Abstract
Background and Objective
Failure of treatment for high-grade gliomas is usually due to local recurrence at the site of surgical resection indicating that a more aggressive form of local therapy such as photodynamic therapy (PDT) could be of benefit. The increase in brain edema following PDT using endogenous and exogenous photosensitizers was compared in terms of animal survival, MR imaging, and histopathological changes in normal brain.
Materials and Methods
Fischer rats were exposed to increasing laser light treatment following intraperitoneal injection of either the photosensitizers 5-aminolevulinic acid (ALA) or aluminum phthalocyanine disulfonate (AlPcS2a). Light treatment was applied either via an optical fiber inserted directly into the brain parenchyma or through a fiber applied to the surface of the intact skull. Edema development was followed by T2-weighted MR imaging.
Results
ALA and AlPcS2a PDT resulted in a fluence dependent increase in cerebral edema and mortality. AlPcS2a PDT showed significant edema and mortality even at low fluences following interstitial light delivery, which was reduced with surface illumination. The mechanism of edema was determined to be vasogenic by response to steroid therapy and confirmed on histological images.
Conclusions
T2 and contrast enhanced T1 MRI scanning proved to be a highly effective and noninvasive modality in following the development of the edema reaction and the degree and time course of blood–brain barrier dysfunction thus allowing the use of fewer animals. ALA mediated PDT induced a lower edema reaction than that observed with the photosensitizer AlPcS2a.
Keywords: photodynamic therapy, PDT, cerebral edema, ALA, AlPcS2a, vasogenic edema, endogenous photosensitizer, exogenous photosensitizer, amino levulenic acid, aluminum phthalocyanine disulfonate
INTRODUCTION
Recent data analysis by the Central Brain Tumor Registry of the United States has determined that gliomas account for 34% of all primary brain tumors, and 82% of all malignant primary brain tumor cases [1]. Tumor resection is usually the first modality employed in the treatment of gliomas [2]. Employing the improved surgical techniques now available, the incidence of gross tumor resection, as defined by postoperative magnetic resonance imaging (MR Imaging or MRI), has greatly increased [3–5]. Nevertheless, the great majority of glioma patients do suffer a recurrence of their tumors leading to their poor prognosis. The therapeutic goal following surgical resection therefore is the elimination of infiltrating tumor cells and transformed stem cells remaining in or migrating to the margins of the resection cavity, where most tumor recurrences occur [6]. Clearly, a more aggressive form of local therapy that could potentially delay the frequency of these recurrences and while minimizing damage to normal brain in the treatment area would be of great importance. Photodynamic therapy (PDT) is a form of light based antineoplastic treatment with the potential for tumor cell specificity, and has been employed as a local therapy within the glioma resection cavity [7,8]. PDT involves the administration of a tumor localizing photosensitizing agent followed by photoactivation by monochromatic illumination. Photosensitizers can either be exogenous, or preformed, such as Photofrin, aluminum phthalocyanine disulfonate (AlPcS2a), or endogenous requiring conversion before becoming active, such as 5-aminolevulinic acid (ALA). ALA is a prodrug that stimulates endogenous porphyrin production and causes intracellular accumulation of protoporphyrin IX (PpIX), a potent sensitizer via the heme biosynthetic pathway to the tumor site and is suitable for PDT treatment of gliomas [9]. The photosensitizer, AlPcS2a, often used for photochemical internalization (PCI), is lipophilic, while two adjacent sulfonate groups attached to the phthalocyanine molecule contributes to a hydrophilic nature [10–13]. This amphiphilic photosensitizer is designed to localize in the cellular membrane, achieved by inserting the lipophilic phthalocyanine skeleton of AlPcS2a in the lipophilic interior of the cellular membrane and dissolving the sulfonate groups in the hydrophilic outer layer of the membrane. While PDT results in direct phototoxic tumoricidal effect, it also has been shown that PDT can cause a variety of vascular effects ranging from transient vasospasm, increased vessel leakiness, and vessel wall disintegration ending in vessel closedown [14–17]. In this context, PDT induced vessel leakage has been reported to induce significant brain edema by a variety of photosensitizers [18–20].
In previous studies, we have demonstrated that ALA-mediated PDT was effective in a temporary opening of the blood–brain barrier (BBB) in a limited region of the rat brain [21]. In the study reported here the increase in brain edema following PDT mediated by two different photosensitizers was evaluated in terms of animal survival, histopathological changes in normal brain and MRI scanning and correlated with the degree of blood–barrier disruption. The effect of steroid treatment, to reduce posttreatment PDT induced edema, was also examined. The use of MRI has allowed a more detailed study of the dynamics of edema and BBB degradation following PDT than that obtained with more conventional methods.
METHODS
Animal Care
Adult male Fischer rats (Simonsen Laboratories, Inc., Gilroy, CA) weighing about 350 g were used in this study. The animal care and protocol were in accordance with institutional guidelines and were approved by the Institutional Animal Care and Use Committees (IACUC) at the Nevada Cancer Institute and University of Nevada Las Vegas where the studies were performed. Animal holding rooms were maintained at constant temperature and humidity on a 12-hour light and dark schedule at an air exchange rate of 18 changes/hour. For surgical procedures animals were anesthetized with pentobarbital (25 mg/kg intraperitoneal). Buprenorphine (0.08 mg/kg subcutaneous) was used as postoperative analgesia. All animals were euthanized at the end of the study or at the first signs of distress. Euthanasia was accomplished with an overdose of pentobarbital (100 mg/kg intraperitoneal).
SURGICAL PROCEDURES
Intracranial Irradiation
Animals were anesthetized, fixed in a stereotactic frame (David Kopf Instruments, Tujunga, CA) and skin covering the skull was incised. A 1.0-mm burr hole was then made with an 18-gauge needle. For light delivery, a 400-μm bare flat-end quartz optical fiber with numerical aperture of 0.22 was introduced directly into the brain according to the following coordinates: 3 mm posterior to and 2 mm to the right of the bregma and a depth of 5 mm below the dura. Light from a 632 nm (for ALA PDT) or 670 nm (for AlPcS2a PDT) diode laser (Intense, North Brunswick, NJ) was delivered interstitially through the optical fiber to obtain the desired fluence doses. In all cases, moisturizing gel was applied to the animal’s eyes and the rat was wrapped in a towel to prevent hypothermia. At the conclusion of treatment, the fiber was withdrawn from the brain, the burr hole was sealed with bone wax, the wound was closed with sutures, and the rats removed from the frame.
Surface Irradiation
At the time of light treatment, anesthetized rats were fixed in a stereotactic frame. A 1 cm skin incision was made exposing the skull and an optical fiber with a micro-lens at its tip, coupled to a 670 nm diode laser, was placed in contact with the surface of the skull 1 mm posterior to the bregma and 2 mm to the right of the midline. Following treatment, the wound was closed with surgical sutures and the rats removed from the frame.
PDT Treatment
Rats were administered either ALA (250 mg/kg) or AlPcS2a (5 or 1 mg/kg) intraperitoneally. Four to five hours following ALA injection and 18 hours following AlPcS2a injection, the rats were anaesthetized and their heads fixed in the stereotactic frame. Skin incision and laser illumination were performed as outlined above. 632 nm light was delivered interstitially (10 mW fluence rate) for a total light fluence of 54, 26, 17, or 9 J for ALA PDT and 670 nm light was delivered interstitially or by surface illumination to a total fluence of 0.5, 1, or 2.5 J (10 mW fluence rate).
MR Imaging
Following PDT treatment, rats were imaged either in a 3 T human (Philips Medical Systems, Bothell, WA) or a 7 T small-bore animal (Bruker, Billerica, MA) MRI scanner. Scans were typically acquired multiple times between days 1 and 18 posttreatments. Imaging procedures were carried out under general anesthesia. A small surface coil was placed on top of the target area. T1-weighted (T1W) images with and without Gadolinium based contrast (Multihance, administered IP), and T2-weighted (T2W) images were acquired. In keeping with known MR imaging findings, a high T2 signal in the area of treatment was interpreted as cerebral edema while T1 postgadolinium enhancement in the same area was interpreted as evidence of BBB breakdown. The extent of edema formation from each treatment was evaluated on T2W images. The qualitative analyses of contrast and edema volumes were examined using OsiriX VP software. The edema volumes were first manually contoured on each T2W image slice (Fig. 1). Then the total volume was calculated automatically by the software according to the following equation:
where Si represents the contrast area contoured on each slice and T represents the image slice thickness (T = 1.0 mm was used in all MR images acquired in this study).
Fig. 1.
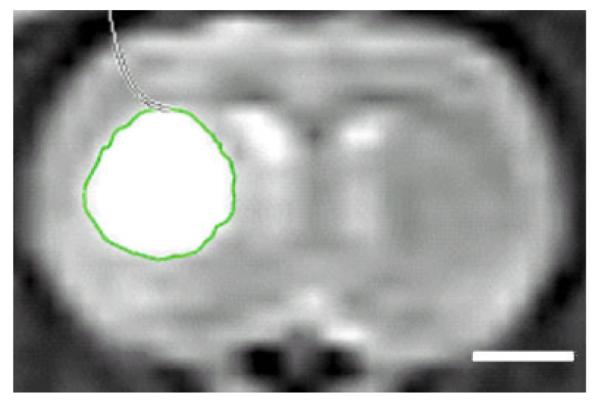
Image analysis methodology. Hyperintense signal regions on the T2W MR image represent cerebral edema. Contouring was performed manually, and edema volume determined using OsiriX VP software. White bar on the bottom right represents a scale of 2 mm.
Histological Preparation
Animals were sacrificed after completion of the in vivo studies and their brains extracted. The brains were sectioned along the fiber injection track and fixed by immersion in 10% buffered (pH 7.2) formalin prior to paraffin embedding. Four-micrometer thick coronal sections were obtained from the original cut surface representing the position of the fiber track and thereafter at 1, 2, and 3 mm depths. The sections were stained with hematoxylin and eosin (H&E) and examined under a light microscope by an independent pathologist blinded to the treatment modes.
RESULTS
Treated rats were followed daily to look for the effects of PDT agent and dosing on survival. As shown in Table 1, rats receiving AlPcS2a showed a significantly greater mortality at lower fluences than rats receiving ALA. Similarly, rats receiving interstitial illumination after receiving 5 mg/kg of AlPcS2a had significantly higher mortality even at low light fluences compared to rats receiving 1 mg/kg. However, all rats receiving surface illumination showed long-term survival at all light fluences and both doses of AlPcS2a. Rats that were alive longer than 20 days following PDT were defined as long-term survivors. Rats receiving 54 J of ALA PDT showed 50% survival, however, when co-administered steroids (solo-medrol 1 mg/kg intraperitoneally) all rats survived indicating that brain edema following PDT was of vasogenic origin.
TABLE 1.
Groups Survival in Each Group Depicted in Figure 2
| Groups | Group | % Survival |
|---|---|---|
| ALA 250 mg/kg + 9 J | A, n = 3 | 100 |
| ALA 250 mg/kg + 18 J | B, n = 3 | 100 |
| ALA 250 mg/kg + 26 J | C, n = 3 | 100 |
| ALA 250 mg/kg + 54 J | D, n = 6 | 50 |
| ALA 250 mg/kg + 54 J + Solo-Medrol | E, n = 6 | 100 |
| AlPcS2a 5 mg/kg + 2.5 J | F, n = 3 | 0 |
| AlPcS2a 5 mg/kg + 1 J | G, n = 3 | 0 |
| AlPcS2a 1 mg/kg + 2.5 J | H, n = 4 | 100 |
| AlPcS2a 1 mg/kg + 1 J | I, n = 4 | 100 |
| Surface illumination, AlPcS2a 1 mg/kg (0.5–2.5 J) | J, n = 16 | 100 |
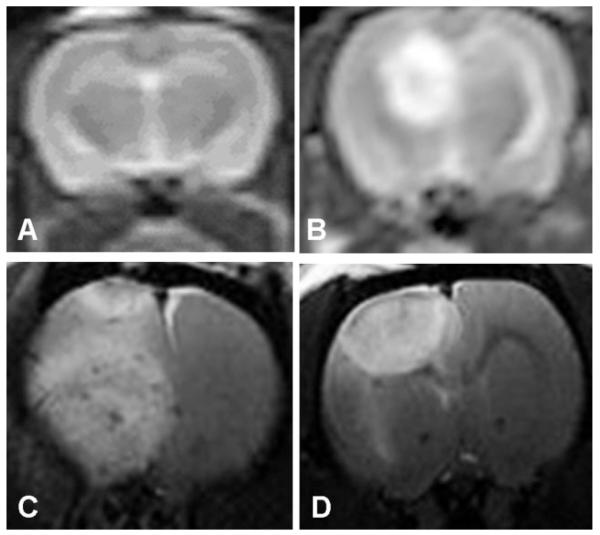
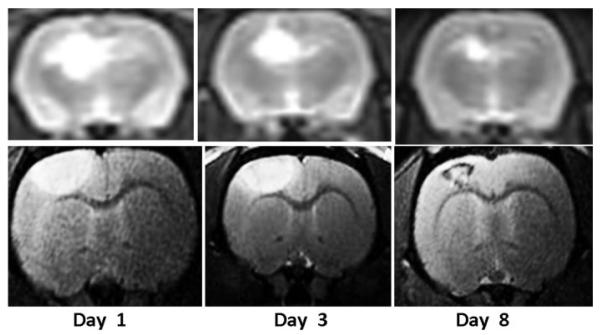
T2W MRI scans obtained posttreatment day 3 following 26 J of ALA PDT or 1 J of interstitial or surface illumination following administration of 5 mg/kg of AlPcS2a is shown in Figure 2A–D. As is evident in the figure, 1 J interstitial illumination with AlPcS2a PDT showed a larger volume of cerebral edema when compared to 26 J ALA PDT or 1 J surface illumination with AlPcS2a PDT. This explains the large mortality seen following interstitial PDT with AlPcS2a. Notice in Figure 2A (control) rats receiving no illumination following administration of ALA showed no evidence of cerebral edema on T2W MRI. Also noticeable was the change in the shape of cerebral edema from spherical to hemispherical with use of surface illumination due to changes in the light distribution (Fig. 2D). Due to the poor survival following interstitial PDT using AlPcS2a, it was decided to perform PDT with AlPcS2a (5 mg/kg) using only surface illumination, while interstitial illumination was continued for ALA PDT. Comparison of cerebral edema volumes on day 3 following ALA/AlPcS2a PDT calculated using T2W MRI is shown in Figure 3. As is evident in both groups, the volume of cerebral edema is clearly fluence dependent. Of note, 2.5 J of surface illumination using AlPcS2a generated a larger volume of cerebral edema than generated by 9 J of ALA PDT delivered interstitially. This demonstrates that AlPcS2a is a much more potent photosensitizer than ALA in opening the BBB with resultant cerebral edema. The time course for cerebral edema formation following 26 J of ALA PDT and 1 J surface illumination of AlPcS2a (5 mg/kg) PDT is shown in Figure 4. T2W MRI obtained on days 1, 3, and 8 following PDT in each case are shown for ALA PDT (upper panel, obtained at 3 T) and AlPcS2a PDT (lower panel, obtained at 7 T). As is evident in each case, edema volume peaked early and gradually decreased over time.
Fig. 2.
T2W MRI images following ALA administration without illumination (A), 26 J ALA PDT (B), 1 J interstitial illumination following 5 mg/kg of AlPcS2a PDT (C), and 1 J surface illumination following 5 mg/kg of AlPcS2a PDT (D). No edema is seen without illumination (A), large spherical edematous reactions is seen following interstitial light delivery (B,C), with change in shape to hemispherical following surface illumination (D).
Fig. 3.
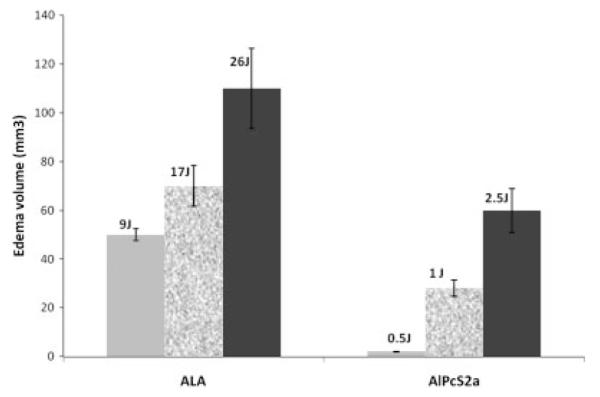
Fluence dependent cerebral edema response following ALA PDT and AlPcS2a PDT (surface illumination). The lower edema volumes following AlPcS2a PDT is likely due to the path of light diffusion with surface illumination that results in a hemispherical shaped edema compared to spherically shaped light distributions with interstitial illumination as seen in Figures 3 and 5.
Fig. 4.
T2W MRI showing the time-course of cerebral edema development following 26 J ALA PDT (upper panel, obtained at 3-T magnetic field strength) and 1 J (surface illumination) AlPcS2a PDT (lower panel, obtained at 7-T magnetic field strength).
Histology
In areas exposed to the highest fluence levels (5–15 μm from the fiber), no significant pathology was observed in coronal histological sections obtained from animals 15 days after being subjected to ALA mediated PDT at a fluence of 9 J. At higher fluence levels of 17 J, extensive infiltration of lymphocytes and macrophages (some loaded with hemosiderin) was apparent (Fig. 5A). Blood vessels in these sections showed hyperplastic endothelial cells. At fluence levels of 26 J a focally extensive area of necrosis with degradation of brain parenchyma and infiltration of lymphocytes, plasma cells, and foamy macrophages (Gitter cells) was seen. Some of the Gitter cells contained hemosiderin, which is suggestive of treatment-induced hemorrhage. Blood vessels in these sections also showed hyperplastic endothelium (images not shown). Histological sections taken from the brains of light-only control animals showed no pathology even at a fluence level of 26 J. Animals fixed and sectioned 15 days after AlPcS2a PDT showed no pathology at 1 J although permanent damage was visible following PDT at 2.5 J (Fig. 5B).
Fig. 5.
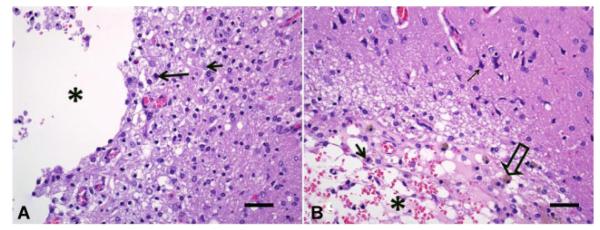
Representative histologic sections of rat brains sectioned 15 days following treatment with ALA PDT at 17 J (A) and AlPcS2a PDT at 2.5 J (B) at ×400 magnification. Figure A from the region of the thalamus shows area of necrosis with proteinaceous perivascular edema (asterisk), foamy macrophages (gitter cells; arrow), and gliosis. Also, large number of reactive astrocytes (short arrow). Chronic hemorrhage with hemosiderin loaded macrophages (hollow arrow). B: Region of the dorsolateral cortex, starting at the meningeal surface and extending deep into the white matter, very close to the hippocampus shows an area with severe malacia, cavitation (asterisk), and large numbers of gitter cells (arrow). Edema and chronic hemorrhage with hemosiderin loaded macrophages (hollow arrow) is present. Pyknotic neurons (thin arrow) are also seen. Bars on the bottom right of each image represents a scale of 50 μm.
The time course for cerebral edema formation as well as contrast uptake following 17 J of ALA PDT is demonstrated in Figure 6. The upper panel (T2W MRI) depicts cerebral edema while the lower panel (postcontrast T1W MRI) depicts opening of the BBB. As is evident from both panels, cerebral edema formation and contrast enhancement is maximum between days 1 and 3 following PDT and then progressively decreases. The effect of steroids on cerebral edema formation following 17 J of ALA PDT is shown in Figure 7. Cerebral edema was significantly decreased following administration of steroids further demonstrating that the edema was vasogenic in nature and responsive to steroid treatment.
Fig. 6.
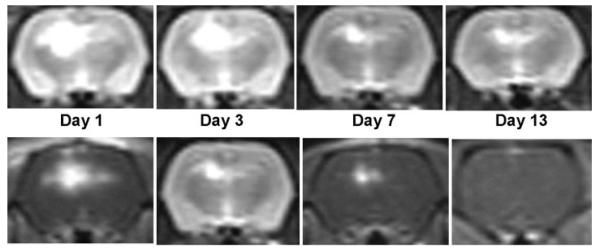
T2W MR images (upper panel) and postcontrast T1W MR images (lower panel) following 17 J of ALA PDT treatment depicting the time-course of cerebral edema development and BBB break down, respectively, following ALA PDT.
Fig. 7.
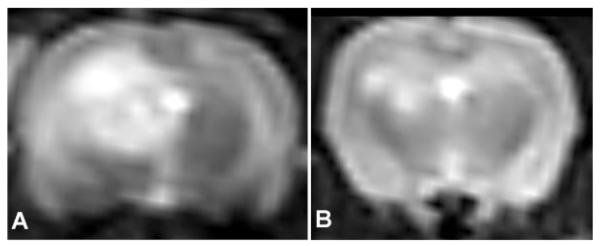
The effect of steroid (solo-medrol) treatment on brain edema following 17 J of ALA PDT. Decreased edema is seen following steroid treatment (B) as compared to untreated brain (A). Similar findings were seen following AlPcS2a PDT at 2.5 J.
DISCUSSION
PDT has developed to become an important approach for cancer treatment. The treatment typically involves systemic administration of a tumor-localizing photosensitizer and its subsequent activation by light of an appropriate wavelength to create a photochemical reaction causing damage to the tumor. Photosensitizers used in PDT are either exogenous and preformed such as AlPcS2a and photofrin, or endogenous and bioconverted such as ALA to protoporphyrin IX (PpIX). Photofrin has been studied worldwide in clinical PDT trials during the past three decades and has been approved in many countries for several medical indications. However, the major side effect associated with exogenous photosensitizers is their persistence in the body and the subsequent risk of skin photosensitization.
ALA used as a photosensitizer in this study is a prodrug that stimulates endogenous porphyrin production and causes intracellular accumulation of PpIX, a potent sensitizer via the heme biosynthetic pathway to the tumor site and is suitable for PDT treatment of gliomas [22–24]. AlPcS2a is amphophillic and perfectly designed to localize in the cellular membrane [10–13]. This is achieved by inserting the lipophilic phthalocyanine skeleton of AlPcS2a in the lipophilic interior of the cellular membrane and dissolving the sulfonate groups in the hydrophilic outer layer of the membrane. AlPcS2a molecules first localize in the cell membrane. During endocytosis, a partial cell membrane with previously localized AlPcS2a molecules pinches inward to form an endocytic vesicle and subsequently, the attached AlPcS2a molecules are transported into the cell via the membrane of the vesicle.
MRI technique is ideally suited for evaluating the extent and time course of edema formation and its noninvasive nature minimizes the number of animals required for the studies. Due to its high water content, edema appears hyperintense (bright) on T2-weighted (T2W) images and hypointense (dark) on T1-weighted (T1W) images. Contrast agents were administered to determine BBB disruption. As gadolinium-based contrast agent (Multihance) does not cross the intact BBB due to its large molecular weight, its appearance on T1W images following contrast administration is indicative of BBB disruption. T2-weighted MR images were used for evaluating the brain edema, which is an indirect result of BBB disruption, induced by PDT. Since water molecules have a long T2 relaxation time, which induces a very high T2 signal, edema appears bright on T2-weighted images and is therefore easily visualized.
ALA and a number of exogenous photosensitizers have been studied extensively for PDT of brain tumors and mediate their effects through direct tumor phototoxicity as well as peritumoral vasculotoxicity. A previous study has demonstrated that ALA-mediated PDT was highly effective in opening the BBB in a limited region of the normal rat brain [21]. The degradation of the BBB was temporary in nature, opening rapidly following treatment and significantly restored during the ensuing 72 hours. The BBB was found to be disrupted as early as 2 hours following PDT and approximately 90% restored 72 hours later. From a therapeutic standpoint, this time window is an opportunity for the systemic administration of anticancer agents. The exact mechanisms by which PDT leads to BBB degradation are uncertain but they likely include direct PDT effects on the endothelial cytoskeleton that lead to cell rounding and contraction, probably mediated by PDT-induced microtubule depolymerization [25]. In addition, the formation and/or enlargement of endothelial gaps, has been observed in response to PDT [14]. Application of surface illumination in our experiment simulates the walls of a resection cavity and the idea was to evaluate the effects of PDT on the more or less normal brain surrounding the resection cavity, that is, the effects on the vasculature (endothelial cells) since the photosensitizer does not pass the intact BBB. For clinical application light delivery should be within tumor resection cavities unless tumors are in unresectable locations in which case stereotactic PDT has been attempted [26].
Results of the present study show that AlPcS2a is a more potent photosensitizer as evidenced by increased mortality and brain edema (Fig. 2) following relatively low light fluences. The increased effectiveness of AlPcS2a PDT in opening the BBB is most likely due to the site of localization of the photosensitizer. Since AlPcS2a is an amphiphilic molecule it accumulates in cellular membranes such as those of the endothelial cells which constitute the BBB. Therefore, AlPcS2a PDT causes direct photochemical damage to cell membranes which can lead to disruption of the BBB. In contrast, ALA is a hydrophilic molecule that tends to accumulate in cellular organelles such as the mitochondria. As a result, ALA-PDT is less likely to produce a direct effect on the vasculature. Thus, ALA PDT can be combined with low-dose exogenous photosensitizer for a synergistic multimodal antitumoral effect. This approach has been previously used successfully in an animal study combining ALA PDT to target tumor cells with photofrin to target the vasculature [27]. The high mortality observed following interstitial AlPcS2a PDT is not only due to localization of AlPcS2a on the capillary endothelial cells, but also due to the light distribution associated with this mode of light delivery. A previous study demonstrated that the light fluence rate at the tip of an optical fiber inserted directly into the rat brain was several orders of magnitude higher than the actual power output [28]. A rapid decrease in the interstitial fluence rate occurs with a light penetration depth of about 6 mm in the rat brain falling to 1% of that measured at the tip. Surface irradiation was chosen in order to avoid the high fluence rates associated with interstitial light delivery and, as shown in Figures 2 and 3, this method of light delivery was well tolerated.
The BBB is formed by brain capillary endothelial cells connected to each other with tight junctions. Substantially different than those found in peripheral microvessels, the endothelial cells lining the brain vessels are connected by much tighter junctional complexes that lack fenestrations that completely seal the pericellular spaces and form a continuous physical barrier between the CNS and blood circulation [29,30]. The brain side of the capillary endothelial cells is completely covered by a basement membrane with astrocyte end-foot processes closely attached to it. The BBB allows diffusion of lipid soluble and small water soluble compounds, but forms a barrier against simple diffusion of larger molecules [31].
The opening of the BBB following PDT results in the formation of vasogenic cerebral edema. The formation of cerebral edema can be dangerous as it can result in increased intracranial pressure, which impairs vascular perfusion and can lead to brain ischemia, herniation, and death [32–34]. Cerebral edema is characterized as being either of vasogenic origin or due to cytotoxic mechanisms [35,36]. Cytotoxic edema occurs due to changes in cellular metabolism resulting in inadequate functioning of the sodium–potassium pump in cell membranes while the BBB remains intact. Lesions leading to disruption of the BBB result in leakage of plasma constituents into surrounding brain tissue as vasogenic edema, with increasing water content thus appearing as increased signal intensity on T2W MRI. The edema caused by PDT in normal brain, due to its rapid reversibility and response to steroid treatment, is likely of the vasogenic type, that is, caused by changes in endothelial fenestrations allowing edema fluid to leave the vascular system. Fortunately, the use of steroids results in a dramatic decrease in PDT-induced edema and results in reduced mortality. The use of steroids for the management of brain tumor related or treatment-induced edema is a common strategy employed clinically and could be effective in managing PDT-induced edema in patients [37–41].
CONCLUSIONS
T2W and contrast-enhanced T1W MRI scanning proved to be a highly effective and noninvasive modality for following the development of the edema reaction and the degree and time course of BBB disruption following PDT. ALA-mediated PDT induced a lower edema reaction than that observed with AlPcS2a for a given fluence. The mechanism of cerebral edema was found to be vasogenic as evidenced by the rapid response to steroid therapy. This was confirmed from histological images.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Nevada Cancer Institute which sponsored this research through the NVCI Collaborative Grant Program. Henry Hirschberg is grateful for the support of the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the Laser-Microbeam and Medical Program (LAMMP) and the Chao Cancer Center Optical Biology Shared Resource at the University of California, Irvine.
REFERENCES
- 1.CBTRUS Statistical Report: Primary Brain Tumors in the United States 2004–2007, the Central Brain Tumor Registry of the United States. [Accessed June 2011].
- 2.Salcman M. Epidemiology and factors affecting survival. In: Apuzzo MLJ, editor. Malignant cerebral glioma. American Association of Neurological Surgeons; IL: 1990. pp. 95–109. [Google Scholar]
- 3.Hirschberg H, Samset E, Hole PK, Lote K. Impact of intra-operative MRI on the results of surgery for high grade gliomas. J Minim Invasive Neurosurg. 2006;8(2):77–84. doi: 10.1055/s-2004-830225. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomized controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 5.Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C, Franz K, Pietsch T, ALA-Glioma Study Group Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 6.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 7.Muller PJ, Wilson BC, Lilge LD, Yang V, Hetzel FW, Chen Q, Selker R, Abrams J. Photofrin photodynamic therapy for malignant brain tumors. Proc SPIE. 2001;4248:34–41. [Google Scholar]
- 8.Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre phase III randomised controlled trial. Lasers Med Sci. 2008;23:361–367. doi: 10.1007/s10103-007-0494-2. [DOI] [PubMed] [Google Scholar]
- 9.Frisen S, Hjortland GO, Hirschberg H, Engebraaten O, Madsen SJ, Nesland JM, Peng Q. 5-Aminolevulinic acid based photodynamic detection and therapy of brain tumors. Int J Oncol. 2002;21(3):577–582. [PubMed] [Google Scholar]
- 10.Maman N, Dhami S, Phillips D, Brault D. Kinetic and equilibrium studies of incorporation of di-sulfonated aluminum phthalocyanine into unilamellar vesicles. Biochim Biophys Acta. 1999;1420:168–178. doi: 10.1016/s0005-2736(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 11.Høgset A, Prasmickaite L, Selbo P, Hellum M, Engesæter B, Bonsted A, Berg K. Photochemical internalisation in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:95–115. doi: 10.1016/j.addr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Berg K, Bommer J, Moan J. Evaluation of sulfonated aluminum phthalocyanines for use in photochemotherapy. Cellular uptake studies. Cancer Lett. 1989;44:7–15. doi: 10.1016/0304-3835(89)90101-8. [DOI] [PubMed] [Google Scholar]
- 13.Paquette B, Ali H, Langlois R, Van Lier J. Biological activities of phthalocyanines—VIII. Cellular distribution in V-79 Chinese Hamster cells and phototoxicity of selectively sulfonated aluminum phthalocyanines. Photochem Photobiol. 1988;47:215–220. doi: 10.1111/j.1751-1097.1988.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 14.Fingar VH. Vascular effects of photodynamic therapy. J Clin Laser Med Surg. 1996;14:323–328. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- 15.Snyder JW, Greco WR, Bellnier DA, Vaughan L, Henderson BW. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res. 2003;63:8126–8131. [PubMed] [Google Scholar]
- 16.Debefve E, Pegaz B, Ballini JP, Konan YN, Van den Bergh H. Combination therapy using aspirin enhanced photodynamic selective drug delivery. Vasc Pharmacol. 2007;46:171–180. doi: 10.1016/j.vph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Debefve E, Pegaz B, van den Bergh H, Wagnieres G, Lange N, Ballini JP. Video monitoring of neovessel occlusion induced by photodynamic therapy with verteporfin (Visudyne), in the CAM model. Angiogenesis. 2008;11:235–243. doi: 10.1007/s10456-008-9106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stummer W, Goetz C, Hassan A, Heimann DVM, Kempski O. Kinetics of Photofrin II in perifocal brain edema. Neurosurgery. 1993;33:1075–1082. doi: 10.1227/00006123-199312000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Goetz1 C, Hasan A, Stummer W, Heimann A, Kempski O. Experimental research photodynamic effects in perifocal, oedematous brain tissue. Acta Neurochir (Wien) 2002;144(2):173–179. doi: 10.1007/s007010200021. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Rachinger W, Stepp H, Reulen HJ, Stummer W. Oedema formation in experimental photo-irradiation therapy of brain tumours using 5-ALA. Acta Neurochir (Wien) 2005;147(1):57–65. doi: 10.1007/s00701-004-0422-1. [DOI] [PubMed] [Google Scholar]
- 21.Hirschberg H, Uzal FA, Chighvinadze D, Zhang MJ, Peng Q, Madsen SJ. Disruption of the blood–brain barrier following ALA-mediated photodynamic therapy. Lasers Surg Med. 2008;40(8):535–542. doi: 10.1002/lsm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem Photobiol. 1997;65:235–251. doi: 10.1111/j.1751-1097.1997.tb08549.x. [DOI] [PubMed] [Google Scholar]
- 23.Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky K-E, Nesland JM. 5-aminolevulinic acid-based photodynamic therapy: Clinical research and future challenges. Cancer. 1997;79:2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Madsen SJ, Sun CH, Tromberg BJ, Hirschberg H. Photodynamic therapy of human glioma spheroids using 5-aminolevulinic acid. Photochem Photobiol. 2000;72:128–134. doi: 10.1562/0031-8655(2000)072<0128:ptohgs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Sporn LA, Foster TH. Photofrin and light induces microtubule depolymerization in cultured human endothelial cells. Cancer Res. 1992;52:3443–3448. [PubMed] [Google Scholar]
- 26.Beck TJ, Kreth FW, Beyer W, Mehrkens JH, Obermeier A, Stepp H, Stummer W, Baumgartner R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med. 2007;39(5):386–393. doi: 10.1002/lsm.20507. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, Warloe T, Moan J, Godal A, Apricena F, Giercksky KE, Nesland JM. Antitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy can be enhanced by the use of a low dose of photofrin in human tumor xenografts. Cancer Res. 2001;61(15):5824–5832. [PubMed] [Google Scholar]
- 28.Angell-Petersen E, Hirschberg H, Madsen SJ. Determination of fluence rate and temperature distributions in the rat brain; implications for photodynamic therapy. J Biomed Opt. 2007;12(1):014003. doi: 10.1117/1.2709882. [DOI] [PubMed] [Google Scholar]
- 29.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Abbott N. Physiology of the blood–brain barrier and its consequences for drug transport to the brain. Int Congr Ser. 2005;1277:3–18. [Google Scholar]
- 31.Abbott N, Romero I. Transporting therapeutics across the blood–brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 32.Gobiet W, Grote W, Bock WJ. The relation between intracrainal pressure, mean arterial pressure and cerebral blood flow in patients with severe head injury. Acta Neurochir (Wien) 1975;32(1–2):13–24. doi: 10.1007/BF01405899. [DOI] [PubMed] [Google Scholar]
- 33.Tweed WA, Overgaard J. Cerebral blood flow in patients with intracranial pressure elevation due to traumatic brain edema. Can J Neurol Sci. 1976;3(1):35–37. doi: 10.1017/s031716710002597x. [DOI] [PubMed] [Google Scholar]
- 34.Graham DI, Lawrence AE, Adams JH, Doyle D, McLellan DR. Brain damage in non-missile head injury secondary to high intracranial pressure. Neuropathol Appl Neurobiol. 1987;13(3):209–217. doi: 10.1111/j.1365-2990.1987.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 35.Klatzo I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987;72(3):236–239. doi: 10.1007/BF00691095. [DOI] [PubMed] [Google Scholar]
- 36.Kuroiwa T, Ueki M, Chen Q, Suemasu H, Taniguchi I, Okeda R. Biomechanical characteristics of brain edema: The difference between vasogenic-type and cytotoxic-type edema. Acta Neurochir Suppl (Wien) 1994;60:158–161. doi: 10.1007/978-3-7091-9334-1_42. [DOI] [PubMed] [Google Scholar]
- 37.Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. Lancet. 1961;81:46–53. [PubMed] [Google Scholar]
- 38.Rasmussen T, Gulati DR. Cortisone in the treatment of postoperative cerebral edema. J Neurosurg. 1962;19:535–544. doi: 10.3171/jns.1962.19.7.0535. [DOI] [PubMed] [Google Scholar]
- 39.Gårde A. Experiences with dexamethasone treatment of intracranial pressure caused by brain tumours. Acta Neurol Scand Suppl. 1965;13(Pt 2):439–443. doi: 10.1111/j.1600-0404.1965.tb01912.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JA, Assam S. Use of betamethasone in reduction of cerebral edema. Mil Med. 1966;131(1):44–47. [PubMed] [Google Scholar]
- 41.Roth P, Wick W, Weller M. Steroids in neurooncology: Actions, indications, side-effects. Curr Opin Neurol. 2010;23(6):597–602. doi: 10.1097/WCO.0b013e32833e5a5d. [DOI] [PubMed] [Google Scholar]