Abstract
Breast-conservation surgery (BCS) is now utilized in patients with stage I and II invasive breast cancer. However, positive surgical margins are associated with recurrence, and therefore some form of localized postoperative therapy (radiation/chemotherapy) is necessary to eliminate remaining cancer cells. Existing modalities have significant treatment-limiting side effects; therefore, alternative forms of localized therapy need to be explored. We studied the ex vivo effects of photochemical internalization (PCI) using 4 chemotherapeutic agents: cisplatin, cisplatin analog [D prostanoid, DP], doxorubicin, and bleomycin) on 3 breast cancer cell lines: MCF-7, MDA-MB-435, and MDA-MB-231. Illumination was carried out using a 670-nm diode laser at 5 mW/cm2 following incubation in the photosensitizer with aluminum phthalocyanine disulfonate. Toxicity was investigated using colony-forming assays and the mechanism of cell death was determined using Annexin flow-cytometry. We found that toxicity of DP and bleomycin was significantly enhanced by PCI compared with drug alone but was unchanged for cisplatin and doxorubicin. PCI treatment caused a decrease in the percentage of viable cells, predominantly by enhancing apoptosis. The action was synergistic across all 3 cell lines tested for DP and bleomycin. Thus, with appropriate delivery devices and choice of chemotherapeutic agents, PCI holds the promise of enhancing tumor cell toxicity surrounding the cavity of BCS resection sites and thereby decreasing local recurrence.
Keywords: breast cancer chemotherapy, photochemical internalization, photodynamic therapy, chemotherapeutic resistance
I. INTRODUCTION
Breast conservation surgery (BCS) is now recognized as the preferred therapy for patients with stage I and II invasive breast cancer.1 Numerous studies have shown that positive surgical margins are associated with tumor recurrence and, therefore, approximately 40% of patients undergoing BCS return for a re-excision surgery.2–4 Following BCS, some form of localized postoperative therapy such as radiation (RT), chemotherapy, or both is used in the hope of eliminating any remaining malignant cells.5–9 The use of ionizing RT, though effective, has several limitations, as does the dose-limiting toxicity of many of the chemotherapeutic agents in use as postoperative therapy.10–14 In this study we investigated the ability of a light-based approach known as photochemical internalization (PCI) to increase the local efficacy of cisplatin (CP) and one of its analogs, dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum (DP) on several breast tumor cell lines in vitro. The mechanism of cellular toxicity was investigated, and these results were compared with the PCI delivery of 2 other chemotherapy agents (doxorubicin [Dox] and bleomycin [BLM]).
Although CP is one of the most effective and most prescribed antitumor agents in use today, its application is limited because of toxic side effects (renal toxicity, emesis, neurotoxicity, bone marrow suppression, and hearing loss).15–18 In addition, cellular resistance to platinum has resulted in its limited spectrum of activity against several types of cancer.19,20 As a consequence of the limitations of CP, numerous analogs have been developed and characterized with the goal of finding compounds that could be more effective and less toxic.21,22 To address these issues and to improve clinical outcome, alternate methods to reduce the limitations of CP and its analogs would be desirable.
PCI is a novel technology that is under development for utilizing the properties of photodynamic therapy (PDT) to enhance the drug delivery of macromolecules in a site-specific manner.23 Many anticancer agents are limited in their ability to penetrate cell membrane structures and are transported into cells by endocytosis, resulting in their accumulation in intracellular endocytic vesicles (endosomes).24 Specially designed photosensitizers, such as aluminum phthalocyanine disulfonate (AlPcS2a), which localize preferentially in the cell membrane, therefore will be incorporated in the endosomal membranes inside the cell.25 Exposure to light leads to endosomal rupture by phototoxic damage and the release of its contents into the cytosol. The released macromolecules can exert their full biological activity instead of being degraded by lysosomal hydrolases.26 PCI-based relocation and activation of macromolecules has the advantage of reducing drug-induced side effects because the effect is localized to the area exposed to light and because of the possibility of using lower drug concentrations due to increased efficacy. PCI of BLM has proved superior to PDT for sterilizing the tumor bed after cytoreductive surgery.27
II. MATERIALS AND METHODS
A. Cell Culture
Human breast cancer cell lines MCF-7 (estrogen receptor–positive breast epithelial adenocarcinoma), MDA-MB-435 (estrogen receptor–negative breast ductal adenocarcinoma), and MDA-MB-231 (estrogen receptor–negative breast epithelial adenocarcinoma) were obtained from the American Culture Type Collection. All cell lines were grown in minimum essential medium (Invitrogen, Carlsbad, California) supplemented with 10% fetal bovine serum (Invitrogen), penicillin- streptomycin (Invitrogen), and 25 mM HEPES buffer (pH 7.4; Sigma-Aldrich, St. Louis, Missouri). Cells were washed with phosphate buffered saline (PBS; Invitrogen) and treated with 0.25% trypsin-ethylenediaminetetraacetic acid (Invitrogen) to allow for detachment from plastic.
B. Drug Preparation
Cis-diamminedichloroplatinum (II) (Sigma) was dissolved in DMSO (≥ 99.9% A.C.S Spectrophotometric grade, Sigma-Aldrich) to obtain a stock solution of 50 mM and diluted in sterile-filtered dimethyl sulfoxide (DMSO) for stock concentrations of 5 mM, 0.5 mM, 0.05 mM, and 0.005 mM.
The CP analog DP (synthesized in-house at the University of Nevada, Las Vegas) was dissolved in DMSO to obtain a stock solution of 5 mM. All solutions were sterile filtered and diluted in sterile-filtered DMSO to obtain stock solutions of 0.5 mM, 0.05 mM, and 0.005 mM. The structures of CP and DP are shown in Fig. 1. Dox and BLM were purchased from Sigma.
FIGURE 1.
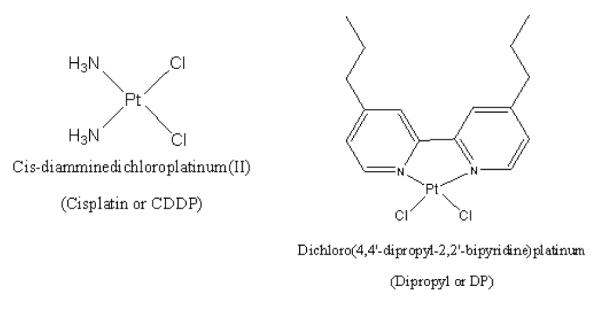
The structure of Cis-diamminedichloroplatinum (II) and cisplatin dipropyl
C. PDT/PCI Treatment
Cells from the various cell lines were plated in 35-mm Petri dishes and incubated overnight to allow them to adhere. The cells were then incubated in 1 μg/mL AlPcS2a (Frontier Scientific, Inc., Logan, UT) and Dulbecco’s modified Eagle medium for 18 hours. Following incubation, cells were washed 3 times with PBS, and 5 mL of fresh medium was added, followed by 1 to 4 hours of incubation with the drug to be tested at various concentrations. PDT controls received no drug. Irradiation was performed with 670-nm light from a diode laser (Intense, North Brunswick, NJ). The cells were exposed to a range of radiant exposures (0.75–3 J/cm2) delivered at a light irradiance of 5 mW/cm2. Following irradiation, cells were washed with PBS, harvested by trypsinization, resuspended in Dulbecco’s modified Eagle medium supplemented with serum, counted, and plated into 60-mm dishes. Cells were allowed to grow for 11 to 14 days, at which time they were stained with 0.5% crystal violet in 95% ethanol. Colonies containing more than 50 cells were scored as survivors. The number of colonies was normalized to a control group consisting of cells incubated in a photosensitizer (dark control) but that received no light treatment. All experiments were performed in quintuplicate and results are a combination of at least 4 separate experiments.
D. Mechanism of Cell Death
Apoptotic or necrotic cell death was evaluated for MDA-MB-435 and MDA-MB-231 48 hours after PCI treatment using Annexin/propidium iodide (PI)–staining flow cytometry. For PI staining, 40% to 80% confluent cells were incubated at 37°C with 5% CO2 for 1 hour with CP or DP. After 1 hour, cells were washed with 5 mL PBS and fresh was medium added. Cells were incubated at 37°C with 5% CO2 for 24 hours and 48 hours, at which point cells were harvested, counted, and centrifuged at 500 g for 5 minutes. Cells were then washed 2 times with 5 mL PBS. After washing, cells were fixed by resuspending in 0.1 mL PBS, and 1 mL of cold 95% ethanol was slowly added drop wise with gentle vortexing. Fixed cells were stored at 4°C until analysis. For analysis, fixed cells were washed once with 1 to 2 mL PBS and centrifuged at 500 g for 5 minutes. Cells were resuspended in 100 mL of 1.0% Triton X-100 buffer solution. Then, 100 mL of a 1.0 mg/mL ribonuclease solution was added and allowed to stand at room temperature for 10 to 15 minutes. While in the dark, 200 mL of a 100 mg/mL PI stain was added to make a final concentration of 50 mg/mL and was gently vortexed. The cell mixture was incubated at room temperature for 30 minutes. Cytometry acquisition was done with a Becton Dickinson FACS Calibur cytometer (Franklin Lakes, NJ) with the argon laser set at 488 nm on the linear flow channel (FL) 2 with doublet discriminatory module and threshold set on the FL2. For Annexin V-FITC/PI staining, the same procedure was used as for PI staining up to the washing point before fixation. After harvesting and counting, cells were centrifuged at 500 g for 5 minutes and washed once with 5 mL Ca2+ and Mg2+-free PBS. Pellets were then washed in 2.0 mL 1X Annexin-V binding buffer (BD Bioscience, San Jose, CA) and centrifuged at 500 g for 5 minutes. The pellets were treated with Annexin V-FITC conjugate (BD Bioscience, San Jose, CA) and incubated in the dark for 15 minutes. Just before acquisition, the volume of the cell–conjugate mixture was adjusted by the addition of 1X Annexin-V binding buffer. Acquisition to discriminate between apoptotic and necrotic cells was done by staining the cell–conjugate mixture with 10 mL PI solution (BD Bioscience). Acquisitions were done with a FACS Calibur cytometer on the FL1 (Annexin) and FL3 (PI) channels with threshold and Doublet discriminating module set at FL1. The level of shift in events distribution in the Annexin-V only and Annexin-V-PI populations compared with control is indicative of the degree of effectiveness of the treatment agents. A quantitative measure of these event shifts was accomplished by gating.
E. Statistical Analysis
Graph Pad Prism 4.0 (Graph Pad Software, San Diego, CA) was used to graph and run nonlinear regressions on the data. Graph Pad Quick Calcs (www.graphpad.com) was used for Student’s t test. P values less than 0.05 were considered significant. All graphical error bars are representative of standard error.
Synergism was calculated when analyzing PCI treatments compared with drug or PDT alone. The equation shown below was used to determine if the PCI effect was synergistic, antagonistic, or additive, where α is the ratio of the cumulative effect of 2 therapies administered independently to the net effect of combining the 2 therapies at a given dose.
In this scheme, SF represents the survival fraction for a specific treatment. If 2 treatments are to be compared, the survival fractions of each separate treatment are multiplied together and then divided by the survival fraction when both treatments were applied together. The interaction is calculated based on the dose of each treatment. The resulting number (α) describes the summative effect as previously described.28 If α > 1, the result is synergistic (supra-additive). If α < 1, the result is antagonistic, and if α = 1 the result is simply additive.
III. RESULTS
A. Effects of Drug Concentration on Clonogenic Survival
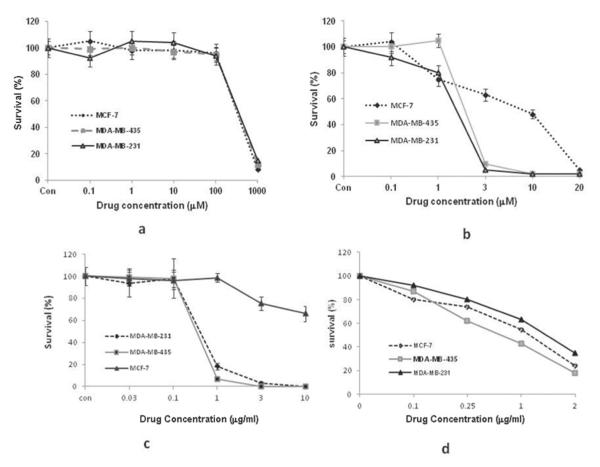
The effects of the 4 chemotherapeutic agents on the 3 cell lines’ ability to form daughter colonies following 1 hour incubation is shown in Fig. 2. The estrogen-positive cell line MCF-7 proved to be significantly more resistant to Dox than the 2 estrogen-negative cell lines. CP had little effect on cell survival at the concentration range of 0.1 μM to 100 μ M. At the highest concentration used (1 mM), cell survival was greatly reduced to less than 12% of control values. In contrast, DP proved to be about 100 times more potent (Fig. 2) than CP on all 3 cell lines. The EC50 values for CP and DP were compared from the results of clonogenic survival assays for the 2 drugs. Student’s t tests were used to verify significant differences between the EC50 values of CP and DP (Table 1). The results for DP efficacy compared with CP were significant (P < 0.05) for all 3 lines; MCF-7, the estrogen-positive cell type, was the most resistant of the 3 to DP.
FIGURE 2.
Effects of drug concentration on clonogenic survival of MDA-MB-435, MDA-MB-231, and MCF-7 cells. Cells were treated for 1 hour with varying concentrations of (a) cisplatin, (b) dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum, (c) doxorubicin, or (d) bleomycin. Cell survival was assessed by normalizing the number of colonies (≥50 cells) formed for treated cells with controls. Data points are the average of 3 individual clonogenic survival experiments performed in triplicate plates. Error bars denote standard error of the mean.
TABLE 1.
EC50 Values determined from clonogenic survival assays
| Drug | Cell Type |
||
|---|---|---|---|
| MCF-7 | MDA-MB-435 | MDA-MB-231 | |
| Cisplatin | 305.00 ± 21.21 | 311.96 ± 57.31 | 329.36 ± 38.74 |
| DP | 6 ± 0.73* | 1.77 ± 0.33* | 1.26 ± 0.31* |
Values presented are drug concentrations in micromoles.
Statistically significant compared with cisplatin within cell line, with P < 0.05. DP, dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum.
B. PDT Effect on Clonogenic Survival Curves
Previous experiments have shown that PCI is most efficient when 70% to 80% cells survive AlPcS2a-mediated PDT treatment. The effects of increasing fluence levels on clonogenic survival for all 3 lines are shown in Fig. 3. As can be seen in Fig. 3, increasing fluence levels produced an increase in the cytotoxic PDT effect. At a fluence level of between 0.75 and 1 J/cm2, between 75% and 85% of the cells survived for all 3 lines. Therefore, these fluence levels were employed in all subsequent PCI experiments.
FIGURE 3.
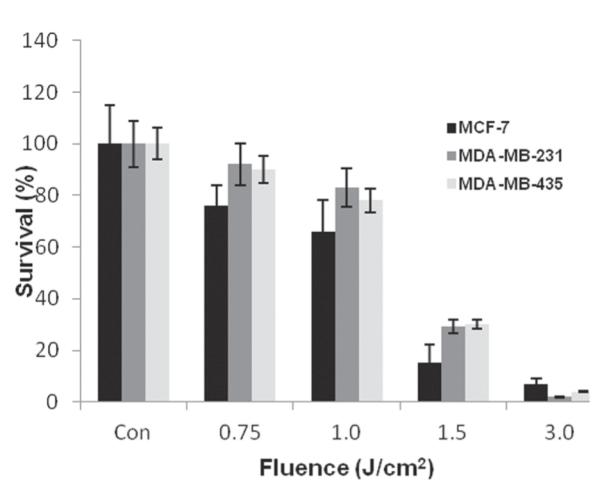
Photodynamic therapy effect on clonogenic survival curves of MDA-MB-435, MDA-MB-231, and MCF-7 cells following 18-hour incubation in 1 μg/mL phthalocyanine disulfonate and illumination (670-nm laser) at 5 mW/cm2.
C. PDT/PCI Effect on Clonogenic Survival
The effects of PCI for the 4 drugs CP, DP, Dox, and BLM on all 3 cell lines were compared with the effects of drug alone for equivalent drug doses. As shown in Fig. 4, PCI of CP or Dox gave no significant increase in efficacy compared with the drug alone. In contrast, PCI of DP or BLM showed significant (P < 0.05) cytotoxic effect for all 3 cell types.
FIGURE 4.
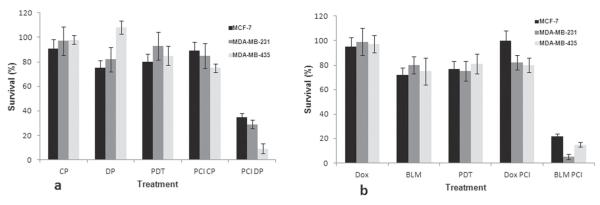
Photodynamic therapy (PDT)/photochemical internalization (PCI) effect on clonogenic survival of MCF-7, MDA-MB-231, MDA-MB-235 cells. PDT, 0.75 J/cm2 for MCF-7 and MDA-MB-435 cells and 1 J/cm2 for MDA-MB-231 cells (at 5 mW/cm2). Drug concentrations: (a) cisplatin (CP), 156 μM and dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum (DP), 1 μM; (b) doxorubicin (Dox), 0.1 μg/ml; bleomycin (BLM), 0.25 μg/ml.
Quantitative evaluation of the PCI effect as determined from the degree of interaction (α) between PDT and drug was calculated from the data shown in Fig. 4 and are shown in Table 2. The α values for the effects of PCI-mediated cytotoxicity for CP and Dox were not significantly larger than 1.0, indicating no synergistic effect (P > 0.1). On the other hand, the α values for the effects of PCI-mediated cytotoxicity for DP and BLM ranged from 1.7 to 9.1 for the different cell line and drug combinations tested. This clearly indicated a synergistic effect of PCI. The PCI-mediated increased efficacy for both drugs proved least for the MCF-7 cell line. Drug titration with and without PCI, therefore, were performed with this cell line for both DP and BLM to compare directly the results obtained with PCI of drug to drug alone over the titration range. As can be seen from Fig. 5, the EC50 value (drug concentration corresponding to 50% survival) with and without PCI was 0.08 μM versus 6 μ M for DP and 0.07 μg/mL versus 1.2 μg/mL for BLM, respectively.
TABLE 2.
Quantitative evaluation of the photochemical Internalization effect as determined from the degree of interaction (α) between photodynamic therapy and the drug
| Drug | Cell Type |
||
|---|---|---|---|
| MDA-MB-231 | MCF-7 | MDA-MB-435 | |
| DP | 2.60 ± 0.26 | 1.7 ± 0.21 | 8.2 ± 0.78 |
| Bleomycin | 9.12 ± 0.85 | 2.8 ± 0.26 | 4.42 ± 0.36 |
| Doxorubicin | 1.16 ± 0.16 | 0.97 ± 0.09 | 0.95 ± 0.1 |
| Cisplatin | 1.06 ± 0.08 | 0.83 ± 0.09 | 1.1 ± 0.08 |
DP, dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum.
FIGURE 5.
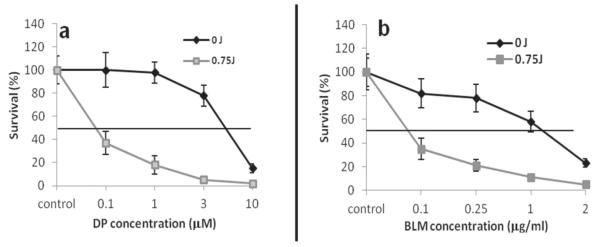
Enhancement of chemotherapeutic effect after photochemical internalization (PCI) with (a) dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum (DP) and (b) bleomycin (BLM) MCF-7 cells. 0 J denotes drug with photosensitizer with no illumination; 0.75 J denotes PCI effect for the same drug. PCI resulted in significantly decreased cell survival at given drug concentrations.
The results of flow cytometry used to determine the fraction of viable, apoptotic, and necrotic cells for CP and DP on the 2 estrogen-negative cell lines are shown in Fig. 6. The 2 different fluorescent labels used were Annexin V-FITC to distinguish apoptotic cells and PI to label necrosis. Unlabeled cells were assumed to be viable. As expected, PCI treatment caused a decrease in the percentage of viable cells, especially for DP-treated cultures. It also significantly increased the percentage of cells in the apoptotic fraction.
FIGURE 6.
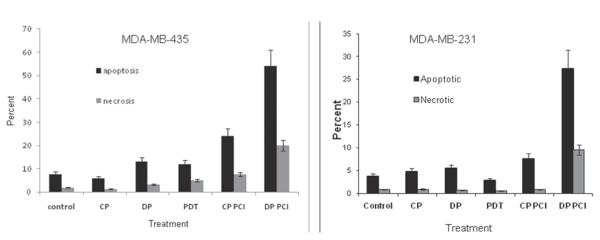
Mechanism of cell death determined by Annexin flow cytometry. MDA-MB −435 (left) and MDA-MB-231 cells (right) 48 hours after photochemical internalization (PCI) (18 hours, 1 μg/mL phthalocyanine disulfonate, 1 hour cisplatin [CP] or dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum [DP]). The predominant mechanism of cell death is apoptotic. PDT, photodynamic therapy.
IV. DISCUSSION
Breast cancer is the most common cancer and the second leading cause of cancer death among women in developed countries.29 Treatment modalities include surgical strategies for tumor removal along with adjuvant or neoadjuvant chemotherapy, local RT, hormone receptor modulators, and specific molecular targeting agents such as human epidermal growth factor receptor-2 inhibitors and vascular endothelial growth factor inhibitors. Although surgical strategies have varied from simple lumpectomies to radical mastectomies, BCS is now recognized as the preferred therapy for patients with stage I and II invasive breast cancer. Despite advances in early detection and understanding of the molecular basis of breast cancer biology, 30% of patients with early stage breast cancer have recurrences.30
The results of this study clearly demonstrate the sensitivity of 3 breast cancer cell lines to PCI-mediated drug delivery. The cell lines were selected because of their established roles in preclinical breast cancer research.31–34 The 4 chemotherapeutic agents were chosen because of their varied mechanisms of cellular uptake and toxicity. The effects of PCI for the 4 drugs—CP, DP, Dox, and BLM—on all 3 cell lines were compared with the effects of drug alone for equivalent drug doses.
PCI of CP or Dox gave no significant increase in efficacy compared with drug alone. CP is an inorganic alkylating agent used for the treatment of a wide variety of malignancies including those of the brain, ovary, breast, head and neck, cervix, and testicles. It is thought to enter the cell by passive diffusion, but there is also evidence to suggest the involvement of transport proteins.35 Cytotoxicity is due to the formation of DNA adducts.35–37 Formation of CP-DNA cross-links distorts the DNA double helix, leading to unwinding and kinking of the DNA. This prevents effective repair, leading to inhibition of DNA replication and transcription and ultimately death.
Dox is an anthracycline antibiotic commonly used for the treatment of a number of cancers including breast, ovarian, small cell lung carcinoma, and acute leukemias. It interacts with membranes containing acidic phospholipids and enters the cell by passive diffusion or carrier-mediated transport.38–41 Its mechanism of action is complex and is believed to be due to intercalation with DNA, resulting in inhibition of DNA and RNA synthesis. Because these 2 drugs do not enter cells by endocytosis, PCI would not be expected to have an enhancing effect, which is what was observed. This is in agreement with the negative results obtained with PCI of Dox on the uterine fibrosarcoma cell line MES-SA,42 as well as that on the MCF-7 cell line reported by Lou et al.43 On the other hand, Lou et al did find that PCI of Dox could reverse the resistance to the drug of the resistant cell line MCF-7/ADR.43 Multiple drug resistance (MDR) is a major clinical problem that seriously reduces the efficacy of many chemotherapeutic agents for breast cancer. The establishment of an MDR phenotype by cancer cells is a result of complex molecular events and is enhanced by genetic amplification through natural selection of resistant cancer cells following repeated chemotherapy cycles.30 The dominant mechanism includes increased drug sequestration in acidic vesicles, followed by transport to the outer cell wall and extrusion into the external medium coupled with over-expression of cell surface efflux pumps that purge a wide spectrum of chemotherapeutic agents from cells, thereby decreasing their intracellular accumulation.44–46 Pharmacologic inhibition of cell surface efflux pumps as a method to reverse MDR in cancer patients has been studied extensively, but the results generally have been disappointing.30 PCI of a variety of anticancer agents, however, has been reported as a therapeutic strategy to kill MDR cancer cells.42,43,47
In contrast to the results obtained with CP, PCI of CP’s analog DP showed a highly significant (P < 0.05) increase in its cytotoxic effect on all 3 cell types (Figs. 4 and 5). DP is a novel CP analog that was synthesized at the University of Nevada, Las Vegas, in 2007. The addition of the bipyridine ring system to CP doubles its molecular weight and results in a 10-fold increase in lethality in prostate and breast cancer cell lines.37,48 The results shown in Fig. 2 clearly demonstrate a significantly improved efficacy compared with CP. The intrastrand lesions caused by CP typically are repaired by the nucleotide excision repair pathway. The introduction of the 2,2′-bipyridine ligand in DP might inhibit the binding of the nucleotide excision repair pathway proteins, thus preventing or inhibiting the repair of the platinum adducts. Because DP is a larger molecule than CP, it likely depends on active uptake by endocytosis for intracellular transport.48 This would explain the increase in its cytotoxic effect by PCI, which greatly enhances endosomal escape. Even though apoptosis was the dominant method of cell death, its occurrence (versus necrotic cell death) was related primarily to the chemotherapeutic agent used in PCI. A significant increase in apoptosis was observed in response to PCI-mediated delivery of DP in the 2 cell lines examined. One of the cell lines used in this study, MDA-MB-435, is known to have a mutant p53 gene. P53-dependent apoptosis following DNA damage by CP and its analogs has been suggested as an explanation for the sensitivity of this cell line to this drug. Flow cytometry data demonstrated a higher level of apoptosis in MDA-MB-435 cells compared with MDA-MB-231 cells following PCI of DP, suggesting that p53 plays a protective role against the action of the drugs or that the p53 pathway antagonizes the drug mechanism.
The effects of BLM have been shown to be enhanced significantly by PCI on a number of cell types, which is in agreement with the results presented here for breast tumor cell lines.26,27,49BLM is a large, water-soluble glycopeptide that exerts its cytotoxic effects by inducing single- and double-stranded DNA breaks.50 BLM commonly is used in a number of standard cancer therapies including treatment of squamous cell carcinoma of the head and neck, esophagus, bronchus, skin, and testis and malignant lymphomas. Because of its hydrophilic nature, BLM does not easily penetrate plasma membranes and is therefore transported into cells by endocytosis. Its limited ability to escape from the resulting intracellular endosomes leads to its inactivation, resulting in a relatively low sensitivity to bleomycin.51 However, once in the cytosol, BLM has a significant toxic effect. The ability of PCI to allow the endosomal escape of BLM into the cytosol is a probable explanation for the high α values obtained (Table 2). It has been estimated that as few as 500 BLM molecules can induce cell death.52
Effective PCI-mediated drug therapy in the BCS resection cavity will depend on the development of specialized light delivery devices using balloon applicators and optical fibers. The feasibility of such an approach has been demonstrated previously for brain tumor resection and can be modified easily for use following breast surgery.53–56 Such an indwelling delivery device would be similar to interstitial brachytherapy balloons already in use following BCS with established safety and efficacy profiles.57,58 This type of device would offer the following advantages: it would define the cavity to be irradiated and provide uniform light distribution to the margins; it could be completely implanted under the skin for chronic light delivery, minimizing infection risk and allowing repeated access to the cavity via simple skin puncture; and it could be modified to allow direct delivery of photosensitizer to the target tissue.
V. CONCLUSION
The efficacy of chemotherapeutic agents such as DP and BLM can be enhanced significantly in a site-specific manner using PCI. PCI treatment causes a decrease in the percentage of viable cells predominantly by apoptosis. With appropriately designed delivery devices, PCI holds the promise of enhancing tumor cell toxicity surrounding the cavity of BCS resection sites and decreasing local recurrence.
ACKNOWLEDGMENTS
The authors are grateful for institutional support from the Beckman Laser Institute (Irvine, CA). This work was supported by grants from the Norwegian Radium Hospital Foundation, Laser Microbeam and Medical Foundation (LAMMP) as well as Chao Family Cancer Center Optical Biology Shared Resource at University of California, Irvine.
ABBREVIATIONS
- AlPcS2a
phthalocyanine disulfonate
- BCS
breast conservation surgery
- BLM
bleomycin
- CP
cisplatin
- DMSO
dimethyl sulfoxide
- Dox
doxorubicin
- DP
dichloro(4,4′-dipropyl-2,2′-bipyridine) platinum
- FL
linear flow channel
- MDR
multiple drug resistance
- PBS
phosphate-buffered saline
- PCI
photochemical internalization
- PDT
photodynamic therapy
- PI
propidium iodide
- DDM
doublet discriminatory module
- RT
radiation
REFERENCES
- 1.Bellon JR, Harris EE, Arthur DW, Bailey L, Carey L, Goyal S, Halyard MY, Horst KC, Moran MS, Macdonald SM, Haffty BG. ACR Appropriateness Criteria® conservative surgery and radiation–stage I and II breast carcinoma: expert panel on radiation oncology: breast. Breast J. 2011;17:448–455. doi: 10.1111/j.1524-4741.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 2.Subhas G, Shah AJ, Gupta A, Cook J, Dubay L, Silapaswan S, Kolachalam R, Kestenberg W, Ferguson L, Jacobs MJ, Goriel Y, Mittal VK. Review of third and fourth re-excision for narrow or positive margins of invasive and intraductal carcinoma. Int Surg. 2011;96(1):18–20. doi: 10.9738/1340.1. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez C, Brem RF, McSwain AP, Rapelyea JA, Torrente J, Teal CB. Factors associated with re-excision in patients with early-stage breast cancer treated with breast conservation therapy. Am Surg. 2010;76(3):331–334. [PubMed] [Google Scholar]
- 4.Horst KC, Smitt MC, Goffinet DR, Carlson RW. Predictors of local recurrence after breast-conservation therapy. Clin Breast Cancer. 2005;5(6):425–438. doi: 10.3816/cbc.2005.n.001. [DOI] [PubMed] [Google Scholar]
- 5.Jalali R, Singh S, Budrukkar A. Techniques of tumour bed boost irradiation in breast conserving therapy: current evidence and suggested guidelines. Acta Oncol. 2007;46(7):879–892. doi: 10.1080/02841860701441798. [DOI] [PubMed] [Google Scholar]
- 6.Graham P, Fourquet A. Placing the boost in breast-conservation radiotherapy: areview of the role, indications and techniques for breast-boost radiotherapy. Clin Oncol (R Coll Radiol) 2006;18(3):210219. doi: 10.1016/j.clon.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Fitzal F, Mittlboeck M, Steger G, Bartsch R, Rudas M, Dubsky P, Riedl O, Jakesz R, Gnant M. Neoadjuvant chemotherapy increases the rate of breast conservation in lobular-type breast cancer patients. Ann Surg Oncol. 2011;19:519–526. doi: 10.1245/s10434-011-1879-9. [DOI] [PubMed] [Google Scholar]
- 8.Bear HD. Neoadjuvant chemotherapy for operable breast cancer: individualizing locoregional and systemic therapy. Surg Oncol Clin N Am. 2010;19(3):607–626. doi: 10.1016/j.soc.2010.04.001. Review. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Shi M, Ling R, Xia Y, Luo S, Fu X, Xiao F, Li J, Long X, Wang J, Hou Z, Chen Y, Zhou B, Xu M. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: a prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100:200–204. doi: 10.1016/j.radonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Li FY, He ZY, Xue M, Chen LX, Wu SG, Guan XX. Feasibility and acute toxicity of 3-dimensional conformal external-beam accelerated partial-breast irradiation for early-stage breast cancer after breast-conserving surgery in Chinese female patients. Chin Med J (Engl) 2011;124(9):1305–1309. [PubMed] [Google Scholar]
- 11.Sharp L, Johansson H, Landin Y, Moegelin IM, Bergenmar M. Frequency and severity of skin reactions in patients with breast cancer undergoing adjuvant radiotherapy, the usefulness of two assessment instruments - a pilot study. Eur J Cancer. 2011;47:2665–2672. doi: 10.1016/j.ejca.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Han HS, Reis IM, Zhao W, Kuroi K, Toi M, Suzuki E, Syme R, Chow L, Yip AY, Glück S. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer. 2011;47:2537–2545. doi: 10.1016/j.ejca.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Yaal-Hahoshen N, Maimon Y, Siegelmann-Danieli N, Lev-Ari S, Ron IG, Sperber F, Samuels N, Shoham J, Merimsky O. A prospective, controlled study of the botanical compound mixture LCS101 for chemotherapy-induced hematological complications in breast cancer. Oncologist. 2011;16:1197–1202. doi: 10.1634/theoncologist.2011-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madarnas Y, Dent SF, Husain SF, Robinson A, Alkhayyat S, Hopman WM, Verreault JL, Vandenberg T. Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres. Curr Oncol. 2011;18:119–125. doi: 10.3747/co.v18i3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staudacher L, Cottu PH, Diéras V, Vincent-Salomon A, Guilhaume MN, Escalup L, Dorval T, Beuzeboc P, Mignot L, Pierga JY. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011;22(4):848–856. doi: 10.1093/annonc/mdq461. [DOI] [PubMed] [Google Scholar]
- 16.Ostrow S, Egorin M, Aisner J, Bachur N, Wiernik PH. High-dose cis-diamminedichloro-platinum therapy in patients with advanced breast cancer: pharmacokinetics, toxicity, and therapeutic efficacy. Cancer Clin Trials. 1980;3(1):23–27. [PubMed] [Google Scholar]
- 17.Forastiere AA, Hakes TB, Wittes JT, Wittes RE. Cisplatin in the treatment of metastatic breast carcinoma: A prospective randomized trial of two dosage schedules. Am J Clin Oncol. 1982;5(3):243–247. doi: 10.1097/00000421-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wood PA, Hrushesky WJ. Cisplatin-associated anemia: an erythropoietin deficiency syndrome. J Clin Invest. 1995;95(4):1650–1659. doi: 10.1172/JCI117840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62(17):4899–4902. [PubMed] [Google Scholar]
- 20.Liedert B, Materna V, Schadendorf D, Thomale J, Lage H. Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol. 2003;121(1):172–176. doi: 10.1046/j.1523-1747.2003.12313.x. [DOI] [PubMed] [Google Scholar]
- 21.Feng Z, Lai Y, Ye H, Huang J, Xi XG, Wu Z. Poly (γ, L-glutamic acid)-cisplatin bioconjugate exhibits potent antitumor activity with low toxicity: a comparative study with clinically used platinum derivatives. Cancer Sci. 2010;101(11):2476–2482. doi: 10.1111/j.1349-7006.2010.01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrzal R, Starha P, Dvorák Z, Trávnícek Z. Evaluation of in vitro cytotoxicity and hepatotoxicity of platinum(II) and palladium(II) oxalato complexes with adenine derivatives as carrier ligands. J Inorg Biochem. 2010;104(10):1130–1132. doi: 10.1016/j.jinorgbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sand-vig K, Moan J, Gaudernack G, Fodstad O, Kjølsrud S, Anholt H, Rodal GH, Rodal SK, Høgset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59(6):1180–1183. [PubMed] [Google Scholar]
- 24.Martinez M, Pramanik A, Moto-Ndje S, Moore CW. Overexpression of genes involved in vesicular trafficking to the vacuole defends against lethal effects of oxidative damage. Cell Mol Biol (Noisy-le-grand) 2003;49(7):1025–1035. [PubMed] [Google Scholar]
- 25.Selbo PK, Sivam G, Fodstad O, Sandvig K, Berg K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int J Cancer. 2001;92(5):761–766. doi: 10.1002/1097-0215(20010601)92:5<761::aid-ijc1238>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Berg K, Dietze A, Kaalhus O, Høgset A. Site-specific drug delivery by photochemical internalization enhances the antitumor effect of bleomycin. Clin Cancer Res. 2005;11(23):8476–8485. doi: 10.1158/1078-0432.CCR-05-1245. [DOI] [PubMed] [Google Scholar]
- 27.Norum OJ, Giercksky KE, Berg K. Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model. Photochem Photobiol Sci. 2009;8(6):758–762. doi: 10.1039/b821129a. [DOI] [PubMed] [Google Scholar]
- 28.Drewinko B, Loo TL, Brown B, Gottlieb JA, Freireich EJ. Combination chemotherapy in vitro with adriamycin. Cancer Biochem Biophys. 1976;1(4):187–195. [PubMed] [Google Scholar]
- 29.Breast cancer statistics [page on the Internet] Centers for Disease Control and Prevention; Atlanta, GA: [cited Nov 2011]. Nov 23, 2010. Available from: http://www.cdc.gov/cancer/breast/statistics. [Google Scholar]
- 30.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 31.Soudy R, Gill A, Sprules T, Lavasanifar A, Kaur K. Proteolytically stable cancer targeting peptides with high affinity for breast cancer cells. J Med Chem. 2011;54(21):7523–7534. doi: 10.1021/jm200750x. [DOI] [PubMed] [Google Scholar]
- 32.Musa MA, Khan MO, Cooperwood JS. Synthesis and antiproliferative activity of coumarin-estrogen conjugates against breast cancer cell lines. Lett Drug Des Discov. 2009;6(2):133–138. doi: 10.2174/157018009787582624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox BP, Kandpal RP. EphB6 receptor significantly alters invasiveness and other phenotypic characteristics of human breast carcinoma cells. Oncogene. 2009;28(14):1706–1713. doi: 10.1038/onc.2009.18. [DOI] [PubMed] [Google Scholar]
- 34.Zen K, Liu DQ, Guo YL, Wang C, Shan J, Fang M, Zhang CY, Liu Y. CD44v4 is a major E-selectin ligand that mediates breast cancer cell transendothelial migration. PLoS One. 2008;3(3):e1826. doi: 10.1371/journal.pone.0001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 36.Danford AJ, Wang D, Wang Q, Tullius TD, Lippard SJ. Platinum anticancer drug damage enforces a particular rotational setting of DNA in nucleosomes. Proc Natl Acad Sci U S A. 2005;102(35):12311–12316. doi: 10.1073/pnas.0506025102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vo V, Kabuloglu-Karayusuf ZG, Carper SW, Bennett BL, Evilia C. Novel 4,4′-diether-2,2′-bipyridine cisplatin analogues are more effective than cisplatin at inducing apoptosis in cancer cell lines. Bioorg Med Chem. 2010;18(3):1163–1170. doi: 10.1016/j.bmc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 38.Mayer LD, Bally MB, Cullis PR. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim Biophys Acta. 1986;857(1):123–126. doi: 10.1016/0005-2736(86)90105-7. [DOI] [PubMed] [Google Scholar]
- 39.Coley HM, Amos WB, Twentyman PR, Workman P. Examination by laser scanning confocal fluorescence imaging microscopy of the subcellular local-isation of anthra-cyclines in parent and multidrug resistant cell lines. Br J Cancer. 1993;67:1316–1323. doi: 10.1038/bjc.1993.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gervasoni JE, Fields SZ, Krishna S, Baker MA, Rosado M, Thuraisamy K, Hindenburg AA, Taub RN. Subcellular distribution of daunorubicin in P-glycoprotein-positive and -negative drug-resistant cell lines using laser-assisted confocal microscopy. Cancer Res. 1991;51:4955–4963. [PubMed] [Google Scholar]
- 41.Weaver JL, Pine PS, Aszalos A, Schoenlein PV, Currier SJ, Padmanabhan R, Gottesman MM. Laser scanning and confocal microscopy of daunorubicin, doxorubicin, and rhodamine 123 in multidrug-resistant cells. Exp Cell Res. 1991;196:323–332. doi: 10.1016/0014-4827(91)90267-x. [DOI] [PubMed] [Google Scholar]
- 42.Selbo PK, Weyergang A, Bonsted A, Bown SG, Berg K. Photochemical internalization of therapeutic macromolecular agents: a novel strategy to kill multidrug-resistant cancer cells. J Pharmacol Exp Ther. 2006;319(2):604–612. doi: 10.1124/jpet.106.109165. [DOI] [PubMed] [Google Scholar]
- 43.Lou PJ, Lai PS, Shieh MJ, Macrobert AJ, Berg K, Bown SG. Reversal of doxorubicin resistance in breast cancer cells by photochemical internalization. Int J Cancer. 2006;119(11):2692–2698. doi: 10.1002/ijc.22098. [DOI] [PubMed] [Google Scholar]
- 44.Larsen AK, Escargueil AE, Skladanowski A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol Ther. 2000;85:217–229. doi: 10.1016/s0163-7258(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 45.Gottesman MM, Pastan I. Biochemistry of multi-drug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 46.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–183. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 47.Adigbli DK, Wilson DGG, Farooqui N, Sousi E, Risley P, Taylor I, MacRobert AJ, Loizidou M. Photochemical internalisation of chemotherapy potentiates killing of multidrug-resistant breast and bladder cancer cells. Br J Cancer. 2007;97:502–512. doi: 10.1038/sj.bjc.6603895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van V. Cytotoxicity of novel cisplatin analogs in lung, prostate and breast cancer cells [Masters thesis] University of Nevada; Las Vegas (NV): 2007. [Google Scholar]
- 49.Norum OJ, Gaustad JV, Angell-Petersen E, Rofstad EK, Peng Q, Giercksky KE, Berg K. Photo-chemical internalization of bleomycin is superior to photodynamic therapy due to the therapeutic effect in the tumor periphery. Photochem Photobiol. 2009;85(3):740–749. doi: 10.1111/j.1751-1097.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 50.Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anesthetic drugs: too little is known. Lancet. 2005;6:176–181. doi: 10.1016/S1470-2045(05)01768-7. [DOI] [PubMed] [Google Scholar]
- 51.Pron G, Mahrour N, Orlowski S. Internalization of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol. 1999;57:45–56. doi: 10.1016/s0006-2952(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 52.Poddevin B, Orlowski S, Belehradek J, Mir LM. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem Pharmacol. 1991;42:S67–75. doi: 10.1016/0006-2952(91)90394-k. [DOI] [PubMed] [Google Scholar]
- 53.Hirschberg H, Madsen S, Lote K, Chen T, Tromberg B. An indweling balloon catheter for combined postoperative intracavity photodynamic and brachytherapy. J Neurooncol. 1999;44:15–21. doi: 10.1023/a:1006215002764. [DOI] [PubMed] [Google Scholar]
- 54.Johannesen TB, Watne K, Lote K, Norum J, Henning R, Tvera K, Hirschberg H. Intracavity fractionated balloon brachytherapy in glioblastoma. Acta Neurchiurica. 1999;141(2):127–133. doi: 10.1007/s007010050276. [DOI] [PubMed] [Google Scholar]
- 55.Madsen SJ, Svaasand LO, Tromberg BJ, H Hirsch-berg H. Characterization of the light distribution from an intracranial balloon applicator for photodynamic therapy. In: Duncan DD, Jacques SL, Johnson PC, editors. Laser-tissue interaction XII: photochemical, photothermal and photomechanical (proceedings volume) Vol. 4257. SPIE; Bellingham (WA): 2001. [Google Scholar]
- 56.Madsen SJ, Sun CH, Tromberg BJ, Hirschberg H. Development of a novel indwelling balloon applicator for optimizing light delivery in photodynamic therapy. Lasers Surg Med. 2001;29(5):406–412. doi: 10.1002/lsm.10005. [DOI] [PubMed] [Google Scholar]
- 57.Wilder RB, Curcio LD, Khanijou RK, Eisner ME, Kakkis JL, Chittenden L, Agustin J, Lizarde J, Mesa AV, Macedo JC, Ravera J, Tokita KM. Preliminary results in 173 breast cancer patients treated with post-lumpectomy mammosite single-lumen brachytherapy or multi-catheter brachytherapy. Breast J. 2010;16(6):581–586. doi: 10.1111/j.1524-4741.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 58.Harper JL, Watkins JM, Zauls AJ, Wahlquist AE, Garrett-Mayer E, Baker MK, Cole DJ, Dragun AE, Jenrette JM., 3rd Six-year experience: long-term disease control outcomes for partial breast irradiation using MammoSite balloon brachytherapy. Am J Surg. 2010;199(2):204–209. doi: 10.1016/j.amjsurg.2009.03.005. [DOI] [PubMed] [Google Scholar]