Abstract
The diagnosis of metastatic clear cell renal cell carcinoma (CC-RCC) can be difficult because of its morphologic heterogeneity and the increasing use of small image-guided biopsies that yield scant diagnostic material. This is further complicated by the degree of morphologic and immunophenotypic overlap with non-renal neoplasms and tissues, such as adrenal cortex. In this study, a detailed immunoprofile of 63 adrenal cortical lesions, which included 54 cortical neoplasms, was compared with 185 metastatic CC-RCC using traditional [anti-calretinin, CD10, anti-chromogranin, anti-EMA, anti-inhibin, anti-melanA, anti-cytokeratins (AE1/AE3 and AE1/CAM5.2), anti-renal cell carcinoma marker (RCCma), and anti-synaptophysin)] and novel [anti-carbonic anhydrase-IX (CAIX), anti-hepatocyte nuclear factor-1b (HNF-1b), anti-human kidney injury molecule-1 (hKIM-1), anti-PAX-2, anti-PAX-8, anti-steroidogenic factor-1 (SF-1), and anti-T cell immunoglobulin mucin-1 (TIM-1)] antibodies. Tissue microarray methodology was used to simulate small image-guided biopsies. Staining extent and intensity were scored semiquantitatively for each antibody. In comparing different intensity thresholds required for a ‘‘positive’’ result, ≥2+ was identified as optimal for diagnostic sensitivity/specificity. For the distinction of adrenal cortical lesions from metastatic CC-RCC, immunoreactivity for the adrenal cortical antigens SF-1 (86% adrenal; 0% CC-RCC), calretinin (89% adrenal; 10% CC-RCC), inhibin (86% adrenal; 9% CC-RCC), and melanA (86% adrenal; 10% CC-RCC) and the renal epithelial antigens hKIM-1 (0% adrenal; 83% CC-RCC), PAX-8 (0% adrenal; 83% CC-RCC), HNF-1b (0% adrenal; 76% CC-RCC), EMA (0% adrenal; 78% CC-RCC), and CAIX (3% adrenal; 87% CC-RCC) had the most potential utility. The use of the novel renal epithelial markers hKIM-1 (clone AKG7) and/or PAX-8, and the adrenocortical marker SF-1 in an immunohistochemical panel for distinguishing adrenal cortical lesions from metastatic CC-RCC offers improved diagnostic sensitivity and specificity.
Keywords: Adrenal cortical, metastatic renal cell carcinoma, immunohistochemistry, calretinin, CAIX, CD10, EMA, inhibin, hKIM-1, HNF-1b, melanA, PAX-2, PAX-8, RCC, SF-1, synaptophysin
INTRODUCTION
Clear cell renal cell carcinoma (CC-RCC) metastasizes frequently and may involve virtually any body site.14, 37, 48 The increasing use of image-guided biopsies15, 17, 38, 46 that yield very scant tissue samples often complicates the diagnosis at a metastatic location. In addition, CC-RCC has considerable morphologic overlap with other non-renal neoplasms and normal tissues,31, 39 especially primary lesions of the adrenal cortex such as adenoma, carcinoma, hyperplasia, and even adrenal-renal fusion. The histologic distinction of CC-RCC from an adrenal cortical lesion, either at the time of staging or in a patient with a remote history of CC-RCC, is a well-recognized diagnostic difficulty39, 51 that has significant therapeutic and prognostic implications.18, 26, 32, 43
Although immunohistochemistry can be helpful in difficult cases, we have encountered problems of weak immunoreactivity with both presumed adrenal cortical specific markers (calretinin, inhibin, and melanA) and presumed CC-RCC-specific markers [CD10, cytokeratins (CK), EMA, renal cell carcinoma marker (RCCma)] in cases of metastatic CC-RCC and adrenal cortical lesions, respectively. Clinically, the occurrence of steroid-inactive adrenal cortical lesions may further confound histologic interpretation.8, 30, 40 While earlier studies have compared immunostaining results in this setting,2-6, 9-12, 16, 25, 27, 33, 34, 36, 41, 42, 44, 45, 50, 53, 54 our goal was to characterize the immunoprofile of primary lesions of the adrenal cortex and metastatic CC-RCC using tissue microarray technology to simulate the small biopsy samples obtained in routine practice, with a focus on the effect of varying intensity thresholds on sensitivity and specificity. In addition, the expression profile with anti-carbonic anhydrase IX (CAIX), anti-human kidney injury molecule-1 (hKIM-1), anti-hepatocyte nuclear factor-1b (HNF-1b), anti-PAX-8, and anti-T cell immunoglobulin mucin-1 (TIM-1), which are proposed markers of renal cell carcinoma7, 13, 21, 23, 29, 47 that to our knowledge have not been fully characterized in adrenal cortical lesions, was studied to determine diagnostic utility in this differential diagnostic setting.
MATERIALS AND METHODS
Sixty-three adrenal cortical lesions and 185 metastatic CC-RCC were retrieved from the pathology archives of Stanford University Medical Center and Veterans Affairs Palo Alto Health Care System from 1992-2008 after IRB approval. Only metastatic CC-RCC cases with a documented prior primary renal cell carcinoma history (by chart review or archival pathology database review) were included in the study. We studied only metastases in order to avoid the possibility of inflating the sensitivity of the renal markers by using primary renal tumors and to expand knowledge of the immunophenotypic spectrum of metastatic renal cell carcinoma. In some cases with synchronous metastasis in other organs/sites (i.e. lung plus multiple lymph node metastasis), one representative block per unique organ/site was selected. Nineteen primary CC-RCC (concurrent or prior) corresponding to a metastatic CC-RCC were also available and retrieved to investigate immunostaining constancy between the metastasis and primary tumor.
All H&E stained sections were reviewed by two authors (A.R.S. and J.K.M.) and diagnoses for all cases were confirmed. Adult adrenal cortical neoplasms were classified by the modified Weiss criteria;1, 20, 49 pediatric adrenal cortical neoplasms were classified separately.52 Prior to staining, cases of metastatic CC-RCC were classified as well-differentiated or poorly-differentiated, based on whether morphologic features were suggestive of CC-RCC (well-differentiated) or whether the diagnosis of CC-RCC would not have been suspected by morphology alone (poorly-differentiated). Relevant clinicopathologic parameters evaluated for all cases included patient age and gender, tumor site, and tumor laterality.
Unique tissue microarrays for the 63 adrenal cortical lesions (Stanford TMA #240) and 185 metastatic CC-RCC (Stanford TMA #238) were prepared in duplicate and evaluated as described elsewhere19, 40 using 1.3 mm and 0.7 mm diameter tissue cores, respectively. The 19 corresponding primary renal cell carcinomas were included on the metastatic CC-RCC tissue microarray. Incomplete or missing cores were excluded from evaluation. Immunohistochemical staining using antibodies against calretinin, CAIX, CD10, CKAE1/AE3, CKAE1/CAM5.2, EMA, inhibin, hKIM-1, HNF-1b, melanA, PAX-2, PAX-8, RCCma, SF-1, synaptophysin, and TIM-1 was performed on 4-mm thick formalin-fixed, paraffin-embedded duplicate sections mounted on charged slides and baked at 60°C for 1 hour. Because immunoreactivity for calretinin, inhibin, melanA, SF-1, and synaptophysin in adrenal cortical lesions had been previously performed and reported by our group,40 these markers were not repeated for the current study. Antibody sources and dilutions for the study are listed in Table 1.
Table 1.
Antibody sources and dilutions
| Antigen | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| Calretinin | Rabbit Polyclonal | 1:100 | Benchmark standard | Cell Marque (Rocklin, CA) |
| CAIX | Polyclonal | 1:500 | Citrate | Novus Biologicals, Inc. (Littleton, CO) |
| CD10 | 56E6 | 1:20 | Benchmark standard | Novacastra (Burlingame, CA) |
| Cytokeratins1 | AE1/AE3; CAM5.2 | 1:50; 1:50 | Protease 2 | Dako (Carpinteria, CA); Becton Dickinson (San Jose, CA) |
| Cytokeratins | AE1/AE3 | 1:50 | Protease 2 | Dako (Carpinteria, CA) |
| EMA | E29 | 1:50 | Benchmark standard | Dako (Carpinteria, CA) |
| Inhibin | R1 | 1:100 | Benchmark standard | Dako (Carpinteria, CA) |
| hKIM-12 | AKG7 | Prediluted | Citrate | Laboratory of Dr. J. Bonventre |
| HNF-1b2 | C-20 | 1:800 | Citrate | Santa Cruz Biotechnology (Santa Cruz, CA) |
| MelanA | A103 | 1:60 | Benchmark standard | Dako (Carpinteria, CA) |
| PAX-2 | Z-RX2 | 1:100 | Citrate | Zymed (San Francisco, CA) |
| PAX-8 | Polyclonal | 1:20 | Citrate | Proteintech Group, Inc. (Chicago, IL) |
| RCCma | PN-15 | Prediluted | None | Vision (Fremont, CA) |
| SF-1 | N1665 | 1:100 | Citrate | Dako (Carpinteria, CA) |
| Synaptophysin2 | Polyclonal | 1:25 | Citrate, Dako Envision+ | Cell Marque (Rocklin, CA) |
| TIM-12 | 219211 | 1:500 | Citrate, Dako Envision+ | R&D Systems (Minneapolis, MN) |
In-house cocktail.
All stains were performed on the Ventana Benchmark, except synaptophysin, h-KIM1, HNF-1b, and TIM-1. Synaptophysin was run on the Dako Autostainer using the Envision+ detection system and hKIM-1, HNF-1b, and TIM-1 were performed manually.
Whole sections of CC-RCC were used as a positive control for the antibodies against CAIX, CD10, CKAE1/AE3, CKAE1/CAM5.2, EMA, hKIM-1, HNF-1b, PAX-2, PAX-8, RCCma, and TIM-1, while whole sections of normal adrenal gland were used as positive control for the antibodies against calretinin, inhibin, melanA, SF-1, and synaptophysin. The following patterns of immunostaining were considered positive: anti-HNF-1b, anti-PAX-2, anti-PAX-8, anti-SF-1: nuclear; anti-calretinin: nuclear and cytoplasmic; anti-inhibin, anti-melanA, anti-synaptophysin: cytoplasmic; anti-CAIX, anti-CKAE1/AE3, anti-CKAE1/CAM5.2, CD10, anti-EMA, anti-hKIM-1, anti-RCCma, anti-TIM-1: membranous and/or cytoplasmic. Staining extent was semiquantitatively scored as negative (0, <5% cells stained), focally positive (1+, 5-10% cells stained), positive (2+, >10-50% cells stained), or diffusely positive (3+, >50% cells stained), and a mean extent (range 0-3) calculated. Staining intensity was semiquantitatively scored from 0 to 3+ and a mean intensity (range 0-3) calculated.
RESULTS
Clinical Features: Adrenal Cortical Lesions versus metastatic CC-RCC
On the TMA, 63 adrenal cortical lesions were intact/complete and available for evaluation. The adrenal cortical lesions consisted of cortical adenomas (43), cortical neoplasms of uncertain malignant potential (4), cortical carcinomas (7), cortical hyperplasias (6), and cortical rests (3). For adrenal cortical lesions, the patient age ranged from 1-86 (mean: 48.7 years) and the size of the lesions ranged from 0.3 to 18 cm (mean: 4.2 cm). Thirty-one patients were male and 32 female; 35 were left sided and 28 were right sided.
One hundred and eight-five metastatic CC-RCC were intact/complete and available for evaluation on the TMA. One hundred thirty-three were well-differentiated and 52 were poorly-differentiated as defined for this study. For some antibody tests, one of the represented tumors may have not been evaluable; therefore, a sample size of 184 cases is reported in some staining results. Metastatic CC-RCC was identified in 27 unique sites: bone (21%), lung (21%), lymph node (16%), soft tissue (9%), brain (6%), blood vessel (4%), skin (3%), adrenal (3%), parotid (3%), pleura (2%), sinonasal (2%), and 16 other miscellaneous sites (10% combined). The patient age ranged from 34-85 years (mean: 60.8 years). One hundred fifty-four patients were male and 31 were female.
Immunohistochemical Results: Adrenal Cortical Lesions versus metastatic CC-RCC
Previously reported studies of immunohistochemical staining in adrenal cortical lesions and CC-RCC were reviewed and summarized in Figure 1. Our staining results are summarized in Tables 2 and 3.
Figure 1.
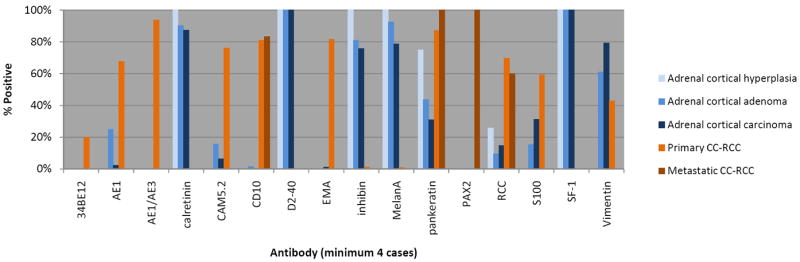
Review of previously reported immunohistochemical findings in adrenal cortical lesions and clear cell renal cell carcinoma. 2-6, 9-12, 16, 25, 27, 33, 34, 36, 41, 42, 44, 45, 50, 53, 54
Table 2.
Immunohistochemical Staining Results* for Metastatic Clear Cell Renal Cell Carcinoma versus Adrenal Cortical Lesion with Adrenocortical Markers
| Calretinin (C, N) | Inhibin (C) | MelanA (C) | SF-1 (N) | Synaptophysin (C) | |
|---|---|---|---|---|---|
| CC-RCC (overall) | 18/184 (10%) | 17/184 (9%) | 18/184 (10%) | 0/184 (0%) | 3/184 (2%) |
| CC-RCC (WD) | 13/133 (10%) | 11/133 (8%) | 11/133 (8%) | 0/133 (0%) | 2/133 (2%) |
| CC-RCC (PD) | 5/51 (10%) | 6/51 (12%) | 7/51 (14%) | 0/51 (0%) | 1/51 (2%) |
| ACL | 56/63 (89%) | 54/63 (86%) | 54/63 (86%) | 54/63 (86%) | 37/63 (59%) |
≥2+ staining intensity considered positive
CC-RCC = metastatic clear cell renal cell carcinoma, WD = well-differentiated, PD = poorly-differentiated, ACL = adrenal cortical lesion, C = cytoplasmic, N = nuclear
Table 3.
Immunohistochemical Staining Results* for Metastatic Clear Cell Renal Cell Carcinoma versus Adrenal Cortical Lesions with Renal Epithelial Markers
| AE1/AE3 (M/C) | CAM5.2/AE1 (M/C) | CD10 (M/C) | EMA (M/C) | RCC (M/C) | CAIX (M/C) | hKIM-1 (M/C) | HNF-1b (N) | PAX-2 (N) | PAX-8 (N) | TIM-1 (M/C) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC-RCC (overall) | 101/184 (55%) | 110/184 (60%) | 142/185 (77%) | 144/185 (78%) | 33/185 (18%) | 160/184 (87%) | 153/185 (83%) | 139/184 (76%) | 91/185 (49%) | 152/184 (83%) | 68/184 (37%) |
| CC-RCC (WD) | 64/133 (48%) | 72/133 (54%) | 103/133 (77%) | 108/133 (81%) | 28/133 (21%) | 119/133 (89%) | 110/133 (83%) | 98/133 (74%) | 70/133 (53%) | 108/133 (81%) | 50/131 (38%) |
| CC-RCC (PD) | 37/51 (73%) | 38/51 (75%) | 39/52 (75%) | 36/52 (69%) | 5/52 (10%) | 41/51 (80%) | 43/52 (83%) | 41/51 (80%) | 21/52 (40%) | 44/51 (86%) | 18/53 (34%) |
| ACL | 6/63 (10%) | 5/63 (8%) | 6/63 (10%) | 0/63 (0%) | 0/63 (0%) | 2/63 (3%) | 0/63 (0%) | 0/63 (0%) | 0/63 (0%) | 0/63 (0%) | 0/63 (0%) |
≥2+ staining intensity considered positive
CC-RCC = metastatic clear cell renal cell carcinoma, WD = well-differentiated, PD = poorly-differentiated, ACL = adrenal cortical lesion, C = cytoplasmic, M = membranous, N = nuclear
Anti-CAIX demonstrated the highest overall sensitivity for CC-RCC using ≥2+ staining intensity as the threshold for a positive result (87%), but it was slightly less sensitive in the poorly differentiated tumors (80%) compared to hKIM-1 (83%) and PAX-8 (86%). Anti-hKIM-1 and anti-PAX-8 also demonstrated slightly better overall specificity for CC-RCC versus adrenal cortical lesions than anti-CAIX (100% for both anti-hKIM-1 and anti-PAX-8) as 2 of 63 (3%) adrenal cortical lesions stained for CAIX (one adrenal cortical adenoma and one carcinoma). The other potential renal markers in this differential diagnostic setting had lower sensitivities: anti-PAX-2 (49%), anti-CKAE1/CAM5.2 (60%), anti-CKAE1/AE3 (55%), CD10 (77%), anti-EMA (78%), anti-HNF-1b (76%), anti-RCCma (18%), and anti-TIM-1 (37%).
Photomicrographs depicting the prototypical immunophenotypes of adrenal cortex and CC-RCC are shown in Figures 2. Figure 3 demonstrates the intensity threshold definitions employed for immunohistochemical interpretation in the study (i.e. 1+ and ≥2+). Sensitivities and specificities for both adrenal cortical lesions and metastatic CC-CC, including a comparison of different results by staining intensity thresholds, are shown in Figures 4 and 5.
Figure 2.
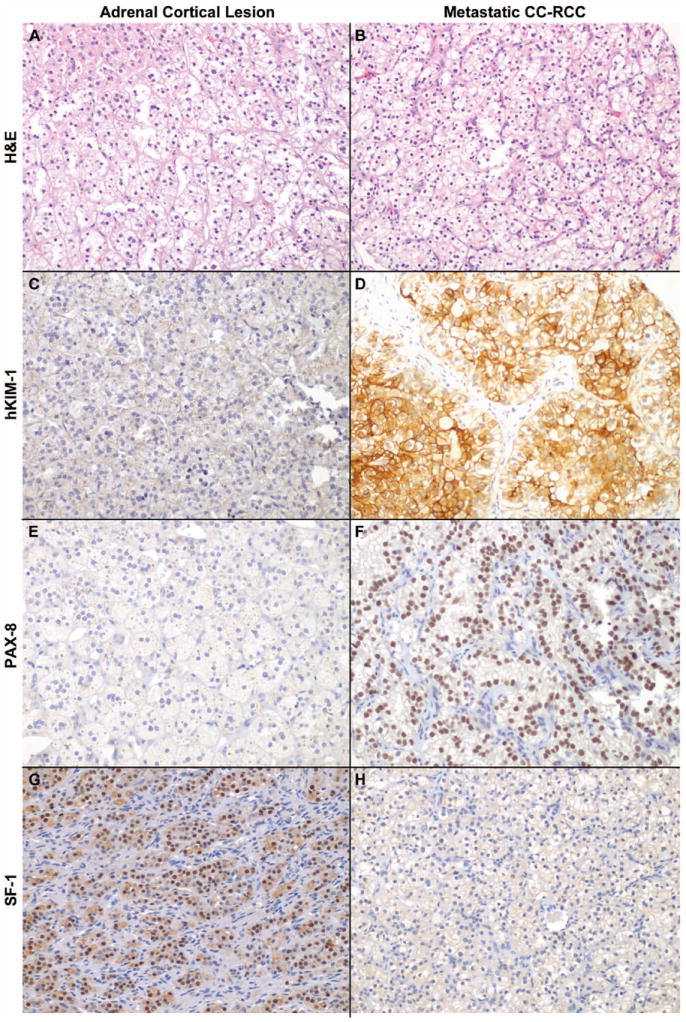
Immunophenotype of adrenal cortex versus metastatic clear cell renal cell carcinoma (CC-RCC). Significant morphologic overlap between (A) Adrenal cortical lesions (in this case, cortical adenoma) from (B) metastatic CC-RCC makes differentiation by H&E staining alone challenging. Adrenal cortical lesions showed only faint background cytoplasmic pigment staining for (C) hKIM-1 and (E) PAX-8, while metastatic CC-RCC showed diffuse (D) membranous/cytoplasmic hKIM-1 and (F) nuclear PAX-8 reactivity. Diffuse SF-1 nuclear staining was positive in (G) Adrenal cortical lesions and negative in (H) metastatic CC-RCC.
Figure 3.
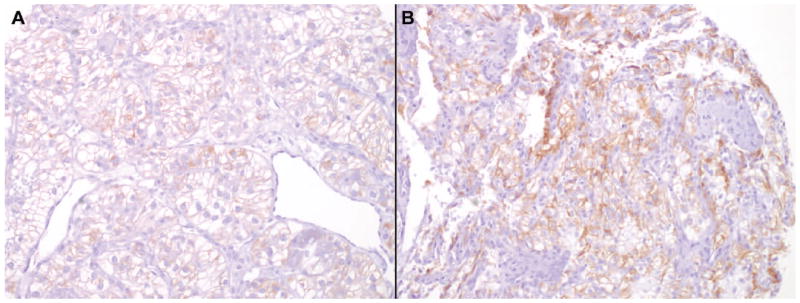
MelanA immunoreactivity in metastatic clear cell renal cell carcinoma (CC-RCC). Weak cytoplasmic staining (1+) in several cases of metastatic CC-RCC was seen with MelanA, calretinin, and (A) inhibin. This may cause significant interpretation problems in small biopsies if a ≥2+ staining threshold is not required. Given that occasional cases of metastatic CC-RCC may show stronger staining (2+) with these 3 markers (B, melanA in this case), the addition of the nuclear marker SF-1 helps improve diagnostic specificity for adrenal cortical lesions versus metastatic CC-RCC.
FIGURE 4.
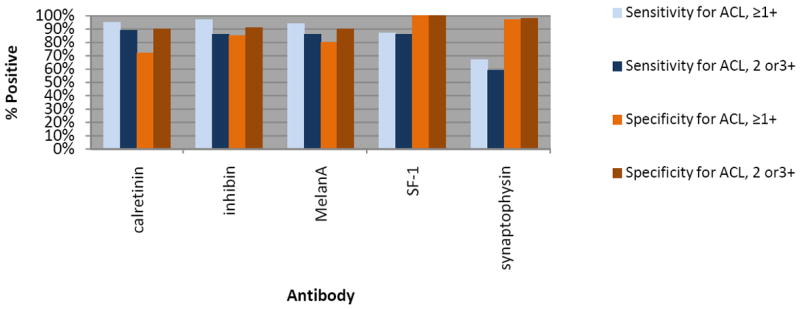
Sensitivity and specificity for adrenal cortical lesions versus metastatic CC-RCC by staining intensity threshold.
FIGURE 5.
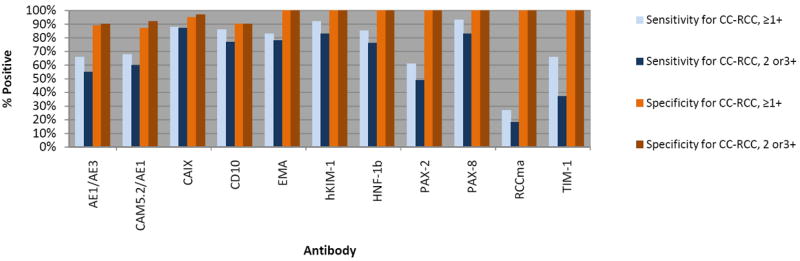
Sensitivity and specificity for metastatic CC-RCC versus adrenal cortical lesions by staining intensity threshold.
Among the presumed adrenal cortex-specific markers, anti-calretinin showed a slightly higher sensitivity for adrenal cortical lesions than anti-SF-1 (89% versus 86%, respectively); however, it lacked the high specificity of anti-SF-1 (100%), as anti-calretinin also stained 10% of metastatic CC-RCC using a ≥2+ positivity cut-point. Anti-SF-1 maintained strong consistency in staining intensity, with 54 of 55 (98%) positive adrenal cortical lesions cases demonstrating ≥2+ staining intensity. Both anti-inhibin and anti-melanA showed an identical sensitivity to anti-SF-1 (86%), but with lower specificities (90% and 91%, respectively, compared to 100% for SF-1). While anti-synaptophysin reactivity was relatively specific for adrenal cortical lesions (98% using a ≥2+ positivity cut-point), sensitivity was much lower than other markers tested (59% using a ≥2+ positivity cut-point). No significant difference in staining was seen among the subtypes of adrenal cortical lesions evaluated.
Immunohistochemical Results: Primary versus Metastatic CC-RCC
For the 19 metastatic CC-RCC with available paired primary CC-RCC, immunoreactivity for all proposed renal epithelial markers studied were compared between the primary and metastatic carcinomas. Anti-hKIM-1 had the highest sensitivity among primary CC-RCC markers (100%), while also showing strong constancy with metastatic CC-RCC (95%), including the poorly-differentiated metastatic carcinomas (80%). While anti-CKAE1/AE3 showed the highest constancy between the primary and metastatic tumors (100%), it had the lowest sensitivity among primary CC-RCC markers (37%).
DISCUSSION
Given the common use of image-guided biopsies performed for the diagnosis of deep-seated mass lesions,15, 17, 38, 46 the amount of tissue samples available for pathologic evaluation has become increasingly smaller. Since some of the morphologic clues to diagnosis, such as architectural growth pattern, are not evaluable in many of these small samples, immunohistochemistry is often required to determine tissue type or tumor lineage. Adjunctive studies are even more commonly needed when considering a diagnosis of metastatic CC-RCC because of its overlapping morphologic appearance with a variety of other tissues and neoplasms. Distinction of CC-RCC from adrenal cortical tissue, whether normal, hyperplastic, or neoplastic, can be a major diagnostic problem. Anecdotally, we have seen a few cases of adrenal-renal fusion initially interpreted as CC-RCC on biopsy. Although there are many previous studies of immunohistochemical markers used for the diagnosis of RCC,2-6, 9-12, 16, 25, 27, 33, 34, 36, 41, 42, 44, 45, 50, 53, 54 it is our experience that overlapping immunophenotypes are more commonly seen in routine practice than would be expected from the reported literature. Also, the appropriate diagnostic threshold for regarding a stain as “positive” has not been fully addressed in this setting. We sought to address several of these unresolved issues: threshold requirements (i.e. minimal staining intensity) for accurate diagnosis, comparative expression within the spectrum of primary adrenal cortical mass lesions and metastatic CC-RCC, comparison of sensitivities in both well-differentiated and poorly differentiated metastatic CC-RCC, and an evaluation of novel markers that have not been fully tested in this setting. All of these issues were addressed using tissue microarray methodology to simulate small biopsy samples.
In the differential diagnostic setting of CC-RCC versus adrenal cortical lesions, this current comparison of traditional and novel renal specific antibodies showed that anti-hKIM-1 (clone AKG7), anti-PAX-8, and anti-CAIX were the superior markers in terms of sensitivity for metastatic CC-RCC using either the ≥1+ or ≥2+ positivity cut-point. Setting a higher threshold (≥2+) was more important for the traditional CC-RCC markers, which had more frequent weak staining in adrenal tissues (using ≥2+ cut-point; Figure 5). Both anti-hKIM-1 and anti-PAX-8 also demonstrate the highest consistency in staining intensity across well and poorly differentiated metastatic CC-RCC. This maintained sensitivity in poorly differentiated CC-RCC is important because the sensitivity of many tissue-specific antibodies markedly declines with increasing tumor grade. The antibodies anti-hKIM-1, anti-PAX-8, and anti-CAIX also show excellent staining constancy between the primary and concurrent/corresponding metastatic CC-RCC. Although HNF-1b results were very promising, we have had trouble obtaining adequate staining with subsequently purchased antibody of a different lot number.
While one would expect similar immunoreactivity for PAX-2 and PAX-8 given their shared role as transcription factors in renal organogenesis and the fact that PAX-8 is always co-expressed with PAX-2 in embryonal and renal tissues,35, 47 PAX-8 had a much higher sensitivity for metastatic CC-RCC as well as higher staining constancy between primary and metastatic CC-RCC. Although lowering the dilution utilized for PAX-2 increases sensitivity to a level comparable to PAX-8, we have previously demonstrated that this reduces specificity for metastatic CC-RCC versus adrenal cortical lesions (43% versus 100% for 1:50 and 1:100 PAX-2 dilution, respectively).28 Over the past several years, we have anecdotally noted a decreasing sensitivity of the anti-PAX-2 antibody for CC-RCC in our daily clinical practice, compared with results we observed in our prior studies.12
The diagnostic specificity of anti-calretinin, anti-inhibin, and anti-melanA for adrenal cortical lesions in a small biopsy sample can be particularly problematic if the ≥2+ staining requirement is not maintained, similar to findings we reported for discrimination from pheochromocytoma.40 Patchy cytoplasmic staining with anti-calretinin, anti-inhibin, and anti-melanA was seen in 28, 15, and 20% of metastatic CC-RCCs, respectively (Figure 3 and 4). The addition of the nuclear marker anti-SF-1 to a diagnostic panel offers improved specificity for adrenal cortical lesions (100%) and may also counter the issues of non-specific cytoplasmic staining.
Although previous immunohistochemical studies have incorporated staining for the antigens S-100 protein and vimentin in the differential diagnosis of adrenal cortical lesions versus CC-RCC (along with other markers),6, 10, 33, 50 these markers were excluded from the current study given the known overlap in immunoreactivity (Figure 1). In addition, one study has reported 100% specificity of the antibody D2-40 (anti-podoplanin) for adrenal cortical lesions,3 but we were unable to achieve satisfactory staining and it was therefore excluded from further evaluation.
Human kidney injury molecule-1 (hKIM-1) is a biomarker for renal proximal tubules undergoing regeneration from tubular injury and has been recently recognized as a sensitive immunohistochemical marker of CC-RCC2, 13 The specificity of this antibody in non-renal tumors has only been explored in a few previous studies, and reactivity has been reported in one of two hepatocellular carcinomas,13 in 13% (5/40) of colonic adenocarcinomas, 32% (12/38) of uterine clear cell carcinomas, 94% of ovarian clear cell carcinomas,2, 24 and 16% (10/64) of papillary urothelial carcinomas.22 At present, the anti-hKIM-1 clone AKG7 is not commercially available, but commercial antibodies are under development. We did test a commercial antibody (anti-TIM-1, clone 219211) that should share significant homology with the anti-hKIM-1 (AKG7) antibody; however, it had a very low sensitivity in this study that would preclude its routine use in the diagnosis of CC-RCC.
In summary, we show that incorporating the novel renal epithelial markers anti-hKIM-1 (as it becomes commercially available) and/or anti-PAX-8, and the adrenocortical marker anti-SF-1 in an immunohistochemical panel for the diagnostic distinction of metastatic CC-RCC from adrenal cortical lesions offers improved diagnostic sensitivity and specificity over traditional markers used in this differential diagnostic setting.
Acknowledgments
The authors would like to acknowledge Shirley Kwok, Linda Lu, and Shuchan Zhao for excellence in tissue microarray construction as well as Dr. Yaso Natkunam for assistance in coordination of tissue microarray preparation.
Footnotes
Presented in part at the 98th meeting of the United States and Canadian Academy of Pathology, Boston, MA, March 2009
References
- 1.Aubert S, Wacrenier A, Leroy X, et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26:1612–1619. doi: 10.1097/00000478-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi N, Kunju LP, Giordano T, et al. Expression of renal cell carcinoma antigen (RCC) in renal epithelial and nonrenal tumors: diagnostic Implications. Appl Immunohistochem Mol Morphol. 2007;15:310–315. doi: 10.1097/01.pai.0000213144.70148.8e. [DOI] [PubMed] [Google Scholar]
- 3.Browning L, Bailey D, Parker A. D2-40 is a sensitive and specific marker in differentiating primary adrenal cortical tumours from both metastatic clear cell renal cell carcinoma and phaeochromocytoma. J Clin Pathol. 2008;61:293–296. doi: 10.1136/jcp.2007.049544. [DOI] [PubMed] [Google Scholar]
- 4.Busam KJ, Iversen K, Coplan KA, et al. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998;22:57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cho EY, Ahn GH. Immunoexpression of inhibin alpha-subunit in adrenal neoplasms. Appl Immunohistochem Mol Morphol. 2001;9:222–228. doi: 10.1097/00129039-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cote RJ, Cordon-Cardo C, Reuter VE, et al. Immunopathology of adrenal and renal cortical tumors. Coordinated change in antigen expression is associated with neoplastic conversion in the adrenal cortex. Am J Pathol. 1990;136:1077–1084. [PMC free article] [PubMed] [Google Scholar]
- 7.Cuff J, Huang S, Higgins JP, et al. CpG Island Methylation within the TCF2 Promoter May Enable Epigenetic Modulation of HNF1-beta and Clear Cell Phenotype in Ovarian Clear Cell Carcinoma. Mod Pathol. 2009;22:210A. [Google Scholar]
- 8.Duenschede F, Bittinger F, Heintz A, et al. Malignant and unclear histological findings in incidentalomas. Eur Surg Res. 2008;40:235–238. doi: 10.1159/000111147. [DOI] [PubMed] [Google Scholar]
- 9.Fetsch PA, Powers CN, Zakowski MF, et al. Anti-alpha-inhibin: marker of choice for the consistent distinction between adrenocortical carcinoma and renal cell carcinoma in fine-needle aspiration. Cancer. 1999;87:168–172. [PubMed] [Google Scholar]
- 10.Gaffey MJ, Traweek ST, Mills SE, et al. Cytokeratin expression in adrenocortical neoplasia: an immunohistochemical and biochemical study with implications for the differential diagnosis of adrenocortical, hepatocellular, and renal cell carcinoma. Hum Pathol. 1992;23:144–153. doi: 10.1016/0046-8177(92)90235-u. [DOI] [PubMed] [Google Scholar]
- 11.Ghorab Z, Jorda M, Ganjei P, et al. Melan A (A103) is expressed in adrenocortical neoplasms but not in renal cell and hepatocellular carcinomas. Appl Immunohistochem Mol Morphol. 2003;11:330–333. doi: 10.1097/00129039-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Gokden N, Gokden M, Phan DC, et al. The utility of PAX-2 in distinguishing metastatic clear cell renal cell carcinoma from its morphologic mimics: an immunohistochemical study with comparison to renal cell carcinoma marker. Am J Surg Pathol. 2008;32:1462–1467. doi: 10.1097/PAS.0b013e318176dba7. [DOI] [PubMed] [Google Scholar]
- 13.Han WK, Alinani A, Wu CL, et al. Human kidney injury molecule-1 is a tissue and urinary tumor marker of renal cell carcinoma. J Am Soc Nephrol. 2005;16:1126–1134. doi: 10.1681/ASN.2004070530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann NE, Gillett MD, Cheville JC, et al. Differences in organ system of distant metastasis by renal cell carcinoma subtype. J Urol. 2008;179:474–477. doi: 10.1016/j.juro.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JH, Jensen CS, Donnelly AD, et al. The role of fine-needle aspiration cytology in the evaluation of metastatic clear cell tumors. Cancer. 1999;87:380–389. doi: 10.1002/(sici)1097-0142(19991225)87:6<380::aid-cncr9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Jalali M, Krishnamurthy S. Comparison of immunomarkers for the identification of adrenocortical cells in cytology specimens. Diagn Cytopathol. 2005;33:78–82. doi: 10.1002/dc.20310. [DOI] [PubMed] [Google Scholar]
- 17.Jhala NC, Jhala D, Eloubeidi MA, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of the adrenal glands: analysis of 24 patients. Cancer. 2004;102:308–314. doi: 10.1002/cncr.20498. [DOI] [PubMed] [Google Scholar]
- 18.Jung SJ, Ro JY, Truong LD, et al. Reappraisal of T3N0/NxM0 renal cell carcinoma: significance of extent of fat invasion, renal vein invasion, and adrenal invasion. Hum Pathol. 2008;39:1689–1694. doi: 10.1016/j.humpath.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 20.Lau SK, Weiss LM. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum Pathol. 2009;40:757–768. doi: 10.1016/j.humpath.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Liao SY, Aurelio ON, Jan K, et al. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–2831. [PubMed] [Google Scholar]
- 22.Lin F, Shi J, Yang XJ, et al. A Useful Panel of Immunohistochemical Markers in Differentiating Papillary Renal Cell Carcinoma from Papillary Urothelial Carcinoma. Mod Pathol. 2008;21:166A. [Google Scholar]
- 23.Lin F, Zhang PL, Yang XJ, et al. Human kidney injury molecule-1 (hKIM-1): a useful immunohistochemical marker for diagnosing renal cell carcinoma and ovarian clear cell carcinoma. Am J Surg Pathol. 2007;31:371–381. doi: 10.1097/01.pas.0000213353.95508.67. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Prichard JW, Shi J, et al. The von Hippel-Lindau Gene Product (pVHL) and Kidney Injury Molecule-1 (KIM-1) Are Useful Diagnostic Markers for Identifying Focal Clear Cell Carcinoma of the Uterus. Mod Pathol. 2009;22:225A. [Google Scholar]
- 25.Loy TS, Phillips RW, Linder CL. A103 immunostaining in the diagnosis of adrenal cortical tumors: an immunohistochemical study of 316 cases. Arch Pathol Lab Med. 2002;126:170–172. doi: 10.5858/2002-126-0170-AIITDO. [DOI] [PubMed] [Google Scholar]
- 26.Marangos IP, Kazaryan AM, Rosseland AR, et al. Should we use laparoscopic adrenalectomy for metastases? Scandinavian multicenter study. J Surg Oncol. 2009;100:43–47. doi: 10.1002/jso.21293. [DOI] [PubMed] [Google Scholar]
- 27.McGregor DK, Khurana KK, Cao C, et al. Diagnosing primary and metastatic renal cell carcinoma: the use of the monoclonal antibody ‘Renal Cell Carcinoma Marker’. Am J Surg Pathol. 2001;25:1485–1492. doi: 10.1097/00000478-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 28.McKenney JK, Fujiwara M, Higgins JP, et al. A cautionary note regarding the use of PAX-2 immunohistochemistry in differentiating metastatic clear cell renal cell carcinoma from adrenal cortical lesions : a tissue microarray study of 245 cases. Mod Pathol. 2009;22:182A. [Google Scholar]
- 29.McKiernan JM, Buttyan R, Bander NH, et al. Expression of the tumor-associated gene MN: a potential biomarker for human renal cell carcinoma. Cancer Res. 1997;57:2362–2365. [PubMed] [Google Scholar]
- 30.Midorikawa S, Sanada H, Hashimoto S, et al. Analysis of cortisol secretion in hormonally inactive adrenocortical incidentalomas: study of in vitro steroid secretion and immunohistochemical localization of steroidogenic enzymes. Endocr J. 2001;48:167–174. doi: 10.1507/endocrj.48.167. [DOI] [PubMed] [Google Scholar]
- 31.Nappi O, Mills SE, Swanson PE, et al. Clear cell tumors of unknown nature and origin: a systematic approach to diagnosis. Semin Diagn Pathol. 1997;14:164–174. [PubMed] [Google Scholar]
- 32.O’Malley RL, Godoy G, Kanofsky JA, et al. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol. 2009;181:2009–2017. doi: 10.1016/j.juro.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Pan CC, Chen PC, Tsay SH, et al. Differential immunoprofiles of hepatocellular carcinoma, renal cell carcinoma, and adrenocortical carcinoma: a systemic immunohistochemical survey using tissue array technique. Appl Immunohistochem Mol Morphol. 2005;13:347–352. doi: 10.1097/01.pai.0000146525.72531.19. [DOI] [PubMed] [Google Scholar]
- 34.Pinkus GS, Etheridge CL, O’Connor EM. Are keratin proteins a better tumor marker than epithelial membrane antigen? A comparative immunohistochemical study of various paraffin-embedded neoplasms using monoclonal and polyclonal antibodies. Am J Clin Pathol. 1986;85:269–277. doi: 10.1093/ajcp/85.3.269. [DOI] [PubMed] [Google Scholar]
- 35.Poleev A, Fickenscher H, Mundlos S, et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development. 1992;116:611–623. doi: 10.1242/dev.116.3.611. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw AA, Granter SR. A comparison of A103 and inhibin reactivity in adrenal cortical tumors: distinction from hepatocellular carcinoma and renal tumors. Mod Pathol. 1998;11:1160–1164. [PubMed] [Google Scholar]
- 37.Renshaw AA, Richie JP. Subtypes of renal cell carcinoma. Different onset and sites of metastatic disease. Am J Clin Pathol. 1999;111:539–543. doi: 10.1093/ajcp/111.4.539. [DOI] [PubMed] [Google Scholar]
- 38.Saeger W, Fassnacht M, Chita R, et al. High diagnostic accuracy of adrenal core biopsy: results of the German and Austrian adrenal network multicenter trial in 220 consecutive patients. Hum Pathol. 2003;34:180–186. doi: 10.1053/hupa.2003.24. [DOI] [PubMed] [Google Scholar]
- 39.Sangoi AR, Karamchandani J, Kim J, et al. The use of immunohistochemistry in the diagnosis of metastatic clear cell renal cell carcinoma: a review of PAX-8, PAX-2, hKIM-1, RCCma, and CD10. Advances in anatomic pathology. 2010;17:377–393. doi: 10.1097/PAP.0b013e3181f89400. [DOI] [PubMed] [Google Scholar]
- 40.Sangoi AR, McKenney JK. A tissue microarray-based comparative analysis of novel and traditional immunohistochemical markers in the distinction between adrenal cortical lesions and pheochromocytoma. Am J Surg Pathol. 2010;34:423–432. doi: 10.1097/PAS.0b013e3181cfb506. [DOI] [PubMed] [Google Scholar]
- 41.Sasano H, Shizawa S, Nagura H. Adrenocortical cytopathology. Am J Clin Pathol. 1995;104:161–166. doi: 10.1093/ajcp/104.2.161. [DOI] [PubMed] [Google Scholar]
- 42.Sasano H, Shizawa S, Suzuki T, et al. Transcription factor adrenal 4 binding protein as a marker of adrenocortical malignancy. Hum Pathol. 1995;26:1154–1156. doi: 10.1016/0046-8177(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 43.Siemer S, Lehmann J, Kamradt J, et al. Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol. 2004;171:2155–2159. doi: 10.1097/01.ju.0000125340.84492.a7. discussion 2159. [DOI] [PubMed] [Google Scholar]
- 44.Sloane JP, Ormerod MG. Distribution of epithelial membrane antigen in normal and neoplastic tissues and it value in diagnostic tumor pathology. Cancer. 1981;47:1786–1795. doi: 10.1002/1097-0142(19810401)47:7<1786::aid-cncr2820470711>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Spagnolo DV, Michie SA, Crabtree GS, et al. Monoclonal anti-keratin (AE1) reactivity in routinely processed tissue from 166 human neoplasms. Am J Clin Pathol. 1985;84:697–704. doi: 10.1093/ajcp/84.6.697. [DOI] [PubMed] [Google Scholar]
- 46.Stelow EB, Debol SM, Stanley MW, et al. Sampling of the adrenal glands by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2005;33:26–30. doi: 10.1002/dc.20273. [DOI] [PubMed] [Google Scholar]
- 47.Tong GX, Yu WM, Beaubier NT, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol. 2009;22:1218–1227. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- 48.Wahner-Roedler DL, Sebo TJ. Renal cell carcinoma: diagnosis based on metastatic manifestations. Mayo Clin Proc. 1997;72:935–941. doi: 10.4065/72.10.935. [DOI] [PubMed] [Google Scholar]
- 49.Weiss LM, Medeiros LJ, Vickery AL., Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13:202–206. doi: 10.1097/00000478-198903000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Wick MR, Cherwitz DL, McGlennen RC, et al. Adrenocortical carcinoma. An immunohistochemical comparison with renal cell carcinoma. Am J Pathol. 1986;122:343–352. [PMC free article] [PubMed] [Google Scholar]
- 51.Wick MR, Ritter JH, Humphrey PA, et al. Clear cell neoplasms of the endocrine system and thymus. Seminars in diagnostic pathology. 1997;14:183–202. [PubMed] [Google Scholar]
- 52.Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003;27:867–881. doi: 10.1097/00000478-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Yang B, Ali SZ, Rosenthal DL. CD10 facilitates the diagnosis of metastatic renal cell carcinoma from primary adrenal cortical neoplasm in adrenal fine-needle aspiration. Diagn Cytopathol. 2002;27:149–152. doi: 10.1002/dc.10153. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Bu H, Chen H, et al. Comparison of immunohistochemical markers in the differential diagnosis of adrenocortical tumors: immunohistochemical analysis of adrenocortical tumors. Appl Immunohistochem Mol Morphol. 2008;16:32–39. doi: 10.1097/PAI.0b013e318032cf56. [DOI] [PubMed] [Google Scholar]


