Abstract
Background
The demand for a large amount of osteogenic cells required in bone tissue engineering warranted exploration of a new source of osteoprogenitor cells. Recent studies have demonstrated that adipose-derived stromal cells possess multiple differentiation capacities, including osteogenic potential, as bone marrow mesenchymal stem cells. In the present study, the authors compared the osteogenic potentials of adipose-derived stromal cells from different anatomical sites of rabbits.
Methods
Different adipose-derived stromal cells were isolated from rabbit visceral and subcutaneous adipose tissues by enzymatic digestion and in vitro differentiation into osteogenic lineage. Osteogenic markers representing differentiation potentials of adipose-derived stromal cells from different anatomical sites were compared by biochemical and immunohistochemical assessment (n = 3).
Results
Fibroblast-like cells were digested from both visceral and subcutaneous adipose tissues. After exposure to osteogenic differentiation medium, visceral adipose-derived cells were found to possess greater osteogenic potentials than cells isolated from subcutaneous adipose tissues, evidenced by significantly different amounts of osteogenic markers including alkaline phosphatase, osteocalcin, and mineral deposition.
Conclusion
This study indicates that osteogenic potentials of adipose-derived cells vary by their anatomical sites, with visceral adipose-derived cells exhibiting higher osteogenic potential than those isolated from subcutis. However, the mechanism is still unidentified.
Because of low self-regenerative ability, bone defects caused by trauma, osteosarcoma, and congenital deficiency are not usually restored spontaneously. Plastic and reconstructive surgeons conventionally fill and repair the defects using autologous tissues.1 Because of major problems associated with autograft transplantation such as insufficient supply, donor-site injury, and surgical risks, alternative approaches have been attempted, including the use of allografts and biosynthetic substitutes. However, these substitutes are limited by their immunogenicity and inertness.2–4 Tissue-engineering strategies combining the techniques of biology, engineering, and medicine represent a promising approach for regenerating defective tissues by means of application of autologous osteoprogenitor cells.5–8
Bone marrow stromal cells, a heterogeneous cell population also known as mesenchymal stem cells, contain osteoprogenitor cells capable of differentiating into osteoblasts.9–14 Bone marrow is considered a main resource of osteoprogenitor cells for therapeutic purposes of bone tissue engineering because it has high differentiation potentials and low morbidity during harvesting.15–17 However, the osteoprogenitor population tends to decrease in aged and osteoporotic humans.18–20 Also, excessive proliferation in vitro would significantly decrease the osteogenic potential.21 Because a high cell density is required to yield a successful outcome of tissue engineered bone, a new source of osteoprogenitor cells would be useful as an alternative or supplement to bone marrow cells.
Recently, fibroblast-like cells with multiple differentiation potentials have been found in adipose tissues of several species, including humans.22,23 Osteogenic potentials were observed in adipose-derived stromal cells isolated from rat, mouse, and human adipose tissues under specific differentiation stimulation. The adipose-derived stromal cells are heterogeneous populations, potentially mixed with fibroblasts, adipocytes, preadipocytes, myoblasts, and epithelial and multifunctional progenitor cells.24 It is assumed that different histomorphologies of adipose tissues located at different anatomical sites such as subcutaneous and visceral sites would cause different ratios of cell types within the mixtures, which might affect the osteogenic differentiation potentials.25 In the present study, we enzymatically digested and isolated cells from rabbit visceral and subcutaneous adipose tissues and compared their osteogenic potentials by various histochemical and immunohistochemical techniques after in vitro osteoinductive stimulation.
Materials and Methods
Adipose Tissue Collection and Cell Isolation
Six healthy, male, adult, New Zealand White rabbits weighing approximately 3.5 kg were killed by intravenous administration of an overdose of pentobarbital. All the procedures were performed under an animal protocol approved by the Animal Care Committee of the University of Illinois at Chicago. Immediately after the animals were killed, the abdominal fur was clipped and a sterile midline incision was made in the epigastric region of the animal, exposing the subcutaneous and visceral adipose tissues. Visceral adipose samples were harvested from the gonads. Visceral and subcutaneous fat tissues were separately and carefully dissected away and finely minced with scissors.26 The fat tissue was washed three times with phosphate-buffered saline and centrifuged at 500 g for 5 minutes after each wash to remove contaminated tissues. The samples were digested with 0.075 wt% collagenase type I (Worthington, Lake-wood, N.J.) for 45 minutes at 37°C with intermittent shaking. The tissue solution was then neutralized and filtered through a cell strainer with a pore size of 100 μm to remove undigested tissues. The sample was centrifuged again and the pellet was resuspended in basic cell culture medium consisting of Dulbecco's minimal essential medium (Gibco-BRL, Carlsbad, Calif), 10% fetal bovine serum, and 1% antibiotic/antimycotic. The harvested cells were then plated at a density of 106 cells per 100-mm Petri dish and cultured in basic medium at 37°C incubator with 5% carbon dioxide. Culture medium was changed every third day. On reaching 80 to 90 percent confluence, cells were trypsinized using 0.25 wt% trypsin/ethylenediaminetetraacetic acid (Gibco-BRL), and collected for the osteogenic differentiation study.
In Vitro Osteogenic Differentiation
First passage adipose-derived stromal cells were subcultured in six-well plates at a density of 106 cells per well and evaluated for their differentiation potential by exposing the cells to osteoinductive medium for 6 weeks. Osteogenic differentiation medium consisted of basic medium supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate. The cells cultured in basic medium served as controls. On days 3, 7, 14, 21, 28, and 42, cells from both osteoinductive and control groups were washed twice with phosphate-buffered saline, suspended, and lysed in 1% Triton-X100 solution (Sigma, St. Louis, Mo.). The collected cells were subsequently homogenized using sonication (Dismembrator Model 100; Fisher Scientific, Pittsburgh, Pa.). Sample size was three for quantitative measurement in the present study.
Assessment of Cell Proliferation
Cell proliferation was quantitatively evaluated by fluorometric assessment of genomic DNA content using Hoechst dye 33258 (Bio-Rad Laboratories, Hercules, Calif.). The fluorescent optical density of each sample was measured using a fluorometer with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. The amount of DNA in each sample was determined by using a prepared standard curve.27
Osteogenic Differentiation Potentials of Adipose-Derived Stromal Cells
Osteogenic differentiation potentials of adipose-derived stromal cells were indicated by alkaline phosphatase activity, mineral deposition of extracellular matrix, and osteocalcin content. At each defined time point, the plated cells were fixed with 10% paraformaldehyde and stained using alkaline phosphatase and von Kossa stain to evaluate osteogenic differentiation of adipose-derived cells. Alkaline phosphatase activity within cytoplasm was quantitatively measured with an alkaline phosphatase diagnostics kit (Sigma).
Extracellular matrix mineral deposition was reflected by the presence of black nodules using von Kossa staining. Under von Kossa staining, darker cellular nodules have more calcium deposition in the extracellular matrix. Quantitative assessment of mineral deposition was performed on photographs of osteodifferentiated adipose-derived stromal cells stained with von Kossa dye. An image of stained differentiated adipose-derived stromal cells was captured by a computer-connected digital camera for each experimental group. Using Adobe Photoshop software (Adobe Systems, San Jose, Calif.) and a gray-scale setting, the experimental samples were quantified and compared with the controls. Absolute white represents 0 percent mineral deposition, whereas pure black represents 100 percent. More than 10 areas, randomly selected by the software, were selected and analyzed for darkness. Osteocalcin as a late bone turnover marker only secreted by terminally differentiated osteoblasts was quantitatively measured by an osteocalcin enzyme-linked immunosorbent assay kit (Quidel Co., San Diego, Calif). All procedures were performed according to the manufacturer's protocols.
Statistical Analysis
All data were expressed as mean ± SD. The data were analyzed by one-way analysis of variance and Bonferroni post hoc was used to compare the various parameters, with a value of p < 0.05 indicating significance.
Results
During tissue processing, macroscopically dissected visceral fat pads showed more vasculature, indicated by numerous blood vessels, as compared with subcutaneous fat pads. A larger fibrous tissue fraction was observed and eliminated in minced subcutaneous fat pads. Fibroblast-like cells were isolated from rabbit visceral and subcutaneous adipose tissues (Figs. 1, above, left and 2, above, left). When plated and cultured in osteogenic medium, changes in cell shape after 4 days were observed. Microscopically, cells changed from an elongated fibroblastic appearance to a more round, cuboidal shape. After treatment with osteogenic differentiation medium, both visceral adipose-derived cells and subcutaneous adipose-derived cells proliferated and reached high confluence in monolayer culture, and cellular nodules were formed (Figs. 1, above, right and 2, above, right). After 1 week of osteogenic differentiation stimulation, microscopic differences in cellular nodule density were observed between visceral adipose-derived cells and subcutaneous adipose-derived cells, visceral adipose-derived cells forming apparently a higher population of cellular nodules than subcutaneous adipose-derived cells (Figs. 1, above, right and 2, above, right). At 2 weeks, visceral adipose-derived cells expressed an intense positive reaction to alkaline phosphatase staining (Fig. 1, center, right), and by the fourth week, well-defined nodules of mineral deposition were observed by positive von Kossa staining (Fig. 1, below, right). By contrast, corresponding control visceral adipose-derived cells exposed to basic medium showed mostly negative reactions to alkaline phosphatase and von Kossa staining at weeks 2 and 4 (Fig. 1, center, left and below, left, respectively). Subcutaneous adipose-derived cells exposed to osteogenic differentiation medium exhibited weak positive reaction to alkaline phosphatase and von Kossa staining compared with visceral adipose-derived cells after 2 and 4 weeks (Fig. 2, center, right and below, right). Corresponding control subcutaneous adipose-derived cells exposed to basic medium also exhibited mostly negative reactions to alkaline phosphatase and von Kossa staining after the same time periods (Fig. 2, center, left and right, respectively).
Fig. 1.
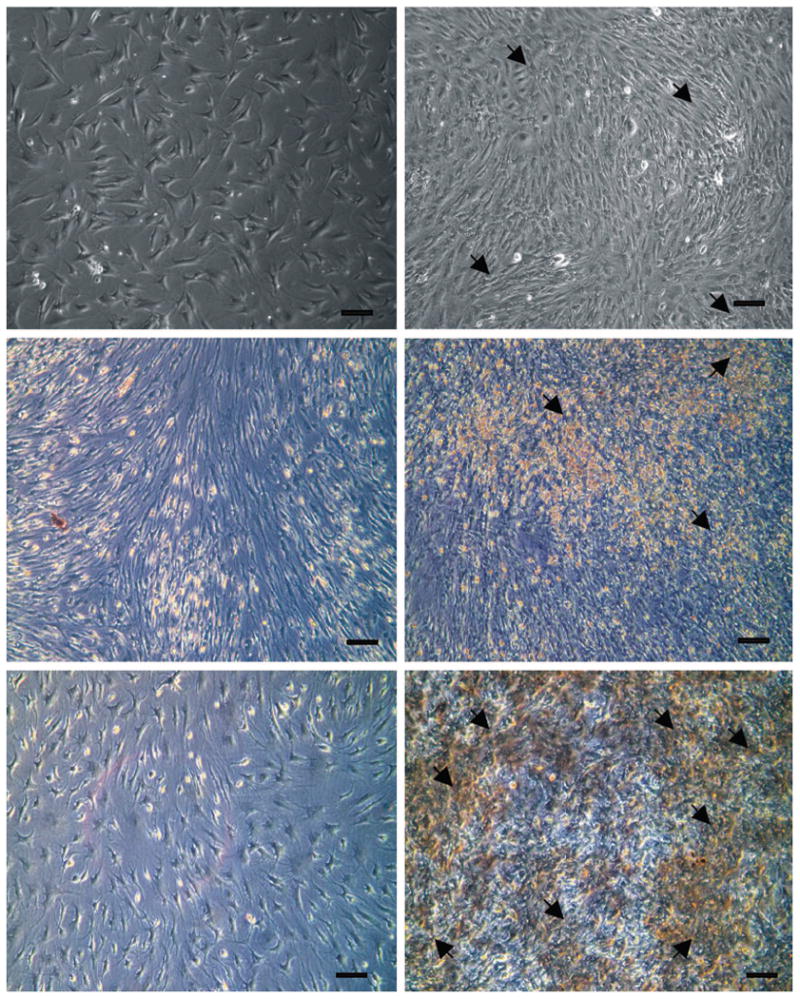
Osteogenic differentiation of rabbit visceral adipose-derived cells. (Above, left) Fibroblastic cells isolated from visceral adipose tissue. (Above, right) One week after osteogenic differentiation, nodules characterized by a high density of cells formed (arrow). (Center, left) After 2-weekculture of basic medium, visceral adipose-derived cells exhibited a negative reaction to alkaline phosphatase staining. (Center, right) Positive alkaline phosphatase staining indicated by red color was observed at visceral adipose-derived cells after 2 weeks' exposure to osteogenic medium (arrow). (Below, left) On 4 weeks of culture, negative staining of von Kossa was observed at visceral adipose-derived cells exposed to basic medium. (Below, right) Mineral depositions indicated by black color positively stained by von Kossa were observed at visceral adipose-derived cells after 4-week exposure to osteogenic medium. Bar = 100 μm.
Fig. 2.
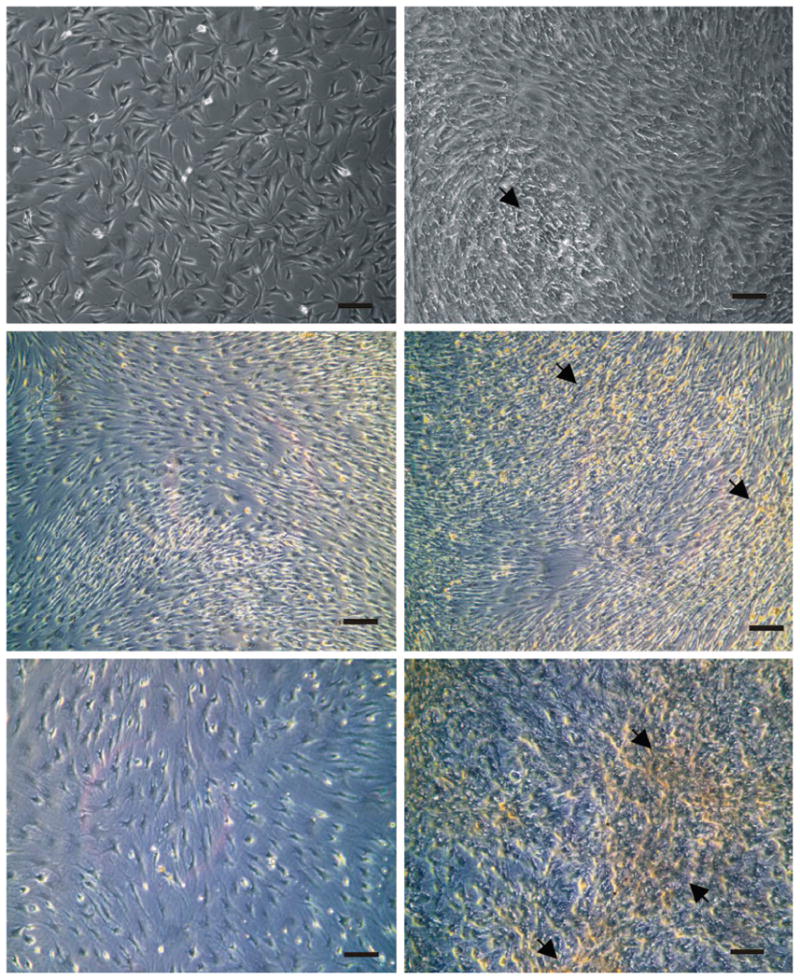
Osteogenic differentiation of rabbit subcutaneous adipose-derived cells. (Above, left) Fibroblastic cells isolated from subcutaneous adipose tissue. (Above, right) One week after osteogenic differentiation, nodules characterized by a high density of cells formed (arrow). The nodule number seemed smaller than that of visceral adipose-derived cells. (Center, left) After 2-week culture with basic medium, subcutaneous adipose-derived cells exhibited a negative reaction to alkaline phosphatase staining. (Center, right) Weak positive alkaline phosphatase staining indicated by red color was observed at subcutaneous adipose-derived cells 2 weeks after exposure to osteogenic medium (arrow). (Below, left) On 4 weeks of culture, negative staining of von Kossa was observed at subcutaneous adipose-derived cells exposed to basic medium. (Below, right) Mineral depositions indicated by black color positively stained by von Kossa were observed at subcutaneous adipose-derived cells after 4-week exposure to osteogenic medium. However, the density of von Kossa staining and black-stained nodule number was smaller than that of visceral adipose-derived cells. Bar = 100 μm.
The adipose-derived cells' proliferation rate represented by quantitative DNA contents revealed that subcutaneous adipose-derived cells proliferate faster than visceral adipose-derived cells in both osteogenic and basic medium. Both visceral adipose-derived cells and subcutaneous adipose-derived cells showed higher proliferation rates after treatment with osteogenic medium compared with those exposed to basic medium (Fig. 3). Intracellular alkaline phosphatase activity of visceral adipose-derived cells under treatment with osteogenic differentiation medium increased over time, peaking at 2 weeks and then declining gradually. Consistent with qualitative observations, alkaline phosphatase activity was significantly higher than that of cells treated with basic medium at 2, 3, and 4 weeks of culture (Fig. 4). However, subcutaneous adipose-derived cells presented no significant difference of alkaline phosphatase activity after exposure to osteogenic differentiation medium compared with the subcutaneous adipose-derived cells treated with basic medium. The amount of alkaline phosphatase activity of osteogenic differentiated subcutaneous adipose-derived cells was significantly lower than that of corresponding visceral adipose-derived cells (Fig. 4).
Fig. 3.

Proliferation rates of rabbit adipose-derived cells after exposure to osteogenic medium. Both subcutaneous adipose-derived cells (filled squares) and visceral adipose-derived cells (filled circles) exposed to osteogenic medium proliferated faster than those corresponding groups exposed to basic medium (open squares and open circles represent subcutaneous adipose-derived cells and visceral adipose-derived cells in basic medium, respectively). ***p < 0.01, osteogenic differentiated subcutaneous adipose-derived cells versus all other groups at the same time point (n = 3); **p < 0.05, osteogenic differentiated visceral adipose-derived cells versus the control group exposed to basic medium at the same time point (n = 3);*p < 0.05, subcutaneous adipose-derived cells versus visceral adipose-derived cells both in basic medium without osteogenic supplements at the same time point (n = 3).
Fig. 4.
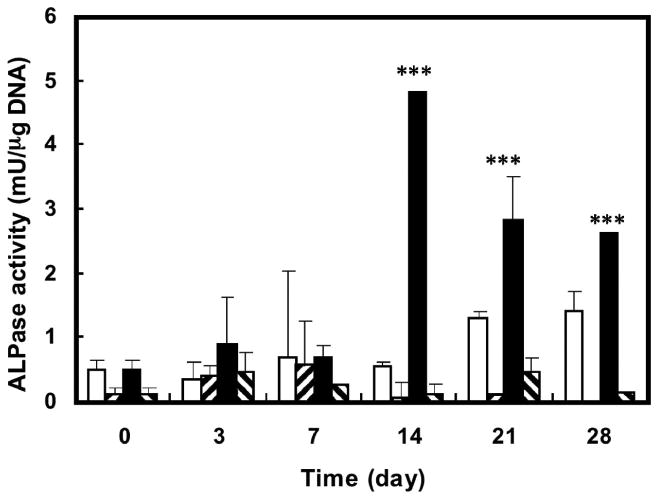
Alkaline phosphatase activities of rabbit adipose-derived cells after exposure to osteogenic medium. Open and filled squares represent the visceral adipose-derived cells exposed to basic medium and osteogenic medium, respectively. Right-hatched bars and left-hatched bars represent the subcutaneous adipose-derived cells exposed to basic medium and osteogenic medium, respectively. ***p < 0.01, osteogenic differentiated visceral adipose-derived cells versus all other groups at the same time point (n = 3).
The osteocalcin content of 0.24 and 0.42 ng/μg DNA for visceral adipose-derived cells treated with osteoinductive medium was quantitatively measured by enzyme-linked immunosorbent assay at weeks 4 and 6, respectively, in comparison with undetectable osteocalcin amounts in the cells treated with basic medium. However, osteocalcin content of subcutaneous adipose-derived cells exposed to osteogenic medium was undetectable also after 4 weeks and only 0.12 (ng/μg DNA) after 6 weeks of differentiation, values that were significantly lower than those of visceral adipose-derived cells exposed to osteogenic medium. Like visceral adipose-derived cells exposed to the basic medium, the osteocalcin content of corresponding subcutaneous adipose-derived cells were undetectable after 4 and 6 weeks (Fig. 5).
Fig. 5.
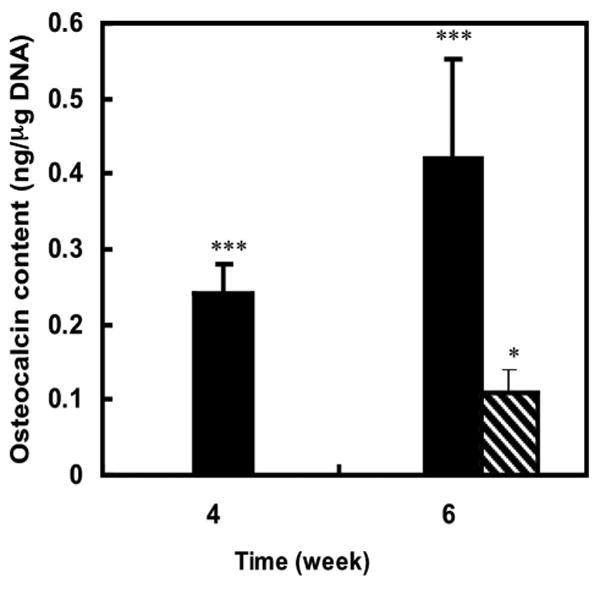
Osteocalcin content of rabbit adipose-derived cells 4 and 6 weeks after exposure to osteogenic medium. The osteocalcin was not detected in visceral adipose-derived cells, subcutaneous adipose-derived cells exposed to basic medium, or subcutaneous adipose-derived cells exposed to osteogenic medium for 4 weeks. Filled bars represent the visceral adipose-derived cells exposed to osteogenic medium. Hatched bar represents subcutaneous adipose-derived cells exposed to osteogenic visceral adipose-derived cells. ***p < 0.01, osteogenic differentiated visceral adipose-derived cells versus all other groups at the same time point (n = 3);*p < 0.01, osteogenic differentiated subcutaneous adipose-derived cells versus subcutaneous adipose-derived cells exposed to basic medium at the same time point (n = 3).
Mineral deposition was observed in rabbit adipose-derived cells 4 weeks after exposure to the osteogenic medium (Fig. 6). The well-defined nodules of mineral deposition stained by von Kossa were observed in both visceral adipose-derived cells and subcutaneous adipose-derived cells exposed to osteogenic medium. No color change was found in the adipose-derived cells treated with basic medium under von Kossa staining (Fig. 6). By computing the intensity of the darkness of von Kossa staining, visceral adipose-derived cells of osteogenic differentiation possessed a significantly higher content of mineral deposition (35.25 ± 6.36 percent) than that of subcutaneous adipose-derived cells (15.16 ± 3.51 percent) and cells treated with basic media (8.33 ± 1.55 percent and 7.58 ± 2.23 percent, respectively) (Fig. 7).
Fig. 6.
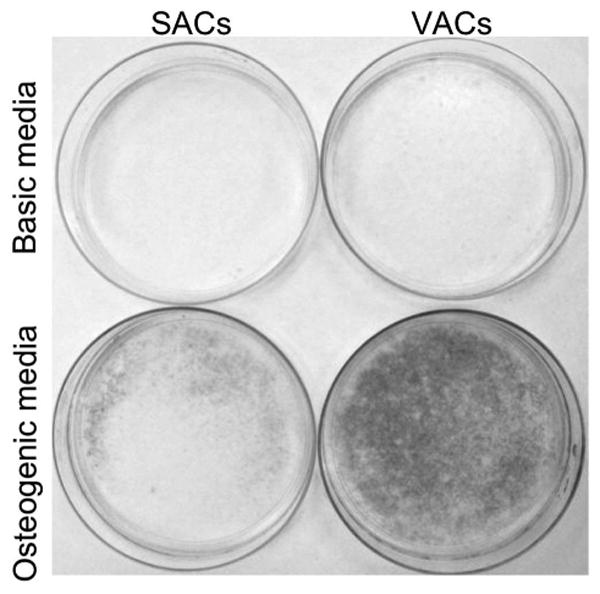
Qualitative and quantitative measurement of mineral deposition of rabbit adipose-derived cells 4 weeks after exposure to osteogenic medium. (Above) Photograph of von Kossa staining on subcutaneous adipose-derived cells and visceral adipose-derived cells exposed to basic (left and right, respectively) and osteogenic medium (Below, left and right, respectively).
Fig. 7.
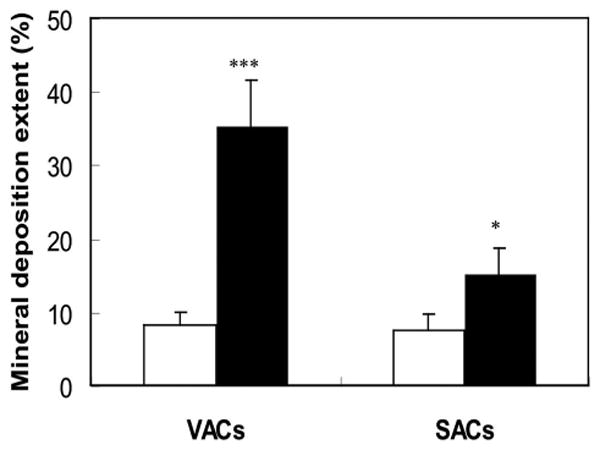
Quantitative measurement of mineral deposition extent of visceral adipose-derived cells and subcutaneous adipose-derived cells exposed to basic (open bars) and osteogenic medium (filled bars).*** p < 0.01 versus all other groups at the same time point (n = 3); *p < 0.05 versus the control groups exposed to basic medium at the same time point (n = 3).
Discussion
The present study compared the osteogenic differentiation potentials of adipose-derived stromal cells harvested from different anatomical sites, namely, viscus and subcutis. This study demonstrated that, after exposure to osteogenic differentiation medium, visceral adipose-derived cells exhibited significantly higher osteogenic potentials than those isolated from subcutaneous adipose tissue, whereas the cell proliferation rate was slower. Although the mechanism is unknown, it probably could be explained by histologic characteristics of adipose tissues located at different anatomical sites, specifically, the visceral adipose containing more vascular supplies and less fibrous encapsulation compared with subcutaneous adipose. These factors might contribute to the different populations of osteoprogenitor cells isolated from these adipose tissues, resulting in different potentials of osteogenesis.
Lennon et al. revealed that bone marrow-derived stromal cells would lose or decrease their osteogenic differentiation potentials if they mixed with dermal fibroblasts.25 This indicated that the population of osteoprogenitor cells within heterogeneous cells critically influences their differentiation functions. This finding probably could be used to explain why the subcutaneous adipose-derived cells possessed lower osteogenic differentiation potential than that of cells isolated from visceral tissue. Although adipose-derived stromal cells were recently demonstrated to possess multiple differentiation potentials including osteogenic potential, the process of adipose-derived stromal cell isolation was not able to select the multiple functional cells from adipose tissue. More fibrous tissue mixed in subcutaneous adipose tissue results in more populations of fibroblasts within adipose-derived stromal cells than that from visceral adipose. We observed in another parallel study (unpublished data) that the proliferation rate of subcutaneous fibroblasts was much higher than that of osteoprogenitor cells isolated from bone marrow. Subcutaneous adipose-derived cells containing more fibroblasts than were isolated from visceral adipose also could be evidenced by its higher cell proliferation rate. The osteogenic potential was assessed by measuring osteogenic markers normalized on the cell amount. Thus, highly proliferative cells of subcutaneous adipose-derived cells likely resulted in a much lower osteogenic potential. We assumed that an original high population of fibroblasts within the heterogeneous cells isolated from subcutaneous adipose-derived cells increased after a certain period of expansion, resulting in inhibition of adipose-derived stromal cell differentiation ability.
The difference in osteogenic potentials of visceral and subcutaneous adipose-derived cells could also be attributed to the difference in the tissue vasculature. The better the blood supply present in visceral adipose tissue, the higher the population of osteoprogenitor cells within the harvested heterogeneous cells. Microvascular pericytes have been demonstrated to potentially serve the role of multi-potential bone marrow stromal cells,28,29 expressing type I collagen, alkaline phosphatase enzyme activity, and osteocalcin immunoreactivity under osteogenic differentiation.29 Also, bone marrow stromal cells existing in the circulation might contribute to the difference of multiple functional cell populations in these two types of adipose tissue.30 Accordingly, although the mechanism needs to be further studied, different histologic characteristics caused by anatomical site vary the osteogenic potentials of adipose-derived stromal cells. This study indicates that a critical process for purifying osteoprogenitor cells from heterogeneous cells digested from adipose tissue with the long-term aim of exploring adipose-derived cells as a cell resource for bone tissue engineering and regeneration is needed.
Acknowledgments
This research was supported by Whitaker Foundation biomedical engineering research grant RG-02-0701 (L. Hong), March of Dimes Foundation grant 5-FY-03-160 (L. Hong), and National Institutes of Health grants DE13964 and EB02332 (J. J. Mao). The authors are indebted to Carla Evans for constructive comments on an earlier version of the article. They gratefully acknowledge Paul Clark and Aurora Lopez for their technical assistance.
References
- 1.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop. 1987;225:16. [PubMed] [Google Scholar]
- 2.Boyce RG, Toriumi DM. Considerations in the use of biologic grafts and alloplastic implants in facial plastic and reconstructive surgery. J Long Term Eff Med Implants. 1992;2:220. [PubMed] [Google Scholar]
- 3.Betz RR. Limitations of autograft and allograft: New synthetic solutions. Orthopedics. 2002;25(5 Suppl):70. doi: 10.3928/0147-7447-20020502-04. [DOI] [PubMed] [Google Scholar]
- 4.Seiler JG, III, Johnson J. Iliac crest autogenous bone grafting: Donor site complications. J South Orthop Assoc. 2000;9:97. [PubMed] [Google Scholar]
- 5.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop. 1998;355:256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 6.Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Invest Drugs. 2002;3:1004. [PubMed] [Google Scholar]
- 7.Ohgushi H, Caplan AI. Stem cell technology and bioceramics: From cell to gene engineering. J Biomed Mater Res. 1999;48:927. doi: 10.1002/(sici)1097-4636(1999)48:6<913::aid-jbm22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Calvert JW, Weiss LE, Sundine MJ. New frontiers in bone tissue engineering. Clin Plast Surg. 2003;30:648. doi: 10.1016/s0094-1298(03)00081-6. [DOI] [PubMed] [Google Scholar]
- 9.Dennis JE, Merriam A, Awadallah A, Yoo JU, John-stone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 13.Bruder SP, Jaiswal N, Ricalton NS, Mosca JD, Kraus KH, Kadiyala S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop. 1998;355(Suppl):S247. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 14.Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Invest Drugs. 2002;3:1000. [PubMed] [Google Scholar]
- 15.Goshima J, Goldberg VM, Caplan AI. The osteogenic potential of culture-expanded rat marrow mesenchymal cells assayed in vivo in calcium phosphate ceramic blocks. Clin Orthop. 1991;262:311. [PubMed] [Google Scholar]
- 16.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg (Am) 1998;80:996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expended human mesenchymal stem cells. J Orthop Res. 1998;16:162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 18.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 19.Huibregtse BA, Johnstone B, Goldberg VM, Caplan AI. Effect of age and sampling site on the chondroosteogenic potential of rabbit marrow-derived mesenchymal progenitor cells. J Orthop Res. 2000;18:24. doi: 10.1002/jor.1100180104. [DOI] [PubMed] [Google Scholar]
- 20.Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 21.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive sub-cultivation and following cryopreservation. J Cell Biochem. 1997;64:231. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R, Mizuno H, Watanabe A, Migita M, Shimada T, Hyakusoku H. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 2004;313:877. doi: 10.1016/j.bbrc.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Pettersson P, Cigolini M, Sjoestroem L, Smith U, Bjoerntorp P. Cells in human adipose tissue developing into adipocytes. Acta Med Scand. 1984;215:453. doi: 10.1111/j.0954-6820.1984.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 25.Lennon DP, Haynesworth SE, Arm DM, Baber MA, Caplan AI. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn. 2000;219:62. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1037>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Tholpady SS, Katz AJ, Ogle RC. Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat Rec. 2003;272:402. doi: 10.1002/ar.a.10039. [DOI] [PubMed] [Google Scholar]
- 27.Rengarajan K, Cristol SM, Mehta M, Nickerson JM. Quantifying DNA concentrations using fluorometry: A comparison of fluorophores. Mol Vis. 2002;6:421. [PubMed] [Google Scholar]
- 28.Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97:454. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- 29.Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 30.Lazarus HM, Haynesworth SE, Gerson SL, Caplan AI. Human bone marrow-derived mesenchymal (stromal) progenitors (MPCs) cannot be recovered from peripheral blood progenitor cell collections. J Hematother. 1997;6:452. doi: 10.1089/scd.1.1997.6.447. [DOI] [PubMed] [Google Scholar]


