Abstract
Many outbreaks of A(H1N1)pdm09 influenza have occurred in schools with a high population density. Containment of school outbreaks is predicted to help mitigate pandemic influenza. Understanding disease transmission characteristics within the school setting is critical to implementing effective control measures. Based on a school outbreak survey, we found almost all (93.7%) disease transmission occurred within a single grade, only 6.3% crossed grades. Transmissions originating from freshmen exhibited a star-shaped network; other grades exhibited branch- or line-shaped networks, indicating freshmen have higher activity and are more likely to cause infection. R0 for freshmen, calculated as 2.04, estimated as 2.76, was greater than for other grades (P < 0.01). Without intervention, the estimated number of cases was much greater when the outbreak was initiated by freshmen than by other grades. Furthermore, the estimated number of cases required to be under quarantine and isolation for freshmen was less than that of equivalent other grades. So we concluded that different grades have different transmission mode. Freshmen were the main facilitators of the spread of A(H1N1)pdm09 influenza during this school outbreak, so control measures (e.g. close contact isolation) priority used for freshmen would likely have effectively reduced spread of influenza in school settings.
The 2009 H1N1 influenza virus pandemic (A(H1N1)pdm09) emerged in Mexico and rapidly spread globally, resulting in millions of cases and approximately 18,000 deaths in nearly 200 countries22,34. Surveillance data from many countries have shown that school-aged children and young adults most likely to be infected by A(H1N1)pdm09 influenza16,23,24,26. Many outbreaks occurred in schools, presumably because of a higher density of susceptible persons10,11,18,20,29,30,31. Surveillance data on A(H1N1)pdm09 influenza in mainland China from May 7 to November 30, 2009 indicates that 39% (27,806/71,665) of reported A(H1N1)pdm09 cases were reported to be part of a recognized outbreak and 94% of those cases associated with an outbreak were 5–24 years of age36. Based on surveillance data from Beijing city, 81% (47/58) of outbreaks with 10 or more laboratory-confirmed cases occurred in schools, and most of school outbreak was initiated by freshmen. Studies have also suggested that school-aged individuals facilitated the introduction and spread of A(H1N1)pdm09 influenza in households2,6. Curbing school outbreaks would therefore help to mitigate pandemic influenza. Understanding disease transmission characteristics within school settings would therefore be helpful in defining effective control measures for A(H1N1)pdm09 influenza.
Compared to Western countries, there have been few descriptions of the transmission dynamics of A(H1N1)pdm09 influenza in school outbreaks in mainland China, where campus quarantines (fengxiao) rather than school closures represent the primary public health intervention4,5,10,11,16,18,20,31. Studies have suggested that social networks and population structures affect the spread of communicable diseases and the selection of optimal control strategies7,9,19,21. To identify optimal control interventions, we used an epidemiological survey and disease transmission dynamic model to explore the disease transmission characteristics among students in a school involved in a outbreak of A(H1N1)pdm09 influenza.
Results
Epidemiological Features
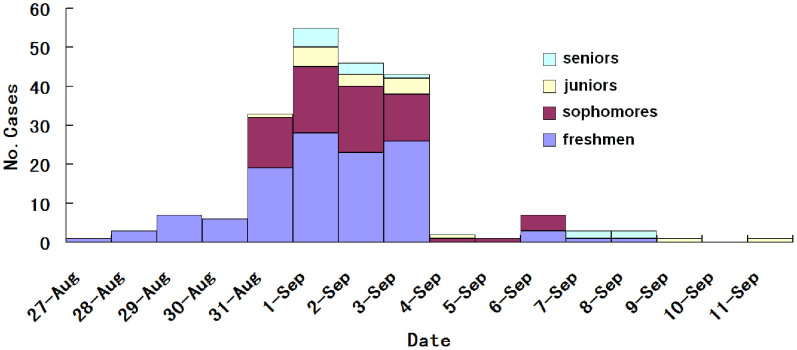
The epidemiological investigation showed that this outbreak was initiated by one infected freshman. A total of 226 confirmed cases were found, comprising 212 students and 14 teachers. Among confirmed cases, 198 were male (93.3%) and 14 were female (6.7%). This study focused on disease transmission among the student population, so 212 students data were used to analysis below. Of the 212 infected students, 24 (11%) were also excluded from the transmission chain analysis because they could not recall the source of their infection or contacts. Among 212 infected students, 118 (63%) were freshmen, 65 (35%) sophomores, 16 (9%) juniors, and 13 (7%) seniors (Figure 1). The infection rate was 6.8% among freshmen, 3.0% among sophomores, 0.9% among juniors and 0.95% seniors. Freshmen were the most affected group, with the highest infection rate among the four grades (Chi square test, p < 0.0001).
Figure 1. Number of confirmed H1N1 cases in four grades based on the illness onset.
Transmission Characteristics
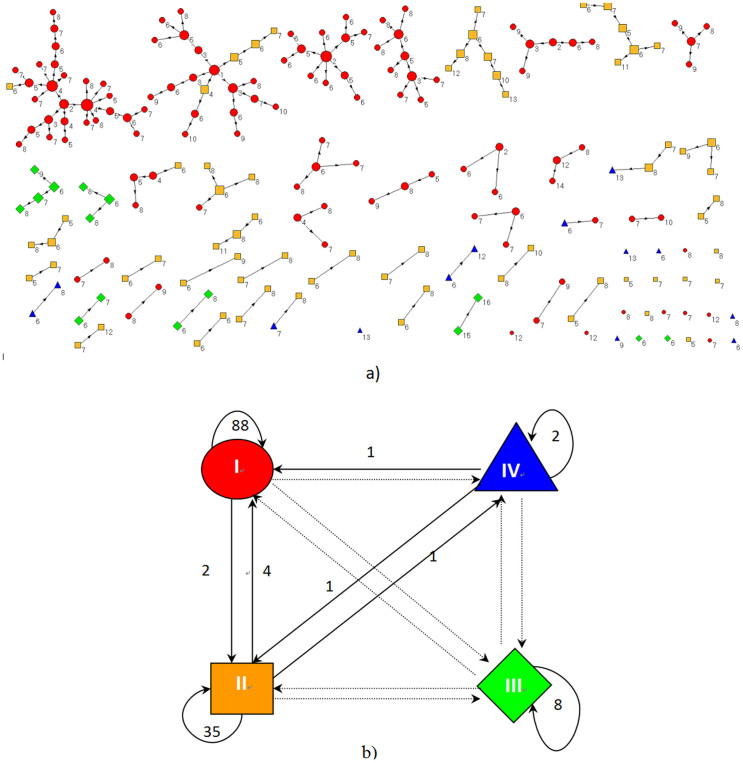
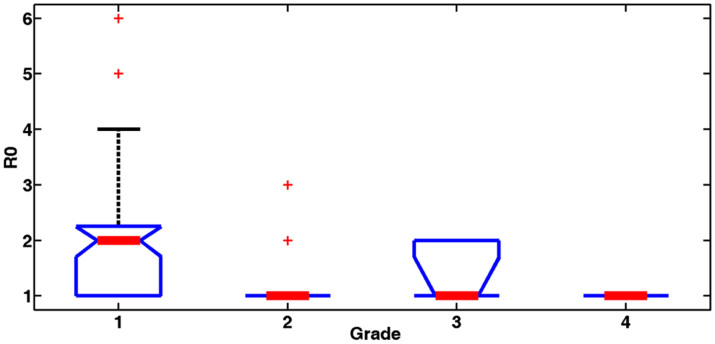
The transmission tree of A(H1N1)pdm09 influenza among the confirmed cases is shown in Figure 2a. A total of 142 transmission chains were identified, comprising 133 (93.7%) within-grade chains and 9 (6.3%) chains across grades (Figure 2b). Moreover, disease transmission with a freshman as the infection source exhibited star-shaped networks; and transmission with students from the other three grades as the infection source exhibited branch- or line-shaped networks (Figure 2a). This indicates that freshmen were the important determinant of social mixing between the students. An investigation of whether freshmen affected transmission dynamics of A(H1N1)pdm09 influenza in the school was performed by calculating basic reproductive numbers (R0) from the different grades. The basic reproductive number of an infection, R0, is defined as the expected number of secondary infectious cases generated by an average infectious case in an entirely susceptible population39, and it was calculated from epidemiological data using the average number of individuals directly infected by a confirmed case during her or his entire infectious period40. The R0 values were 2.04, 1.18, 1.40, and 1.00 for freshmen, sophomores, juniors and seniors, respectively. The 95% confidence intervals of R0 are [1.61, 2.45], [0.99, 1.38], [0.7199, 2.08] and [1, 1] for freshmen, sophomores, juniors and seniors, respectively. Because the numbers of secondary infectious cases resulting from seniors were all 1 based on our epidemiological investigation, so the confidence interval of the R0 for seniors is [1, 1].The R0 value of the seniors was calculated based on data from the epidemiological investigation, the standard estimate of R0 is always an underestimate of the true value40. So the actual R0 of seniors should be higher than 1.0. The R0 value for freshmen was significantly higher than those for the other grades (single factor analysis of variance, P < 0.01; Figure 3).
Figure 2. Disease transmission among confirmed cases.
(a) Disease transmission between confirmed cases. Red ellipse: freshmen, yellow box: sophomores, green diamond: juniors, blue triangle: seniors. The numbers means illness onset, 1 refers to August 27, 2 refers to August 28, and so forth. (b) The disease transmission across the grades. I: freshmen, II: sophomores, III: juniors, and IV: seniors.
Figure 3. Comparison of actual R0 values for different grades.
† single factor analysis of variance (P < 0.01), the median of actual R0 values for freshmen is 2, and is 1 for other grades.
Estimated Transmission Dynamics
Initial dynamics of disease transmission among the different grades were reproduced using the constructed model. The parameters were estimated using our model: α1 = 1.1, α2 = 0.8, α3 = 0.70, α4 = 0.69, α5 = 0.30 and C = 0.05. The estimated R0 values determined by the model were 2.76, 2.01, 1.76, and 1.73 for freshmen, sophomores, juniors and seniors, respectively. The 95% confidence intervals of R0 are [2.73, 2.77], [1.97, 2.04], [1.73, 1.79] and [1.72, 1.75] for freshmen, sophomores, juniors and seniors, respectively. The R0 estimated value from model was higher than that from epidemiological investigation because the close contacts cannot be completely traced. The average goodness of fit between the simulation and observation data was 76.6% (Appendix figure 1). Also, the estimated R0 value for freshmen was higher than those for the other grades.
Effectiveness of Different Interventions
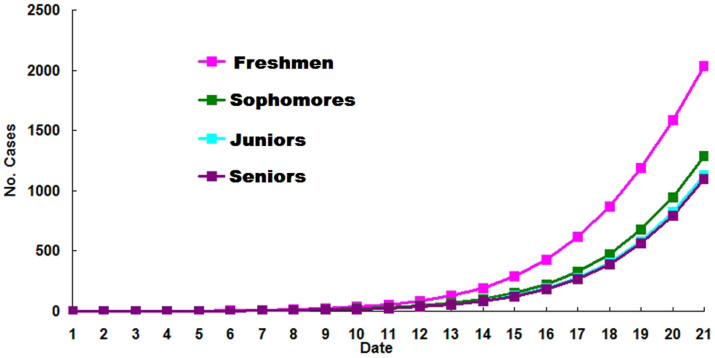
By using the transmission dynamic model, it was estimated that a total of 2,035 cases would have resulted by the 20th day if no control measures were implemented if the outbreak was initiated by a freshman. The total number of cases estimated in the same day of the outbreak was greater when the outbreak was initiated by a freshman than if the outbreak was initiated by a student in any other grade (Figure 4).
Figure 4. Estimated numbers of cases of A(H1N1)pdm09 when no control measures are used to control the outbreak, varying the source of the index case.
The model shows predicted cases if the outbreak was initiated by a freshman, sophomore, junior, or senior, respectively.
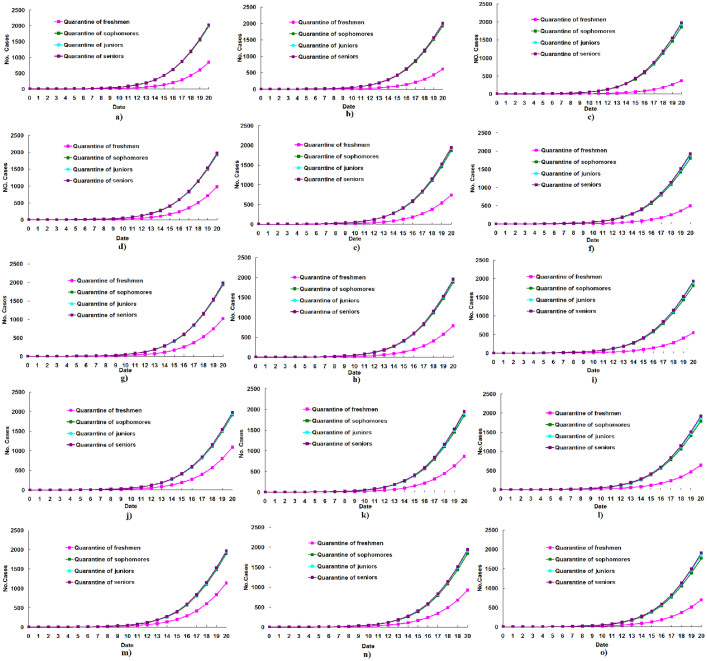
The total number of cases infected by the 20th day of the outbreak under different quarantine and isolation situations was estimated using the constructed model (Table). Assuming that this outbreak was initiated by a freshman, the estimated number of freshmen cases under quarantine and isolation was less than the number under the quarantine and isolation of equal number of other grades, regardless of when quarantine and isolation occurred during the first to fifth days of the outbreak (Table; Figure 5). Assuming that this outbreak was initiated by a sophomore, the estimated number of cases under quarantine and isolation of sophomores was less than the number under the quarantine and isolation of equal number of other grades if quarantine and isolation occurred on the first, second and third day of the outbreak. However, the estimated number of cases under quarantine and isolation of freshmen was less than the number under quarantine and isolation of equal number of other grades, when the quarantine and isolation occurred on the fourth or fifth day of the outbreak (Table; Appendix figure 2). Assuming that this outbreak was initiated by a junior, the estimated number of cases under quarantine and isolation of juniors was less than the number under quarantine and isolation of equal number of other grades, when the quarantine and isolation were performed on the first, second and third day of the outbreak. However the estimated number of cases under quarantine and isolation of freshmen was less than the number under quarantine and isolation of equal number of other grades if quarantine and isolation occurred on the fourth or fifth day of the outbreak (Table; Appendix figure 3). Assuming that this outbreak was initiated by a senior, the estimated number of cases under quarantine and isolation of seniors was less than the number under quarantine and isolation of equal number of other grades, when the quarantine and isolation were performed on the first, second and third day of the outbreak. However the estimated number of cases under quarantine and isolation of freshmen was less than the number under quarantine and isolation of equal number of other grades if quarantine and isolation occurred on the fourth or fifth day of the outbreak (Table; Appendix figure 4).
Table 1. Estimated number of cases involved on the 20th day of this outbreak under the quarantine and isolation of different proportional students from different grades on the first day, second day, third day, fourth day and fifth day of the outbreak, assuming the outbreak was initiated by different student grades respectively.
| Estimated number of cases | ||||||||
|---|---|---|---|---|---|---|---|---|
| First case | Population intervened | Proportion intervened | Onset date of intervention: | First day | Second day | Third day | Fourth day | Fifth day |
| Freshman | Freshmen | 25% | 845 | 981 | 1025 | 1090 | 1136 | |
| Sophomore | 25% | 1903 | 1916 | 1931 | 1942 | 1992 | ||
| Juniors | 25% | 1942 | 1946 | 1959 | 1970 | 2017 | ||
| Seniors | 25% | 1966 | 1971 | 1976 | 1982 | 2026 | ||
| Freshmen | 50% | 609 | 744 | 791 | 868 | 922 | ||
| Sophomore | 50% | 1842 | 1856 | 1870 | 1882 | 1929 | ||
| Juniors | 50% | 1903 | 1909 | 1923 | 1934 | 1981 | ||
| Seniors | 50% | 1929 | 1939 | 1949 | 1958 | 2001 | ||
| Freshmen | 75% | 366 | 498 | 549 | 637 | 701 | ||
| Sophomore | 75% | 1776 | 1790 | 1804 | 1815 | 1861 | ||
| Juniors | 75% | 1861 | 1868 | 1886 | 1894 | 1943 | ||
| Seniors | 75% | 1910 | 1921 | 1927 | 1932 | 1976 | ||
| Sophomore | Freshmen | 25% | 988 | 1011 | 1045 | 1109 | 1156 | |
| Sophomore | 25% | 549 | 749 | 815 | 916 | 939 | ||
| Juniors | 25% | 1211 | 1218 | 1229 | 1280 | 1282 | ||
| Seniors | 25% | 1221 | 1227 | 1250 | 1282 | 1283 | ||
| Freshmen | 50% | 880 | 899 | 927 | 978 | 1020 | ||
| Sophomore | 50% | 469 | 680 | 751 | 860 | 988 | ||
| Juniors | 50% | 1200 | 1217 | 1221 | 1278 | 1280 | ||
| Seniors | 50% | 1220 | 1221 | 1247 | 1280 | 1282 | ||
| Freshmen | 75% | 763 | 777 | 796 | 833 | 846 | ||
| Sophomore | 75% | 388 | 612 | 687 | 805 | 858 | ||
| Juniors | 75% | 1191 | 1215 | 1218 | 1276 | 1279 | ||
| Seniors | 75% | 1214 | 1220 | 1244 | 1278 | 1280 | ||
| Junior | Freshmen | 25% | 818 | 869 | 912 | 957 | 982 | |
| Sophomore | 25% | 1018 | 1025 | 1060 | 1102 | 1107 | ||
| Juniors | 25% | 488 | 718 | 786 | 856 | 1101 | ||
| Seniors | 25% | 1075 | 1076 | 1089 | 1126 | 1129 | ||
| Freshmen | 50% | 722 | 765 | 802 | 829 | 865 | ||
| Sophomore | 50% | 994 | 1003 | 1043 | 1089 | 1093 | ||
| Juniors | 50% | 440 | 679 | 752 | 836 | 881 | ||
| Seniors | 50% | 1071 | 1075 | 1088 | 1125 | 1127 | ||
| Freshmen | 75% | 619 | 653 | 680 | 702 | 739 | ||
| Sophomore | 75% | 970 | 981 | 1026 | 1075 | 1080 | ||
| Juniors | 75% | 394 | 641 | 721 | 804 | 842 | ||
| Seniors | 75% | 1069 | 1074 | 1086 | 1121 | 1124 | ||
| Senior | Freshmen | 25% | 810 | 826 | 858 | 863 | 883 | |
| Sophomore | 25% | 985 | 1003 | 1005 | 1021 | 1081 | ||
| Juniors | 25% | 1025 | 1031 | 1032 | 1046 | 1101 | ||
| Seniors | 25% | 483 | 636 | 771 | 874 | 981 | ||
| Freshmen | 50% | 715 | 726 | 752 | 789 | 856 | ||
| Sophomore | 50% | 961 | 981 | 988 | 1009 | 1066 | ||
| Juniors | 50% | 1019 | 1029 | 1031 | 1044 | 1100 | ||
| Seniors | 50% | 437 | 595 | 743 | 802 | 861 | ||
| Freshmen | 75% | 612 | 617 | 636 | 673 | 722 | ||
| Sophomore | 75% | 937 | 958 | 971 | 997 | 1052 | ||
| Juniors | 75% | 1015 | 1028 | 1030 | 1040 | 1099 | ||
| Seniors | 75% | 391 | 556 | 718 | 780 | 837 | ||
Figure 5. Estimated numbers of cases under quarantine and isolation of equal freshmen, sophomores, juniors, or seniors over the first 20 days of the outbreak, if the outbreak was initiated by freshman.
(a), quarantine and isolation of 25% freshmen, sophomores, juniors, or seniors on the first day of outbreak; (b), quarantine of 50% freshmen, sophomores, juniors, or seniors on the first day of outbreak; (c), quarantine of 75% freshmen, sophomores, juniors, or seniors on the first day of outbreak; (d), quarantine of 25% freshmen, sophomores, juniors, or seniors on the second day of outbreak; (e), quarantine of 50% freshmen, sophomores, juniors, or seniors on the second day of outbreak; (f), quarantine of 75% freshmen, sophomores, juniors, or seniors on the second day of outbreak; (g), quarantine of 25% freshmen, sophomores, juniors, or seniors on the third day of outbreak; (h), quarantine of 50% freshmen, sophomores, juniors, or seniors on the third day of outbreak; (i), quarantine of 75% freshmen, sophomores, juniors, or seniors on the third day of outbreak; (j), quarantine of 25% freshmen, sophomores, juniors, or seniors on the fourth day of outbreak; (k), quarantine of 50% freshmen, sophomores, juniors, or seniors on the fourth day of outbreak; (l), quarantine of 75% freshmen, sophomores, juniors, or seniors on the fourth day of outbreak; (m), quarantine of 25% freshmen, sophomores, juniors, or seniors on the fifth day of outbreak; (n), quarantine of 50% freshmen, sophomores, juniors, or seniors on the fifth day of outbreak; (o), quarantine of 75% freshmen, sophomores, juniors, or seniors on the fifth day of outbreak.
Discussion
During the characterization of disease transmission among students in a college involved in an A(H1N1)pdm09 influenza virus outbreak, the data showed that most (93.7%) of the disease transmission chains constructed on the basis of laboratory-confirmed cases occurred within the same school grade. Cauchemez et al also found similar transmission dynamics when investigating an elementary school outbreak of A(H1N1)pdm09 influenza among a semirural population in the United States using Bayesian statistical methods2. This transmission pattern may be caused by participation in more clustering activities among students of the same grade. Other school outbreak investigations showed that A(H1N1)pdm09 influenza spread in the school was likely facilitated by increased student interactions in the activities, including religious and club activities, close contact with friends, and lectures or laboratory work10,11,29. Thus interventions to reduce transmission in collective activity may play a role in curbing and mitigating future school outbreaks.
Investigations at the country- or region- level indicated that seasonal changes in patterns of social contacts have a marked influence on the spread of A(H1N1)pdm09 influenza8,25,32,35. In particular, the patterns of school terms and holidays affect the incidence36. Our finding of star-shaped rather than branch- and line-shaped transmission trees with a freshman student as the infectious source showed that, even in a micro-environment like this school, patterns of social mixing also impacted disease transmission. Freshmen, as newcomers to the school and lacking stable social circles, are more likely to make new contacts by increasing their peer-to-peer activities thus creating their own social networks. The calculated R0 also indicated that freshmen facilitated the spread of A(H1N1)pdm09 influenza between the students. Therefore, infectious disease transmission modes in the school were established by social contacts of freshmen with other students.
Furthermore, the R0 value for freshmen calculated from the investigation data or estimated from a model, was greater than those for other grades. These findings indicate that freshmen are an important determinant of social mixing among students and disease transmission was increased by the degree of social mixing between freshmen and other students. This was further supported by the model estimation that many more cases were involved if the outbreak was initiated by a freshman than if the outbreak was initiated by a member of another grade. Studies using mathematical models also showed that patterns of social mixing played a key role in shaping disease transmission2,5.
Studies have shown that many outbreaks of A(H1N1)pdm09 influenza occurred in schools and school-aged individuals were more likely to be infected10,11,16,18,20,23,24,26,29,30,31,36. School-aged individuals facilitated the introduction and spread of A(H1N1)pdm09 influenza in households2,6. It was inferred that the butterfly effect of disease transmission in A(H1N1)pdm09 influenza would be observed as the disease transmission pattern in school outbreaks; this is influenced by social mixing patterns of newcomers in schools.
During the early stage of A(H1N1)pdm09 influenza pandemic, fengxiao (campus quarantine), a reversal of the usual strategy of school closures adopted in many other countries, is usually implemented in mainland China to prevent movement of infected cases from the community. This measure was put in place to prevent explosive school outbreaks and decelerate disease spread. Studies have shown that the early implementation of fengxiao can delay the epidemic peak significantly and prevent the disease spread to the general population27,28. Once a school outbreak has occurred, further quarantine and isolation of students is necessary to decrease the effect of outbreak without school closure. A key question that remains to be answered is how to implement the quarantine and isolation measures to effectively mitigate school outbreaks, especially in a situation when not all of the contacts can be traced due to complex mixing of students in many school activities.
By using a transmission dynamic model to estimate the case number involved in this outbreak under different quarantine and isolation methods, we found that the quarantine and isolation of freshmen more effectively mitigated the school outbreak, if the outbreak was initiated by a freshman and no matter when quarantine and isolation measures were conducted. The same model was used to estimate the number of cases if the outbreak was initiated by other students in other grades and if quarantine and isolation was conducted on days two through five of the outbreak. The model demonstrated that quarantine and isolation of freshmen without school closure can effectively contain a school outbreak when not all of the contacts can be traced. As mentioned above, school-aged individuals facilitated the introduction and spread of A(H1N1)pdm09 influenza in households2,6. Therefore, the quarantine and isolation of freshmen without school closure is predicted to effectively reduce the spread and impact of pandemic influenza in the community. From another perspective, this finding suggested that freshmen were the key determinant shaping the disease transmission in this school outbreak.
In this study we ignored the heterogeneity of innate immunity to A(H1N1)pdm09 influenza among the students, since most people were expected to lack innate immunity to the novel strain of influenza. Additionally, the dynamic model assumed homogenous mixing of the students from the same grade. Although these limitations mean that the mathematical model used in this study represents a simplified version of a complex infectious disease system, they may provide insight into transmission characteristics of A(H1N1)pdm09 influenza among students in school outbreaks and serve as a tool for decision making when there is uncertainty. We conclude that, different grades have different transmission modes, and freshmen have higher activity and are more likely to cause infection. Freshmen were the main facilitators of the spread of influenza during this school outbreak, so control measures (e.g. close contact isolation) priority used for freshmen would likely have effectively reduced spread of influenza in school settings. It will provide the useful suggestions on making effective and economic control measures in future school outbreak of influenza or other respiratory infectious diseases.
Methods
Ethics Statement
The institutional review board of the Academy of Military Medical Sciences waived the need for written informed consent from the participants. This study is approved by the Academy of Military Medical Sciences Review Board. The study is entirely based on epidemiological survey data which is required by Chinese Ministry of Health to record during the epidemic control. There was no request for an ethical permission according to Chinese law. Even so, we took the following steps to protect our survey respondents and anyone mentioned in the survey. Firstly, we kept all completed questionnaires under strict anonymity and confidentiality, left no identifiable personal information (such as names of respondents or anyone listed in the contact diaries) in our electronic data set, and destroyed the completed questionnaires after data cleaning. Secondly, we used the personal information from our sample list only to locate and identify our targeted respondents and then destroyed the name list after the interviews.
Outbreak Survey
The outbreak began on a college grounds near Beijing in northern China on August 27, 2009 and an epidemiological investigation was conducted by the team from the Institution of Disease Control and Prevention. The college awards a four-year degree; all students reside at the college with a total enrollment of 6721 students (1742 freshman, 1805 sophomores, 1808 juniors, 1366 seniors) ranging in age from 19 to 24. Influenza-like illness (ILI) was defined as the sudden onset of a fever of >38°C with cough or sore throat in the absence of other diagnoses; a confirmed case of influenza was defined as a case of ILI with laboratory-confirmed A(H1N1)pdm09 influenza virus infection using the polymerase chain reaction (PCR) according to WHO guidelines33. We detected the antibody levels against seasonal influenza A (H1N1), H3N2 and influenza B, and found different grades had no difference. Each confirmed case was interviewed face-to-face to obtain demographic information, clinical symptoms, onset date of influenza-like symptoms and contacts. A close contact was defined as a person having cared for, lived with, or who had direct contact with respiratory secretions or body fluids of a probable or confirmed case, or conversation with probable cases more than 20 minutes continuously in one encounter within 3 days after symptom onset33. Public health control measures implemented by the response team, led by the Institute of Disease Control and Prevention of the Academy of Military Medical Sciences, followed the guidelines from the Ministry of Health of China. From September 1, interventions, including individual isolation of confirmed cases and quarantining of contacts (home stay or dormitory) were taken. All students and staff were not permitted to leave the school grounds. Interventions were sustained during the whole period of the epidemic. Before the intervention measures were taken, all four grades had confirmed cases.
Transmission Chains
We constructed disease transmission pathways among the confirmed A(H1N1)pdm09 influenza cases. If student A and student B are close contacts and symptom onset appeared in student A prior to student B, we assume that student A infected student B. We used the expression “A → B” to show the transmission relationship between A and B. If there were several possible routes of transmission, we assumed the “earlier infector” is the “real infector”. Disease transmission pathways were constructed using the program Pajek12. Pajek is a Windows program for analysis and visualization of large networks having some thousands or millions of vertices.
Transmission Dynamic Model
The susceptible-exposed-infected-removed (SEIR) model was used to simulate transmission dynamic processes. The SEIR model has been widely used in epidemic simulations13. The SEIR model was used to simulate infectious diseases transmission dynamics, estimate epidemiological parameters and effectively evaluate interventions37,38. The SEIR model separates the population of a region into four compartments, susceptible, exposed, infected, and removed, and simulates the time evolution of each of these subpopulations. We assumed the school to be a closed setting and the students from the same grade (e.g. freshmen, sophomores, juniors, seniors) to be homogenously mixed in school activities due to students from same grade with almost same age, and also they have same schedule, and ignored the heterogeneity of innate immunity to A(H1N1)pdm09 influenza between the students. Students from each grade were divided into four groups (Susceptible, Exposed, Infected and Removed (or Recovered)). Under these circumstances, a transmission dynamic model was constructed (Figure 6).
Figure 6. The transmission dynamics model of A(H1N1)pdm09 influenza among students attending a college in northern China.
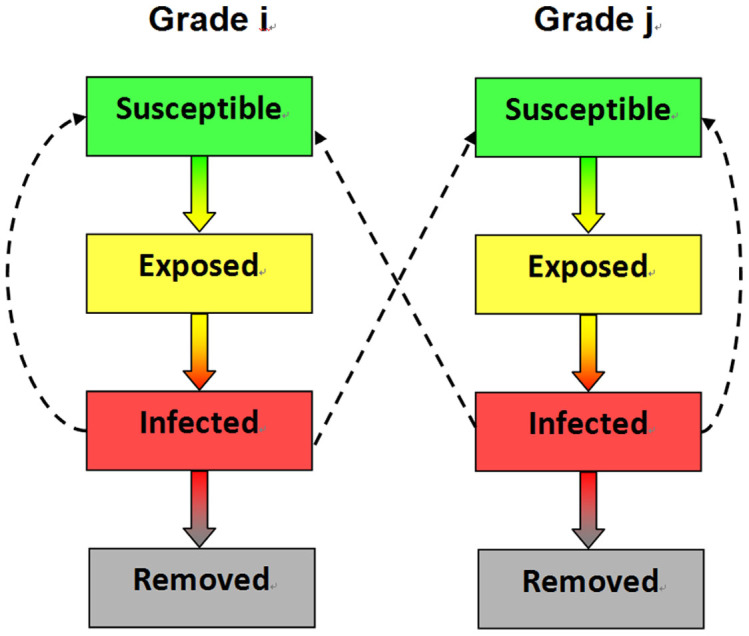
† i = 1,2,3,4, j = 1,2,3,4, i ≠ j. ‡Each grade was divided into four groups (Susceptible, Exposed, Infected and Removed).
There were Ni individuals in grade i, i = 1, 2, 3, 4. Therefore the number of susceptible students from grade i in the t day of outbreak; is denoted as Si(t); Ei(t), is the number of exposed students from grade i in the day t of outbreak; Ii(t), is the number of infected students from grade i in the t day of outbreak; and Ri(t) is the number of recovered students from grade i in the t day of outbreak. We also denoted αi (i = 1, 2, 3, 4), as the average number of secondary cases per primary case in a susceptible population from the same grade in the infectious period of primary cases per unit time; α5, as the average number of secondary cases per primary case in a susceptible population from the different grade in the infectious period of primary cases per unit time. A parameter (C) was set to control the change of αi (i = 1,2,3,4,5) under the control measures taken. The parameter C is equal to 1 when no interventions are taken, it is smaller than 1 when interventions are taken, and the more stringent the control measures, the smaller the C value. From the literature-derived parameter values of the incubation and infectious periods1, the incubation period was assumed to be 1/β = 1.1 days and the infectious period was 1/η = 2.5 days. The αi/η is the R0 value of grade i (i = 1,2,3,4). The newly infected cases per day were used to fit our model. The ANNEAL algorithm was used to optimize parameters of function, which is a stochastic method currently in wide use for difficult optimization problems14,17. The model was constructed by using ordinary differential equations15:
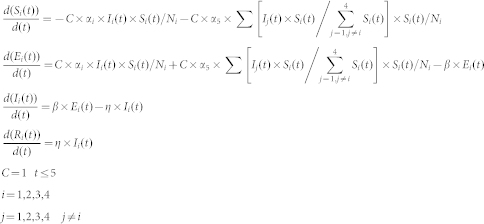 |
Using the constructed model, the initial dynamics of disease transmission among the different grades were reproduced, and used to estimate R0 values for freshman, sophomores, juniors or seniors We simulated 100 times using the ANNEAL algorithm that minimizes a function using the method of simulated annealing17. Simulated annealing (SA) is a generic probabilistic metaheuristic for the global optimization problem of locating a good approximation to the global optimum of a given function in a large search space. We simulate effectiveness of intervention through control numbers of four compartments (Susceptible, Exposed, Infected, and Removed).
Estimation of Intervention Effectiveness
By using the constructed model, estimates were derived for the total numbers of cases involved on the 20th day of the outbreak. Assuming that the first case of this outbreak was a freshman, estimates were made of the number of infected students involved in the outbreak on the 20th day, when 25%, 50%, or 75% of freshmen, sophomores, juniors, or seniors were quarantined and isolated on the first, second, third, fourth and fifth day of the outbreak. Similar estimates were made assuming the first case was a sophomore, junior, or senior, respectively. For these estimates, we applied the same stringent intervention measures to contain the epidemic of A(H1N1)pdm09 influenza, including isolation of confirmed cases and quarantining of contacts, which were recommended by the Chinese Ministry of Health on May 29, 20093. The susceptible students involved in this outbreak were assumed to be close contacts. Interventions were sustained through the whole epidemic in order to avoid creating a population of individuals who were protected from infection but would become infected after the intervention was stopped. The effectiveness of different quarantine methods under the different conditions were compared according to the estimated number of cases involved.
Author Contributions
H.B.S. and L.G.W. designed the study. Y.W., C.Y.C., S.F.Q., P.L., Z.H.W., Z.Q.Y., Z.D.C., G.Y. and Y.Y.X. collected the epidemiological data. H.B.S., L.G.W., R.Z.H., D.J.Z. and Z.J.L. analyzed and interpreted the data. H.B.S., L.G.W. and C.Y.C. wrote the manuscript. All authors reviewed the manuscript.
Supplementary Material
supplementary information
Acknowledgments
We are grateful to the staff of the school medical services for data collection and record and the staff of local CDC for virus detection and our colleagues involved in this study for their field work. We are grateful to Dr. John D. Klena (United States Centers for Disease Control and Prevention, International Emerging Infections Program, Beijing, China) for critical review of the manuscript. This study was supported by China Special Grant for the Prevention and Control of Infectious Diseases (2013ZX10004218, 2012ZX10004801-004 and 2012ZX10004215) and Natural Science Foundation of China (81001266).
References
- Balcan D. et al. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility. BMC Med 7, 45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S. et al. Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci U S A 108, 2825–30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Center for Disease Control and Prevention: Technical guidance of epidemical survey and intervention of influenza A pandemic (H1N1). 2009; Available from http://www.chinacdc.cn/n272442/n272530/n273736/n273781/n4624704/n4624714/31611.html. Date of access: 11/4/2013.
- Chu C. Y. et al. Quarantine methods and prevention of secondary outbreak of pandemic (H1N1) 2009. Emerg Infect Dis 16, 1300–02 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames K. T., Tilston N. L., Brooks-Pollock E. & Edmunds W. J. Measured dynamic social contact patterns explain the spread of H1N1v influenza. PLoS Comput Biol 8, e1002425 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earn D. J., He D., Loeb M. B., Fonseca K., Lee B. E. & Dushoff J. Effects of school closure on incidence of pandemic influenza in Alberta, Canada. Ann Intern Med 156, 173–81 (2012). [DOI] [PubMed] [Google Scholar]
- Eubank S. et al. Modelling disease outbreaks in realistic urban social networks. Nature 429, 180–84 (2004). [DOI] [PubMed] [Google Scholar]
- Fang L. Q. et al. Distribution and risk factors of 2009 pandemic influenza A (H1N1) in mainland China. Am J Epidemiol 175, 890–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N. M., Cummings D. A., Fraser C., Cajka J. C., Cooley P. C. & Burke D. S. Strategies for mitigating an influenza pandemic. Nature 442, 448–52 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh A. et al. Transmission of 2009 pandemic influenza A (H1N1) at a Public University--Delaware, April-May 2009. Clin Infect Dis 52, S131–7 (2011). [DOI] [PubMed] [Google Scholar]
- Iuliano A. D. et al. Notes from the field: outbreak of 2009 pandemic influenza A (H1N1) virus at a large public university in Delaware, April-May 2009. Clin Infect Dis 49, 1811–20 (2009). [DOI] [PubMed] [Google Scholar]
- Batagelj V. & Mrvar A. Pajek - analysis and visualization of large networks. in Graph Drawing Software (ed. Jünger, M. & Mutzel, P.) 77–103 (Springer, Berlin, 2003). [Google Scholar]
- Saito M. M. et al. Extension and verification of the SEIR model on the 2009 influenza A (H1N1) pandemic in Japan. Math Biosci 246, 47–54 (2013). [DOI] [PubMed] [Google Scholar]
- Sartakhti J. S., Zangooei M. H. & Mozafari K. Hepatitis disease diagnosis using a novel hybrid method based on support vector machine and simulated annealing (SVM-SA). Comput Methods Programs Biomed 108, 570–9 (2012). [DOI] [PubMed] [Google Scholar]
- Li M. Y., Graef J. R., Wang L. & Karsai J. Global dynamics of a SEIR model with varying total population size. Math Biosci 160, 191–213 (1999). [DOI] [PubMed] [Google Scholar]
- Kang S. H., Choi H. M. & Lee E. H. Positive rates of 2009 novel influenza A (H1N1) was high in school-aged individuals: significance in pandemic control. J Korean Med Sci 27, 332–4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick S., Gelatt C. D. Jr & Vecchi M. P. Optimization by simulated annealing. Science 220, 671–80 (1983). [DOI] [PubMed] [Google Scholar]
- Lessler J. et al. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med 361, 2628–36 (2009). [DOI] [PubMed] [Google Scholar]
- Meyers L. A., Pourbohloul B., Newman M. E., Skowronski D. M. & Brunham R. C. Network theory and SARS: Predicting outbreak diversity. J Theor Biol 232, 71–81 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. et al. Non-pharmaceutical interventions during an outbreak of 2009 pandemic influenza A (H1N1) virus infection at a large public university, April-May 2009. Clin Infect Dis 52, S138–45 (2011). [DOI] [PubMed] [Google Scholar]
- Newman M. E. J. Spread of epidemic disease on networks. Phys Rev E Stat Nonlin Soft Matter Phys 66, 016128 (2002). [DOI] [PubMed] [Google Scholar]
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360, 2605–15 (2009). [DOI] [PubMed] [Google Scholar]
- Opatowski L. et al. Transmission characteristics of the 2009 H1N1 influenza pandemic: comparison of 8 Southern hemisphere countries. PLoS Pathog 7, e1002225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine S. et al. Transmissibility of 2009 pandemic influenza A(H1N1) in New Zealand: effective reproduction number and influence of age, ethnicity and importations. Euro Surveill 15, 19591 (2010). [PubMed] [Google Scholar]
- Shu Y. L., Fang L. Q., de Vlas S. J., Gao Y., Richardus J. H. & Cao W. C. Dual seasonal patterns for influenza, China. Emerg Infect Dis 16, 725–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón Méndez L. et al. Transmissibility and severity of the pandemic influenza A (H1N1) 2009 virus in Spain. Gac Sanit 25, 296–302 (2011). [DOI] [PubMed] [Google Scholar]
- Tang S., Xiao Y., Yuan L., Cheke R. A. & Wu J. Campus quarantine (Fengxiao) for curbing emergent infectious diseases: lessons from mitigating A/H1N1 in Xi'an, China. J Theor Biol 295, 47–58 (2012). [DOI] [PubMed] [Google Scholar]
- Tang S., Xiao Y., Yang Y., Zhou Y., Wu J. & Ma Z. Community-based measures for mitigating the 2009 H1N1 pandemic in China. PLoS One 5, e10911 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M., Tsukahara T., Kaneko M., Washizuka S. & Kawa S. How the H1N1 influenza epidemic spread among university students in Japan: experience from Shinshu University. Am J Infect Control 40, 218–20 (2012). [DOI] [PubMed] [Google Scholar]
- Uchida M., Tsukahara T., Kaneko M., Washizuka S. & Kawa S. Swine-origin influenza a outbreak 2009 at Shinshu University, Japan. BMC Public Health 11, 79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. Epidemiological and clinical characteristics of the outbreak of 2009 pandemic influenza A (H1N1) at a middle school in Luoyang, China. Public Health 126, 289–94 (2012). [DOI] [PubMed] [Google Scholar]
- Western Pacific Region Global Influenza Surveillance and Response System. Epidemiological and virological characteristics of influenza in the Western Pacific Region of the World Health Organization, 2006-2010. PLoS One 7, e37568 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Human infection with pandemic (H1N1) 2009 virus: updated interim WHO guidance on global surveillance, 2009. http://www.who.int/csr/resources/publications/swineflu/interim_guidance/en/index.html. Date of access: 11/4/2013.
- World Health Organization. Pandemic (H1N1) 2009—update 99, 2010. http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html. Date of access: 11/4/2013.
- Xiao H. et al. Study on sensitivity of climatic factors on influenza A (H1N1) based on classification and regression tree and wavelet analysis. Chin J Epidemiol 46, 430–5 (2012). [PubMed] [Google Scholar]
- Yu H. et al. Transmission dynamics, border entry screening, and school holidays during the 2009 influenza A (H1N1) pandemic, China. Emerg Infect Dis 18, 758–66 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock J. & Galvani A. P. Optimizing influenza vaccine distribution. Science 325, 1705 (2009). [DOI] [PubMed] [Google Scholar]
- Lee B. Y. et al. Simulating school closure strategies to mitigate an influenza epidemic. J Public Health Manag Pract 16, 252–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breban R., Vardavas R. & Blower S. Theory versus data: how to calculate R0? PLoS One 2, e282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M. J. & Grenfell B. T. Individual-based perspectives on R0. J. Theor. Biol 203, 51–61 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information