1. Introduction
Androgens, and the transcriptional programs activated via binding of androgens to the androgen receptor (AR), are critical for normal prostate development, growth, and the maintenance of mature physiologic functions. Signaling via the AR axis is also thought to be important in facilitating prostate carcinogenesis, although the precise mechanisms driving initiation and progression of prostate cancer (PCa) are still being elucidated. PCa risk and outcome have been variably associated with serum androgen levels, and (more consistently) with polymorphisms in the androgen signaling pathway. More recently, widely prevalent androgen sensitive genomic rearrangements involving ETS family transcription factors have been identified in prostate tumors, and appear to play a key role in PCa invasion and progression. Importantly, up-regulated expression of the AR and AR target genes, indicative of ongoing AR-mediated signaling and cellular activity, is almost uniformly observed in advanced prostate tumors recurring despite castration.
Thus, the AR signaling axis provides a critical target for PCa prevention and the treatment of hormone naïve and castration resistant prostate cancer (CRPC). Androgen deprivation therapy (ADT) remains the single most effective therapy for the initial treatment of advanced PCa. Moreover, novel agents targeting the AR pathway, such as abiraterone and MDV3100, have demonstrated remarkable clinical efficacy in Phase II and III studies, underscoring the sensitivity of castration resistant disease to subsequent, more stringent AR pathway inhibition. In this report, we provide an overview of androgen action and metabolism in the prostate, with a focus on receptor and ligand-mediated mechanisms by which castration resistant prostate tumors promote and retain dependence on the AR axis.
2. Androgen Receptor: Structure and function in normal physiology
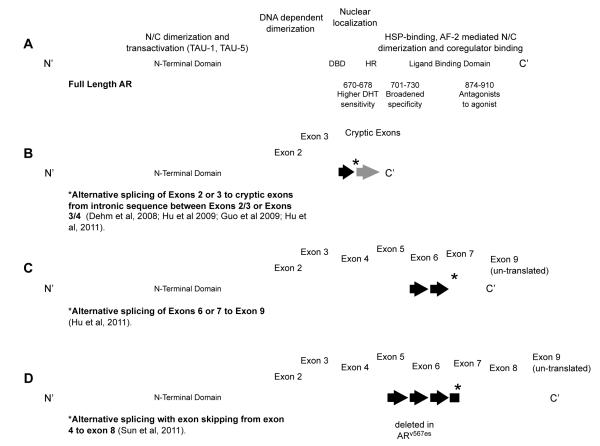
The AR is a member of the nuclear receptor transcription factor superfamily and is a key regulator of normal prostate development and physiology. The AR contains several distinct functional motifs and displays a structure similar to many other steroid hormone receptors. These motifs are organized as the amino-terminal domain (NTD), DNA-binding domain (DBD), the carboxy-terminal ligand-binding domain (LBD), and transactivation domains (AF-1, 2 and 5) (Figure 1A). A hinge region separates the DBD and the LBD, and contains a nuclear localization signal. The DBD is comprised of two zinc finger motifs. The first zinc finger defines DNA binding specificity, whereas the second zinc finger facilitates receptor dimerization and stabilization of the DNA-receptor complex. Co-regulator binding and transcriptional activation is defined by AF-1, 2 and 5, of which AF-1 appears to be the major contributor. Contrary to the highly conserved DBD and LBD, the NTD is the least conserved region of the AR. This region is comprised of poly-glutamine (Poly-Q), poly-proline (Poly-P) and poly-glycine (Poly-G) repeats. Notably, the Poly-Q displays high variability, ranging in length from 18-22 repeats (normal) to over 40 repeats, which is linked to spinal and bulbar muscular atrophy (Walcott and Merry 2002). Studies suggest that shorter tracts correlate with a more active AR, which may correlate with PCa risk, however the results are conflicting (Linja and Visakorpi 2004).
Figure 1. Androgen receptor domains affected by mutation in prostate cancer and exon structure of major splice variants.
(A) Domains of the full length androgen receptor (AR), include the amino (N) terminal domain (NTD), the DNA binding domain (DBD), the hinge region (HR) and the carboxy (C) terminal ligand binding domain (LBD), involved in the indicated functions in AR transactivation. Three codon regions of the AR in which mutations have been identified in prostate tumors are denoted, along with the indicated changes in function. Generation of recently reported truncated AR variants lacking the LBD occurs via alternative splicing to cryptic exons (B, C), or alternative splicing with exon skipping, resulting in exon deletion (D). In all cases the N’ terminal domain is lost, with consequent loss of ligand binding activity.
In the absence of hormone ligand, the AR is cytoplasmic and bound by heat shock protein (HSP) complexes. Although inactive, the HSPs maintain the AR in a “poised state” capable of binding hormone. Upon binding hormone, the HSPs dissociate from the complex, the AR dimerizes and translocates to the nucleus (Edwards and Bartlett 2005). In the canonical pathway, AR dimers bind to AR-response elements (AREs) in the DNA, recruit coactivator complexes that modify chromatin structure, recruit RNA polymerase II and induce transcription (Heinlein and Chang 2004). Other evidence suggests that the AR activates transcription by binding to other transcription factors and essentially acts as a coactivator via the recruitment of other coactivators (Lu et al. 2000). Additional studies have revealed that activated AR can stimulate downstream kinases (e.g. p42/p44 MAPK and PI3K) via interactions with modulator of nongenomic action of estrogen receptor (MNAR) and src (Castoria et al. 2004; Unni et al. 2004). Furthermore, the AR has been shown to be involved in the repression of some genes (Baniwal et al. 2009).
3. AR: Alterations in structure, function and ligand binding associated with PCa
In the normal prostate, the predominant role of AR is to promote differentiation of luminal epithelial cells and to regulate the transcription of genes encoding proteins necessary for prostate function, such as prostate specific antigen (PSA). In PCa, the significant functions of AR are less clear, although presumably AR activity modulates the expression of genes associated with cell cycle regulation, survival and growth (Knudsen et al. 1998; Xu et al. 2006; Wang et al. 2007; Wang et al. 2009). Furthermore, AR is known to stimulate the expression of TMPRSS2:ERG, a common gene fusion associated with PCa (Cerveira et al. 2006; Perner et al. 2006; Tomlins et al. 2006; Cai et al. 2009). High levels of ERG, and other members of the Ets oncogene family, may contribute to PCa development, however the exact biological functions remain to be fully understood (Tomlins et al. 2008). Regardless of the mechanism, it is clear that AR signaling plays a critical role in the development and progression of PCa.
ADT remains the primary modality for treating locally advanced or metastatic disease. That ADT is initially highly effective is irrefutable, as evidenced by marked improvement in cancer-related symptoms and radiographic tumor regression. However, the clinical course is uniformly marked by progression to the castration resistant disease state, at which point the median survival is between 18 and 24 months (Gleave and Small 2004). Although ADT, and additional therapeutics targeting AR activity such as abiraterone (Attard et al. 2008) prolong survival, long-term responses or cures are exceedingly rare. Mechanisms responsible for the incomplete response to ADT and progression to CRPC have not been fully elucidated. However, increasing serum PSA levels are almost invariably associated with the detection of recurrent tumors, strongly suggesting that AR is reactivated in PCa progression (Ryan et al. 2006).
In recent years, analyses of CRPC tumor samples have revealed several mechanisms by which AR is reactivated in the presence of ADT. Examples include:
3.1. Increased expression/amplification of AR
The vast majority of CRPC patients display a marked increase in AR mRNA and protein (van der Kwast et al. 1991; Ruizeveld de Winter et al. 1994; Taplin et al. 1995; Visakorpi et al. 1995; Bubendorf et al. 1999; Latil et al. 2001; Linja et al. 2001; Ford et al. 2003; Holzbeierlein et al. 2004; Mohler et al. 2004; Stanbrough et al. 2006). In nearly one-third of CRPC patients, the mechanism for increased AR expression is through amplification of the AR locus itself (Visakorpi, Hyytinen et al. 1995; Koivisto et al. 1997; Brown et al. 2002; Edwards et al. 2003; Ford, Gregory et al. 2003). Other mechanisms promoting AR reactivation are less understood, but may include increased transcription rates, or stabilization of the mRNA or protein. Regardless of the mechanism, increased AR expression may contribute to PCa growth in a castrate setting by compensating for low androgen levels. Indeed, xenograft studies demonstrated that PCa growth in castrate animals is augmented by AR overexpression (Chen et al. 2004). Furthermore, transgenic mice overexpressing AR in a prostate-specific manner developed hyperplasia and carcinoma in situ (Stanbrough et al. 2001). Clearly, enhanced AR expression plays a major role in AR reactivation and CRPC formation.
3.2. AR mutations
Several studies have revealed a number of AR alterations that can either widen the spectrum of ligands that function as AR agonists or bypass the requirement for ligand altogether. A large number of AR somatic mutations have been identified which result in promiscuous binding and activation of the AR by weak adrenal androgens and other steroid hormones, including DHEA, progesterone, estrogens, and cortisol (Culig et al. 1993; Tan et al. 1997; Zhao et al. 2000; Taplin 2007; Brooke and Bevan 2009; Yuan and Balk 2009). Other mutations convert AR antagonists (flutamide and bicalutamide) into potent agonists, and it has been proposed that treatment with specific AR antagonists actually selects for tumors expressing AR mutants activated by the therapeutic agent (Steinkamp et al. 2009). Mutations in three areas of the AR appear to impart specific properties (Figure 1A): 1. codon 701-730 mutations broaden ligand specificity to adrenal androgens, glucocorticoids and progesterone; 2. codon 874-910 mutations in the AF-2 region convert AR antagonists to agonists (eg T877A); 3. codon 670-678 mutations in the hinge and LBD impart increased sensitivity to DHT (Veldscholte et al. 1990; Middleman et al. 1996; Taplin et al. 1999; Hara et al. 2003; Taplin et al. 2003; Yoshida et al. 2005; Taplin 2007). Other mutations in the amino terminus (Han et al. 2005) and the DNA binding domain (Wu et al. 2007) of the AR have been shown to have oncogenic properties. Although the frequency of AR mutation in CRPC tumors is low (8-25%), it is clear that specific mutations are selected for by a subset of tumors and that these mutations may promote the progression to CRPC.
3.3. AR splice variants
Recent studies have identified several splice variants of the AR mRNA that are able to bypass the requirement for ligand (Dehm et al. 2008; Guo et al. 2009; Hu et al. 2009; Steinkamp, O’Mahony et al. 2009; Sun et al. 2010; Hornberg et al. 2011; Hu et al. 2011) Although each variant displays a slightly different structure, they each lack portions of the carboxy-terminal LBD, resulting in a constitutively active AR (Figure 1B-D) (Jenster et al. 1995). Because of the altered structure, it is possible that AR splice variants also display altered interactions with coregulators and altered binding to AREs. Indeed, studies have shown that AR isoforms have unique and overlapping transcriptional programs compared to the wild-type AR (Guo, Yang et al. 2009; Hu, Dunn et al. 2009; Sun, Sprenger et al. 2010). Importantly, the expression of certain variants (e.g. AR-V7, which can be detected in hormone-naïve PCa) has been associated with a shorter time to disease recurrence following radical prostatectomy (Guo, Yang et al. 2009; Hu, Dunn et al. 2009; Hornberg, Ylitalo et al. 2011). Furthermore, the expression of these constitutively active splice variants is enhanced in CRPC tumors relative to primary tumors, but is generally absent in normal prostate epithelium, also suggesting a role in prostate cancer progression (Hu, Dunn et al. 2009; Sun, Sprenger et al. 2010). Although the precise number and frequency of these alternatively spliced variants associated with CRPC are unknown, tumors expressing these variants may present a significant clinical challenge depending on their sensitivity to AR antagonists designed to target the AR LBD (e.g. bicalutamide, TOK-001 or MDV3100). Interestingly, emerging data suggest that truncated AR variants function in large part via binding and promoting nuclear localization of full length AR, and thus, the presence of carboxy-terminal AR variants does not necessarily preclude a response to ligand binding inhibitors such as MDV. (Watson et al. 2010) Moreover, a novel small molecule specifically targeting the AR NTD (EPI-001) has recently been reported and its efficacy against prostate tumors expressing AR splice variants is under investigation. (Andersen et al. 2010)
3.4. Alterations in cofactor recruitment
As a transcription factor, the AR recruits cis-acting cofactors that significantly influence how the AR regulates target genes. It is generally accepted that AR agonists induce the recruitment of coactivators, whereas AR antagonists influence conformational changes that promote the recruitment of corepressors. Therefore, dysregulation of expression and/or turnover of coactivators and corepressors may promote aberrant AR activity and disease progression. Indeed, several coactivators, including TIF2, SRC1, SRC2, SRC3, TIP60 and ARA70 are enhanced in recurrent prostate cancer (Gregory et al. 2001; Halkidou et al. 2003; Agoulnik et al. 2005; Chmelar et al. 2007; Xu et al. 2009). Additionally, loss of AR corepressor function via the exclusion of NCoR from the AR complex (Zhu et al. 2006), the down-regulation of prohibitin (Dai et al. 2008; Dart et al. 2009) and the aberrant mislocalization of Hey1 (Belandia et al. 2005) has been shown to promote AR-mediated transcription in PCa. Together, the gain of coactivators and loss of corepressors may provide CRPC tumors a compensatory mechanism in a low-hormone environment.
3.5. Ligand-independent activation via cross-talk with signal transduction pathways
Activation of several signal transduction pathways in CRPC have been shown to enhance AR-activity, either directly or via coactivators, in an environment where androgen levels are low or even absent. Specifically, EGF, IGF, IL-6, Wnt and Stat5a/b signaling pathways have been implicated in enhancing AR transcriptional responses to low androgen levels (Hernes et al. 2004; Krueckl et al. 2004; Bartlett et al. 2005; Wu et al. 2006; Aaronson et al. 2007; Schweizer et al. 2008; Tan et al. 2008). Likewise, activation of certain kinases and kinase signal transduction programs, including Cdk1, protein kinase A, PI3 kinase/Akt and the Ras-Raf-MAP kinase pathway have been shown to enhance AR activation in response to low levels of androgen (Culig et al. 1995; Nazareth and Weigel 1996; Craft et al. 1999; Gioeli et al. 1999; Sadar 1999; Yeh et al. 1999; Gregory et al. 2001; Bakin et al. 2003; Bakin et al. 2003; Gregory et al. 2004; Mellinghoff et al. 2004; Weber and Gioeli 2004; Chen et al. 2006). Furthermore, activation of the Ras-Raf-MAP kinase and PI3 kinase/Akt pathways as well as the increased expression of the HER2/Neu receptor tyrosine kinase are all associated with aggressive primary PCa and CRPC (Craft, Shostak et al. 1999; Gioeli, Mandell et al. 1999; Signoretti et al. 2000; Yuan et al. 2006). In general, these kinase pathways enhance AR activity indirectly, by mediating the phosphorylation of coactivators, rather than via direct phosphorylation of the AR itself (Gregory, Fei et al. 2004). Collectively, these studies suggest that cross-talk signaling is an important mechanism for PCa progression in a castrate environment.
4. Androgen Metabolism in Prostate Cancer
Prostate cancer growth in castrate animal models is characterized by AR overexpression and remains ligand sensitive (Chen, Welsbie et al. 2004). Furthermore, several studies have demonstrated that intratumoral androgens in CRPC tumors are maintained at levels sufficient to activate the AR signaling pathway (Geller et al. 1979; Mohler, Gregory et al. 2004; Nishiyama et al. 2004; Page et al. 2006; Montgomery et al. 2008). One hypothesis is that activated steroidogenesis pathways provide an adaptive response to ADT, facilitating CRPC tumor survival in a low exogenous androgen environment. Indeed, recent studies have suggested that the maintenance of intratumoral androgens can be accounted for in part by intratumoral or intracrine biosynthesis of steroid hormones, either via the uptake and conversion of adrenal androgens or potentially via de novo steroidogenesis (Stanbrough, Bubley et al. 2006; Locke et al. 2008; Montgomery, Mostaghel et al. 2008; Mostaghel et al. 2009; Hofland et al. 2010).
4.1. Classical and backdoor pathways of androgen metabolism
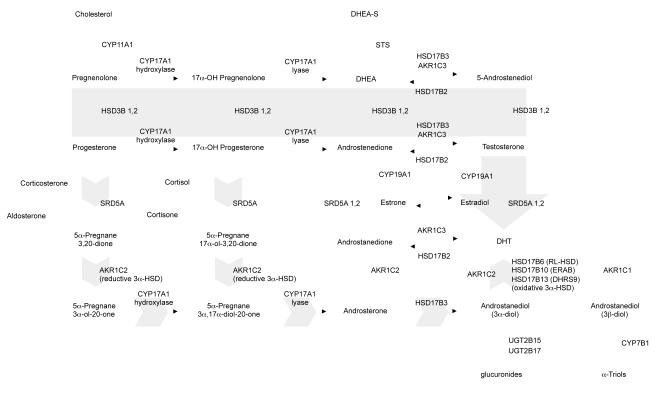
Testosterone is normally produced by Leydig cells in the testes and is converted in the prostate to the more potent androgen 5α-DHT by steroid 5α-reductase type 2 (SRD5A2) (Russell and Wilson 1994). DHT can also be derived from the prostatic uptake and conversion of the adrenal androgen dehydroepiandrosterone (DHEA) by the sequential actions of three enzyme families: 3β-hydroxysteroid dehydrogenase (3β-HSD type 1 and type 2 (HSD3B1, HSD3B2)), 17β-HSD type 3 (HSD17B3) and type 5 (HSD17B5, known as AKR1C3), and SRD5A type 1 or 2 (Figure 2) (Labrie et al. 1995; Labrie et al. 2000; Penning et al. 2006). Recently, a ‘backdoor pathway’ to 5α-DHT has been proposed in which DHT is generated in a sequence of steps that bypass the generation of testosterone and DHEA, such that DHT is not derived from the forward conversion of testosterone by SRD5A, but from the ‘back’ conversion of 3α-androstanediol (3α-diol) (Auchus 2004; Bauman et al. 2006).
Figure 2. The classical and backdoor pathways of androgen biosynthesis.
In the classical pathway (solid gray arrow), C21 precursors (pregnenolone and progesterone) are converted to the C19 adrenal androgens DHEA and androstenedione (AED) by the sequential hydroxylase and lyase activity of CYP17A1. Circulating adrenal androgens (including the sulfated form of DHEA, DHEA-S), enter the prostate and can be converted to testosterone by a series of reactions involving the activity of HSD3B, HSD17B and AKR1C enzymes. Testosterone is then converted to the potent androgen DHT by the activity of SRD5A. In the backdoor pathway to DHT synthesis (short gray arrows), C21 precursors are first acted upon by SRD5A and the reductive 3α-HSD activity of the AKR1C family member, AKR1C2, followed by conversion to C19 androgens via the lyase activity of CYP17A. DHT is subsequently generated by the action of HSD17B3 and an oxidative 3 α -HSD enzyme, including HSD17B6, HSD17B10 or HSD17B13 (as well as RODH4, RODH5 and NT 3 α-HSD, not shown). Adapted from Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22:243, with permission.
4.2. Altered expression of steroidogenic enzymes in CRPC
Alterations in critical genes involved in androgen biosynthesis have been identified in CRPC. In a study comparing CRPC with primary tumors, changes in the relative expression of numerous transcripts involved in androgen biosynthesis were observed, including CYP17A1 (16.9-fold increase), HSD3B2 (7.5-fold increase), AKR1C3 (8.0-fold increase), SRD5A1 (2.6-fold increase), and SRD5A2 (9.4-fold decrease) (Montgomery, Mostaghel et al. 2008). Other studies of CRPC tumors have demonstrated similar findings suggestive of intracrine steroidogenesis, including the expression of HSD17B3 and AKR1C3 (Stanbrough, Bubley et al. 2006; Hofland, van Weerden et al. 2010), as well the expression of cholesterol biosynthetic enzymes including squalene epoxidase (SQLE), the rate limiting enzyme in cholesterol biosynthesis (Holzbeierlein, Lal et al. 2004), and the loss of enzymes mediating DHT catabolism such as AKR1C1 and AKR1C2 (Ji et al. 2007; Penning et al. 2007). These studies suggest that upregulated intratumoral steroidogenesis plays a role in facilitating CRPC survival in a castrate environment.
4.3. Intracrine steroidogenesis in human prostate tumors
A limited number of studies have attempted to directly examine the ability of prostatic tumors to mediate the de novo synthesis of steroid hormones from progesterone, or the conversion of adrenal androgens to T or DHT. Klein et al evaluated the presence of adrenal androgens and steroid metabolizing activity (including SRD5A, HSD3B, and HSD17B) ex vivo in untreated primary tumors and hormone naive lymph node metastases. They demonstrated that benign and neoplastic prostate tissues had qualitatively similar androgen levels and steroid metabolizing capacity, although malignant tissue had a sub-total loss of SRD5A activity, and concluded that primary tumors and metastases possessed the capacity to metabolize adrenal androgen precursors along the pathway to DHT (Klein et al. 1988). Di Silverio et al demonstrated the conversion of DHEA-S (the primary circulating form of DHEA) to DHEA within prostate cancer tissue extracts from both eugonadal and castrate men (Di Silverio et al. 1976), and Klein et al subsequently confirmed the presence of the steroid sulfatase required for conversion of DHEA-S to DHEA within prostate epithelial tissue (Klein et al. 1989).
In the mid 1960’s, Acevedo et al investigated the metabolism of C14 progesterone in primary prostate cancer tissues, but did not observe significant metabolic conversion beyond the formation of immediate progesterone derivatives (Acevedo and Goldzieher 1965). This finding is not necessarily unexpected, as several studies have now clearly demonstrated upregulated expression of steroidogenic genes in CRPC tumors compared to primary prostate tumors.
4.3.1. de novo steroidogenesis in model systems
More recently, studies in model systems demonstrate that CRPC tumor cells are capable of de novo synthesis of their own androgens. For example, the expression of steroid metabolic machinery, including steroidogenic acute regulatory (StAR) protein, cytochrome P450 cholesterol side chain cleavage (P450scc) and CYP17A1 was found to be significantly higher in an androgen-independent LNCaP derivative (C81), compared to its androgen-dependent counterpart (C33) (Dillard et al. 2008). Importantly, the C81 cell line was able to directly convert cholesterol into testosterone. Studies using a LNCaP mouse xenograft model for CRPC identified increases in the expression of genes responsible for the accumulation of free cholesterol and cholesterol synthesis: low density lipoprotein receptor (LDLR), scavenger receptor (SR)B1, ATP-binding cassette (ABC)A1, StAR, acyl-coenzyme A:cholesterol acyltransferase-(ACAT) 1 and 2, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA), and the side-chain cleavage enzyme (CYP11A1) (Locke, Wasan et al. 2008; Leon et al. 2009; Locke et al. 2009). Additionally, increases in transcripts encoding CYP17A1, AKR1C1, AKR1C2, AKR1C3, HSD17B2, and SRD5A1 were detected (Locke, Wasan et al. 2008). Collectively, these data suggest that these xenografts may be capable of de novo steroidogenesis from cholesterol. Indeed, conversion of acetic acid to 5α-DHT was observed in these xenografts (Locke, Wasan et al. 2008). Furthermore, these tumors were shown to metabolize progesterone to six different intermediates upstream of 5α-DHT, via both the classic and “backdoor” pathways (Locke, Wasan et al. 2008). The importance of this work is the demonstration that CRPC tumors synthesize androgens, potentially via de novo metabolism from cholesterol.
4.4. Stromal-epithelial interactions and intratumoral androgen biosynthesis
Several studies have indicated that bone-marrow and PCa-derived stromal cells may play an important role in facilitating androgen biosynthesis in prostate cancer cells. Whereas DHEA induced little or no PSA expression in monocultures of LAPC-4 PCa cells, co-culture with PCa-associated stromal cells resulted in marked stimulation of PSA expression, likely mediated by stromal cell generation of T from DHEA (as T was detected in a time and dose-dependent manner in PCa-stromal cell monocultures treated with DHEA) (Arnold et al. 2008) Similarly, the impact of DHEA on PSA promoter activity in LNCaP cells was markedly enhanced in the presence of PCa-derived stromal cells (Mizokami et al. 2009). Knockdown of AR in the LNCaP cells abrogated this effect, while coculture with PCa-stromal cells transfected with AR shRNA did not, suggesting paracrine factors secreted by the stromal cells act on the LNCaP AR. Furthermore, following DHEA treatment, testosterone and DHT concentrations were ~5-fold higher in the PCa-stromal/LNCaP coculture vs. the LNCaP monoculture. Interestingly, normal-prostate stroma, bone-marrow stroma, lung stroma and bone-derived stromal cells also induced an increase in PSA expression, although the strongest effects were noted with PCa-stromal cells. In a separate study of bone-marrow stromal cells, resting mesenchymal cells were found to express HSD3B and SRD5A protein, while incubation with DHEA resulted in the additional expression of HSD17B5(Sillat et al. 2009). These findings indicate that metabolism of androgen precursors in PCa-associated stromal cells may facilitate and/or potentiate the maintenance of intratumoral androgen levels in CRPC tumors. Together these studies provide evidence supporting the role of steroidogenesis in reactivating AR signaling in CRPC, and highlight the interplay between stromal and epithelial cells in this mechanism.
4.5. Alterations in androgen transport
While the transit of steroid hormones from the circulation to intracellular compartments is generally attributed to free diffusion across the lipid membrane, recent studies suggest a potential role for steroid transport proteins in actively mediating the uptake of androgen into PCa cells. The organic anion-transporting polypeptides (OATP; encoded by the SLCO gene family) are variably expressed throughout the liver, kidney and steroidogenic tissues, and several SLCO-genes are overexpressed in CRPC metastases vs. untreated PCa (Mostaghel, 2011 in press). These transporters mediate the uptake of substrates such as bile acids, xenobiotics and steroidogenic precursors, and it has been demonstrated that single nucleotide polymorphisms (SNPs) in SLCO genes can markedly alter substrate-specific transport efficiency. Notably, OATP1B3 has been shown to actively transport testosterone in transiently transfected COS-7 cells (Hamada et al. 2008). Furthermore, a non-synonymous SNP of OATP1B3 displayed a 2-fold decrease in testosterone uptake activity, which correlated with a longer median survival, improved 10 year survival, and a longer time to androgen independence in two small studies of men with CRPC (Hamada, Sissung et al. 2008; Sharifi et al. 2008). In a parallel study, it was found that OATP2B1 mediates the uptake of DHEA-S in transiently transfected LNCaP cells, and a non-synonymous SNP which displayed impaired DHEAS uptake was correlated with a longer time to progression in men with CRPC receiving ADT (Yang et al. 2010). Together, these studies imply that active hormone uptake may contribute to the elevated androgen levels observed in CRPC tumors and the progression of advanced disease.
5. Therapeutic Approaches Targeting Androgen Action and Metabolism in PCa
The biochemical goal of ADT is to deprive the AR of its ligand and/or to block the ligand-mediated interaction of AR with DNA, thereby suppressing its transactivation potential. Such strategies include surgical castration, chemical castration via GnRH agonists or antagonists, inhibition of steroidogenic enzymes with agents such as SRD5A inhibitors, and the use of antiandrogens that block the binding of androgens to the AR. Until recently, the therapeutic armamentarium following the initial development of castration resistant disease was limited to second line hormonal therapies with relatively limited clinical benefit. This included anti-androgens such as flutamide and bicalutamide, or nilutamide after progression on bicalutamide, estrogenic agents such as DES, and the non-specific CYP17A inhibitor ketoconazole.
Accumulating evidence suggests that CRPC remains a ligand- and AR-driven disease, and that residual tumor androgens play a prominent role in mediating CRPC progression. Thus, emerging therapies (reviewed more extensively elsewhere in this issue) have been designed to more effectively target ligand engagement and activation of the AR, as well as the metabolic pathways mediating androgen biosynthesis. Among these, the novel anti-androgens MDV3100 and EPI-001, and the CYP17A inhibitors abiraterone, Tak-700 and TOK-001 have shown promising pre-clinical activity, and are in various stages of clinical development, with abiraterone recently receiving FDA approval for metastatic CRPC following docetaxel treatment (Table 1) (de Bono et al. 2011).
Table 1.
Novel Androgen Receptor Antagonists and Steroid Synthesis Inhibitors in Clinical Development
| Agent | Company | Target | Mechanism of Action | Phase | Major Trials | Identifier* | Efficacy | References |
|---|---|---|---|---|---|---|---|---|
| MDV3100 | Medivation | AR (LBD) | Competitive AR antagonist. Impairs nuclear translocation, DNA binding and coactivator recruitment without partial agonist activity |
Phase III metastatic CRPC |
AFFIRM - docetaxel treated PREVAIL - docetaxel naïve |
NCT00974311 (chemo-treated) NCT01212991 (chemo-naïve) |
>50% PSA declines in 62% and 51% of chemo- treated and chemo-naïve patients, with median time to PSA progression of 41 and 21 weeks, respectively, reported in phase I/II study |
Tran et al. 2009, Scher et al. 2010 |
| Abiraterone (Zytiga) |
Johnson & Johnson |
CYP17A | Inhibitor of hydroxylase and lyase activities of CYP17A |
Phase III metastatic CRPC |
COU-AA01 - docetaxel treated COU-AA02 docetaxel naïve |
COU-AA01 completed NCT00887198 (chemo-naïve) |
FDA approved in post-docetaxel setting based on improvement in overall survival from 10.9 (placebo) to 14.8 months (abiraterone) in COU-AA01 |
de Bono et al. 2011 |
| TAK-700 (Orteronel) |
Millenium/ Takeda Oncology |
CYP17A | Inhibitor of CYP17 lyase activity | Phase II non- metastatic CRPC Phase III metastatic CRPC |
NCT01046916 (non-metastatic) NCT01193257 (chemo-treated) NCT01193244 (chemo-naïve) |
11 of 20 patients with metastatic CRPC showed PSA declines >50% in phase I data. |
Dreicer et al. 2010 | |
| VN/124-1 (TOK-001) |
Tokai | CYP17A, AR (LBD) |
Dual CYP17A inhibitor and competitive AR antagonist |
Phase I/II metastatic and non-metastatic CRPC |
ARMOR1 - docetaxel naïve |
NCT00959959 | not available | Vasaitis et al. 2008 |
| EPI-001 | AR (NTD) | Inhbits AR transactivation by disrupting AR N/C interaction and co-factor recruitment. Does not prevent ligand binding or nucelar translocation |
Phase I/II under development |
not available | Anderson et al. 2010 |
all ongoing clincial trials can be accessed at http://clinicaltrials.gov
5.1. Novel Agents Targeting the Androgen Receptor
MDV3100 is a competitive AR antagonist which binds to the AR with 5-8 fold greater affinity than bicalutamide and potently decreases the nuclear translocation of AR, as well as reducing chromatin occupancy at canonical ARE’s.(Tran et al. 2009) In preclinical studies, MDV3100 did not elicit agonist activity against LNCaP tumors overexpressing the AR, or against AR with the T877A or W741C mutations, situations in which bicalutamide demonstrates agonist activity. In a Phase I/II study of men with CRPC, MDV3100 led to >50% PSA declines in 62% and 51% of chemo-therapy naïve patients and docetaxel-treated patients, and a median time to PSA progression of 41 and 21 weeks, respectively(Scher et al.). Thirty-seven percent of patients with prior ketoconazole treatment achieved a PSA decline >50% vs. 71% of those without. Phase III, randomized placebo-controlled trials of MDV3100 in docetaxel-treated men (AFFIRM) and in docetaxel-naïve men (PREVAIL) with metastatic CRPC are currently ongoing.
An alternative to targeting the AR LBD is targeting the NTC, which is essential for both ligand dependent and independent AR activation. Among the most promising compounds is EPI-001, a degradation product of bisphenyl-A isolated from a product library of marine sponges (Andersen, Mawji et al. 2010). EPI-001 binds to the amino terminus of the AR without altering ligand binding or AR nuclear translocation. Instead EPI-001 inhibits AR transactivation by disrupting the AR N/C interaction and inhibiting co-factor recruitment. No apparent toxicity has been noted in animals and a phase I/II study is under development.
5.1. Novel Agents Targeting Steroid Synthesis
Abiraterone (Zytiga, Johnson & Johnson) is a selective irreversible inhibitor of both the 17 alpha-hydroxylase and C17,20-lyase activity of CYP17, and has shown promising efficacy in reducing circulating androgen levels and achieving PSA responses in men with CRPC. Phase I/II studies have demonstrated PSA responses and clinical activity in chemotherapy naïve and post-docetaxel treated CRPC patients (including patients who have progressed on ketoconazole). (Attard, Reid et al. 2008; Attard et al. 2009; Danila et al. 2010; Reid et al. 2010; Ryan et al. 2010) Phase III studies of abiraterone in the chemotherapy naïve (COU-AA-302) and post docetaxel setting (COU AA-301) are ongoing; early results of the Phase III COU-AA-301 study in the post-chemotherapy setting were recently published, demonstrating an overall survival of 14.8 months compared to 10.9 months in the placebo-treated group (hazard ratio 0.646, p<0.0001), representing a 35% reduction in risk of death with abiraterone treatment (de Bono, Logothetis et al. 2011).
VN/124-1 is a potent steroidal CYP17 and AR inhibitor currently under evaluation in Phase I/II study (ARMOR1) under the trade name TOK-001 (Tokai Pharmaceuticals) (Vasaitis et al. 2008). VN/124-1 exhibits 3 and 4 fold stronger inhibition of CYP17 activity than abiraterone and ketoconazole, respectively, and is also a potent inhibitor of the AR, both as a competitive antagonist (with a binding affinity comparable to bicalutamide) and as a dose-dependent inhibitor of AR protein expression, mediated in part via an increase in AR degradation (Vasaitis, Belosay et al. 2008). TAK-700 (Orteronel, Millenium, Takeda Oncology Company) a non-steroidal CYP17 inhibitor, has shown promising efficacy in a Phase I/II dose-escalation study in men with metastatic CRPC (Dreicer et al. 2010), and has been expanded to phase III studies in metastatic CRPC (Dreicer, Agus et al. 2010) and a phase II study in non-metastatic CRPC.
7. Conclusions
Recent advances in basic and clinical sciences have demonstrated that prostate cancers resist AR inhibition through multiple mechanisms. The presence of residual androgens and persistent activation of AR signaling in CRPC suggest that a majority of prostate cancers progress through pathways that continue to activate the cellular AR program. While the introduction of potent steroidogenic inhibitors such as abiraterone, in combination with novel AR inhibitors such as MDV3100, holds significant promise for the concept of multi-targeted AR pathway blockade, the optimal timing, sequence, and potential combinatorial strategies using new AR pathway inhibitors are critical unanswered questions in the treatment of men with CRPC.
Importantly, while novel agents targeting ligand synthesis and AR activation have demonstrated striking responses in men with CRPC, not all patients respond and many eventually demonstrate progression. Mechanisms of resistance in men who develop progression while on abiraterone or MDV3100 have not been established. It is unknown whether these tumors represent cancers that are entirely independent of AR pathway activity or still retain dependence on the AR signaling axis. Clinical studies with biopsy of tumor tissue at progression will be a critical resource in delineating mechanisms of resistance and in developing biomarkers for rational stratification of men to optimal treatment strategies. However, until AR signaling is completely extinguished, and tumors progress with no evidence of AR activity, the ‘AR addiction’ pathway remains the most attractive target for the continued development of effective therapies.
Highlights.
Prostate cancer is a malignancy fueled by androgens and driven by the cellular program regulated by the androgen receptor (AR). This review details the mechanisms by which the AR is activated including those that motivate signaling in advanced prostate cancer that has progressed in the setting of castrate levels of serum testosterone. These mechanisms include mutation of the receptor, intracrine androgen biosynthesis, induction of AR splice variants, and cross-talk with other signaling programs. Therapeutic approaches designed to target these resistance mechanisms are discussed.
Acknowledgments
Support: Pacific Northwest Prostate Cancer SPORE P50 CA97186; Prostate Cancer Foundation; Damon Runyon Cancer Research Foundation (Damon Runyon-Genentech Clinical Investigator Award CI-40-08 to EAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- Aaronson DS, Muller M, Neves SR, Chung WC, Jayaram G, Iyengar R, Ram PT. An androgen-IL-6-Stat3 autocrine loop re-routes EGF signal in prostate cancer cells. Mol Cell Endocrinol. 2007;270(1-2):50–56. doi: 10.1016/j.mce.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Acevedo HF, Goldzieher JW. The metabolism of [4-14C] progesterone by hypertrophic and carcinomatous human prostate tissue. Biochim Biophys Acta. 1965;111(1):294–298. doi: 10.1016/0304-4165(65)90495-2. [DOI] [PubMed] [Google Scholar]
- Agoulnik IU, Vaid A, Bingman WE, 3rd, Erdeme H, Frolov A, Smith CL, Ayala G, Ittmann MM, Weigel NL. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65(17):7959–7967. doi: 10.1158/0008-5472.CAN-04-3541. [DOI] [PubMed] [Google Scholar]
- Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Banuelos CA, Williams DE, McEwan IJ, Wang Y, Sadar MD. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Arnold JT, Gray NE, Jacobowitz K, Viswanathan L, Cheung PW, McFann KK, Le H, Blackman MR. Human prostate stromal cells stimulate increased PSA production in DHEA-treated prostate cancer epithelial cells. J Steroid Biochem Mol Biol. 2008;111(3-5):240–246. doi: 10.1016/j.jsbmb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4-2 prostate cancer cells. Cancer Res. 2003;63(8):1975–1980. [PubMed] [Google Scholar]
- Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63(8):1981–1989. [PubMed] [Google Scholar]
- Baniwal SK, Khalid O, Sir D, Buchanan G, Coetzee GA, Frenkel B. Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol. 2009;23(8):1203–1214. doi: 10.1210/me.2008-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JM, Brawley D, Grigor K, Munro AF, Dunne B, Edwards J. Type I receptor tyrosine kinases are associated with hormone escape in prostate cancer. J Pathol. 2005;205(4):522–529. doi: 10.1002/path.1735. [DOI] [PubMed] [Google Scholar]
- Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the Major Oxidative 3{alpha}-Hydroxysteroid Dehydrogenase in Human Prostate That Converts 5{alpha}-Androstane-3{alpha},17{beta}-diol to 5{alpha}-Dihydrotestosterone: A Potential Therapeutic Target for Androgen-Dependent Disease. Mol Endocrinol. 2006;20(2):444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- Belandia B, Powell SM, García-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25(4):1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke GN, Bevan CL. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics. 2009;10(1):18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RS, Edwards J, Dogan A, Payne H, Harland SJ, Bartlett JM, Masters JR. Amplification of the androgen receptor gene in bone metastases from hormone-refractory prostate cancer. J Pathol. 2002;198(2):237–244. doi: 10.1002/path.1206. [DOI] [PubMed] [Google Scholar]
- Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59(4):803–806. [PubMed] [Google Scholar]
- Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res. 2009;69(15):6027–6032. doi: 10.1158/0008-5472.CAN-09-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, De Falco A, Varricchio L, Bottero D, Nanayakkara M, Migliaccio A, Auricchio F. Rapid signalling pathway activation by androgens in epithelial and stromal cells. Steroids. 2004;69(8-9):517–522. doi: 10.1016/j.steroids.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Cerveira N, Ribeiro FR, Peixoto A, Costa V, Henrique R, Jerónimo C, Teixeira MR. TMPRSS2-ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8(10):826–832. doi: 10.1593/neo.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. PNAS. 2006;103(43):15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int. J. Cancer. 2007;120:719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7(12):1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Hittmair A, Radmayr C, Bartsch G, Klocker H. Activation of the androgen receptor by polypeptide growth factors and cellular regulators. World J Urol. 1995;13(5):285–289. doi: 10.1007/BF00185971. [DOI] [PubMed] [Google Scholar]
- Dai Y, Ngo D, Jacob J, Forman LW, Faller DV. Prohibitin and the SWI/SNF ATPase subunit BRG1 are required for effective androgen antagonist-mediated transcriptional repression of androgen receptor-regulated genes. Carcinogenesis. 2008;29(9):1725–1733. doi: 10.1093/carcin/bgn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28(9):1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart DA, Spencer-Dene B, Gamble SC, Waxman J, Bevan CL. Manipulating prohibitin levels provides evidence for an in vivo role in androgen regulation of prostate tumours. Endocr Relat Cancer. 2009;16(4):1157–1169. doi: 10.1677/ERC-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Silverio F, Gagliardi V, Sorcini G, Sciarra F. Biosynthesis and metabolism of androgenic hormones in cancer of the prostate. Invest Urol. 1976;13(4):286–288. [PubMed] [Google Scholar]
- Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295(1-2):115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreicer R, Agus DB, MacVicar GR, MacLean D, Zhang T, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in castration-resistant, metastatic prostate cancer: A phase I/II, open-label study; 2010 Genitourinary Cancers Symposium.2010. [Google Scholar]
- Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU Int. 2005;95(9):1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89(3):552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford OH, 3rd, Gregory CW, Kim D, Smitherman AB, Mohler JL. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003;170(5):1817–1821. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- Geller J, Albert J, Nachtsheim D, Loza D, Lippman S. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res. 1979;33:103–111. [PubMed] [Google Scholar]
- Gioeli D, Mandell JW, Petroni GR, Frierson HFJ, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59(2):279–284. [PubMed] [Google Scholar]
- Gleave ME, Small EJ. Androgen deprivation therapy for prostate cancer. Report to the Nation on Prostate Cancer 2004. Medscape. 2004:27–36. [Google Scholar]
- Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279(8):7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61(11):4315–4319. [PubMed] [Google Scholar]
- Gregory CW, Johnson RT, Jr., Presnell SC, Mohler JL, French FS. Androgen receptor regulation of G1 cyclin and cyclin-dependent kinase function in the CWR22 human prostate cancer xenograft. J Androl. 2001;22(4):537–548. [PubMed] [Google Scholar]
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkidou K, Gnanapragasam VJ, Mehta PB, Logan IR, Brady ME, Cook S, Leung HY, Neal DE, Robson CN. Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene. 2003;22(16):2466–2477. doi: 10.1038/sj.onc.1206342. [DOI] [PubMed] [Google Scholar]
- Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, Venzon D, Maeda K, Nagao K, Sparreboom A, Mitsuya H, Dahut WL, Figg WD. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14(11):3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Buchanan G, Ittmann M, Harris JM, Yu X, DeMayo FJ, Tilley W, Greenberg NM. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, Miyamoto M. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63(1):149–153. [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Hernes E, Fosså SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer. 2004;90(2):449–454. doi: 10.1038/sj.bjc.6601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, Schroder FH, de Jong FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70(3):1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikstrom P. Expression of Androgen Receptor Splice Variants in Prostate Cancer Bone Metastases is Associated with Castration-Resistance and Short Survival. PLoS One. 2011;6(4):e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011 doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270(13):7341–7346. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- Ji Q, Chang L, Stanczyk FZ, Ookhtens M, Sherrod A, Stolz A. Impaired dihydrotestosterone catabolism in human prostate cancer: critical role of AKR1C2 as a pre-receptor regulator of androgen receptor signaling. Cancer Res. 2007;67(3):1361–1369. doi: 10.1158/0008-5472.CAN-06-1593. [DOI] [PubMed] [Google Scholar]
- Klein H, Bressel M, Kastendieck H, Voigt KD. Androgens, adrenal androgen precursors, and their metabolism in untreated primary tumors and lymph node metastases of human prostatic cancer. Am J Clin Oncol. 1988;11(Suppl 2):S30–36. doi: 10.1097/00000421-198801102-00008. [DOI] [PubMed] [Google Scholar]
- Klein H, Molwitz T, Bartsch W. Steroid sulfate sulfatase in human benign prostatic hyperplasia: characterization and quantification of the enzyme in epithelium and stroma. J Steroid Biochem. 1989;33(2):195–200. doi: 10.1016/0022-4731(89)90294-x. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273(32):20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314–319. [PubMed] [Google Scholar]
- Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64(23):8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Simard J, Van L-T, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Ann N Y Acad Sci. 1995;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin S, Simard J, Labrie C, El-Alfy M, Pelletier G, Belanger A. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25(1):1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, Vidaud M. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61(5):1919–1926. [PubMed] [Google Scholar]
- Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, Nelson CC, Guns ES, Wasan KM. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2009;70(4):390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- Linja MJ, Savinainen KJ, Saramaki OR, Tammela TLJ, Vessella RL, Visakorpi T. Amplification and Overexpression of Androgen Receptor Gene in Hormone-Refractory Prostate Cancer. Cancer Res. 2001;61(9):3550–3555. [PubMed] [Google Scholar]
- Linja MJ, Visakorpi T. Alterations of androgen receptor in prostate cancer. J Steroid Biochem Mol Biol. 2004;92(4):255–264. doi: 10.1016/j.jsbmb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Locke JA, Nelson CC, Adomat HH, Hendy SC, Gleave ME, Guns ES. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol. 2009 doi: 10.1016/j.jsbmb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Locke JA, Wasan KM, Nelson CC, Guns ES, Leon CG. Androgen-mediated cholesterol metabolism in LNCaP and PC-3 cell lines is regulated through two different isoforms of acyl-coenzyme A:Cholesterol Acyltransferase (ACAT) Prostate. 2008;68(1):20–33. doi: 10.1002/pros.20674. [DOI] [PubMed] [Google Scholar]
- Lu S, Jenster G, Epner DE. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: role of androgen receptor and transcription factor Sp1 complex. Mol Endocrinol. 2000;14(5):753–760. doi: 10.1210/mend.14.5.0461. [DOI] [PubMed] [Google Scholar]
- Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6(5):517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Middleman MN, Lush RM, Figg WD. The mutated androgen receptor and its implications for the treatment of metastatic carcinoma of the prostate. Pharmacotherapy. 1996;16(3):376–381. [PubMed] [Google Scholar]
- Mizokami A, Koh E, Izumi K, Narimoto K, Takeda M, Honma S, Dai J, Keller ET, Namiki M. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr Relat Cancer. 2009;16(4):1139–1155. doi: 10.1677/ERC-09-0070. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghel EA, Montgomery B, Nelson PS. Castration-resistant prostate cancer: Targeting androgen metabolic pathways in recurrent disease. Urol Oncol. 2009;27(3):251–257. doi: 10.1016/j.urolonc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271(33):19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Hashimoto Y, Takahashi K. The Influence of Androgen Deprivation Therapy on Dihydrotestosterone Levels in the Prostatic Tissue of Patients with Prostate Cancer. Clin Cancer Res. 2004;10(21):7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- Penning TM, Bauman DR, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5[alpha]-DHT to the androgen receptor. Molecular and Cellular Endocrinology. 2007;265-266:77–82. doi: 10.1016/j.mce.2006.12.007. (Adrenal/Molecular Steroidogenesis Conference 2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, Fung K-M, Lin H-K. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Molecular and Cellular Endocrinology, The International Workshop on 11beta and 17beta-Hydroxysteroid Dehydrogenases. 2006;248(1-2):182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, Tchinda J, Tomlins SA, Hofer MD, Pienta KG, Kuefer R, Vessella R, Sun XW, Meyerson M, Lee C, Sellers WR, Chinnaiyan AM, Rubin MA. TMPRSS2:ERG Fusion-Associated Deletions Provide Insight into the Heterogeneity of Prostate Cancer. Cancer Res. 2006;66(17):8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, Dearnaley D, Swennenhuis JF, Terstappen LW, Lee G, Kheoh T, Molina A, Ryan CJ, Small E, Scher HI, de Bono JS. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, Santerse AB, Schroder FH, van der Kwast TH. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144(4):735–746. [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5alpha-Reductase: Two Genes/Two Enzymes. Annual Review of Biochemistry. 1994;63(1):25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Smith A, Lal P, Satagopan J, Reuter V, Scardino P, Gerald W, Scher HI. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: An early predictor of relapse or incomplete androgen suppression. Urology. 2006;68(4):834–839. doi: 10.1016/j.urology.2006.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadar MD. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J Biol Chem. 1999;274(12):7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer L, Rizzo CA, Spires TE, Platero JS, Wu Q, Lin TA, Gottardis MM, Attar RM. The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol. 2008 Jan 24;9:4. 2008;9(4) doi: 10.1186/1471-2121-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Hamada A, Sissung T, Danesi R, Venzon D, Baum C, Gulley JL, Price DK, Dahut WL, Figg WD. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008;102(5):617–621. doi: 10.1111/j.1464-410X.2008.07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, Hahnfeldt P, Kantoff P, Loda M. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92(23):1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- Sillat T, Pöllänen R, Lopes JR, Porola P, Ma G, Korhonen M, Konttinen YT. Intracrine androgenic apparatus in human bone marrow stromal cells. J Cell Mol Med. 2009;13(9B):3296–3302. doi: 10.1111/j.1582-4934.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- Stanbrough M, Leav I, Kwan PW, Bubley GJ, Balk SP. Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc Natl Acad Sci U S A. 2001;98(19):10823–10828. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp MP, O’Mahony OA, Brogley M, Rehman H, Lapensee EW, Dhanasekaran S, Hofer MD, Kuefer R, Chinnaiyan A, Rubin MA, Pienta KJ, Robins DM. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69(10):4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, deVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11(4):450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- Tan SH, Dagvadorj A, Shen F, Gu L, Liao Z, Abdulghani J, Zhang Y, Gelmann EP, Zellweger T, Culig Z, Visakorpi T, Bubendorf L, Kirken RA, Karras J, Nevalainen MT. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68(1):236–248. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
- Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4(4):236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4(4):236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59(11):2511–2515. [PubMed] [Google Scholar]
- Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ. Androgen receptor mutations in androgen-independent prostate cancer. J Clin Oncol. 2003;21(14):2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, Mehra R, Chinnaiyan AM. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10(2):177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, Cao X, Wei JT, Rubin MA, Shah RB, Chinnaiyan AM. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66(7):3396–3400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64(19):7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48(2):189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, Guo Z, Fang HB, Njar VC, Brodie AM. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7(8):2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Walcott JL, Merry DE. Trinucleotide repeat disease. The androgen receptor in spinal and bulbar muscular atrophy.” Vitam Horm. 2002;65:127–147. doi: 10.1016/s0083-6729(02)65062-9. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proceedings of the National Academy of Sciences. 2010 doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91(1):13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99(2):392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhao W, Zhao J, Pan J, Wu Q, Zhang Y, Bauman WA, Cardozo CP. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology. 2007;148(6):2984–2993. doi: 10.1210/en.2006-1653. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9(9):615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66(15):7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- Yang M, Oh WK, Xie W, Mostaghel EA, Sun T, Sharifi N, Regan MM, Figg WD, Lee GM, Kantoff P. Genetic variations in SLCO2B1 and SLCO1B3 and the efficacy of androgen-deprivation therapy in prostate cancer patients; 2010 Genitourinary Cancers Symposium.2010. [Google Scholar]
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96(10):5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Kinoshita H, Segawa T, Nakamura E, Inoue T, Shimizu Y, Kamoto T, Ogawa O. Antiandrogen Bicalutamide Promotes Tumor Growth in a Novel Androgen-Dependent Prostate Cancer Xenograft Model Derived from a Bicalutamide-Treated Patient. Cancer Res. 2005;65(21):9611–9616. doi: 10.1158/0008-5472.CAN-05-0817. [DOI] [PubMed] [Google Scholar]
- Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27(1):36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, Bubley GJ, Lu ML, Balk SP. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169(2):682–696. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124(3):615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]