Abstract
Mantle cell lymphoma (MCL) is one of the most aggressive B cell non-Hodgkin lymphomas with a median survival of about five years. Currently, there is no curative therapy available for refractory MCL because of relapse from therapy-resistant tumor cells. The NF-κB and mTOR pathways are constitutively active in refractory MCL leading to increased proliferation and survival. Targeting these pathways is an ideal strategy to improve therapy for refractory MCL. Therefore, we investigated the in vitro and in vivo antilymphoma activity and associated molecular mechanism of action of a novel compound 13-197, a quinoxaline analog that specifically perturbs IκB kinase (IKK) β, a key regulator of the NF-κB pathway. 13-197 decreased the proliferation and induced apoptosis in MCL cells including therapy-resistant cells compared to control cells. Furthermore, we observed down-regulation of IκBα phosphorylation and inhibition of NF-κB nuclear translocation by 13-197 in MCL cells. In addition, NF-κB regulated genes such as cyclin D1, Bcl-XL and Mcl-1 were down-regulated in 13-197-treated cells. 13-197 also inhibited the phosphorylation of S6K and 4E-BP1, the downstream molecules of mTOR pathway that are also activated in refractory MCL. Further, 13-197 reduced the tumor burden in vivo in the kidney, liver, and lungs of therapy-resistant MCL bearing NOD-SCID mice compared to vehicle treated mice; indeed, 13-197 significantly increased the survival of MCL transplanted mice. Together, results suggest that 13-197 as a single agent disrupts the NF-κB and mTOR pathways leading suppression of proliferation and increased apoptosis in malignant MCL cells including reduction in tumor burden in mice.
Keywords: Refractory Mantle cell lymphoma, NF-κB, mTOR
Introduction
Mantle cell lymphoma (MCL) is one of the most aggressive B cell malignancies with a median survival of about five years due to relapse from therapy-resistant tumor cells (1–5). Despite availability of new agents to combine with high dose therapy followed by stem cell transplantation, disease-free survival rate in MCL is disappointing (5–8). Therefore, identification of key cellular/molecular pathways that can be targeted to refractory state of MCL and the development of an effective therapeutic strategy for targeting those pathways is needed.
MCL has a characteristic chromosomal translocation t(11;14)(q13;q32), a hallmark of this disease which results in cyclin D1 overexpression, which is believed to contribute to deregulated proliferation in MCL (9). Although it is widely accepted that cyclin D1 has an important role in the pathogenesis of MCL, accumulating evidence suggests that MCL often has defects in many other cellular processes, such as those involved in cell cycle regulation, apoptosis and DNA repair. MCL is known to be resistant to apoptosis with existing therapy. Recent studies have revealed a number of biochemical defects that may contribute to its relatively high resistance to apoptosis due to overexpression of several anti-apoptotic proteins. Several lines of evidence indicate that NF-κB pathway is constitutively active in MCL which leads to the overexpression of several anti-apoptotic molecules (9–15). Also, gene expression analyses of MCL patient samples and therapy-resistant MCL cells derived from different tissue sites have frequently shown high expression of NF-κB target genes (12, 16, 17). Consequently, inhibition of this constitutive activation of NF-κB has been shown to elicit cell cycle arrest and cell death in MCL cells (10, 13–15, 18). Strong evidence implicating the NF-κB pathway in the onset and progression of MCL has made it an attractive target for therapeutic intervention. Aberrant cellular signaling, such as PI3K/mTOR pathway, may also contribute to the chemoresistance of MCL (19, 20). Targeting these pathways can be a novel approach for developing a new therapeutic strategy for improved treatment of refractory MCL.
IκB kinase β (IKKβ) is a key upstream kinase in the NF-κB pathway that is activated by immune and inflammatory responses regulating cell growth and survival in the pathogenesis of various solid and hematological malignancies (21). In general, NF-κB is transcriptionally inactive in the cytoplasm of most cells through interaction with its cytoplasmic inhibitor protein called IκBα. Upon activation, the IKKβ becomes phosphorylated and activated. Activated IKKβ phosphorylates NF-κB-bound IκBα and targets it for polyubiquitination and proteasomal mediated degradation (21–23). As a consequence, free NF-κB dimers are further activated through post-translational modifications and translocate to the nucleus resulting in transcriptional activation of several hundred survival, proliferation and apoptosis associated genes (21–25). The IKKβ is also known to result in the activation of tuberous sclerosis 1 (TSC1). The phosphorylation mediated suppression of TSC1 results in the activation of the mTOR pathway (26, 27). Together, these data suggest that IKKβ is a key kinase and upon activation it regulates transcription of genes through the NF-κB pathway and translation of the gene products through the mTOR pathway. Recently, IKKβ has made an attractive target for therapeutic development (28–30). Although several IKKβ inhibitors have been used for the treatment of diseases in preclinical studies and so far none have received FDA approval (31). One possible reason for this is the observed toxicity (ML-120B and TPCA1) in preclinical models (32, 33). Therefore, the development of IKKβ inhibitors without toxicity in clinics is needed.
Compounds containing the quinoxaline core are found in a number of natural products as validated hits from high throughput screens, clinical candidates and drugs on the market (34). Synthesis and screening of a focused library of quinoxaline compounds led to a well-defined structure activity relationship and the identification of a quinoxaline urea analog that inhibited growth of cancer cells with low micromolar potency (35, 36). Recent studies by us also suggest that the quinoxaline urea analog 13–197 inhibits the constitutively active form of IKKβ which is an emerging target for therapeutic development (28–30). In cell-based NF-κB driven dual luciferase assay, the potency of 13-197 is comparable to the reported NF-κB inhibitors, Parthenolide, Curcumin and Noscapine (37). Considering IKKβ and the NF-κB pathway are constitutively active in the therapy-resistant MCL, we speculated that 13-197 might be a therapeutic option for refractory MCL.
The effect of this novel inhibitor 13-197 and its molecular mechanism(s) against MCL has not been described. Therefore, we evaluated the efficacy and potency of 13-197 as single agent targeting NF-κB and mTOR pathways in MCL and therapy-resistant MCL cell lines. We found that 13-197 treated cells showed inhibition of IκB phosphorylation and phosphorylation of S6K and 4E-BP1 which are downstream of IKKβ in the NF-κB and mTOR pathways, respectively. This resulted in significant suppression of proliferation and increased apoptosis in MCL cells including therapy-resistant cells, and also reduction of tumor burden in mice. Our data strongly supports 13-197 as a novel and potential therapeutic agent against malignant MCL to be further explored to take to clinic.
Materials and Methods
Cell lines and Maintenance
The MCL cell line Granta 519 was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Germany) and Rec-1 and Mino were obtained from ATCC (Manassas, VA). These cell lines were authenticated by ATCC in 2012. These cell lines were maintained in RPMI media (Invitrogen, CA) containing 10% FBS (Atlanta Biologicals, GA), 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen, CA) and 2 mM L-Glutamine (Invitrogen, CA). This medium with supplements is referred to as RF-10 medium in this report. The cultures were maintained in a humidified incubator adjusted at 5% CO2 and 95% air atmosphere at 37°C. All cultures were split twice weekly, cell counting was performed using hemocytometer, and cell viability was assayed by trypan blue staining method.
Isolation of therapy-resistant MCL cells
The therapy-resistant MCL cell lines were characterized and established after transplanting the Granta 519 into the NOD-SCID mice following treatment with CHOP chemotherapy and bortezomib as described previously (16, 38). These therapy-resistant tumor cells derived from liver, kidney and lungs were described as Granta 519 resistant from liver (GRL), Granta 519 resistant from kidney (GRK), and Granta 519 resistant from lungs (GRR), respectively, and compared to parental Granta 519 cells (GP). These cells have been shown to be resistant to chemotherapy as confirmed both in vitro and in vivo studies. The additional properties of these therapy-resistant cell lines have been recently published (16, 38).
The therapeutic agent 13-197
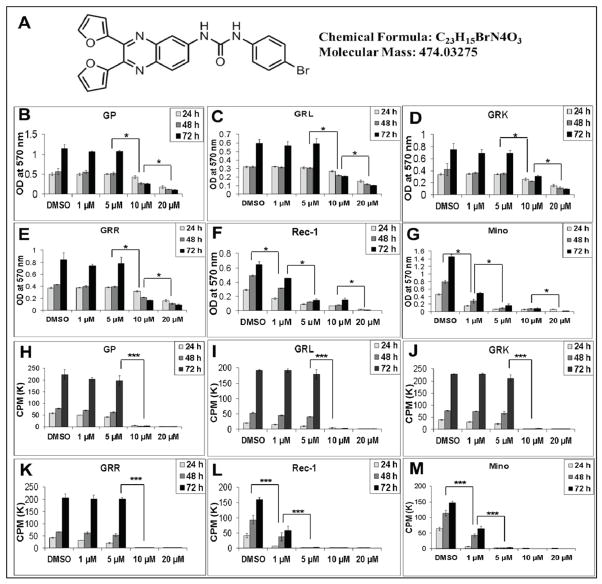
In this study, we used a quinoxaline urea analog called 13-197 which inhibits NF-κB and mTOR pathways via IKKβ in pancreatic cancer cell lines in vitro and in vivo (28). The molecular structure of 13-197 is described in Fig. 1A. The toxicity and pharmacokinetics (PK)-properties of this compound has been reported by Gautam, et al (39). IC50 of 13-197 in different MCL cell lines are described in supplementary Table 1.
Figure 1. Effect of 13-197 on therapy-resistant MCL cells growth/proliferation in-vitro.
Ten thousand of each MCL cells indicated were cultured in RF-10 media containing 1, 5, 10 and 20 μM 13-197 in 96-well plates for 24, 48 and 72 hours. A: shows the chemical structure and molecular properties of 13-197. B–G: MTT assay was used to determine the cell viability in control and treated cells. The values represent the means ± SD from four wells of the 96-well plates. H–M: The proliferation levels of control and treated cells were determined using 3[H]-thymidine uptake method. The values represent the means ± SD from triplicate wells of the 96-well plates. Similar results were obtained from three sets of independent experiments. * -and *** indicate the significance at p<0.01 and p<0.001, respectively.
In vitro growth assay
Ten thousand GP, GRL, GRK, GRR, Rec-1 and Mino MCL cells were cultured in RPMI media containing 0.5, 1.0, 5.0, 10, 20, and 50 μM 13-197 or DMSO (vehicle) in 96-well plates and the growth of these cells were determined at 24, 48 and 72 hours using MTT and 3[H]-thymidine uptake assays. Briefly, 25 μl of MTT reagent (5 mg/ml in PBS) was added to the culture and incubated for 2 hours before the respective time point, and the cells were lysed using an SDS-based lysing reagent. The intensity of the color developed was determined at 570 nm using a plate reader (Biotek). In another set of experiments, 0.5 μCi of 3[H]-thymidine was added 15 hours prior to cell harvest. The cells were harvested at 24, 48 and 72 hours using a PHD cell harvester (Cambridge Technologies, MA). The incorporated radioactivity was counted using a liquid scintillation counter (Packard Instruments, IL).
Apoptosis assay
The MCL cell lines were cultured at a concentration of 1 × 106 cells/ml in RF-10 media containing 10 μM 13-197 or DMSO for 48 hours. The percent of the cells undergoing apoptosis was then assessed using the Annexin-V:FITC apoptosis assay kit (BD Biosciences, CA), following the manufacturer’s instructions and flow cytometry.
Cytomorphology
Control and 13-197 treated cells were washed twice with PBS. Cytospin preparations were made from different MCL cells used in this study and stained with Wright-Giemsa stain in the UNMC pathology core lab and the cytomorphology was compared by light microscopy.
Western Blotting
Western blot analysis was performed using a standardized protocol in the laboratory. Briefly, the cells were harvested after the indicated time, washed with ice-cold PBS and lysed in a buffer containing 50 mM Tris-Hcl (pH 7.0), 150 mM Nacl, 0.1% SDS, 1% Na-deoxycholate, 1% NP-40 and a complete protease and phosphatase inhibitor cocktail (Pierce, IL). For cytoyplasmic and nuclear protein extraction, a NE-PER kit (Pierce, IL) was used following the manufacturer’s instructions. Protein concentration was determined with a protein assay kit (Bio-Rad, CA). These protein lysates were subjected to 10–12% SDS-polyacrylamide gel electrophoresis. After electrophoresis and proteins transfer to PVDF membrane, the membrane was blocked with 5% non-fat dry milk and probed with specific primary antibodies. The primary antibodies used in this study included NF-κB (p65), Mcl-1, β-actin (Santacruz, CA), IκBα, phospho-IκBα, phospho-NF-κB (p65), Bcl-XL, S6K, phospho-S6K, 4E-BP1, phospho-4E-BP1, PARP, α-tubulin (Cell Signaling Technology, MA) and an anti-cyclin D1 (BD Biosciences, CA). Immunoreactivity was detected using appropriate peroxidase-conjugated secondary antibodies (Santacruz, CA) and visualized using enhanced chemiluminescence (ECL) detection system (Pierce, IL).
MCL patient blood samples
Primary MCL cells were obtained from four MCL patients in leukemic phase using an UNMC Institutional Review Board approved protocol and informed consent. Peripheral blood mononuclear cells (PBMCs) from MCL patients with high lymphocyte count were isolated using lymphocyte separation medium (Accurate Chemical and Scientific, NY) described previously (40) and used to evaluate the efficacy of 13-197. The immunophenotypic analysis of these cells using flow cytometry showed over 90% CD5 and CD20 positivity. These MCL cells were treated with vehicle control (DMSO) or different concentrations of 13-197 for 24 and 48 hours to determine the efficacy of 13-197 as described earlier for the established cell lines. As an additional control, normal B cells from three healthy donors purified using Miltenyi magnetic bead separation method were used. These healthy donor samples were provided by the apheresis core unit of UNMC clinic.
In vivo studies
All animal experiments were approved by the UNMC Institutional Animal Care and Use Committee (IACUC). For in vivo studies, 6–8 weeks old NOD.CB17-Prkdcscid/J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the UNMC Comparative Medicine Animal Facilities. The NOD/SCID mice were irradiated with 215 gy using the UNMC experimental irradiator facility. One million of therapy-resistant MCL (GRL) cells suspended in 100 μl sterile media were transplanted into NOD-SCID mice via lateral tail vein injection. After 7 days, one set of 7 mice was treated with cremophor (vehicle) orally and served as a control group, and another set of 10 mice was treated with 30 consecutive daily oral doses of 13-197 (120 mg/kg/body weight) and served as an experimental group. When mice became moribund as evidenced by weight loss, hunching back, ruffled fur, excessive dehydration, and/or hind-limb paralysis, they were euthanized using the CO2 chamber method. Liver, lungs, and kidneys were harvested and fixed in 10% buffered formalin solution for further histological and immunohistological analyses. The survival of the vehicle or 13-197 treated mice was determined by the Kaplan-Meier method and analyzed using the log rank test.
Immunohistochemistry
The H&E stained histological sections and immunohistochemical analyses for CD20, phospho-p65 and phospho-S6K expression of tissues harvested from control and 13-197 treated mice were performed at the Histology Core Facility of the Nebraska Medical Center. For tumor burden analyses, the histological sections were scanned under virtual microscopy and the tumor area was quantitated using Neuroinformatica software.
Statistical analysis
Each experiment was performed in triplicate and repeated for an additional 2–3 times and the mean and standard error values were calculated. The significance of difference (p-value) was calculated using independent Student t-test and the p-value, less than 0.05 was considered significant.
Results
Growth inhibitory effect of 13-197 against MCL and therapy-resistant MCL cells
As 13-197 has been reported to be effective in micromolar (μM) ranges of concentration to kill pancreatic cancer cells in vitro (28), we used the μM concentrations of 13-197 to determine its efficacy in MCL cell lines. To examine the ability of 13-197 to inhibit proliferation of therapy-resistant MCL cells in vitro, the MCL cells were incubated with 1, 5, 10 and 20 μM 13-197 for 24, 48 and 72 hours, and the growth of the cells was assessed using MTT and 3[H]-thymidine uptake assays. The MTT result showed a dose- and time-dependent growth inhibition of MCL cells as shown in Fig. 1B–G. Similarly, 3[H]-thymidine uptake assay also showed significant inhibition of MCL cells proliferation at 1 μM 13-197. A dose- and time-dependent inhibition of MCL cells was observed as shown in Fig. 1H–M. At 10 μM, 13-197 completely suppressed the proliferation in all MCL cell lines used in the present study. Together, these results suggest that 13-197 suppresses the aggressive, MCL and therapy-resistant MCL cells growth/proliferation in vitro at the μM potency. To address the toxicity issue of this compound, we also determined the effect of 13-197 on the viability of normal B cells of healthy donors (N=3). The MTT assay results clearly showed no significant effect of 13-197 (μM ranges 1–100) on the viability of normal B cells (Fig. S1), suggesting 13-197 specificity to kill tumor cells only, not normal or untransformed cells.
Induction of increased apoptosis by 13-197 in MCL and therapy-resistant MCL cells
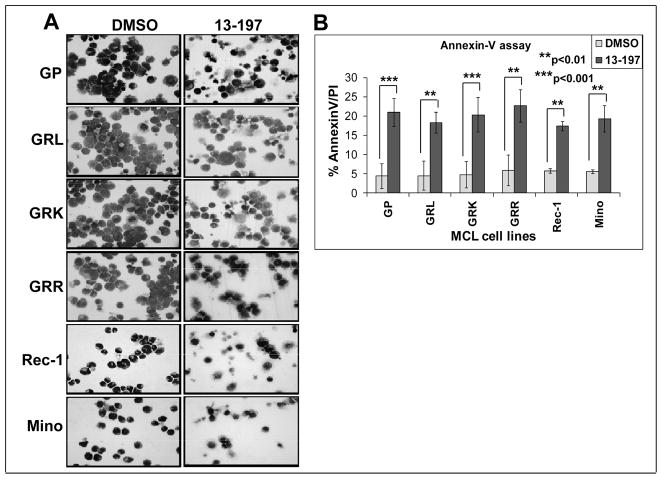
To investigate the ability of 13-197 to induce apoptosis in GP, GRL, GRK, GRR, Rec-1 and Mino MCL cells, cytomorphological analyses and AnnexinV staining method were used in 13-197-treated cells for 48 hours. Light microscopic appearance of Wright-Giemsa stained control and treated cells are shown in Fig. 2A. Results from cytomorphology of therapy-resistant MCL cells clearly showed a significant increase in apoptotic cells in therapy-resistant MCL cells treated with 13-197 compared to vehicle only treated control (Fig. 2A). To confirm this observation, Annexin-V assay was performed where MCL cells were treated with 13-197 using the same conditions as in their cytomorphological analyses and then the cells were analyzed by flow cytometry for the binding of Annexin-V to indicate induction of apoptosis. As shown in Fig. 2B, treatment with 13-197 greatly induced (~ four fold) apoptosis in all MCL cell lines compared to vehicle (DMSO)-treated cells and further confirms ability of 13-197 to induce apoptosis in these cell lines. These results are consistent with previous studies (Fig. 1) exhibiting the ability of 13-197 to inhibit proliferation and growth in malignant cells of MCL.
Figure 2. Effect of 13-197 on therapy-resistant MCL cells morphology/apoptosis.
Exponentially growing therapy-resistant and other MCL cells were treated with 10 μM 13-197 for 48 hours. Following treatment, cells were stained with Wright-Giemsa staining using a cytospin preparation. The cells were examined under bright field microscope for the apoptotic cells and images were captured at 40X magnification; A: cytomorphology of different MCL cells indicated above following treatment with 13-197 specifically examining apoptotic bodies; B: Annexin-V apoptosis detection assay was used to access percent of cells undergoing apoptosis in those MCL cell lines following treatment with 10 μM 13-197 for 48 hours. The values represent the means ± SD of three separate experiments.
Down-regulation of NF-κB signaling molecules in MCL cells following treatment with 13-197
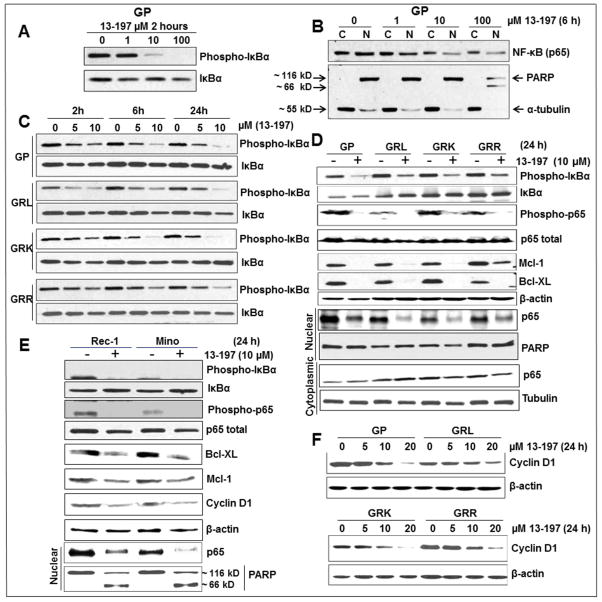
It has been established that NF-κB is constitutively active in malignant MCL (9–13, 41). Therefore, initially, we confirmed the constitutive activation of NF-κB pathway using western blotting. Our results clearly showed the increased level of nuclear NF-κB (p65) and phosphorylation of IκBα in MCL and therapy-resistant-MCL cell lines as well as primary cells of a MCL patient compared to normal B cells (Fig. S2). We then explored whether 13-197 down-regulates NF-κB signaling molecules in the MCL and therapy-resistant MCL cells. Initially, we examined the effect of 13-197 on IκBα phosphorylation, a major target molecule of the NF-κB signaling pathway using western blot method. The results indicated a significant down-regulation of IκBα phosphorylation at the 5 μM concentration of 13-197 and the down-regulation of IκBα phosphorylation followed dose- and time-dependent manner in MCL cell lines including therapy-resistant cell lines examined (Fig. 3A, C). It is well known that phosphorylated IκBα is rapidly ubiquitinated which leads to its proteasomal degradation and this allows the release of NF-κB (p65) from the complex. Released p65 undergoes posttranslational modifications including phosphorylation which leads to its nuclear translocation (25). We next performed western blotting for NF-κB phosphorylation and nuclear translocation in DMSO (vehicle) and 13-197-treated MCL cells following cytoplasmic and nuclear fractionation of the cells. Fig. 3B and D show a decreased level of NF-κB (p65) phosphorylation and inhibition of its nuclear retention by 13-197 in the therapy-resistant MCL cells. The inhibition of p65 nuclear level by 13-197 was also dose-dependent (Fig. 3B). Further, our western blot results showed decreased expression of NF-κB regulated anti-apoptotic molecules including Bcl-XL, a member of Bcl-2 ant-apoptotic gene family, overexpressed in MCL, and Mcl-1 in all 13-197-treated MCL cells (Fig. 3D, E). Cyclin D1 is a molecule which is overexpressed in MCL and is also regulated by NF-κB (9). We next examined the expression level of cyclin D1 by western blotting following treatment of MCL cells with 13-197. As expected, the expression level of cyclin D1 was significantly decreased by 13-197 at 10 μM in MCL cells examined and the decreased level of cyclin D1 followed in a dose-dependent fashion, as shown in Fig. 3F. These results were consistent with other MCL cell lines including Rec-1 and Mino (Fig. 3E). Overall, these results suggest that 13-197 suppresses the cell growth and induced the apoptosis by abrogating NF-κB signaling pathway in MCL and therapy-resistant MCL cell lines.
Figure 3. Effect of 13-197 on the NF-kB pathway associated molecules in therapy-resistant MCL cells.
MCL cells were cultured in RF-10 media containing vehicle (DMSO) or indicated concentration of 13-197 for different time points. After incubation, cells were harvested and whole cell lysate or cytoplasmic/nuclear fraction was prepared and subjected to western blot for the expression of NF-kB pathway associated proteins. A: shows the levels of IkBα phosphorylation by 13-197 in GP cells in a dose-dependent manner; B: shows the decrease levels of NF-kB nuclear translocation in GP MCL cells following treatment with different concentrations of 13-197. PARP and α-tubulin were also detected to confirm cytoplasmic and nuclear fractionation of proteins; C: phosphorylation status of IkBα by 13-197 in therapy-resistant and their parental GP cells in a time- and dose-dependent manner; D: status of IkBα and p65 phosphorylations, p65 nuclear translocation, Mcl-1 and Bcl-XL in therapy-resistant and their parental GP cells following treatment with 10 μM 13-197; E: levels of IkBα and p65 phosphorylations, p65 nuclear translocation, Mcl-1, Bcl-XL and cyclin D1 in Rec-1 and Mino MCL cell lines following treatment with 10 μM 13-197; F: expression levels of cyclin D1 in therapy-resistant and parental GP cell lines following treatment with different concentrations of 13-197. β-actin was used as an internal control in all these experiments. The results shown are a representative of three sets of independent experiments.
13-197 inhibits the activation of mTOR pathway molecules
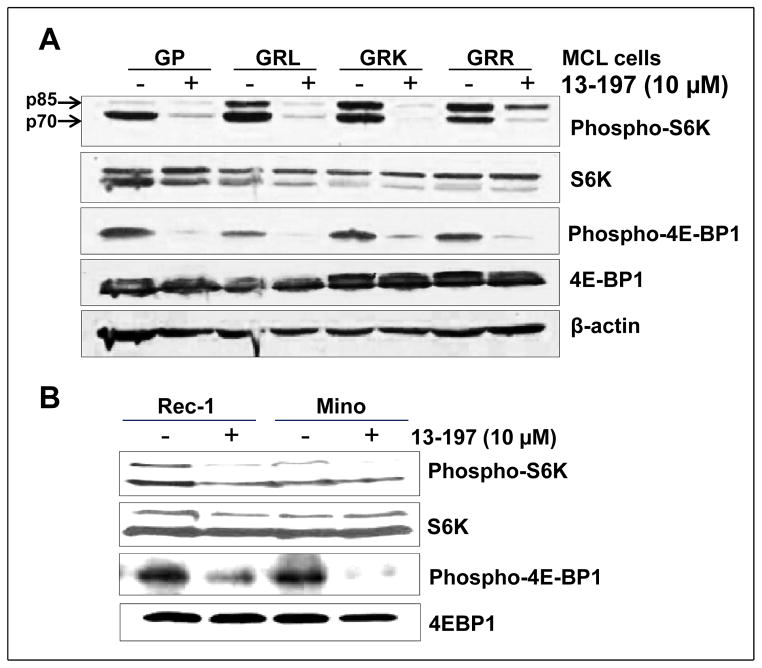
Constitutive activation of mTOR signaling pathway has also been implicated in the development of various malignancies including MCL (9, 19, 42). Regarding constitutive activation of mTOR pathway in MCL, our western blot results are also in agreement where we observed the increased phosphorylations of the mTOR downstrem molecules such as S6K and 4E-BP1 in MCL cell lines including therapy-resistant cell lines and primary cells of a MCL patient compared to normal B cells (Fig. S2). Activation of S6K and inactivation of 4E-BP1 through phosphorylation by mTOR lead to increased protein synthesis, cell proliferation, and VEGF secretion and ultimately to tumorigenesis (26). Since IKKβ is the major upstream kinase in the NF-κB signaling pathway which is known to activate mTOR signaling pathway (27), we examined the ability of 13-197 in modulating mTOR pathway molecules by targeting IKKβ. In this regard, we analyzed the principal molecules of mTOR pathway especially S6K and 4E-BP1 phosphorylation in the 6 and 24 hours 13-197 treated MCL cells using western blot. The results shown in Fig. 4 clearly demonstrate that 13-197 treatment for 24 hours, significantly down-regulated the phosphorylation of S6K and 4E-BP1 in all MCL cell lines examined. The results for 6 hours treatment with 13-197 in parental GP and therapy-resistant GRL cell lines are shown in supplementary Fig. S3 where 13-197 significantly decreased the levels of mTOR activated molecules in both parental and therapy-resistant MCL. These results suggest that 13-197 can abrogate both NF-κB and mTOR signaling pathways by targeting central player IKKβ. Together, the results demonstrated that 13-197 is an effective compound that has all the desirable properties to inhibit the constitutively active NF-κB pathway which results in significantly decreased proliferation and increased apoptosis in MCL as well as therapy-resistant MCL variants isolated from liver, lungs and kidney.
Figure 4. Effect of 13-197 on the mTOR target molecules in the therapy-resistant MCL cells.
MCL cells were cultured in RF-10 medium containing vehicle (DMSO) or 13-197 (10 μM) for 24 hours. Following incubation, cells were harvested and whole cell lysate was prepared and subjected to western blot for the expression of phosphorylation status of mTOR pathway molecules namely S6K and 4E-BP1. A: status of mTOR pathway molecules in therapy-resistant and GP parental MCL cell lines following treatment with 13-197; B: status of mTOR pathway molecules in Rec-1 and Mino MCL cell lines following treatment with 13-197. β-actin was used as an internal control in all these experiments. Similar results were obtained from three sets of independent experiments.
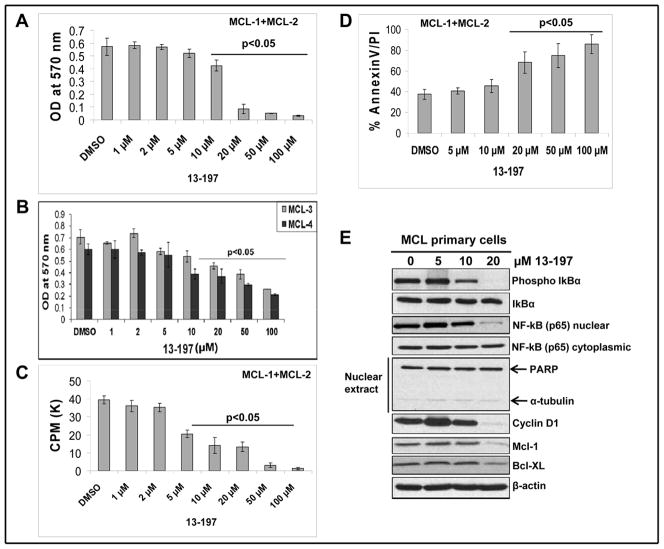
Effect of 13-197 in MCL primary cells
To authenticate our previous observations in established MCL cell lines, we further investigated the anti-lymphoma activity of 13-197 using primary cells of MCL patients. For this purpose, we treated MCL primary cells with increasing concentrations of 13-197. After 48 hours of treatment, cell growth and apoptosis were determined using MTT/3-H-thymidine uptake and Annexin-V assays, respectively. As shown in Fig. 5A–D, 13-197 treatment induced a decreased cell growth/proliferation and an increase in the apoptotic-appearing cells of primary MCL cells in a dose-dependent manner. Further, we determined effects of 13-197 on NF-κB signaling molecules using western blot analyses as shown in Fig. 5E. Following 24 hours treatment with increasing concentration of 13-197, western blot result clearly showed that 13-197 at 20 μM concentration decreased the phosphorylation of IκB, reduced the level of nuclear NF-κB (p65) by inhibiting its nuclear translocation and subsequently down-regulated the expression level of NF-κB target molecules including Cyclin D1, Bcl-XL and Mcl-1. Together, these results suggest that 13-197 also suppresses the cell growth and induces the apoptosis by abrogating NF-κB signaling pathway in primary cells of MCL patients, substantiating our previous finding in established MCL cell lines.
Figure 5. Effect of 13-197 in primary MCL cells.
Primary MCL cells from MCL patients samples (N=4) were cultured in RF-10 medium containing vehicle (DMSO) or indicated concentrations of 13-197 for 24 and 48 hours. Following incubation, cells were subjected for growth/proliferation, apoptosis and western blot analyses. A: MTT assay was used to determine the cell viability in control and 13-197 treated combined cells of two MCL patients (MCL-1 and MCL-2) for 48 hours. The values represent the means ± SD from four wells of the 96-well plates. B: MTT assay was used to determine the cell viability in control and 13-197 treated cells of two additional MCL patients (MCL-3 and MCL-4) for 48 hours. The values represent the means ± SD from four wells of the 96-well plates. C: The proliferation levels in control and treated cells of MCL patients (N=2) were determined using 3[H]-thymidine uptake method following treatment with 13-197 for 48 hours. The values represent the means ± SD from triplicate wells of the 96-well plates. D: Annexin-V apoptosis detection assay was used to access percent of cells undergoing apoptosis in those MCL primary cells (N=2) following treatment with 13-197 for 48 hours. The values represent the means ± SD from triplicate wells of the 6-well plates. E: Following 24 hours of 13-197 treatments, cells were harvested and whole cell lysate or cytoplasmic/nuclear fraction was prepared and subjected to western blot for the expression of indicated molecules of NF-κB pathway. β-actin was used as an internal control in this experiment. This Fig. shows a representative data of two MCL patients.
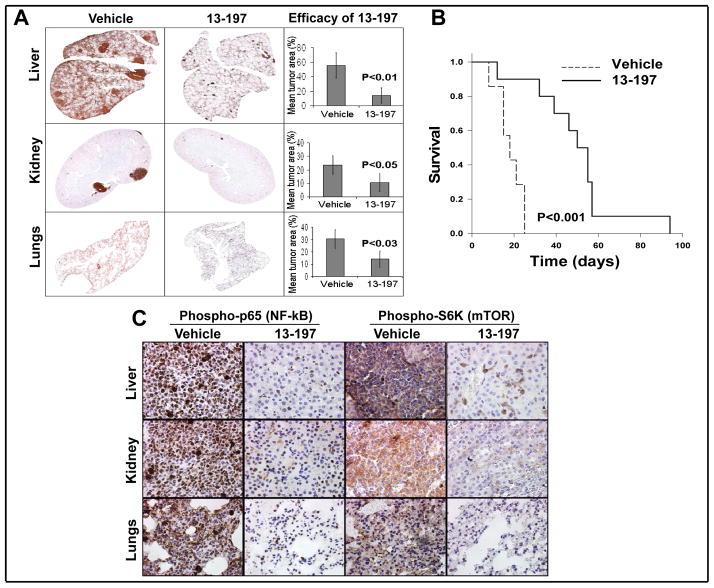
Anti-tumor effect of 13-197 against therapy-resistant MCL in vivo
To determine the anti-tumor efficacy of 13-197 against therapy-resistant MCL tumor cells in vivo, we used 17 immune-compromised NOD-SCID mice which were transplanted with GRL. We selected GRL for this study because in a previous study, we showed that among the stable therapy-resistant MCL cell lines we developed, GRL is the most aggressive cell line (16, 38). Seven days following tumor transplantation, the mice were randomized and divided into two groups. One group of mice (N=7) were given vehicle (cremophor, p.o.) only, while another group of mice (N=10) received 13-197 (120 mg/kg/day, p.o.) for 30 consecutive days. The dose of 13-197 for the treatment of mice was achieved from the reports described previously (28, 39). The dose and schedule for 13-197 were well tolerated. On days 12 and 20 after initiation of treatment, we randomly selected 3 mice from each treatment group for necropsy and tumor burden analyses in different organs including liver, kidney and lungs; this was performed using CD20 immunohistochemical analyses. As shown in Fig. 6A, there was significantly decreased tumor burden in the liver, lungs and kidney from mice treated with 13-197 compared to control. Indeed, 13-197 significantly increased disease-free survival (p<0.001) of mice transplanted with MCL compared to control mice (Fig. 6B) indicating that 13-197 decreases tumor burden and thereby increases the survival of mice. Further, we validated the 13-197 effect on targeting NF-kB and mTOR pathways in vivo. In, this regard, our immunohistochemistry results with phosphorylated NF-kB (p-65) and mTOR (p-S6K) molecules showed significantly decreased levels in 13-197 treated liver, kidney and lungs of MCL bearing NOD-SCID mice (Fig. 6C), confirming 13-197 antilymphoma efficacy targeting NF-kB and mTOR in vivo as well. Together, these results suggested that 13-197 has potential therapeutic efficacy against therapy-resistant MCL and warrants further preclinical and clinical investigations to take this approach to clinical setting.
Figure 6. Anti-lymphoma efficacy of 13-197 in a NOD-SCID mouse model in vivo.
NOD-SCID mice bearing therapy-resistant GRL MCL cells were treated with vehicle (cremophor) or 13-197 (120 mg/kg/) for 30 days. A: Immunohistochemical analysis of the liver, kidney, and lungs of MCL bearing NOD-SCID mice following treatment with 13-197. The histological sections from liver, lungs and kidney were stained with anti-CD20 antibody, imaged and quantitated, and analyzed under a digital scanner microscope using Neuroinformatica software. The images of tissue sections from different treatment groups were captured using light microscopy at 10X magnification. B: Kaplan-Meier analyses for the survival of mice using log-rank test. Total number of mice, N=17. C: Immunhistochemical (IHC) analysis for the level of NF-κB (phospho-p65) and mTOR (phospho-S6K) in the liver, kidney, and lungs of MCL bearing NOD-SCID mice following treatment with 13-197. The IHC images were captured using a light microscope at 40X magnification.
Discussion
In the present study, single agent treatment of MCL with oral compound 13-197 not only suppressed cell growth and induced apoptosis in vitro, but also reduced the tumor burden and increased the survival of MCL bearing mice. In addition to the significant antilymphoma efficacy as a single agent, the 13-197 also targeted NF-κB and mTOR signaling pathways by specifically inhibiting the nuclear retention of NF-κB (p65) and phosphorylation of IκB, S6K and 4E-BP1 in MCL cells including therapy-resistant cells. Intracellular signaling pathways that control cell growth and survival mechanisms are complex, interactive, and often cross-talk with each other in various types of human hematologic malignancies. Frequently, however, these pathways including NF-κB and PI3K-mTOR are used and constitutively activated in these tumors (3, 12, 13, 19, 42). Targeting these pathways with new and novel therapeutic agents that have molecular specificity is likely to provide improved treatment outcomes (8, 9, 43). Novel therapies ideally should be based on a basic understanding of the biology of therapy-resistant MCL, especially molecular aspects of the disease progression, which translate into the clinical phenotype. Therefore, MCL cells from refractory patients needed to be evaluated, particularly tumor cells from different tissues, to understand the influence of tissue microenvironment on molecular phenotype of therapy-resistance. It is not feasible to perform all these studies in patients with resistant MCL; therefore, to determine the efficacy and understand the molecular basis of therapy-resistance in MCL, we used the therapy-resistant MCL cell lines which we have already established from relapsed tumors from different organs of MCL-bearing NOD-SCID mice, following high dose therapy combined with Bortezomib, a proteosome inhibitor (16, 38). These therapy-resistant cell lines served as ideal preclinical models in the present study. In this regard, targeting NF-κB and mTOR pathways in these therapy-resistant MCL cells further attest the novelty and therapeutic efficacy of 13-197.
The oral compound 13-197 showed a significant anti-MCL efficacy in our study. Recently, Radhakrishnan et al (28) have demonstrated that 13-197 has strong therapeutic efficacy against human pancreatic adenocarcinoma via targeting NF-κB and mTOR pathways. They have identified 13-197 as an IKKβ inhibitor with the screening of 13-197 against a panel of kinases. Their study shows that 13-197 targets IKKβ and inhibits NF-κB and mTOR signaling pathways in pancreatic cancer cell lines. Regarding toxicity and pharmacokinetics properties of 13-197, Gautam et al (39) have shown that 13-197 does not have significant toxicity to normal tissues of mice and rats.
Our results and reports by others have shown that NF-κB is constitutively expressed in refractory MCL (9–13, 41). In this regard, our therapy-resistant MCL cell lines showed constitutive activation of NK-κB (p65) and IκBα. These MCL cells also overexpressed Mcl-1, Bcl-XL and cyclin D1 that are known to be regulated by NF-κB (9, 13, 14). Treatment of MCL cells with 13-197 downregulated the constitutively active NF-κB and inhibited the phosphorylation of IκBα and p65, and ultimately suppressed the expression of proliferation (cyclin D1) and survival (Bcl-xL, Mcl-1) molecules. Cyclin D1, a NF-κB-regulated gene, is overexpressed in MCL as a result of a t(11;14) chromosomal translocation, a hallmark of MCL (9). Cyclin D1 has been previously shown to be involved in the regulation of MCL cell proliferation (9, 12). In the present report, we show that inhibition of proliferation of MCL correlated with the down-regulation of the expression of cyclin D1 protein. The suppression of cyclin D1 by 13-197 resulted in the suppression of MCL cell proliferation. Because NF-κB is well known to mediate anti-apoptotic effects (21), we examined whether suppression of NF-κB by 13-197 could lead to apoptosis. We found that 13-197 significantly induced the apoptosis in MCL cells including therapy-resistant cells and primary cells of MCL patients examined (Fig. 2, 5D). To further elucidate the mechanism of 13-197-mediated apoptosis in MCL cells, we investigated the important apoptotic regulators, i.e. Bcl-XL, Mcl-1, which are known to be regulated by NF-κB and overexpressed in MCL cells. Our results show that 13-197 downregulated the anti-apoptotic genes Bcl-XL and Mcl-1 in MCL cells including therapy-resistant cells and also in MCL primary cells. Further, our data also showed that 13-197 induced the cleavage of PARP protein, a marker of apoptosis, in these MCL cells (Fig. 3B and E). Together, results suggest that 13-197 exerts its antiproliferative and apoptotic effects in MCL cells by inhibiting the NF-κB pathway.
IKKβ, the major upstream kinase required for NF-κB activation, is constitutively phosphorylated in refractory MCL (14, 22, 44). We found that 13-197 treatment abrogated the constitutive IKKβ of NF-κB pathway activation through the inhibition of IkBα phosphorylation. Inhibition of IkBα phosphorylation resulted in the suppression of constitutive phosphorylation of p65 and its nuclear translocation (Fig. 3, 5). Several lines of evidence indicate that activation of IKKβ leads to the activation of another downstream signaling pathway like mTOR signaling pathway molecules, i.e. S6K, 4E-BP1, that increase the protein synthesis and thereby abnormal cell proliferation in various malignancies (20, 41, 42). In this context, our results showed that 13-197 can perturb mTOR signaling pathway by also inhibiting S6K and 4E-BP1 phosphorylation (Fig. 4). These data suggest that 13-197 principally targets IKKβ that leads to abrogation of NF-kB (transcriptionally) and mTOR (translationally) and thus suppress the cell proliferation and induce apoptosis in malignant cells of MCL.
Having shown the in vitro effect of 13-197, as a next logical step we examined efficacy of 13-197 in vivo in a mouse tumor model. NOD-SCID mice bearing therapy-resistant MCL tumor showed a significant decrease in tumor burden following treatment with 13-197 (Fig. 6A). Further, this reduced tumor burden significantly reflected in increasing disease-free survival of NOD-SCID mice transplanted with MCL (Fig. 6B), showing the ability of 13-197 as a single agent to reduce tumor burden and increase survival in an animal model. Also, 13-197 showed antilymphoma efficacy by dual targeting NF-kB and mTOR pathways against therapy-resistant MCL bearing a mouse model (Fig. 6C). We also observed no significant synergistic effect in reducing tumor burden when 13-197 was used in combination with CHOP chemotherapy (data not shown). This observation reaffirms that NOD-SCID mice bearing GRL tumor are already resistant to CHOP chemotherapy which we developed as a therapy-resistant model; thus, we did not observe added effect of CHOP chemotherapy on 13-197-mediated tumor burden in mice. These findings indicate that 13-197 alone has therapeutic efficacy against therapy-resistant MCL.
In summary, this is the first report evaluating the effects of a novel inhibitor 13-197 on MCL including therapy-resistant MCL. The current work has shown the single agent activity of 13-197 against MCL. Our results show that 13-197 can block the constitutive activation of IKKβ that perturb NF-κB and mTOR signaling pathways, suppresses the proliferation of therapy-resistant MCL and leads the MCL cells to apoptosis through the inhibition of NF-κB regulated anti-apoptotic genes in vitro. Also, results demonstrate the anti-lymphoma activity of 13-197 in primary cells of MCL patient samples by targeting NF-κB pathway. In addition, 13-197 alone has shown antitumor effect in reducing tumor burden and increasing disease-free survival of NOD-SCID mice transplanted with MCL in vivo. Thus, our findings highlight the dual targeting of NF-κB and mTOR pathways by which 13-197 modulates proliferation/apoptosis and exerts its antilymphoma activity while also warranting further preclinical and clinical investigations to take this approach to clinical setting.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the Lymphoma Research Foundation, New York, NY (S.S. Joshi). The present study was also supported in part by NIH grant (CA127239) (A. Natarajan).
The authors thank the flow cytometry, histology and virtual microscopy core facilities at UNMC for their help in these studies.
Footnotes
Disclosure of Conflicts of Interest: Authors have no conflict-of-interest to disclose.
References
- 1.Gianni AM, Magni M, Martelli M, Di Nicola M, Carlo-Stella C, Pilotti S, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen) Blood. 2003;102:749–55. doi: 10.1182/blood-2002-08-2476. [DOI] [PubMed] [Google Scholar]
- 2.Mangel J, Leitch HA, Connors JM, Buckstein R, Imrie K, Spaner D, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15:283–90. doi: 10.1093/annonc/mdh069. [DOI] [PubMed] [Google Scholar]
- 3.Nogai H, Dörken B, Lenz G. Pathogenesis of non-Hodgkin’s lymphoma. J Clin Oncol. 2011;29:1803–11. doi: 10.1200/JCO.2010.33.3252. [DOI] [PubMed] [Google Scholar]
- 4.Williams ME, Densmore JJ. Biology and therapy of mantle cell lymphoma. Curr Opin Oncol. 2005;17:425–31. doi: 10.1097/01.cco.0000174039.69656.2b. [DOI] [PubMed] [Google Scholar]
- 5.Williams ME, Dreyling M, Winter J, Muneer S, Leonard JP. Management of mantle cell lymphoma: key challenges and next steps. Clin Lymphoma Myeloma Leuk. 2010;10:336–46. doi: 10.3816/CLML.2010.n.066. [DOI] [PubMed] [Google Scholar]
- 6.Diefenbach CS, O’Connor OA. Mantle cell lymphoma in relapse: the role of emerging new drugs. Curr Opin Oncol. 2010;22:419–23. doi: 10.1097/CCO.0b013e32833d58f2. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen E, Freedman A. An update on the role of high-dose therapy with autologous or allogeneic stem cell transplantation in mantle cell lymphoma. Curr Opin Oncol. 2004;16:106–13. doi: 10.1097/00001622-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Leonard JP, Williams ME, Goy A, Grant S, Pfreundschuh M, Rosen ST, et al. Mantle cell lymphoma: biological insights and treatment advances. Clin Lymphoma Myeloma. 2009;9:267–77. doi: 10.3816/CLM.2009.n.055. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ. Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood. 2006;107:4540–8. doi: 10.1182/blood-2005-10-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–7. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 12.Martinez N, Camacho FI, Algara P, Rodriguez A, Dopazo A, Ruiz-Ballesteros E, et al. The molecular signature of mantle cell lymphoma reveals multiple signals favoring cell survival. Cancer Res. 2003;63:8226–32. [PubMed] [Google Scholar]
- 13.Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 15.Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008;7:40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrens AK, Chaturvedi NK, Nordgren TM, Dave BJ, Joshi SS. Establishment and Characterization of Therapy-Resistant Mantle Cell lymphoma Cell Lines Derived from Different Tissue Sites. Leuk Lymphoma. 2012;53:2269–78. doi: 10.3109/10428194.2012.691481. [DOI] [PubMed] [Google Scholar]
- 17.Tracey L, Perez-Rosado A, Artiga MJ, Camacho FI, Rodriguez A, Martinez N, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol. 2005;206:123–34. doi: 10.1002/path.1768. [DOI] [PubMed] [Google Scholar]
- 18.Zak Z, Gelebart P, Lai R. Fenofibrate induces effective apoptosis in mantle cell lymphoma by inhibiting the TNFalpha/NF-kappaB signaling axis. Leukemia. 2010;24:1476–86. doi: 10.1038/leu.2010.117. [DOI] [PubMed] [Google Scholar]
- 19.Dal Col J, Zancai P, Terrin L, Guidoboni M, Ponzoni M, Pavan A, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111:5142–51. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 20.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–47. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 23.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 25.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 27.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 28.Radharkrishnan P, Bryant VC, Blowers EC, Rajule R, Gautham N, Anwar MM, et al. Targeting the NF-κB and mTOR pathways with a quinoxaline urea analog that inhibits IKKβ for pancreas cancer therapy. Clin Cancer Res. 2013;19:2025–35. doi: 10.1158/1078-0432.CCR-12-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roue G, Perez-Galan P, Lopez-Guerra M, Villamor N, Campo E, Colomer D. Selective inhibition of IkappaB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol. 2007;178:1923–30. doi: 10.4049/jimmunol.178.3.1923. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Guerra M, Roue G, Perez-Galan P, Alonso R, Villamor N, Montserrat E, et al. p65 activity and ZAP-70 status predict the sensitivity of chronic lymphocytic leukemia cells to the selective IkappaB kinase inhibitor BMS-345541. Clin Cancer Res. 2009;15:2767–76. doi: 10.1158/1078-0432.CCR-08-2382. [DOI] [PubMed] [Google Scholar]
- 31.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y, Yeddula N, Leblanc M, Ke E, Zhang Y, Oldfield E, et al. Reduced cell proliferation by IKK2 depletion in a mouse lung-cancer model. Nat Cell Biol. 2012;14:257–65. doi: 10.1038/ncb2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greten FR, Arkan MC, Bollrath J, Hsu JC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol. 2010;14:347–61. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajule R, Bryant VC, Lopez H, Luo X, Natarajan A. Perturbing pro-survival proteins using quinoxaline derivatives: a structure-activity relationship study. Bioorg Med Chem. 2012;20:2227–34. doi: 10.1016/j.bmc.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Q, Bryant VC, Lopez H, Kelly DL, Luo X, Natarajan A. 2,3-Substituted quinoxalin-6-amine analogs as antiproliferatives: a structure-activity relationship study. Bioorg Med Chem Lett. 2011;21:1929–32. doi: 10.1016/j.bmcl.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryant VC, Kishore Kumar GD, Nyong AM, Natarajan A. Synthesis and evaluation of macrocyclic diarylether heptanoid natural products and their analogs. Bioorg Med Chem Lett. 2012;22:245–8. doi: 10.1016/j.bmcl.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegde GV, Nordgren TM, Munger CM, Mittal AK, Bierman PJ, Weisenburger DD, et al. Novel therapy for therapy-resistant mantle cell lymphoma: Multipronged approach with targeting of hedgehog signaling. Int J Cancer. 2012;131:2951–60. doi: 10.1002/ijc.27602. [DOI] [PubMed] [Google Scholar]
- 39.Gautam N, Bathena SP, Chen Q, Natarajan A, Alnouti Y. Pharmacokinetics, protein binding and metabolism of a quinoxaline urea analog as an NF-κB inhibitor in mice and rats by LC-MS/MS. Biomed Chromatogr. 2013;27:900–9. doi: 10.1002/bmc.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegde GV, Munger CM, Emanuel K, Joshi AD, Greiner TC, Weisenburger DD, et al. Targeting of sonic hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphoma. Mol Cancer Ther. 2008;7:1450–60. doi: 10.1158/1535-7163.MCT-07-2118. [DOI] [PubMed] [Google Scholar]
- 41.Cecconi D, Zamo A, Bianchi E, Parisi A, Barbi S, Milli A, et al. Signal transduction pathways of mantle cell lymphoma: a phosphoproteome-based study. Proteomics. 2008;8:4495–506. doi: 10.1002/pmic.200800080. [DOI] [PubMed] [Google Scholar]
- 42.Peponi E, Drakos E, Reyes G, Leventaki V, Rassidakis GZ, Medeiros LJ. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169:2171–80. doi: 10.2353/ajpath.2006.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 44.Guo R, Chang L, Liu Z, Li AX, Huang Q, Ann DK, et al. Canonical nuclear factor κB pathway links tumorigenesis of synchronous mantle-cell lymphoma, clear-cell renal-cell carcinoma, and GI stromal tumor. J Clin Oncol. 2011;29:257–61. doi: 10.1200/JCO.2010.32.1802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.