and regional interplay between risk factors and subclinical disease (22–24). In particular, using myocardial tagging as the reference standard to evaluate regional myocardial function, we previously demonstrated a regional relationship between coronary artery calcification and systolic circumferential strain in the corresponding perfusion territory among MESA participants who were free of clinical cardiovascular disease (22). Similarly, a regional relationship exists between subclinical reduction in myocardial perfusion reserve and impaired systolic circumferential strain among asymptomatic MESA participants (23). These cross-sectional observations provide supporting evidence for a regional basis underlying the inception of myocardial dysfunction along the subclinical disease continuum, which importantly, as demonstrated in this prospective analysis on our larger entire MESA MRI cohort, is associated with incident cardiovascular events.
The precise etiology underlying asymptomatic RMD is not completely understood. It is likely multifactorial and related in part to the cumulative exposure to various atherosclerotic and nonatherosclerotic risk factors leading to diverse intermediate disease processes including endothelial dysfunction (25,26), progressive myocyte overload (27,28), inflammatory (29,30) and oxidative (31) myocardial injury, microvascular disease (32), and microembolization (33). All of these pathophysiologic plausibilities are conceptually regional in nature and concordant with the hypothesis that early myocardial dysfunction may commence in a regional manner before progression to global ventricular dysfunction.
Endeavors to elucidate the disease processes that precede global ventricular dysfunction and its clinical implications have been undertaken mainly in patients with ischemic heart disease (34–36) and heart failure (37). Among these selected symptomatic individuals, regional wall motion abnormalities identified by echocardiography have been shown to predict death and heart failure hospitalization. More recently, regional wall motion abnormalities detected visually by echocardiography among a subset of 2,864 clinically apparent disease-free individuals enrolled in the SHS (Strong Heart Study) have been shown to be independently associated with adverse cardiovascular outcomes (38). Of note, the predominantly American-Indian population of the SHS study also exhibited elevated risk for cardiovascular events secondary to high prevalence of diabetes and obesity. In contrast to the SHS cohort, MESA participants represent a larger, ethnically diverse, exclusively asymptomatic cohort by study design and thus are a substantially lower-risk population. Moreover, our present analysis demonstrates that RMD, objectively quantified by MRI, can be detected in as many as one-quarter of this even lower-risk asymptomatic population and represents a strong independent predictor of incident heart failure and atherosclerotic events. Our observations lend further credence to the prognostic importance of even more subtle yet quantifiable RMD by MRI among apparently healthy individuals.
As a measure of overall ventricular function, LV ejection fraction may not reflect regional depression in contractile function, which can be offset to a variable degree by compensating hypercontractile segments. Expectedly, participants with RMD had slightly lower but still normal ejection fractions. It is thus anticipated, and confirmed in our primary analyses of the entire asymptomatic cohort, that specific evaluation of regional myocardial function probes earlier manifestations of subclinical disease and confers incremental risk prediction beyond assessment of global LV systolic function that may be insensitive to incipient regional myocardial functional impairment. Our ancillary subgroup analysis is the first to corroborate the significant independent association between RMD and incident adverse cardiovascular events in asymptomatic adults with accurately measured normal LV ejection fraction. These individuals would not have been contemporarily considered as harboring subclinical ventricular dysfunction. This reinforces the pathophysiologic link and incremental prognostic value of RMD as an earlier subclinical phenotype of potential preventive and therapeutic implications.
Study strengths and limitations
MESA was the first epidemiologic study to use cardiac MRI in a large cohort to evaluate incident cardiovascular events. To our best knowledge, this analysis of 4,510 apparently healthy individuals is the first and largest multiethnic investigation of asymptomatic RMD in relation to clinical outcome. The use of cardiac MRI, an accurate and reproducible technique for assessing LV morphology and function, permitted objective quantification of subtle impairment in regional myocardial function among lower-risk individuals in whom the epidemiologic evaluation of early subclinical disease is particularly important and its implication on risk stratification most clinically relevant. Furthermore, our study cohort was diverse in ethnicity, and our results are therefore applicable to the general population.
Conversely, our study also had important limitations. The complete analysis of all myocardial segments was not possible in 490 participants (9.8%) who had MRI examinations. The exclusion of these participants may have introduced unknown bias. However, they were not systematically different than those who entered the analysis with respect to risk factors and clinical outcome (data not shown). Because all MESA participants had no known baseline cardiovascular disease, older individuals represented a particularly healthy sample of the population at large, and our results may be subjected to survival bias. Our low-risk cohort afforded only sufficient power to demonstrate a comprehensively adjusted independent association between RMD and composite cardiovascular events but not heart failure among the very low-risk subgroup with normal ejection fraction. Nevertheless, our adequately powered primary analyses establishing significant RMD associations with all a priori endpoints examined, as well as the demonstrated prognostic homogeneity of RMD across different levels of ejection fraction, are sufficient to have explicitly tested and robustly confirmed our hypothesis that RMD independently confers adverse prognosis beyond ejection fraction among apparently asymptomatic low-risk individuals.
LV ejection fraction measurements were not available in a minority of cases of incident heart failure. On the other hand, regardless of this limitation, the low overall incidence of heart failure would have precluded detailed examination of such etiologic distinction. It is also important to note that although RMD portends a statistically significant increased risk for incident cardiovascular events, the absolute event rates were still low (<2%/year) among individuals harboring this subclinical abnormality. The therapeutic implications of early detection of this subclinical disease phenotype among otherwise low-risk individuals remain to be determined. Therefore, our present findings should not be construed as support for routine screening of RMD to refine cardiovascular risk stratification. Nevertheless, our results afford novel pathogenetic insights into the initiation of disease processes and identification of potential subclinical therapeutic targets to halt early disease progression. Although the precise mechanisms underlying RMD and its relationship to the development of global ventricular dysfunction are not elucidated in this present analysis, longitudinal investigation of potential pathophysiologic mechanisms remain underway in MESA and other prospective studies. At the time of baseline imaging data collection, MRI cine images were obtained using fast gradient–echo pulse sequence, which has been superseded by the newer-generation, steady-state free precession pulse sequence capable of providing higher signal-to-noise ratio and temporal resolution. Nevertheless, because RMD was defined with reference to a normal population distribution using the same MRI technique, our findings are robust and generalizable to other accurate and sensitive imaging modalities.
Conclusions
Among an asymptomatic multiethnic American cohort, RMD was independently predictive of incident heart failure and atherosclerotic cardiovascular events beyond traditional risk factors and global LV assessment. Therefore, RMD may be a useful marker of subclinical disease. The clinical utility of early recognition of RMD to refine risk stratification and to optimize primary disease prevention warrants further investigation.
Acknowledgments
This study was supported in part by National, Heart, Lung and Blood Institute grants RO1-HL66075-01 and MESA study contracts NO1-HC-95159 through NO1-HC-95168. Dr. Yan was supported by Fellowship Awards from the Canadian Institutes of Health Research and the Detweiler Travelling Fellowship Award from the Royal College of Physicians and Surgeons of Canada.
The authors thank all of the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions is available (20).
Abbreviations and Acronyms
- CI
confidence interval
- HR
hazard ratio
- LAD
left anterior descending artery
- LCx
left circumflex artery
- LV
left ventricle
- MRI
magnetic resonance imaging
- RCA
right coronary artery
- RMD
regional myocardial dysfunction
- SWT
systolic wall thickening
Footnotes
The authors have reported that they have no relationships to disclose. Kim Allan Williams, Sr, MD, served as Guest Editor for this paper.
References
- 1.Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol. 1987;10 (Suppl 6):S135–40. [PubMed] [Google Scholar]
- 2.Lauer MS, Evans JC, Levy D. Prognostic implications of subclinical left ventricular dilatation and systolic dysfunction in men free of overt cardiovascular disease (the Framingham Heart Study) Am J Cardiol. 1992;70:1180– 4. doi: 10.1016/0002-9149(92)90052-z. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 4.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Ho K, Thom T. Changing epidemiological features of cardiac failure. Br Heart J. 1994;72:S3–9. doi: 10.1136/hrt.72.2_suppl.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–12. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 9.Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA guidelines for the clinical application of echocardiography: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography) J Am Coll Cardiol. 1997;29:862–79. doi: 10.1016/s0735-1097(96)90000-5. [DOI] [PubMed] [Google Scholar]
- 10.Constantine G, Shan K, Flamm SD, Sivananthan MU. Role of MRI in clinical cardiology. Lancet. 2004;363:2162–71. doi: 10.1016/S0140-6736(04)16509-4. [DOI] [PubMed] [Google Scholar]
- 11.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Nasir K, Tsai M, Rosen BD, et al. Elevated homocysteine is associated with reduced regional left ventricular function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;115:180–7. doi: 10.1161/CIRCULATIONAHA.106.633750. [DOI] [PubMed] [Google Scholar]
- 14.Natori S, Lai S, Finn JP, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 15.Holman ER, Buller VG, de Roos A, et al. Detection and quantification of dysfunctional myocardium by magnetic resonance imaging: a new three-dimensional method for quantitative wall-thickening analysis. Circulation. 1997;95:924–31. doi: 10.1161/01.cir.95.4.924. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539– 42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–42. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed March 1, 2011];MESA Web. Available at: http://www.mesa-nhlbi.org.
- 21.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 22.Edvardsen T, Detrano R, Rosen BD, et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:206–11. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]
- 23.Rosen BD, Lima JA, Nasir K, et al. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;114:289–97. doi: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:2420– 8. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 25.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 26.Fischer D, Rossa S, Landmesser U, et al. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–9. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 27.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 28.Palmon LC, Reichek N, Yeon SB, et al. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation. 1994;89:122–31. doi: 10.1161/01.cir.89.1.122. [DOI] [PubMed] [Google Scholar]
- 29.Rosen BD, Cushman M, Nasir K, et al. Relationship between C-reactive protein levels and regional left ventricular function in asymptomatic individuals: Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;49:594– 600. doi: 10.1016/j.jacc.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 31.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 32.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 33.Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–3. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 34.Peels KH, Visser CA, Dambrink JH, et al. for the CATS Investigators Group. Left ventricular wall motion score as an early predictor of left ventricular dilation and mortality after first anterior infarction treated with thrombolysis. Am J Cardiol. 1996;77:1149–54. doi: 10.1016/s0002-9149(96)00153-1. [DOI] [PubMed] [Google Scholar]
- 35.Carluccio E, Tommasi S, Bentivoglio M, Buccolieri M, Prosciutti L, Corea L. Usefulness of the severity and extent of wall motion abnormalities as prognostic markers of an adverse outcome after a first myocardial infarction treated with thrombolytic therapy. Am J Cardiol. 2000;85:411–5. doi: 10.1016/s0002-9149(99)00764-x. [DOI] [PubMed] [Google Scholar]
- 36.Stein JH, Neumann A, Preston LM, et al. Improved risk stratification in unstable angina: identification of patients at low risk for in-hospital cardiac events by admission echocardiography. Clin Cardiol. 1998;21:725–30. doi: 10.1002/clc.4960211006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madsen BK, Videbaek R, Stokholm H, Mortensen LS, Hansen JF. Prognostic value of echocardiography in 190 patients with chronic congestive heart failure. A comparison with New York Heart Association functional classes and radionuclide ventriculography. Cardiology. 1996;87:250– 6. doi: 10.1159/000177096. [DOI] [PubMed] [Google Scholar]
- 38.Cicala S, de Simone G, Roman MJ, et al. Prevalence and prognostic significance of wall-motion abnormalities in adults without clinically recognized cardiovascular disease: the Strong Heart Study. Circulation. 2007;116:143–50. doi: 10.1161/CIRCULATIONAHA.106.652149. [DOI] [PubMed] [Google Scholar]