Abstract
Drip irrigation is broadly extended in order to save water in the arid cotton production region of China. Biochar is thought to be a useful soil amendment to reduce greenhouse gas (GHG) emissions. Here, a field study was conducted to compare the emissions of nitrous oxide (N2O) and methane (CH4) under different irrigation methods (drip irrigation (D) and furrow irrigation (F)) and fertilization regimes (conventional fertilization (C) and conventional fertilization + biochar (B)) during the cotton growth season. The accumulated N2O emissions were significantly lower with FB, DC, and DB than with FC by 28.8%, 36.1%, and 37.6%, while accumulated CH4 uptake was 264.5%, 226.7%, and 154.2% higher with DC, DB, and FC than that with FB, respectively. Irrigation methods showed a significant effect on total global warming potential (GWP) and yield-scaled GWP (P < 0.01). DC and DB showed higher cotton yield, water use efficiency (WUE), and lower yield-scaled GWP, as compared with FC and FB. This suggests that in northwestern China mulched-drip irrigation should be a better approach to increase cotton yield with depressed GHG. In addition, biochar addition increased CH4 emissions while it decreased N2O emissions.
1. Introduction
Crop cultivation stimulates greenhouse gas (GHG) emissions from soil to the atmosphere from agricultural practices such as irrigation and fertilization, which in turn influences the biogeochemical process of carbon and nitrogen (N) in the soil. The emissions of GHG from crop land have been estimated to account for 13.5% of the anthropogenic emissions worldwide [1]. How to reduce GHG emissions from agricultural practices without yield loss is an urgent task for crop production. Improving the cropping practices is a recommended strategy to mitigate greenhouse gas emissions from agricultural soil [1]. However, this strategy is highly dependent on the crops, since the cropping practices varied with crop species [2]. Cotton is one of the major cash crops delivering natural fibers to textile industries around the world. Globally, the harvested area of seed cotton is 32 million ha in 2010 [3]. Considerable field experiments have documented large amount of N2O emitted from cotton field due to high N fertilizer input and immoderate irrigation [4–6].
Soil moisture is one of the key factors affecting GHG production in agricultural soil. An optimal irrigation can reduce GHG emissions by regulating the N and carbon turnover process in soil via manipulating soil moisture. Most of the cotton production is situated in the semiarid or arid areas, where water-saving irrigation is a key issue for the cotton cultivation. Drip irrigation is one of the water-saving irrigation approaches broadly extended in semiarid or arid regions, since it can reduce surface evaporation, surface runoff, and deep percolation [7]. Water and mineral N fertilizer are directly supplied to the crop root zone through drip irrigation system to adapt to the crop requirements, hence improving the water and N use efficiency. Therefore, drip irrigation may have a large influence on the nitrogen and carbon turnover in soil and reduce the N fertilizer-induced N2O or carbon-related greenhouse gas (e.g., CH4) production, in relation to conventional furrow irrigation. For instance, several studies showed that drip irrigation significantly decreased the N2O emission from tomato and melon field, as compared with furrow irrigation [8–10]. However, the N fertilizer application rate is much higher in cotton cultivation than that in the aforementioned crops. The N fertilizer application rate is approximately 300 kg N ha−1 in cotton production area of China, which is nearly two times higher than that in previous studies (120–175 kg N ha−1) [8, 9]. Thus, it is still unknown what the impact of drip irrigation would be on N2O emission under high N fertilizer application conditions.
Biochar is the byproduct of biomass pyrolysis, one of the technologies used to produce bioenergy. It has been suggested that biochar can be a useful soil amendment to improve soil physiochemical properties and crop yield, as well as to increase soil carbon storage and reduce GHG emissions [11–13]. Biochar addition could mitigate or inhibit N2O emission in most studies for increased adsorption of NH4 + or changes in pH that alter the N2O-to-N2 ratio during denitrification [14–16]. However, the effects of biochar on CH4 emissions have yet been inconsistent. Previous study showed that biochar addition to the upland soil increased CH4 emissions by 37% [17]. On the other hand, CH4 uptake increased in some studies after biochar additions [18, 19]. The results for the observed changes in CH4 emissions may contradictorily depend on soil water content, soil type, and biochar type. Till now, few data are available to support these conclusions on the field scale especially for uplands.
Drip irrigation with plastic film mulching is widely recommended as a replacement of the traditional furrow irrigation, because seasonal shortage of irrigation water and low temperature have become critical factors limiting the productivity of cotton crop in this area. However, only a few studies investigated the characteristics of CO2, N2O, and CH4 emissions from cotton field under drip irrigation in China [20–22]. To our knowledge, there are no published field studies on the effect of biochar addition on GHG emissions from cotton field. Thus, the objectives of this study are to (a) investigate the characteristics of N2O and CH4 emissions from cotton field under different irrigation methods and fertilization regimes and (b) compare the integrated effects of different irrigation methods and fertilization regimes on the GHG emissions.
2. Materials and Methods
2.1. The Study Site
A field experiment was carried out at the experimental farm of Shihezi University in Xinjiang Province (45°19′ N, 116°34′ E, 433–437 m in elevation), which locates in the primary cotton production region of China. This region has a dry continental climate with mean annual temperature of 8°C and precipitation of 150 mm, most of which occurs from June to September. The main crops in this area are cotton, wheat, and maize. The soil in the experiment site is heavy loam, and the previous crop is cotton. Some chemical properties for the topsoil sampled at 0–15 cm depth were as follows: soil organic matter, 13.1 g·kg−1; total soil nitrogen, 0.9 g·kg−1; available soil phosphorus, 66.3 mg·kg−1; available soil potassium, 169.8 mg·kg−1.
2.2. Treatments and Field Work
The field experiment comprised two factors during the cotton growing seasons of 2011, including different irrigation methods (furrow irrigation and drip irrigation) and fertilization regimes (conventional fertilization and conventional fertilization + biochar). The four treatments included (1) FC, furrow irrigation (mulch-free) with conventional fertilization; (2) DC, drip irrigation (plastic film mulching) with conventional fertilization; (3) FB, furrow irrigation (mulch-free) with conventional fertilization + biochar; (4) DB, drip irrigation (plastic film mulching) with conventional fertilization + biochar. The experiment was a randomized block design with three replicates. The size of each experimental plot was 40 m2 (5 m × 8 m). As shown in Figure 1, the cotton was planted in narrow row spacing of 30 cm and wide row spacing of 60 cm, with plant spacing of 10 cm. For treatments of DC and DB, transplant plastic film in width of 120 cm covered four rows.
Figure 1.
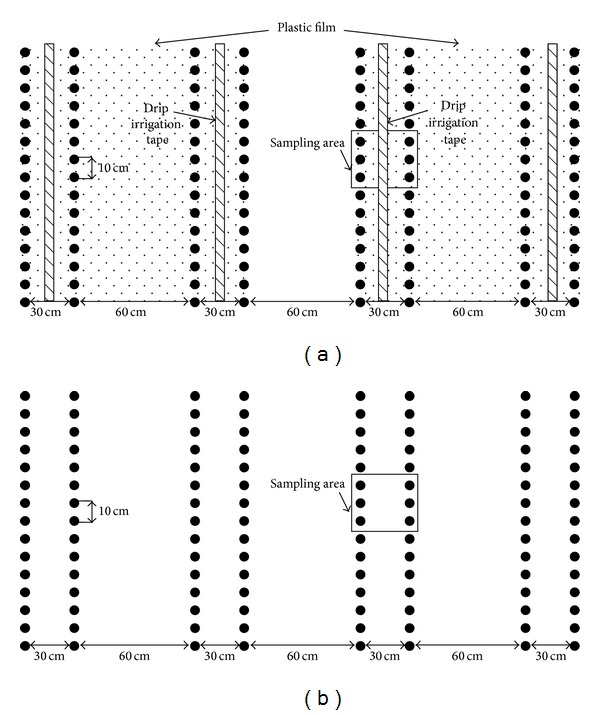
Experimental layout in the cotton field for drip irrigation treatments (DC, DB) (a) and furrow irrigation treatments (FC, FB) (b).
Seeds (Xinjiang cotton cv. number 36) were sown on April 27 and emerged on May 5. The fertilization and irritation were applied according to the local farming regime. The irrigation volumes were 4500 m3 ha−1 and 6000 m3 ha−1 for drip irrigation treatments (DC and DB) and furrow irrigation treatments (FC and FB), respectively. The biochar (Sanli New Energy, China) was applied as basal fertilizer at a rate of 7500 kg hm−2. Chemical fertilizer was applied at the same total rate of diammonium phosphate (300 kg·hm−2), urea (555 kg·hm−2), and potassium dihydrogen phosphate (90 kg·hm−2) for all treatments. Diammonium phosphate was applied as basal fertilizer. The percentages for dressing fertilizer were different for two irrigation methods: the topdressing was applied in three times from June 10 to July 10 for furrow irrigation treatments, while the topdressing was fertigated with the drip irrigation system in several times for the drip irrigation treatments. The detail of fertilization and irrigation for these four treatments was shown in Table 1.
Table 1.
The applications of irrigation and topdressing during the cotton growth period.
| Irrigation date | Drip irrigation treatments | Furrow irrigation treatments | ||||
|---|---|---|---|---|---|---|
| Volume (m3 ·hm−2) | Urea (kg·hm−2) | Potassium dihydrogen phosphate (kg·hm−2) | Volume (m3 ·hm−2) | Urea (kg·hm−2) | Potassium dihydrogen phosphate (kg·hm−2) | |
| 6.10-6.11 | 225 | 30 | 15 | 1050 | 150 | |
| 6.24-6.25 | 300 | 30 | 15 | 1500 | 300 | 60 |
| 7.3 | 300 | 30 | ||||
| 7.9-7.10 | 375 | 60 | 1500 | 105 | 30 | |
| 7.17 | 450 | 75 | ||||
| 7.23-7.24 | 450 | 75 | 1200 | |||
| 8.1 | 450 | 60 | ||||
| 8.5-8.6 | 450 | 60 | 750 | |||
| 8.13 | 375 | 45 | 15 | |||
| 8.21 | 375 | 45 | 15 | |||
| 8.27 | 300 | 30 | 15 | |||
| 9.3 | 225 | 15 | 15 | |||
| 9.10 | 225 | |||||
2.3. Investigation of GHG Emissions
GHG fluxes from cotton field were measured using static chamber and gas chromatography method [23]. The size of chambers was 80 cm × 80 cm × 45 (90) cm (length × width × height); the height of chambers was adapted to cotton plant growth. The gas sampling was carried out between 9:00 and 11:00 hours. Gas samples were drawn from the chambers through a three-way stopcock using an airtight syringe with volume of 50 mL at 0, 10, 20, and 30 min after closure and immediately transferred into 50 mL vacuum glass container. The GHG fluxes from all plots were measured at 7-day interval. The gas samples were analyzed for the concentrations of N2O and CH4 using a gas chromatograph (Agilent 7890, Agilent Technologies, USA) equipped with an electron capture detector (ECD) and a flame ionization detector (FID). The rates of N2O and CH4 flux were calculated by the linear increase of the gas concentration at each sampling time (0, 10, 20, and 30 min); sample sets were rejected unless the correlation coefficient (R 2) for the linear regression is greater than 0.9 (0.7 for small flux rates). All flux rates were adjusted for air temperature, air pressure, and area and volume of the chamber [24]. Average GHG fluxes were calculated by triplicate plots. Seasonal accumulation amounts of GHG emissions were calculated by the emissions between every two adjacent intervals of the measurements.
2.4. Soil Temperature, Moisture, and Mineral N Content
Soil temperature and soil moisture were measured at four different points near the area covered by the chamber. Soil temperature was taken at 5 cm depth. Soil moisture was determined using a TDR (time domain reflectometer) [25].
Surface soil samples (0–20 cm) at the experiment plots close to the chamber covered area were collected for the analysis of soil mineral N (ammonium and nitrate) contents at the same day as the gas sampling during the cotton growth season. Fresh soil samples were extracted with 0.01 M CaCl2 in a 1 : 10 ratio of soil to extractant. The concentrations of ammonium and nitrate in the extract were analyzed using continuous flow analytical system [26]. Cotton yield was recorded at cotton harvest.
2.5. Statistical Analyses
Differences in seasonal N2O and CH4 emissions, soil temperature, soil N mineral contents, and cotton yield as affected by irrigation methods and fertilization regimes were examined by using a two-way analysis of variance (ANOVA). The statistical analysis was carried out using SPSS 20.0 (IBM SPSS Statistics, Chicago, IL, USA).
3. Results
3.1. Soil Characteristics, Cotton Yield, and Water Use Efficiency
The soil moisture under FC and FB was significantly higher than that under DC and DB on June 30, July 14, and July 28, while on other days the soil moisture with FC and FB was close to that with DC and DB (Figure 2(a)). The soil temperatures during cotton growing season were significantly higher with DC and DB than with FC and FB during the bud stage (P < 0.05) (Table 2). As compared with FC, DC showed significantly lower soil temperature during flowering and boll-forming stage (P < 0.05) (Table 2). However, fertilization regimes had no effect on soil temperature. The soil NO3 −-N contents were significantly higher with FC and FB than with DC and DB during the bud stage, and that with FB was significantly higher than those with FC, DC, and DB during the flowering and boll-forming stage (P < 0.05) (Table 2, Figure 3(b)), which was caused by the topdressing of N fertilizer in furrow irrigation plots. This topdressing event also led to a peak in soil NH4 +-N contents with FC and FB on June 23 and June 30 (Figure 3(c)).
Figure 2.
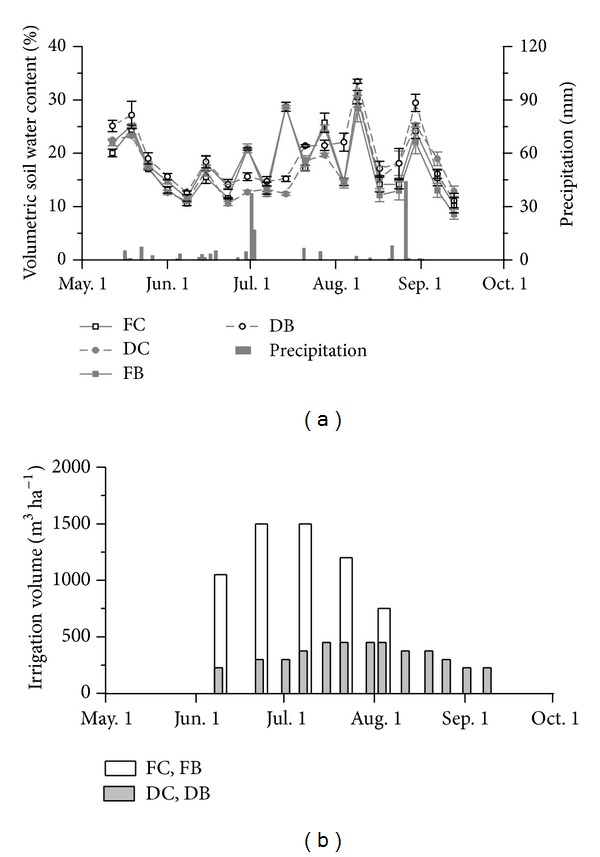
Effects of different irrigation methods and fertilization regimes on soil moisture, precipitation, and volume of irrigation water during cotton growing season.
Table 2.
Effects of different irrigation methods and fertilization regimes on major soil characteristics. Values are means ± standard deviation of three replicates. Different small letters in the same column refer to significant difference between treatments at P < 0.05 level.
| Seedling stage | Bud stage | Flowering and boll-forming stage | Boll opening stage | |
|---|---|---|---|---|
| Soil moisture (%) | ||||
| FC | 16.79 ± 0.37b | 18.63 ± 0.25a | 20.46 ± 1.04a | 16.00 ± 1.25b |
| DC | 17.28 ± 0.05b | 12.24 ± 0.13c | 19.79 ± 1.00a | 18.81 ± 1.13a |
| FB | 18.19 ± 0.35ab | 18.92 ± 0.35a | 19.52 ± 0.85a | 14.16 ± 1.50b |
| DB | 19.65 ± 0.71a | 14.93 ± 0.69b | 23.11 ± 0.82a | 18.70 ± 1.56a |
| Soil temperature (°C) | ||||
| FC | 24.01 ± 0.33a | 24.55 ± 0.12b | 21.2 ± 0.12b | 18.53 ± 0.10a |
| DC | 23.98 ± 0.12a | 26.29 ± 0.25a | 22.55 ± 0.15a | 18.29 ± 0.27a |
| FB | 24.28 ± 0.19a | 24.41 ± 0.36b | 21.78 ± 0.37ab | 18.03 ± 0.37a |
| DB | 23.92 ± 0.09a | 25.80 ± 0.37a | 22.11 ± 0.33ab | 18.00 ± 0.05a |
| Soil NO3 −-N (mg kg−1) | ||||
| FC | 100.82 ± 4.03a | 145.82 ± 3.52a | 56.38 ± 6.68b | 26.94 ± 4.18a |
| DC | 108.44 ± 3.39a | 19.80 ± 1.44b | 46.11 ± 1.29b | 38.77 ± 5.15a |
| FB | 98.24 ± 6.86a | 155.94 ± 12.38a | 76.94 ± 2.49a | 37.26 ± 0.12a |
| DB | 104.75 ± 5.31a | 25.54 ± 0.80b | 41.13 ± 4.90b | 39.19 ± 6.45a |
| Soil NO4 +-N (mg kg−1) | ||||
| FC | 2.14 ± 0.28a | 1.98 ± 0.41ab | 1.38 ± 0.19a | 0.46 ± 0.08a |
| DC | 2.14 ± 0.21a | 1.07 ± 0.13b | 0.96 ± 0.01a | 0.36 ± 0.08a |
| FB | 2.17 ± 0.05a | 2.29 ± 0.16a | 1.24 ± 0.24a | 0.34 ± 0.05a |
| DB | 2.04 ± 0.11a | 1.29 ± 0.06b | 1.01 ± 0.12a | 0.57 ± 0.16a |
Figure 3.
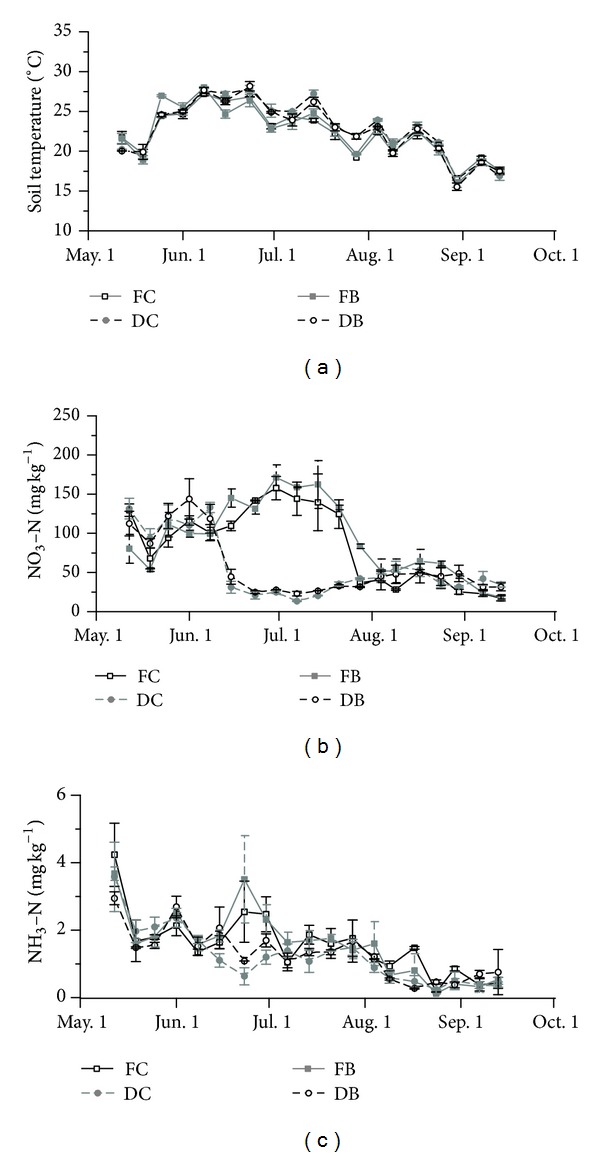
Effects of different irrigation methods and fertilization regimes on soil temperature and mineral N contents during cotton growing season.
Although there was no significant difference between the cotton yield among these four treatments, the water use efficiency (WUE) calculated on cotton yield per unit irrigation volume was significantly higher with DC and DB than that with FC by 53.8% and 60.2%, respectively (P < 0.05) (Table 3).
Table 3.
Effects of different irrigation methods and fertilization regimes on cotton yield and water use efficiency. Values are means ± standard deviation of three replicates. Different small letters in the same column refer to significant difference between treatments at P < 0.05 level.
| FC | DC | FB | DB | |
|---|---|---|---|---|
| Cotton yield (Mg ha−1) | 1.76 ± 0.16a | 2.02 ± 0.10a | 1.94 ± 0.17a | 2.11 ± 0.14a |
| Water use efficiency (kg m−3) | 0.29 ± 0.03b | 0.45 ± 0.02a | 0.32 ± 0.03b | 0.47 ± 0.03a |
3.2. N2O Fluxes
The N2O flux rates with FC varied from 7.0 μg m−2 h−1 to 320.9 μg m−2 h−1 during the cotton growing season, with two flux peaks on June 30 and July 21 (Figure 4(a)). As compared with FC, the N2O flux rates of FB were relatively stable and lower. Under DC, the N2O flux rates were relatively lower among the sampling dates comparing to FC, except a flux peak on August 9. On most days, the N2O flux rates with DB were close to that with DC (Figure 4(a)). The accumulated N2O emissions during the cotton growing season were significantly lower with FB, DC, and DB than that with FC by 28.8%, 36.1%, and 37.6%, respectively (P < 0.05) (Table 4). At different growth stages, the highest N2O flux rate under FC, DC, and DB appeared at the flowering and boll-forming stage, while FB appeared at the bud stage (Figure 4(b)). The N2O flux rate with FC performed differently with other three treatments at the flowering and boll-forming stage and the bud stage (P < 0.05). No significant difference was observed between DC and DB during the whole period.
Figure 4.
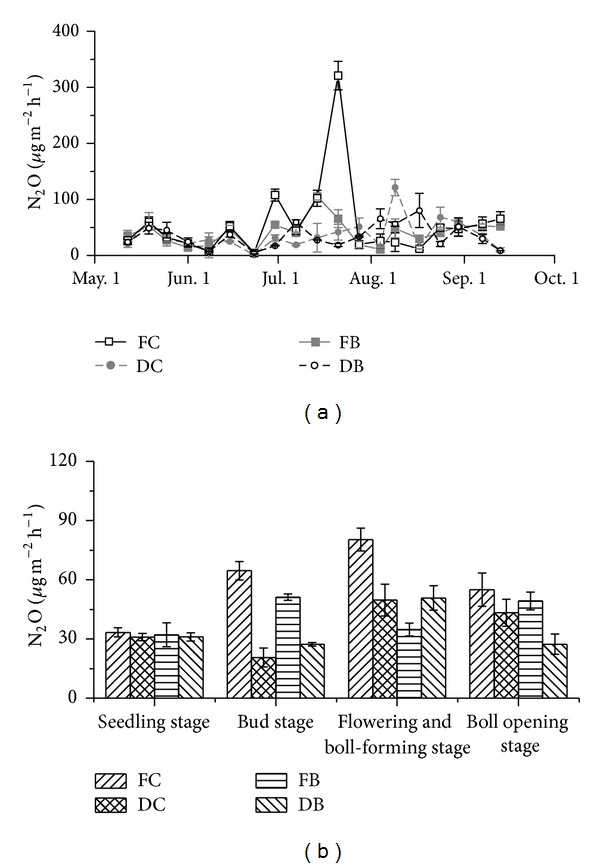
Effects of different irrigation methods and fertilization regimes on N2O flux rates during cotton growing season.
Table 4.
Effects of different irrigation methods and fertilization regimes on accumulated N2O and CH4 emissions during cotton growing season. Values are means ± standard deviation of three replicates. Different small letters in the same column refer to significant difference between treatments at P < 0.05 level.
| N2O (kg ha−1) | CH4 (kg ha−1) | |
|---|---|---|
| FC | 1.71 ± 0.13a | −2.92 ± 0.96ab |
| DC | 1.09 ± 0.11b | −8.87 ± 1.85b |
| FB | 1.21 ± 0.07b | 5.39 ± 4.91a |
| DB | 1.04 ± 0.06b | −6.84 ± 1.07b |
3.3. CH4 Fluxes
The CH4 flux rates under FC, DC, and DB were below zero on most sampling days, indicating that cotton fields were the sink of CH4 except under FB for most of the time of the cotton growing season (Figure 5(a)). The CH4 flux rates were similar under the four treatments in May and June but diverged after June. The highest uptake of CH4 appeared at the flowering and boll-forming stage under FC and DB, while it appeared at the bud stage under DC (Figure 5(b)). The accumulated CH4 emissions during the cotton growing season were significantly lower with DC and DB than that with FB by 264.5% and 226.7%, respectively (P < 0.05) (Table 4). Although the accumulated CH4 emission under FC was 154.2% lower than that with FB, there was little difference between the two treatments.
Figure 5.
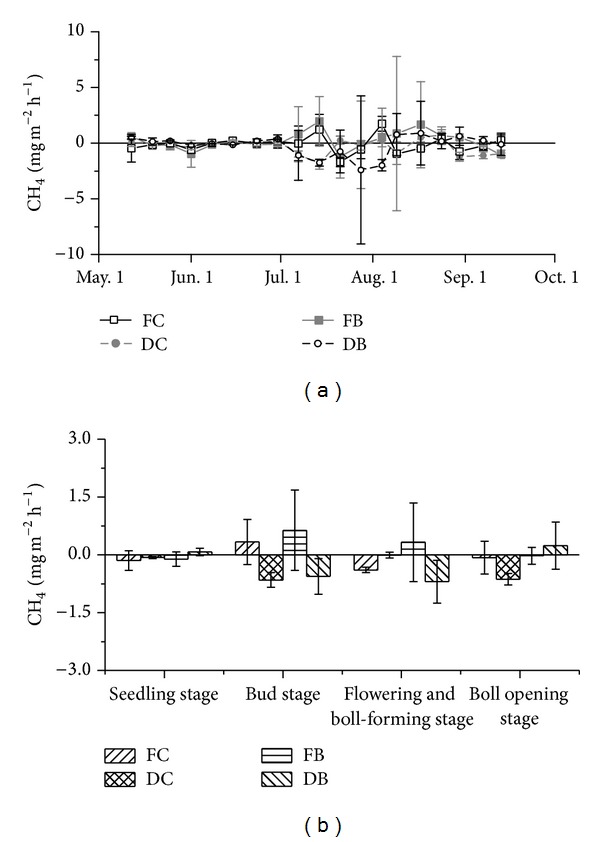
Effects of different irrigation methods and fertilization regimes on CH4 flux rates during cotton growing season.
3.4. Yield-Scaled GWP
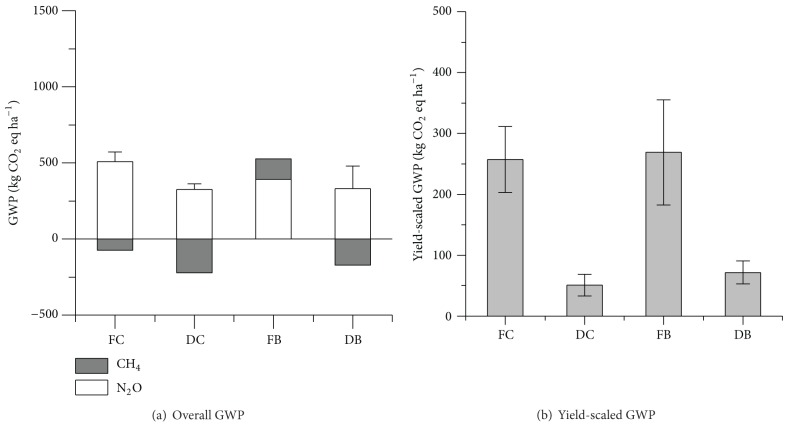
The total GWP of N2O and CH4 emissions during cotton growing season was 102.89, 146.23, 435.09, and 496.49 under treatments of DC, DB, FC, and FB, respectively (Figure 6(a)). Cotton fields under DC, DB, and FC were all sinks for CH4, which reduced the contribution of N2O emission to the overall GWP by 68.3%, 53.9%, and 14.4%, respectively. The yield-scaled GWP calculated by GWP per unit cotton yield with FC and FB were significantly higher than those with DC and DB (P < 0.01) (Figure 6(b)). As compared with FC, DC and DB were 80.1% and 72.2% lower in yield-scaled GWP, respectively. Irrigation methods showed extremely significant effect on the total GWP and yield-scaled GWP (P < 0.01), while fertilization regimes had no effect on both.
Figure 6.
Overall GWP of GHGs (a) and yield-scaled GWP (b) for different treatments. The error bar in (a) was the standard error of overall GWP of N2O and CH4 emissions.
4. Discussion
In the present study, it was observed that the soil temperature during cotton growing season was higher with DC and DB than with FC and FB in most of the time during cotton growing season for the plastic film mulching. A similar result was found in the previous study for different irrigation methods in maize field in China [27]. The plastic film usually prevents the evaporation of soil moisture [22]. However, the difference of the soil moisture between four treatments was primarily attributed to the water supply regimes (Figure 2). The plastic film showed little effect on maintenance of soil moisture in the present study. Biochar can efficiently retain soil moisture due to its special physical structure [18], which was consistent with our results under DC and DB.
The emissions of N2O and CH4 from cotton field were investigated under different irrigation methods and fertilization regimes in an arid area of northwestern China. The accumulated N2O emissions during cotton growth season in this study were lower than that from semiarid cotton field in northern China [4] and arid cotton fields in Uzbekistan [6] but higher than the N2O emissions from semiarid cotton field in Pakistan [5]. The variance in N2O emission among different ecosites might be attributed to the difference in N fertilizer application rate, irrigation, climate factors, and soil properties [28–33]. Here, lower level of N2O emission from cotton field was found under FB, DC, and DB, in relation to FC with a high N fertilizer application rate (300 kg N ha−1) (Table 4). The relatively lower N2O emission from cotton field under drip irrigation treatments was mainly attributed to the water supply regime and N fertilizer dressing method (so-called fertigation) in drip irrigation system, which favored decrease in N2O emission. In detail, the soil moisture was lower in cotton field with drip irrigation treatments than that with FC during the bud stage, which was the main fertilization time of FC. Previous studies reported that soil moisture is a key factor in regulation of N2O emission from agricultural soil. For instance, the N2O emission was enhanced along with increased soil moisture in a given range, due to the improved denitrification [34–36]. Therefore, the relatively lower soil moisture in cotton field with DC and DB during the bud stage could reduce the N2O emission more effectively than that with FC. Furthermore, the decreased N fertilizer was directly fertigated to the rhizosphere of cotton plants in drip irrigation treatments with more times but less application rate per time. This N fertilizer dressing method could also improve N uptake of cotton plant but decrease the soil inorganic N pool (NO3 −-N and NH4 +-N) (Table 2) and hence reduce N source for N2O emission, which was significantly correlated with soil N [37]. However, the observed reduction in N2O emission caused by drip irrigation was less than that in previous studies conducted in the melon and tomato fields [8–10]. This might be related to higher N fertilizer application rate in this study (300 kg N ha−1), suggesting that the mitigation effect of drip irrigation on N2O may be depressed with a higher N fertilizer application rate.
Although FB and FC used the same irrigation system, FB showed lower N2O emission than FC for the addition of biochar. A similar result was found in wheat field [14, 15]. However, the effect of biochar on N2O emissions under DB and DC was covered up by the effect of drip irrigation. Biochar had been shown to efficiently retain NH4 + via cation exchange by its developed specific surface area and surface negative charge density [38]. Then the retained N would be slowly released for plant growth; thus, biochar could coordinate the mineral N availability and plant uptake. This would reduce the amount of N available for denitrification and lost as N2O [18]. On the other hand, decreases in emissions of N2O in soil amended with biochar might be attributed to improved aeration and porosity for its developed microstructure, which might lead to lower denitrification rates and alter the N2O-to-N2 ratio during denitrification [16].
The present study indicated that the arid cotton fields under FC, DC, and DB were the sinks of CH4, while FB was the source of CH4. This was consistent with the results of previous studies with different irrigation methods conducted in upland field [39–41]. However, drip irrigation was a source of CH4 in the study in cotton field [22], which was inconsistent with the present results. This might be related to the different rate of irrigation and fertilization. Upland fields are normally net sinks for CH4, since the consumption exceeds the production in CH4 [42]. The dry soil in upland field limited the CH4 emission; however, it did have a possibility to become a source after rainfall within several days or weeks [43, 44]. Comparing the CH4 emission between DC and FC, it was shown that DC increased the uptake of CH4 for the relative lower soil moisture in most of the time during cotton growing season (Figure 2(a)). The main factor affecting CH4 uptake in summer was soil moisture [45], which plays a critical role in CH4 consumption [46]. Decrease in soil moisture enhanced CH4 oxidation through improving CH4 diffusion from atmosphere into soil pore spaces [47] and through gas diffusivity to control microbial oxidation, which changes inversely with soil moisture [48, 49].
In our study, the addition of biochar increased CH4 emissions by 284.6% higher with FB than that with FC, while the uptake of CH4 with DB was 22.9% lower than that with DC. The result was in good agreement with previous study that biochar addition promoted CH4 emissions of the upland soil [17]. However, other studies had reported that biochar amendment reduces CH4 emissions as compared with the control [18, 19]. The inconsistence might be attributed to the soil type, agricultural management, and biochar type. On one hand, the addition of biochar increased the substrate supply and created a favorable environment for methanogenic activity. On the other hand, the CH4 emission from acid soil was usually much lower than that from neutral soil [50]. Biochar addition could increase soil PH, which was a benefit for methanogenic bacteria. Furthermore, increased soil aeration due to increased porosity could increase CH4 diffusion. Biochar might play a more important role under paddy fields, compared with straw returning directly, which would increase CH4 emission obviously [51].
There was significant difference between drip irrigation treatments and furrow irrigation treatments in the total GWP of N2O and CH4 emissions (P < 0.01), while fertilization regimes showed little effect (Figure 6). The average yield-scaled GWP was 80.1% and 72.2% lower with DC and DB than that with FC, indicating that drip irrigation could mitigate the yield-scaled GHG emissions from cotton field. In previous studies, cotton yield was higher with drip irrigation than that with furrow irrigation [52], which agreed with our study. Although the difference of cotton yield between four treatments was insignificant, WUE was significantly higher with DC and DB than that with FC by 53.8% and 60.2%, respectively (P < 0.05) (Table 3). WUE represented in this study (from 0.263 kg m−3 to 0.527 kg m−3) were lower than that from the studies in Turkey, where it ranged from 0.508 kg m−3 to 0.648 kg m−3 or from 0.76 kg m−3 to 1.46 kg m−3 [52, 53]. The variance in WUE in different ecosites could be related to climate, plant number, varietal differences, and irrigation amount [54]. It indicated that drip irrigation significantly increased WUE of cotton plants, in relation to furrow irrigation. The relative higher WUE with DC and DB was related to improved fertilizer efficiency and depressed leaching potential in drip irrigation system [55].
5. Conclusions
Irrigation methods significantly affected the GHG emissions from cotton field in arid northwestern China. Biochar addition increased CH4 emissions and decreased N2O emissions. The water supply and N fertilizer dressing method played a key role in regulating gas emissions. Drip irrigation treatments (DC and DB) remarkably reduced GHG emissions, compared with furrow irrigation treatments (FC and FB). In addition, drip irrigation treatments (DC and DB) had higher yield and WUE, compared to furrow irrigation treatments (FC and FB). Thus, mulched-drip irrigation with conventional fertilization or conventional fertilization + biochar should be a better approach to increase cotton yield with depressed GHG emissions in arid northwestern China.
Acknowledgment
This work was financially supported by the opened subject of Xinjiang Key Laboratory of Water Cycle and Utilization in Arid Zone (Grant no. XJYS0907-2010-03).
Abbreviations
- GHG:
Greenhouse gas
- GWP:
Global warming potential
- WUE:
Water use efficiency.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.IPCC. Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 2.Bouwman AF, Boumans LJM, Baffes NH. Global Estimates of Gaseous Emissions of NH3, NO and N2O from Agricultural Land. Rome, Italy: Food and Agriculture Organisation; 2001. [Google Scholar]
- 3.FAOSTAT. 2002, http://faostat.fao.org/
- 4.Liu C, Zheng X, Zhou Z, et al. Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China. Plant and Soil. 2010;332(1-2):123–134. [Google Scholar]
- 5.Mahmood T, Ali R, Iqbal J, Robab U. Nitrous oxide emission from an irrigated cotton field under semiarid subtropical conditions. Biology and Fertility of Soils. 2008;44(5):773–781. [Google Scholar]
- 6.Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R. Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: influence of nitrogen applications and irrigation practices. Soil Biology and Biochemistry. 2008;40(2):290–301. [Google Scholar]
- 7.Li J, Zhang J, Ren L. Water and nitrogen distribution as affected by fertigation of ammonium nitrate from a point source. Irrigation Science. 2003;22(1):19–30. [Google Scholar]
- 8.Kallenbach CM, Rolston DE, Horwath WR. Cover cropping affects soil N2O and CO2 emissions differently depending on type of irrigation. Agriculture, Ecosystems and Environment. 2010;137(3-4):251–260. [Google Scholar]
- 9.Sánchez-Martín L, Arce A, Benito A, Garcia-Torres L, Vallejo A. Influence of drip and furrow irrigation systems on nitrogen oxide emissions from a horticultural crop. Soil Biology and Biochemistry. 2008;40(7):1698–1706. [Google Scholar]
- 10.Sanchez-Martín L, Meijide A, Garcia-Torres L, Vallejo A. Combination of drip irrigation and organic fertilizer for mitigating emissions of nitrogen oxides in semiarid climate. Agriculture, Ecosystems and Environment. 2010;137(1-2):99–107. [Google Scholar]
- 11.Lehmann J. Bio-energy in the black. Frontiers in Ecology and the Environment. 2007;5(7):381–387. [Google Scholar]
- 12.Fowles M. Black carbon sequestration as an alternative to bioenergy. Biomass and Bioenergy. 2007;31(6):426–432. [Google Scholar]
- 13.Rondon M, Ramirez JA, Lehmann J. Charcoal additions reduce net emissions of greenhouse gases to the atmosphere. Proceedings of the 3rd USDA Symposium on Greenhouse Gases and Carbon Sequestration; March 2005; Baltimore, Md, USA. p. p. 208. [Google Scholar]
- 14.Aguilar-Chávez Á, Díaz-Rojas M, del Rosario Cárdenas-Aquino M, Dendooven L, Luna-Guido M. Greenhouse gas emissions from a wastewater sludge-amended soil cultivated with wheat (Triticum spp. L.) as affected by different application rates of charcoal. Soil Biology and Biochemistry. 2012;52:90–95. [Google Scholar]
- 15.Castaldi S, Riondino M, Baronti S, et al. Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere. 2011;85(9):1464–1471. doi: 10.1016/j.chemosphere.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. Journal of Environmental Quality. 2010;39(4):1224–1235. doi: 10.2134/jeq2009.0138. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Pan X, Liu Y, Zhang X, Xiong Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant and Soil. 2012;360(1-2):287–298. [Google Scholar]
- 18.Karhu K, Mattila T, Bergström I, Regina K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—results from a short-term pilot field study. Agriculture, Ecosystems and Environment. 2011;140(1-2):309–313. [Google Scholar]
- 19.Scheer C, Grace PR, Rowlings DW, Kimber S, van Zwieten L. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant and Soil. 2011;345(1):47–58. [Google Scholar]
- 20.Li Z, Zhang R, Wang X, Wang J, Zhang C, Tian C. Carbon dioxide fluxes and concentrations in a cotton field in northwestern China: effects of plastic mulching and drip irrigation. Pedosphere. 2011;21(2):178–185. [Google Scholar]
- 21.Zhang Q, Yang L, Wang J, Luo H, Zhang Y, Zhang W. Effects of different irrigation methods and fertilization measures on soil respiration and its component contribution in cotton field in arid region. Scientia Agricultura Sinica. 2012;25(12):2420–2430. [Google Scholar]
- 22.Li Z, Zhang R, Wang X, Chen F, Lai D, Tian C. Effects of plastic film mulching with drip irrigation on N2O and CH4 emissions from cotton fields in arid land. Journal of Agricultural Science. 2013 [Google Scholar]
- 23.FAO/IAEA. Measurement of Methane and Nitrous Oxide Emission from Agriculture, A Joint Undertakong by the Food and Agriculture Organization of United Nations and International Atomic Energy Agency. Vienna, Austria: International Atomic Energy Agency; 1992. [Google Scholar]
- 24.Huang Y, Jiang J, Zong L, Zhou Q, Sass RL, Fisher FM. Influence of planting density and precipitation on N2O emission from a winter wheat field. Environmental Science. 2001;22(6):20–23. [PubMed] [Google Scholar]
- 25.Topp GC, Davis JL, Annan AP. Electromagnetic determination of soil water content: measurements in coaxial transmission lines. Water Resources Research. 1980;16(3):574–582. [Google Scholar]
- 26.Lu R. Soil Agricultural Chemistry Analytical Method. Beijing, China: China Agricultural Science and Technology Press; 2000. [Google Scholar]
- 27.Zhou L, Li F, Jin S, Song Y. How two ridges and the furrow mulched with plastic film affect soil water, soil temperature and yield of maize on the semiarid Loess Plateau of China. Field Crops Research. 2009;113(1):41–47. [Google Scholar]
- 28.Clemens J, Schillinger MP, Goldbach H, Huwe B. Spatial variability of N2O emissions and soil parameters of an arable silt loam—a field study. Biology and Fertility of Soils. 1999;28(4):403–406. [Google Scholar]
- 29.Inubushi K, Barahona MA, Yamakawa K. Effects of salts and moisture content on N2O emission and nitrogen dynamics in Yellow soil and Andosol in model experiments. Biology and Fertility of Soils. 1999;29(4):401–407. [Google Scholar]
- 30.Jørgensen BJ, Jørgensen RN. Field-scale and laboratory study of factors affecting N2O emissions from a rye stubble field on sandy loam soil. Biology and Fertility of Soils. 1997;25(4):366–371. [Google Scholar]
- 31.Mosier AR, Zhu Z. Changes in patterns of fertilizer nitrogen use in Asia and its consequences for N2O emissions from agricultural systems. Nutrient Cycling in Agroecosystems. 2000;57(1):107–117. [Google Scholar]
- 32.Ruser R, Flessa H, Schilling R, Beese F, Munch JC. Effect of crop-specific field management and N fertilization on N2O emissions from a fine-loamy soil. Nutrient Cycling in Agroecosystems. 2001;59(2):177–191. [Google Scholar]
- 33.Song C, Zhang J. Effects of soil moisture, temperature, and nitrogen fertilization on soil respiration and nitrous oxide emission during maize growth period in northeast China. Acta Agriculturae Scandinavica B: Soil and Plant Science. 2009;59(2):97–106. [Google Scholar]
- 34.Linn DM, Doran JW. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Science Society of America Journal. 1984;48(6):1267–1272. [Google Scholar]
- 35.Liu XJ, Mosier AR, Halvorson AD, Reule CA, Zhang FS. Dinitrogen and N2O emissions in arable soils: effect of tillage, N source and soil moisture. Soil Biology and Biochemistry. 2007;39(9):2362–2370. [Google Scholar]
- 36.Sánchez-Martín L, Vallejo A, Dick J, M Skiba U. The influence of soluble carbon and fertilizer nitrogen on nitric oxide and nitrous oxide emissions from two contrasting agricultural soils. Soil Biology and Biochemistry. 2008;40(1):142–151. [Google Scholar]
- 37.Mu Z, Kimura SD, Toma Y, Hatano R. Nitrous oxide fluxes from upland soils in central Hokkaido, Japan. Journal of Environmental Sciences. 2008;20(11):1312–1322. doi: 10.1016/s1001-0742(08)62227-5. [DOI] [PubMed] [Google Scholar]
- 38.Liang B, Lehmann J, Solomon D, et al. Black carbon increases cation exchange capacity in soils. Soil Science Society of America Journal. 2006;70(5):1719–1730. [Google Scholar]
- 39.Boeckx P, Van Cleemput O. Estimates of N2O and CH4 fluxes from agricultural lands in various regions in Europe. Nutrient Cycling in Agroecosystems. 2001;60(1–3):35–47. [Google Scholar]
- 40.MacDonald JA, Skiba U, Sheppard LJ, et al. The effect of nitrogen deposition and seasonal variability on methane oxidation and nitrous oxide emission rates in an upland spruce plantation and moorland. Atmospheric Environment. 1997;31(22):3693–3706. [Google Scholar]
- 41.Whalen SC. Influence of N and non-N salts on atmospheric methane oxidation by upland boreal forest and tundra soils. Biology and Fertility of Soils. 2000;31(3-4):279–287. [Google Scholar]
- 42.Megonigal JP, Guenther AB. Methane emissions from upland forest soils and vegetation. Tree Physiology. 2008;28(4):491–498. doi: 10.1093/treephys/28.4.491. [DOI] [PubMed] [Google Scholar]
- 43.Davidson EA, Ishida FY, Nepstad DC. Effects of an experimental drought on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Global Change Biology. 2004;10(5):718–730. [Google Scholar]
- 44.Wang FL, Bettany JR. Methane emission from Canadian prairie and forest soils under short term flooding conditions. Nutrient Cycling in Agroecosystems. 1997;49(1–3):197–202. [Google Scholar]
- 45.Brumme R, Borken W. Site variation in methane oxidation as affected by atmospheric deposition and type of temperate forest ecosystem. Global Biogeochemical Cycles. 1999;13(2):493–501. [Google Scholar]
- 46.Adamsen APS, King GM. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Applied and Environmental Microbiology. 1993;59(2):485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD. Factors controlling atmospheric methane consumption by temperate forest soils. Global Biogeochemical Cycles. 1995;9(1):1–10. [Google Scholar]
- 48.Ball BC, Smith KA, Klemedtsson L, et al. The influence of soil gas transport properties on methane oxidation in a selection of northern European soils. Journal of Geophysical Research D. 1997;102(19):23309–23317. [Google Scholar]
- 49.Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. European Journal of Soil Science. 2003;54(4):779–791. [Google Scholar]
- 50.Feng H, Cheng G, An L. Microbial-mediated methane cycle in soils and global change: a review. Journal of Glaciology and Geocryology. 2006;26(4):411–419. [Google Scholar]
- 51.Yan Y, Wang D, Zheng J. Advances in effects of biochar on the soil N2O and CH4 emissions. Chinese Agricultural Science Bulletin. 2013;29(8):140–146. [Google Scholar]
- 52.Cetin O, Bilgel L. Effects of different irrigation methods on shedding and yield of cotton. Agricultural Water Management. 2002;54(1):1–15. [Google Scholar]
- 53.Dağdelena N, Başalb H, Yılmaza E, Gürbüza T, Akçaya S. Different drip irrigation regimes affect cotton yield, water use efficiency and fiber quality in western Turkey. Agricultural Water Management. 2009;96(1):110–120. [Google Scholar]
- 54.Ibragimov N, Evett SR, Esanbekov Y, Kamilov BS, Mirzaev L, Lamers JPA. Water use efficiency of irrigated cotton in Uzbekistan under drip and furrow irrigation. Agricultural Water Management. 2007;90(1-2):112–120. [Google Scholar]
- 55.Camp CR, Bauer PJ, Hunt PG. Subsurface drip irrigation lateral spacing and management for cotton in the southeastern Coastal plain. Transactions of the American Society of Agricultural Engineers. 1997;40(4):993–999. [Google Scholar]