Abstract
Cancer metastasis, the process of cancer cell migration from a primary to distal location, typically leads to a poor patient prognosis. Hematogenous metastasis is initiated by intravasation of circulating tumor cells (CTCs) into the bloodstream, which are then believed to adhere to the luminal surface of the endothelium and extravasate into distal locations. Apoptotic agents such as tumor necrosis factor (TNF) apoptosis-inducing ligand (TRAIL), whether in soluble ligand form or expressed on the surface of natural killer (NK) cells, have shown promise in treating CTCs to reduce the probability of metastasis. The role of hemodynamic shear forces in altering the cancer cell response to receptor-mediated apoptosis has not been previously investigated. Here, we report that human colon cancer COLO 205 and prostate cancer PC-3 cells exposed to a uniform fluid shear stress in a cone-and-plate viscometer become sensitized to TRAIL-induced apoptosis. Shear-induced sensitization directly correlated with the application of fluid shear stress, and TRAIL-induced apoptosis increased in a fluid shear stress force- and time-dependent manner. In contrast, TRAIL-induced necrosis was not affected by the application fluid shear stress. Interestingly, fluid shear stress did not sensitize cancer cells to apoptosis when treated with doxorubicin, which also induces apoptosis in cancer cells. Caspase inhibition experiments revealed that shear stress-induced sensitization to TRAIL occurs via caspase-dependent apoptosis. These results suggest that physiological fluid shear force can modulate receptor-mediated apoptosis of cancer cells in the presence of apoptotic agents.
Keywords and phrases: cancer, death receptors, shear stress, mechanotransduction, TRAIL, caspase
1. INTRODUCTION
Approximately 90% of human cancer-related deaths are due to cancer metastasis (1), which consists of a series of discrete steps that allow cancer cell migration from a primary site to a distal location. For hematogenous metastasis to occur, cancer cells must detach from the primary tumor, invade surrounding tissue, and intravasate into the circulation as circulating tumor cells (CTCs) (2). Once in the circulation, CTCs are believed to adhere to the luminal surface of the microvasculature in a manner similar to the leukocyte adhesion cascade (3). The process consists of adhesive interactions with the receptor-bearing endothelial cell wall, including selectin-mediated cell tethering and rolling along the endothelium, followed by firm adhesion or arrest (3,4). Once firmly adhered, CTCs may extravasate into a distal site and proliferate to form secondary tumors (5,6). Radiation therapy, chemotherapy and surgery are generally successful in the treatment of primary tumors, however the treatment of metastases is challenging due to its systemic nature, and typically signals a poor prognosis (7). The targeting and treatment of CTCs within the circulation are a potential solution to reduce the probability of metastasis. Several approaches have been developed to isolate patient CTCs from whole blood (8,9), along with novel strategies to target and treat CTCs with therapeutics under physiological flow (10,11).
One therapeutic that has displayed potential for the treatment of CTCs is tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a type 2 transmembrane protein of the tumor necrosis factor family (12). TRAIL binds to death receptors (DR4 and DR5) expressed on the surface of a range of cancer cells, which can induce cell apoptosis (13). TRAIL also binds to three decoy receptors (DcR1, DcR2, DcR3) expressed on the surface of cells, which do not signal apoptosis and can act as competitive inhibitors to apoptosis (14). Additionally, TRAIL does not exert apoptotic effects on most normal cells (15). Natural killer (NK) cells, which are believed to play a physiological role in natural protection against tumor formation (16), can express TRAIL on the NK cell membrane. NK cell subpopulations in adult mouse liver constitutively expressed TRAIL in an interferon γ- dependent manner, and played a role in the suppression of tumor initiation and metastasis (17,18). Interferon γ-dependent TRAIL expression on NK cells also plays a role in interferon γ-dependent tumor prevention effects of interleukin-12 (IL-12) and α-galactosylceramide (α-GalCer) (19). In addition to its therapeutic effects as a soluble ligand, TRAIL can play a role in NK cell surveillance of CTCs and tumors (18).
The microenvironments of tumors and CTCs are remarkably different, with fluid shear forces being one factor that is dramatically altered once cancer cells enter the vascular microenvironment. In the tumor microenvironment, cancer cells are exposed to shear stresses created by interstitial flows, which range from 0.1 to 1.0 µm/s in normal tissues and higher values in the tumor microenvironment (20,21). Such flows cause an upregulation of transforming growth factor beta (TGFβ) in fibroblasts, which can lead to myofibroblast differentiation, along with TGFβ-dependent alignment and stiffening of the extracellular matrix (22). Interstitial flows can also upregulate matrix metalloproteinase expression to enhance glioma cell invasion (23). Surface shear stress estimates for cancer cells exposed to interstitial flow are difficult to measure, but are estimated to be relatively low in comparison to those experienced in the vasculature. For interstitial flow rates of 1 µm/s, one study estimated a fluid shear stress range of 0.007–0.015 dyn/cm2 (24). This is in contrast to shear stresses in the circulation, which range from approximately 0.5 to 4.0 dyn/cm2 in the venous circulation and 4.0 to 30.0 dyn/cm2 in the arterial circulation, with the maximum shear stress experienced at the vessel wall (25). Increases in fluid shear forces could affect cancer cell survival, as only a small portion of CTCs survive the circulation to generate metastases (26). Conversely, fluid shear forces can aid CTCs in binding to the vascular endothelium via selectin-mediated tethering and rolling, followed by firm adhesion to the endothelium (27). While previous studies have investigated the role of fluid shear forces in CTC adhesion to the microvasculature, little is known about the effects of fluid shear forces on the viability and proliferation of CTCs (28).
The role of hemodynamic shear forces in altering receptor-mediated apoptosis of cancer cells has not yet been investigated. In this study, we investigated the role of physiological shear forces in sensitizing cancer cells to TRAIL-mediated apoptosis.
2. MATERIALS AND METHODS
2.1. Cell culture
Colorectal adenocarcinoma cell line COLO 205 (ATCC #CCL-222) and prostate adenocarcinoma cell line PC-3 (ATCC #CRL-1435) were purchased from American Type Culture Collection (Manassas, VA, USA). COLO 205 and PC-3 cells were cultured in RPMI 1640 and F-12K cell culture medium from Invitrogen (Grand Island, NY, USA). Complete media was supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) PenStrep, all purchased from Invitrogen. COLO 205 and PC-3 cells were incubated under humidified conditions at 37°C and 5% CO2, and were not allowed to exceed 90% confluence.
2.2. Preparation of cells for fluid shear stress studies
COLO 205 and PC-3 cells were washed in Ca2+ and Mg2+ free HBSS (Invitrogen, Carlsbad, CA, USA) and then treated with Accutase (Sigma Aldrich, St. Louis, MO, USA) for 5–10 min at 37°C before handling. COLO 205 and PC-3 cells were washed with Ca2+ and Mg2+ free DPBS (Invitrogen) at 300 × g for 5 min at 23°C. Cells were resuspended in media at a concentration of 0.5 × 106 cells/mL. Prior to performing fluid shear stress studies, 99% cell viability was confirmed by using trypan blue exclusion staining (Gibco, Grand Island, NY, USA).
For TRAIL studies, cells were treated with 0.1 µg/mL recombinant human TRAIL (R&D Systems, Minneapolis, MN, USA) prior to the application of fluid shear stress. For caspase inhibition studies, cells were treated with 50 µM of pan-caspase inhibitor Z-VAD-FMK or negative control compound Z-FA-FMK (R&D Systems) for 4 hours at 37°C prior to TRAIL treatment. For doxorubicin (DOX) studies, COLO 205 cells were treated with doxorubicin hydrochloride (Sigma Aldrich) at a concentration of 20 µM, which has previously been shown to induce COLO 205 cell death prior to the onset of fluid shear stress (10).
2.3. Cone-and-plate viscometer assay
To study the fluid shear stress response of cancer cells in a controlled, uniform environment, studies were conducted using a cone-and-plate device consisting of a stationary plate underneath a rotating cone maintained at 37°C by a circulating water bath (Brookfield, Middleboro, MA) as described previously (29). The design of the cone-and plate-viscometer allows a uniform shear rate to be applied to the cancer cell suspension. The shear rate (G), does not depend on distance from the center of the cone, and is given by:
where ω is the cone angular velocity (rad/s) and θ is the cone angle (rad). Under all experimental conditions, a laminar flow field is expected. For a Newtonian fluid under these conditions, the shear stress, τ, is proportional to the shear rate being applied:
where µ is the viscosity of the medium. Prior to fluid shear stress experiments, the stationary plate and rotating cone were washed thoroughly with 70% ethanol. TRAIL or doxorubic-intreated cancer cell suspensions were introduced to the plate at a concentration of 0.5 × 106 cells/mL, and were allowed to equilibrate for 1 min prior to the onset of fluid shear stress. To identify a shear stress threshold required for cancer cell sensitization to TRAIL, cells were exposed to shear stresses ranging from 0.05–2.0 dyn/cm2 for a duration of 120 min. To determine the characteristic shear stress exposure time required for TRAIL sensitization, cells were exposed to a shear stress of 2.0 dyn/cm2 for increasing time intervals of 1–120 min. COLO 205 sensitization responses were determined by comparing samples exposed to shear and static conditions using the following equation:
The sensitization equation applies to COLO 205 cells labeled for apoptosis and necrosis, for both TRAIL-treated and untreated samples, at all shear stress magnitudes and durations.
After shear stress exposure, cells were washed thoroughly in PBS and analyzed for cell death using an Annexin-V assay. For doxorubicin studies, cells were washed and incubated overnight prior to apoptosis analysis, as a longer duration was required for cells to initiate doxorubicin-induced apoptosis.
2.4. Annexin-V Apoptosis Assay
A FITC-conjugated Annexin-V assay (Trevigen, Gaithersburg, MD, USA) was used to assess cell apoptosis and necrosis. Due to the intrinsic fluorescence of doxorubicin, all doxorubicin treated cells were analyzed using an APC-conjugated Annexin-V assay (BD Pharmingen, San Diego, CA, USA). The manufacturer’s instructions were followed to prepare samples for flow cytometric analysis. Viable cells were identified as being negative for both Annexin-V and propidium iodide (PI), early apoptotic cells as positive for Annexin-V only, late apoptotic cells were positive for both Annexin-V and PI, and necrotic cells were positive for PI only.
2.5. Flow Cytometry
Cells were incubated with Annexin-V reagents for 15 min at room temperature in the absence of light, and immediately analyzed using an Accuri C6 flow cytometer (Accuri Cytometers Incorporated, Ann Arbor, MI, USA). Flow cytometry plots were generated using Accuri CFlow Plus and FCS Express V3 software (De Novo Software, Thornhill, Canada). The following control samples were used to calibrate the instrument: unlabeled cell samples to evaluate the level of autofluorescence and adjust the instrument accordingly, and cell samples labeled individually with Annexin-V and PI to define the boundaries of each cell population.
2.6. Brightfield and Phase Contrast Microscopy
Cell samples were placed into 6 well plates and incubated at 37°C for 60 min to allow cells to adhere to the plate surface. Cells were then imaged by brightfield and phase contrast microscopy using an Olympus IX81 inverted microscope (Olympus America Inc., Center Valley, PA) to observe the presence of viable cells and membrane blebbing characteristic of cells undergoing apoptosis. All images were processed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
2.7. Death Receptor Quantification
The average number of death receptors on the surface of cancer cells was determined using flow cytometry calibration with Quantum simply cellular (QSC) anti-mouse IgG beads (Bangs Laboratories, Inc., Fisher, IN, USA). QSC beads were incubated for 45 min at 4°C with a PE-conjugated antibody specific to the antigen on the beads. A mixture of antibody-conjugated beads with a range of antigen binding capacities (ABCs) was run through a flow cytometer. Bead populations corresponding to increasing numbers of ABCs yield increasingly fluorescent peaks in the PE fluorescence channel. Median values of each fluorescence peak were obtained using Accuri CFlow Plus software. Fluorescence data and ABC values reported by the manufacturer were used to generate a calibration curve using QuickCal v2.3 (Bangs Labs, Fisher, IN).
Following the calibration step, surface expression of death receptors DR4 and DR5 on cancer cells was determined using flow cytometry. COLO 205 cells were exposed to static conditions or fluid shear stress (2.0 dyn/cm2) in a cone-and-plate viscometer for 120 min, followed by immediate incubation with either a PE-conjugated isotype or PE-conjugated DR4 and DR5 antibodies (Biolegend, San Diego, CA, USA) for 45 min at 4°C. Cells were then washed and analyzed for death receptor expression using flow cytometry. Median values of each fluorescence peak were recorded from each sample, and the fluorescence data was converted into the number of receptors using the calibration curve in QuickCal.
2.8. Statistical Analysis
Data sets were plotted and analyzed using Prism 5.0b for Mac OS X (GraphPad software, San Diego, CA, USA). A two-tailed paired t-test was used for comparisons between two groups with p < 0.05 considered significant.
3. RESULTS
3.1. Fluid shear stress increases TRAIL-induced cancer cell apoptosis
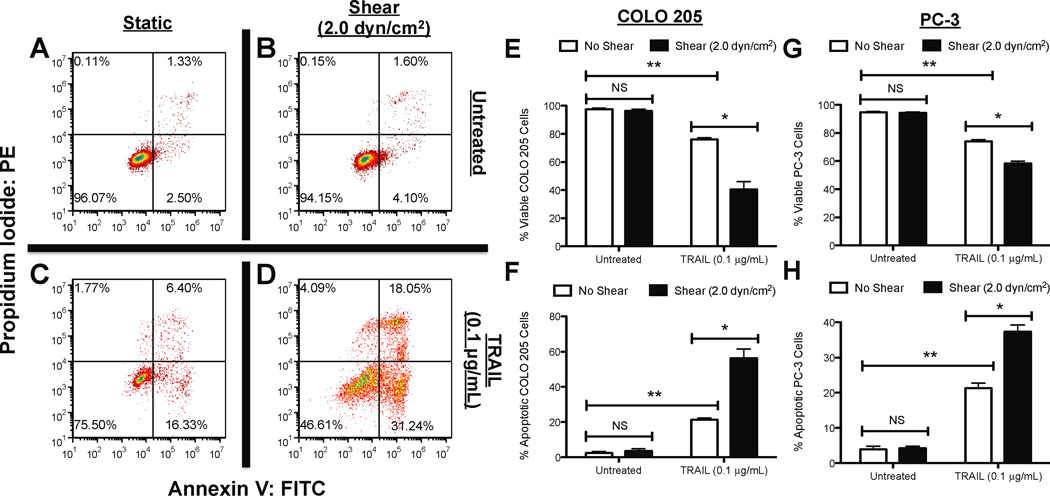
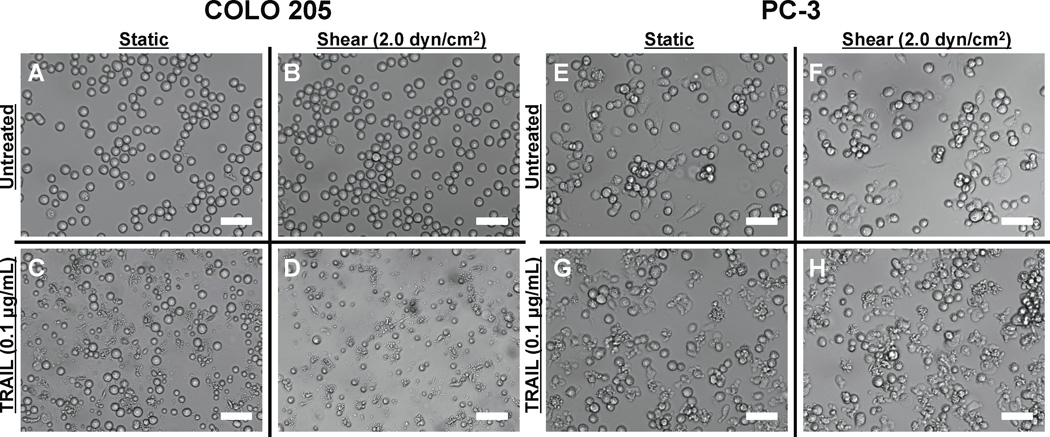
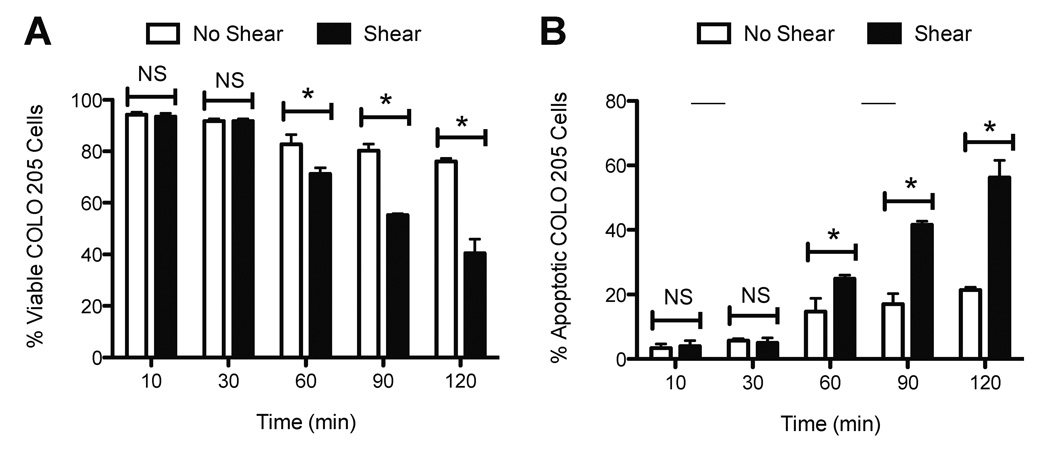
We first characterized the effect of fluid shear stress on TRAIL-treated cancer cells in terms of cell viability. COLO 205 cells were treated with equal amounts of TRAIL (0.1 µg/mL) and then exposed to either static conditions or 2.0 dyn/cm2 of fluid shear stress in a cone-and-plate viscometer for 120 min at 37°C. TRAIL binds to death receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2) on the cell surface, which signal apoptosis (14). Both death receptors are expressed on the surface of COLO 205 cells (30). COLO 205 cells exposed to static conditions (Fig. 1A) or 2.0 dyn/cm2 of fluid shear stress (Fig. 1B) for 120 min maintained high cell viability (>94%) with minimal apoptosis observed in the absence of TRAIL (<6%). As expected, COLO 205 cells treated with TRAIL exposed to static conditions for 120 min caused a ~25% decrease in cell viability, with >22% of the cell population becoming apoptotic (Fig. 1C). However, cells exposed to the same dosage of TRAIL, followed by exposure to fluid shear stress (Fig. 1D) induced a greater decrease in cell viability (>53%) and more than doubled the amount of apoptotic cells (>47%), compared to TRAIL-treated static samples. Experiments performed in triplicate revealed that fluid shear stress alone did not affect cell viability (Fig. 1E) or apoptosis (Fig. 1F), yet induced a significant decrease in cell viability and increase in apoptosis in the presence of TRAIL. Similar effects on cell viability (Fig. 1G) and apoptosis (Fig. 1H) were also found in experiments performed with prostate adenocarcinoma cell line PC-3. Brightfield microscopy images revealed that COLO 205 cells remained viable and retained their characteristic morphology when exposed to static conditions (Fig. 2A) or 2.0 dyn/cm2 of fluid shear stress (Fig. 2B). A greater number of viable cells was observed in TRAIL-treated COLO 205 samples exposed to static conditions (Fig. 2C) compared to samples exposed to 2.0 dyn/cm2 of fluid shear stress (Fig. 2D), with fewer viable cells and a greater degree of membrane blebbing characteristic of cell apoptosis. PC-3 cells also remained healthy under static (Fig. 2E) and shear (Fig. 2F) conditions, while a greater number of apoptotic cells was observed in TRAIL-treated samples exposed to shear (Fig. 2H) compared to TRAIL-treated samples exposed to static conditions (Fig. 2G).
Figure 1.
Fluid shear stress sensitizes cancer cells to TRAIL. COLO 205 cancer cells exposed to static conditions (A) and 2.0 dyn/cm2 of fluid shear stress (B) for 120 min at 37°C, respectively. COLO 205 cells treated with 0.1 µg/mL TRAIL and then exposed to static conditions (C) and 2.0 dyn/cm2 of fluid shear stress (D) for 120 min at 37°C. Percent viable (E) and apoptotic (F) COLO 205 cells after treatment with 0.1 µg/mL TRAIL followed by exposure to static conditions and 2.0 dyn/cm2 of fluid shear stress (n = 3). Percent viable (G) and apoptotic (H) PC-3 cells after treatment with 0.1 µg/mL TRAIL followed by exposure to static conditions and 2.0 dyn/cm2 of fluid shear stress (n = 3). Lower lefthand and righthand quadrants of each flow cytometry plot represent viable and early apoptotic cells, respectively. Upper lefthand and righthand quadrants of each flow cytometry plot represent necrotic and late apoptotic cells, respectively. PI: propidium iodide. FITC: Fluorescein isothiocyanate. Error bars represent 95% confidence intervals. *P < 0.05. **P < 0.01. NS: non-significant.
Figure 2.
Brightfield microscopy images of untreated COLO 205 cells exposed to static conditions (A) and 2.0 dyn/cm2 of fluid shear stress (B) for 120 min at 37°C. COLO 205 cells treated with 0.1 µg/mL TRAIL and then exposed to static conditions (C) and 2.0 dyn/cm2 of fluid shear stress (D) for 120 min at 37°C. Untreated PC-3 cells exposed to static conditions (E) and 2.0 dyn/cm2 of fluid shear stress (F) for 120 min at 37°C. PC-3 cells treated with 0.1 µg/mL TRAIL and then exposed to static conditions (G) and 2.0 dyn/cm2 of fluid shear stress (H) for 120 min at 37°C. Scale bars = 30 µm.
3.2. Fluid shear stress does not alter TRAIL-induced cancer cell necrosis
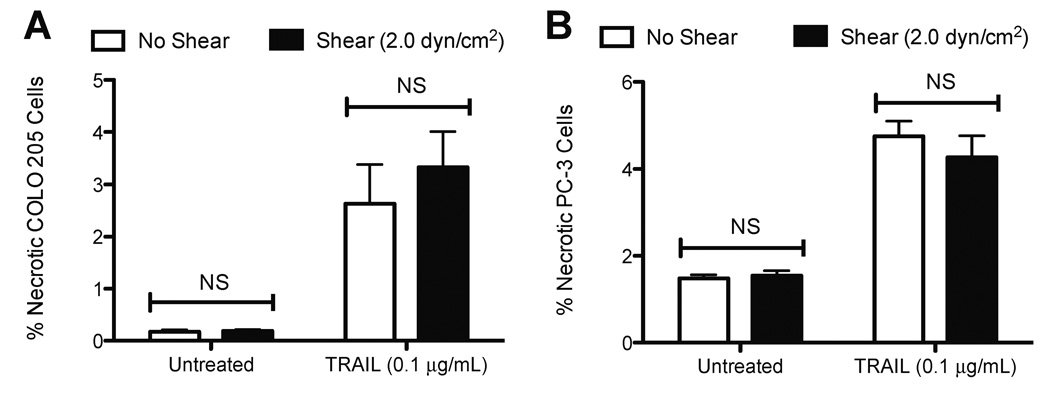
To assess whether fluid shear stress sensitizes cancer cells to TRAIL-induced necrosis, another form of cell death, cells treated with 0.1 µg/mL TRAIL followed by shear stress exposure were stained with propidium iodide (PI) dye and characterized using flow cytometry. Cells positive for PI labeling but negative for Annexin-V were determined to be necrotic, as the cytoplasmic membrane is compromised but lacks the membrane flipping of phosphatidylserine (PS), characteristic of apoptosis. Untreated COLO 205 (Fig. 3A) and PC-3 (Fig. 3B) cells exposed to static conditions or fluid shear stress did not show significant differences in the degree of necrotic cell death. Treatment with 0.1 µg/mL TRAIL induced an increase in COLO 205 and PC-3 necrotic cell death, compared to untreated samples. However, fluid shear stress did not induce significant differences in TRAIL-mediated COLO 205 and PC-3 necrotic cell death, compared to samples exposed to static conditions. These results indicate that the shear stress sensitization response is TRAIL-mediated apoptosis-specific.
Figure 3.
Percent necrotic COLO 205 cells (A) after treatment with 0.1 µg/mL TRAIL followed by exposure to static conditions and 2.0 dyn/cm2 of fluid shear stress (n = 3) 120 min at 37°C. Percent necrotic PC-3 cells (B) after treatment with 0.1 µg/mL TRAIL followed by exposure to static conditions and 2.0 dyn/cm2 of fluid shear stress (n = 3) 120 min at 37°C. Error bars represent 95% confidence intervals. NS: non-significant.
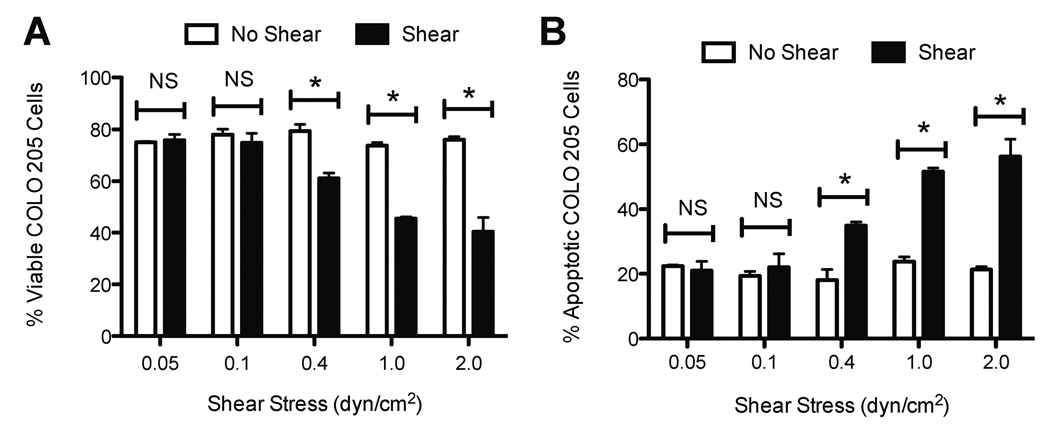
3.3. TRAIL-induced apoptosis in cancer cells is fluid shear stress dose-dependent
The effect of increasing shear force on TRAIL-mediated apoptosis was evaluated by exposing TRAIL-treated COLO 205 cells to a range of shear stress from 0.05 to 2.0 dyn/cm2, for an exposure period of 120 min. The shear stress range is representative of shear stress values experienced in the microcirculation (31), and was used to identify a shear stress threshold that induces sensitization to apoptosis. At shear stresses of 0.05 and 0.1 dyn/cm2, no significant differences in cell viability or apoptosis were found in TRAIL-treated COLO 205 cells, compared to samples exposed to static conditions. Interestingly, a shear stress of 0.4 dyn/cm2 was found to induce a significant decrease in cell viability (Fig. 4A) and increase in apoptosis (Fig. 4B), compared to TRAIL-treated cells exposed to static conditions. A shear stress range of 1.0–2.0 dyn/cm2 induced a more pronounced decrease in cell viability and increase in cell apoptosis, indicating that the sensitization to apoptosis is fluid shear stress dose-dependent.
Figure 4.
Increasing fluid shear stress sensitizes cancer cells to TRAIL. Percent viable (A) and apoptotic (B) COLO 205 cells (n = 3). Shear stress magnitude was varied in separate experiments from 0.05 – 2.0 dyn/cm2 for 120 min at 37°C. COLO 205 cells were treated with 0.1 µg/mL TRAIL prior to the onset of fluid shear stress. Error bars represent 95% confidence intervals. *P < 0.05 for all measurements.
3.4. TRAIL-induced apoptosis in cancer cells is fluid shear stress time-dependent
To assess the kinetics of the sensitization response, TRAIL-treated COLO 205 cells were exposed to a shear stress of 2.0 dyn/cm2, while the duration of fluid shear stress exposure was increased in parallel experiments from 10 to 120 min. The exposure duration was increased to determine if a threshold time period is required to induce TRAIL sensitization in cancer cells. No significant differences in cell viability (Fig. 5A) and apoptosis (Fig. 5B) were observed in TRAIL-treated COLO 205 cells exposed to fluid shear stress for 10–30 min, compared to samples exposed to static conditions for the same duration. Exposure to fluid shear stress for 60 min was found to induce significant decreases in COLO 205 cell viability (Fig. 5A) and increases in apoptosis (Fig. 5B), compared to TRAIL-treated cells exposed to static conditions. Shear stress exposure times of 90–120 min caused further decreases in cell viability and increases in COLO 205 apoptosis, providing evidence that the sensitization to TRAIL-induced apoptosis is fluid shear stress time-dependent.
Figure 5.
Shear-induced sensitization to TRAIL increases with increasing fluid shear stress duration. Percent viable (A) and apoptotic (B) COLO 205 cells (n = 3). Time dependence of shear-induced sensitization was determined by increasing the fluid shear stress exposure time from 10 – 120 min at a uniform shear stress of 2.0 dyn/cm2 at 37°C. COLO 205 cells were treated with 0.1 µg/mL TRAIL prior to the onset of fluid shear stress. Error bars represent 95% confidence intervals. *P < 0.05 for all measurements.
3.5. Cancer cells develop a sensitization to TRAIL-induced apoptosis with increasing shear stress magnitude and shear stress exposure time
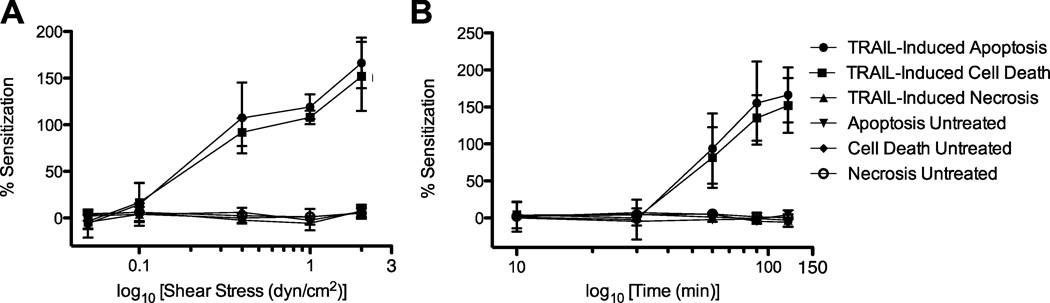
Sensitization to TRAIL was quantified by determining the relative difference in COLO 205 cell death for sheared and nonsheared samples, over a range of shear stress magnitudes and exposure times. By varying the magnitude of fluid shear stress, it is apparent that shear stress values of 0.05–0.10 dyn/cm2 induce minimal sensitization of COLO 205 cells to TRAIL (Fig. 6A) as measured by apoptosis, necrosis, and overall cell death. COLO 205 sensitization to TRAIL is readily apparent at a shear stress value of 0.4 dyn/cm2, as cells are sensitized to overall cell death and apoptosis, but not necrosis. Interestingly, sensitization plots also showed that the average percent sensitization to TRAIL-mediated cell death and apoptosis increases with each increasing shear stress, from 0.4–2.0 dyn/cm2 (Fig. 6A). Untreated control samples do not show sensitization to apoptosis, necrosis, and overall cell death across the range of shear stresses.
Figure 6.
Cancer cells develop sensitization to TRAIL-mediated apoptosis with increasing shear stress magnitude (A) and exposure time (B) (n = 3). Resistance is plotted as a function of the log10 of shear stress (dyn/cm2) or log10 of time (min). Error bars represent 95% confidence intervals.
By varying the exposure time of cells to a fluid shear stress of 2.0 dyn/cm2, a similar trend is observed where short shear stress exposure times of 10 and 30 min do not induce cancer cell sensitization to TRAIL (Fig. 6B). After 60 min of shear stress exposure, COLO 205 cells develop a sensitization to cell death and apoptosis, but not necrosis. From there, sensitization increases linearly with increasing exposure time (Fig. 6B). As expected, untreated control samples do not show sensitization from exposure to fluid shear stress over the time intervals studied.
3.6. Fluid shear stress does not sensitize cancer cells to doxorubicin-induced apoptosis
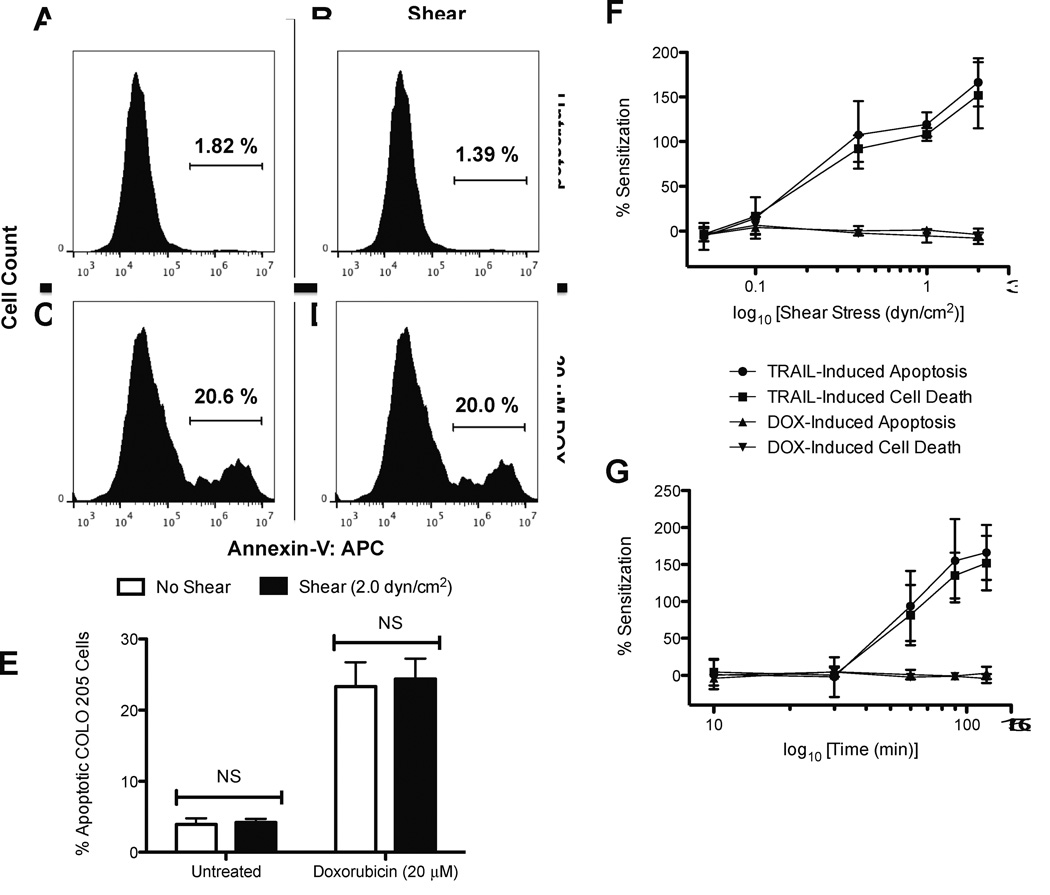
To assess whether shear-induced sensitization to TRAIL is receptor-mediated apoptosis-specific, versus other cancer therapeutics, sensitization experiments were compared to cancer cell treatment with doxorubicin prior to the onset of fluid shear stress. While TRAIL binds to death receptors on the surface of the cancer cell membrane to signal cell death, doxorubicin induces cell death from its function in the inhibition of topo-isomerase II and DNA intercalation (32,33). COLO 205 cells analyzed using an APC-conjugated Annexin-V assay showed that untreated COLO 205 cells exposed to static conditions (Fig. 7A) or fluid shear stress (Fig. 7B) do not show measurable differences in apoptotic cell death. Doxorubicin-treated COLO 205 cells experienced an increase in cell apoptosis (22–23%), however minimal differences were found between doxorubicin treated COLO 205 cells exposed to static conditions (Fig. 7C) and fluid shear stress (Fig. 7D). Experiments performed in triplicate revealed no significant differences in untreated cells exposed to static and shear conditions (Fig. 7E), and doxorubicin-treated cells showed no significant differences in apoptosis. Sensitization plots over varying shear stress magnitudes (Fig. 7F) and exposure times (Fig. 7G) show that while a shear-induced sensitization to TRAIL is apparent, COLO 205 cells are not sensitized to doxorubicin treatment upon exposure to fluid shear stress.
Figure 7.
Fluid shear stress does not sensitize cancer cells to doxorubicin. COLO 205 cells exposed to static conditions (A) and 2.0 dyn/cm2 of fluid shear stress (B) for 120 min at 37°C. COLO 205 cells treated with 20 µM doxorubicin and then exposed to static conditions (C) and 2.0 dyn/cm2 of fluid shear stress (D) for 120 min at 37°C. Percent apoptotic (E) COLO 205 cells after treatment with 20 µM doxorubicin followed by exposure to static conditions and 2.0 dyn/cm2 of fluid shear stress (n = 3). Comparison of cancer cell shear-induced sensitization to TRAIL and doxorubicin with increasing shear stress magnitude (F) and exposure time (G) (n = 3). Resistance is plotted as a function of the log10 of shear stress (dyn/cm2) or log10 of time (min). Error bars represent 95% confidence intervals. Gated region of flow cytometry histograms represent apoptotic COLO 205 cells. Gates determined by labeling viable COLO 205 control samples with Annexin-V APC staining. APC: allophycocyanin. NS: non-significant.
3.7. Cancer cell shear-induced sensitization to cell death occurs via caspase-dependent apoptosis
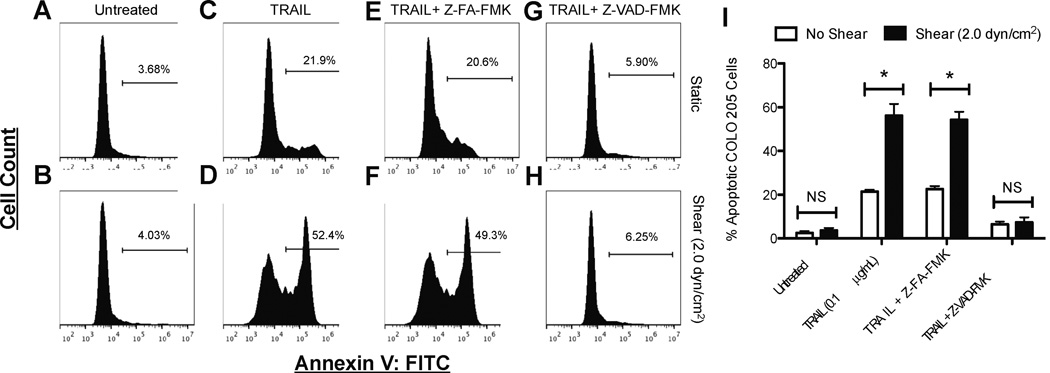
To assess whether shear-induced sensitization to apoptosis is caspase-dependent, COLO 205 cells were incubated with the pan caspase inhibitor Z-VAD-FMK before treatment with TRAIL, followed by exposure to fluid shear stress. The binding of TRAIL to death receptors on the cell surface can activate caspases that initiate the caspase cascade, which triggers cell apoptosis. Z-VAD-FMK is a general caspase inhibitor that irreversibly binds to the catalytic site of caspase proteases, which inhibit apoptosis (34). FITC-conjugated Annexin-V analysis revealed that untreated COLO 205 cells exposed to static conditions (Fig. 8A) or fluid shear stress undergo minimal cell death, while cells treated with TRAIL showed characteristic sensitization to apoptosis when exposed to fluid shear stress (Fig. 8D), compared to exposure to static conditions (Fig. 8C). While the negative control inhibitor Z-FA-FMK did not affect the sensitization response to TRAIL (Fig. 8E,F), treatment with general caspase inhibitor Z-VAD-FMK abolished the sensitization response (Fig. 8G,H), as the differences in apoptosis between TRAIL treated samples exposed to shear and static conditions proved were not significant (Fig. 8I). These results indicate the shear-induced sensitization to TRAIL is in fact caspase-dependent.
Figure 8.
Fluid shear stress sensitization to TRAIL-mediated apoptosis is caspase-dependent. COLO 205 cells (A,B) treated with 0.1 µg/mL TRAIL (C,D), negative control inhibitor Z-FA-FMK followed by 0.1 µg/mL TRAIL (E,F), and pan caspase inhibitor Z-VAD-FMK followed by 0.1 µg/mL TRAIL (G,H), exposed to static conditions and 2.0 dyn/cm2 of fluid shear stress for 120 min at 37°C, respectively. Percent apoptotic COLO 205 cells (n = 3) after exposure to various treatments (I). Gated region of flow cytometry histograms represent apoptotic COLO 205 cells. Gates determined by labeling viable COLO 205 control samples with Annexin-V FITC staining. FITC: fluorescein isothiocyanate. NS: non-significant.
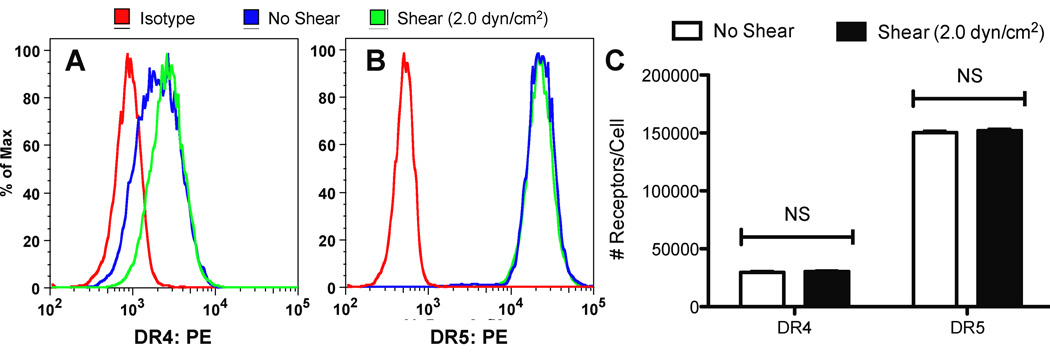
3.8. Fluid shear stress does not alter death receptor expression on the cancer cell surface
To investigate fluid shear stress effects on DR4 and DR5 surface expression, COLO 205 cells were exposed to shear stress (2.0 dyn/cm2) and static conditions at 37°C for 120 min and immediately labeled with anti-DR4 and anti-DR5 antibodies to be analyzed via flow cytometry. COLO 205 cells exposed to either static conditions or fluid shear stress did not show measurable differences in DR4 (Fig. 9A) or DR5 surface expression (Fig. 9B). QSC bead analysis did not show significant differences in COLO 205 DR4 surface expression, with sheared and nonsheared samples averaging approximately 30,000 receptors/cell (Fig. 9C). COLO 205 cells also did not show significant differences in DR5 surface expression, with sheared and nonsheared samples averaging approximately 150,000 receptors/cell.
Figure 9.
Fluid shear stress does not alter death receptor surface expression. COLO 205 cells exposed to static conditions and 2.0 dyn/cm2 of fluid shear stress for 120 min at 37°C were labeled with anti-DR4 (A) and anti-DR5 (B) antibodies, respectively. (C) QSC receptor quantification of DR4 and DR5 on the surface of COLO 205 cells exposed to static conditions and fluid shear stress (n = 3). NS: non-significant.
4. DISCUSSION
The aim of this study was to quantify the role of fluid shear stress in altering the cancer cell response to receptor-mediated apoptosis. TRAIL-treated colon and prostate cancer cells were sensitized to receptor-mediated apoptosis under the presence of physiological fluid shear stresses (Figs. 1,2). Previous studies have shown that cancer cells can become chemically sensitized to TRAIL therapy. TRAIL resistant LNCaP cells treated with aspirin have been sensitized TRAIL treatment via downregulation of NF-κB, a regulator of antiapoptotic proteins (35). Combined treatment of the demethylating agent 5-Aza-20-deoxycytidine (5–dAzaC) and interferon-γ (IFN-γ) sensitize neuroblastoma and medulloblastoma cells to TRAIL-induced apoptosis via upregulation of caspase-8 expression (36). Second mitochondria-derived activator of caspase (SMAC) synthetic peptides sensitized multiple tumor cell types to TRAIL in vitro and enhanced the antitumor effect of TRAIL in vivo in a human glioma xenograft model (37). Our results show that rather than by chemical sensitization, fluid shear forces alone sensitize cancer cells to TRAIL-induced apoptosis.
While fluid shear stress sensitized cancer cells to apoptosis via TRAIL, fluid shear forces did not alter TRAIL-induced cell necrosis (Fig. 3). TRAIL can induce apoptosis, necrosis, or a combination of both in a variety of cancer cell lines. TRAIL has been shown to induce cell death in prostate adenocarcinoma TRAMP-C2 and Jurkat cell lines via necrosis (38,39). In particular, TRAMP-C2 cell death was via necrosis only, as cells lacked apoptotic characteristics such as an annexin V+/PI− population, SAPK/JNK phosphorylation, caspase activation, or cytochrome c release (38). Recently, acidic extracellular pH has been shown to alter the form of TRAIL-induced cell death, from apoptosis to receptor interacting protein kinase 1 (RIPK1)-dependent regulated necrosis in colon adenocarcinoma HT29 and hepatocarcinoma HepG2 cell lines (40,41). Shear-induced sensitization to TRAIL did not show a shift from TRAIL-induced apoptosis to TRAIL-induced necrosis, indicating that the sensitization response is apoptosis-specific.
Cancer cells developed a shear-induced sensitization to TRAIL-induced apoptosis in a fluid shear stress force- and time-dependent manner, directly implicating fluid shear stress in this response (Figs. 4–6). While low shear forces of 0.01–0.10 dyn/cm2 representative of interstitial flow did not sensitize cancer cells to TRAIL, a minimum shear stress of 0.4 dyn/cm2 induced a significant increase in TRAIL-induced apoptosis. In the tumor microenvironment, cancer cells are exposed to slow interstitial flows in and around the tumor tissue (42). The mechanisms behind how cancer cells sense interstitial flow are not well understood, however shear stress values have been estimated in 3D in vitro matrices (24). For flow rates of 1 µm/s, cell surface shear stress estimates are extremely low, ranging from 0.007–0.015 dyn/cm2 (24). Cancer cells are exposed to greater fluid shear forces after entering the circulation, and such conditions may play a role in sensitization to TRAIL-induced apoptosis. It is interesting to note that the cone-and-plate viscometer shear experiments were designed so that fluid shear forces alone would not induce significant cancer cell death, compared to cancer cells exposed to static conditions. Thus, we were able to isolate fluid shear stress effects on receptor-mediated apoptosis, implicating shear-induced sensitization to TRAIL as a synergistic response.
While fluid shear stress sensitized cancer cells to TRAIL-induced apoptosis, cancer cells did not show an increase in doxorubicin-induced apoptosis under the presence of fluid shear forces (Fig. 7). Much like TRAIL, chemical sensitization to doxorubicin has been investigated previously. Gliotoxin, MG132, and Sulfasalazine sensitized typically resistant pancreatic carcinoma Capan-1 and A818-4 cell lines to doxorubicin-induced apoptosis via inhibition of NF-κB (43). Selenium treatment combined with doxorubicin was successful in enhancing apoptosis in MCF-7 breast cancer cells, a doxorubicin-resistant cell line, via depression of Akt phosphorylation (44). Small molecule inhibitors of the Hdm2:p53 complex, allowing for activation of tumor suppressor p53, exerted synergistic effects with doxorubicin in an A375 melanoma cell line xenograft model to decrease tumor growth (45). Due to the fact that shear stress-induced sensitization to apoptosis was not observed with doxorubicin treatment, it is possible that the fluid shear stress effects originate at the cell surface receptor level, where TRAIL ligand binds to death receptors DR4 and DR5 while exposed to fluid shear stress. This is in direct contrast to doxorubicin, which interacts with DNA within the cell to exert its apoptotic effects.
Treatment with Z-VAD-FMK revealed that shear-induced sensitization to TRAIL-induced apoptosis is caspase-dependent (Fig. 8). Caspase activation is a critical step in the apoptotic pathway, induced by TRAIL binding to death receptors (46). In contrast to extrinsic apoptosis pathways such as TRAIL-mediated apoptosis, intrinsic pathways are initiated by DNA and cellular damage, along with the permeabilization of mitochondria (47). During this process, mitochondrial factors including cytochrome c, AIF (apoptosis-inducing factor), and SMAC are released, with AIF-induced apoptosis occurring via a caspase-independent process (48). DNA-damaging agents have previously shown to sensitize hepatic carcinoma cell lines to TRAIL, due to ATM kinase activation (49). ATM kinase activity in turn leads to a downregulation of antiapoptotic protein cFLIP, and subsequent sensitization to TRAIL. Since our sensitization process is caspase-dependent, it is likely that the shear-induced sensitization is not due to DNA-damaging events, providing further support that the sensitization phenomena may occur at the cell surface. Inhibition of WEE1, a cell cycle checkpoint regulator, has been shown to sensitize a variety of basal breast cancer cell lines to TRAIL-induced apoptosis due to increased surface expression of death receptors and increased caspase activation (50). Our results show that COLO 205 surface expression of death receptors DR4 and DR5 is not altered after exposure to fluid shear stress (Fig. 9), and thus sensitization to TRAIL-induced apoptosis is not likely due to shear-induced changes in receptor expression. It is likely that a combination of fluid shear stress effects along with TRAIL stimulation, rather than fluid shear stress alone, cause changes in death receptor trimerization and signaling. Death receptors, upon binding to TRAIL, are known to trimerize and recruit adaptor proteins to form a signaling complex required for TRAIL-induced apoptosis (51). It is possible that mechanical shear forces could enhance death receptor trimerization in the presence of TRAIL, and assist in the formation of signaling complexes for TRAIL-induced apoptosis. The effects of fluid shear stress on death receptor trimerization upon binding to TRAIL could lead to further insight into the mechanistic basis of shear stress-induced TRAIL sensitization.
5. CONCLUSION
Results from this study indicate that hemodynamic shear forces have a significant effect on receptor-mediated apoptosis of cancer cells in the presence of TRAIL. Fluid shear stress was found to sensitize both colon and prostate cancer cell lines to TRAIL-mediated apoptosis. Cancer cells were not sensitized to other means of cell death, such as necrosis. TRAIL sensitization was shown to be shear stress dose-dependent, as sensitization was found to increase with increasing fluid shear stress. TRAIL sensitization was also fluid shear stress time-dependent, as sensitization to apoptosis was enhanced with increasing fluid shear stress exposure time. The response was TRAIL-specific, as shear stress did not sensitize cancer cells to doxorubicin treatment over varying shear stress magnitudes and exposure times. Caspase inhibition assays revealed the sensitization response to be caspase-dependent. These results shed new light on the cancer cell response to soluble apoptotic agents within the circulation. The effects of fluid shear stress on mechanosensing death receptors on the cancer cell surface, along with their signaling pathways, can reveal new strategies for treating circulating cancer cells and reducing the likelihood of metastasis.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grants no. CA143876 and HL018208.
REFERENCES
- 1.Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, et al. Steps in tumor metastasis: new concepts from intravital microscopy. Cancer and Metastasis Reviews. 1995;14:279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald IC, Groom AC, Chambers AF. Cancer spread and micrometastasis development: Quantitative approaches for in vivo models. Bioessays. 2002;24:885–893. doi: 10.1002/bies.10156. [DOI] [PubMed] [Google Scholar]
- 7.Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Frontiers in Oncology. 2012;2:1–9. doi: 10.3389/fonc.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes AD, Mattison J, Western LT, Powderly JD, Greene BT, King MR. Microtube Device for Selectin-Mediated Capture of Viable Circulating Tumor Cells from Blood. Clinical Chemistry. 2012;58:846–853. doi: 10.1373/clinchem.2011.176669. [DOI] [PubMed] [Google Scholar]
- 9.Greene BT, Hughes AD, King MR. Circulating Tumor Cells: The Substrate of Personalized Medicine? Frontiers in Oncology. 2012;2:1–6. doi: 10.3389/fonc.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell MJ, Chen CS, Ponmudi V, Hughes AD, King MR. E-selectin liposomal and nanotube-targeted delivery of doxorubicin to circulating tumor cells. Journal of Controlled Release. 2012;160:609–617. doi: 10.1016/j.jconrel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rana K, Reinhart-King CA, King MR. Inducing Apoptosis in Rolling Cancer Cells: A Combined Therapy with Aspirin and Immobilized TRAIL and E-Selectin. Mol. Pharmaceutics. 2012;9:2219–2227. doi: 10.1021/mp300073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of Apoptosis by Apo-2 Ligand, a New Member of the Tumor Necrosis Factor Cytokine Family. Journal of Biological Chemistry. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 13.Rana K, Liesveld JL, King MR. Delivery of apoptotic signal to rolling cancer cells: A novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol. Bioeng. 2009;102:1692–1702. doi: 10.1002/bit.22204. [DOI] [PubMed] [Google Scholar]
- 14.Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, et al. TRAIL (Apo2L) suppresses growth of primary human leukemia and myelodysplasia progenitors. Leukemia. 2002;16:67–73. doi: 10.1038/sj.leu.2402338. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural killer cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 17.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. The Journal of Immunology. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nature Medicine. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 19.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, et al. Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (Trail) Contributes to Interferon γ–Dependent Natural Killer Cell Protection from Tumor Metastasis. The Journal of Experimental Medicine. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dafni H, Israely T, Bhujwalla ZM, Benjamin LE, Neeman M. Overexpression of Vascular Endothelial Growth Factor 165 Drives Peritumor Interstitial Convection and Induces Lymphatic Drain. Cancer Research. 2002;62:6731–6739. [PubMed] [Google Scholar]
- 21.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-β1. Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qazi H, Shi ZD, Tarbell JM. PLoS ONE: Fluid Shear Stress Regulates the Invasive Potential of Glioma Cells via Modulation of Migratory Activity and Matrix Metalloproteinase Expression. PLoS ONE. 2011;6:e20348. doi: 10.1371/journal.pone.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen JA, Boschetti F, Swartz MA. Effects of extracellular fiber architecture on cell membrane shear stress in a 3D fibrous matrix. J Biomech. 2007;40:1484–1492. doi: 10.1016/j.jbiomech.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Turitto VT. Blood viscosity, mass transport, and thrombogenesis. Prog Hemost Thromb. 1982;6:139–177. [PubMed] [Google Scholar]
- 26.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 27.Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. American Journal of Physiology - Cell Physiology. 2003;284:C977–C987. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell MJ, King MR. Shear-Induced Resistance to Neutrophil Activation via the Formyl Peptide Receptor. Biophysical Journal. Biophysical Society. 2012;102:1804–1814. doi: 10.1016/j.bpj.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbeuval JP, Lambert C, Sabido O, Cottier M, Fournel P, Dy M, et al. Macrophages From Cancer Patients: Analysis of TRAIL, TRAIL Receptors, and Colon Tumor Cell Apoptosis. Journal of the National Cancer Institute. 2003;95:611–621. doi: 10.1093/jnci/95.8.611. [DOI] [PubMed] [Google Scholar]
- 31.Kim MB, Sarelius IH. Distributions of Wall Shear Stress in Venular Convergences of Mouse Cremaster Muscle. Microcirculation. 2003;10:167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 32.Young RC, Ozols RF, Myers CE. The Anthracycline Antineoplastic Drugs. New England Journal of Medicine. 1981;305:139–153. doi: 10.1056/NEJM198107163050305. [DOI] [PubMed] [Google Scholar]
- 33.Osheroff N, Corbett AH, Robinson MJ. Mechanism of action of topoisomerase IItargeted antineoplastic drugs. Advances in Pharmacology. 1994;29:105–126. doi: 10.1016/s1054-3589(08)61134-5. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry NA, Lazebnik YL. Caspases: Enemies Within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 35.Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of Acetylsalicylic Acid Promotes Tumor Necrosis Factor-related Apoptosis-inducing Ligand-induced Apoptosis by Down-regulating BCL-2 Gene Expression. Journal of Biological Chemistry. 2005;280:41047–41056. doi: 10.1074/jbc.M503713200. [DOI] [PubMed] [Google Scholar]
- 36.Fulda S, Debatin KM. 5-Aza-2′-deoxycytidine and IFN-γ cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene. 2006;25:5125–5133. doi: 10.1038/sj.onc.1209518. [DOI] [PubMed] [Google Scholar]
- 37.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature Medicine. 2002;8:808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 38.Kemp TJ, Kim JS, Crist SA, Griffith TS. Induction of necrotic tumor cell death by TRAIL/Apo-2L. Apoptosis. 2003;8:587–599. doi: 10.1023/A:1026286108366. [DOI] [PubMed] [Google Scholar]
- 39.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 40.Meurette O, Rebillard A, Huc L, Le Moigne G, Merino D, Micheau O, et al. TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosislike cell death under acidic extracellular conditions. Cancer Research. 2007;67:218–226. doi: 10.1158/0008-5472.CAN-06-1610. [DOI] [PubMed] [Google Scholar]
- 41.Meurette O, Huc L, Rebillard A, Le Moigne G, Lagadic-Gossmann D, Dimanche-Boitrel M-T. TRAIL (TNF-related apoptosis-inducing ligand) induces necrosis-like cell death in tumor cells at acidic extracellular pH. Ann. N.Y Acad. Sci. 2005;1056:379–387. doi: 10.1196/annals.1352.018. [DOI] [PubMed] [Google Scholar]
- 42.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Arlt A, Vorndamm J, Breitenbroich M, Fölsch UR, Kalthoff H, Schmidt WE, et al. Inhibition of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20:859–868. doi: 10.1038/sj.onc.1204168. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Molecular Cancer Therapeutics. 2007;6:1031–1038. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- 45.Koblish HK, Zhao S, Franks CF, Donatelli RR, Tominovich RM, LaFrance LV, et al. Benzodiazepinedione inhibitors of the Hdm2:p53 complex suppress human tumor cell proliferation in vitro and sensitize tumors to doxorubicin in vivo. Molecular Cancer Therapeutics. 2006;5:160–169. doi: 10.1158/1535-7163.MCT-05-0199. [DOI] [PubMed] [Google Scholar]
- 46.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quast SA, Berger A, Buttstädt N, Friebel K, Schönherr R, Eberle J. General Sensitization of Melanoma Cells for TRAIL-Induced Apoptosis by the Potassium Channel Inhibitor TRAM-34 Depends on Release of SMAC. PLoS ONE. 2012;7:e39290. doi: 10.1371/journal.pone.0039290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF) Biochem. Biophys. Res. Commun. 2010;396:95–100. doi: 10.1016/j.bbrc.2010.02.163. [DOI] [PubMed] [Google Scholar]
- 49.Stagni V, Mingardi M, Santini S, Giaccari D, Barilà D. ATM kinase activity modulates cFLIP protein levels: potential interplay between DNA damage signalling and TRAIL-induced apoptosis. Carcinogenesis. 2010;31:1956–1963. doi: 10.1093/carcin/bgq193. [DOI] [PubMed] [Google Scholar]
- 50.Garimella SV, Rocca A, Lipkowitz S. WEE1 Inhibition Sensitizes Basal Breast Cancer Cells to TRAIL-Induced Apoptosis. Molecular Cancer Research. 2012;10:75–85. doi: 10.1158/1541-7786.MCR-11-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]