Abstract
It is generally assumed that choice between different actions reflects the difference between their action values yet little direct evidence confirming this assumption has been reported. Here we assess whether the brain calculates the absolute difference between action values or their relative advantage, that is, the probability that one action is better than the other alternatives. We use a two-armed bandit task during functional magnetic resonance imaging and modelled responses to determine both the size of the difference between action values (D) and the probability that one action value is better (P). The results show haemodynamic signals corresponding to P in right dorsolateral prefrontal cortex (dlPFC) together with evidence that these signals modulate motor cortex activity in an action-specific manner. We find no significant activity related to D. These findings demonstrate that a distinct neuronal population mediates action-value comparisons, and reveals how these comparisons are implemented to mediate value-based decision-making.
 In humans, choice between actions depends on the ability to compare action–outcome values. Here, the authors show that action–outcome values are compared on the basis of the relative advantage of a particular action over alternative actions, which takes place in the right dorsolateral prefrontal cortex of the brain.
In humans, choice between actions depends on the ability to compare action–outcome values. Here, the authors show that action–outcome values are compared on the basis of the relative advantage of a particular action over alternative actions, which takes place in the right dorsolateral prefrontal cortex of the brain.
For behaviour to remain adaptive a decision-maker must be able to rapidly establish the best action from multiple possible actions. Such ‘multi-armed bandit’ problems are, however, notoriously resistant to analysis and typically hard to solve when employing realistic reward distributions1,2,3. Understanding the variables we compare to make choices and how we select the best option has, therefore, become an important goal for research into adaptive systems in economics, psychology and neuroscience4,5,6,7,8. It is important to note that choosing between different actions often occurs in the absence of cues predicting the probability of success or reward and under such conditions decisions are made on the basis of action values, calculated from the expected probability that a candidate action will lead to reward multiplied by the reward value9,10,11. Choosing between actions requires, therefore, the ability to compare action values, a comparison that should occur, logically, as a precursor to choice, serving as an input into the decision-making process. Nevertheless, despite the importance of this process, it is not known how such comparisons are made, and where in the brain these comparisons are implemented to guide action selection12.
Conventionally, action values have been compared based on a difference score between the two values (for example, QLeft−QRight in reinforcement-learning models10,11)13,14. Although computationally straightforward, this approach can be sub-optimal because it requires the accurate estimation of the value of all available actions before the comparison can be made15. Ultimately, what matters to the agent is not necessarily the absolute difference in action values but which action has the greater value. As such, to make a decision, it is often sufficient to calculate the likelihood of an action being better than alternatives, rather than calculating by how much. As an alternative to the difference score, therefore, actions could be compared based on their relative advantage; that is, the probability that one action’s value is greater than the alternate action16, that is, P(QLeft>QRight). The relative advantage (P) is less informative than the difference because it provides no information regarding the amount by which QLeft is greater than QRight; however, P is also more efficient because it is only necessary to calculate the relative advantage of taking an action without having to determine the value of the inferior action, and this is sufficient to optimally guide choice3,17.
Studies to date have reported neural signals related to action value (that is, QRight, QLeft) in the caudate and efferent motor regions of the cortex14,18,19,20,21. However, few studies have reported neural signals related to the comparison of these values. Single-unit studies in monkeys have gone to some length to isolate action values from stimulus values using free-response tasks involving distinct motor actions instead of visual stimuli to discriminate options18,20. Using this approach, values related to the reward contingency of the separate actions have been distinguished in different striatal projection neurons. However, relatively few caudate neurons appear to represent the difference between action values20. Human neuroimaging studies have distinguished action values in motor regions of the cortex, such as the premotor cortex and supplementary eye field13,14,21; however, only two studies have reported signals representing the difference between options and these studies involved choices between discriminative cues13,14. Consequently, it is unknown the extent to which these neural signals reflect differences in action values or learned stimulus values.
Accordingly, we assessed the comparison of action values in an unsignalled choice situation, using a free-response test, to eliminate any potential influence of stimulus values on the comparison process. In each block, we programmed one response with a slightly higher reward contingency to produce realistic differences in action values, and participants had to learn which of the two actions was superior via feedback (note that because we manipulate reward contingency and not reward magnitude, contingency and value are effectively equivalent in our study). We distinguished two alternate computational signals comparing action values at each response: P representing the probability the left action was more likely to lead to reward than the right action (QLeft>QRight); and D representing the difference between each action’s value (QLeft−QRight). It is important to recognize that, although both models can potentially discriminate the best choice, we were concerned here to establish (i) whether P adds any advantage in predicting choice over D; (ii) which model best predicts both choice performance and the changes in BOLD signal associated with those choices and (iii) whether any such region modulates choice-related activity in the motor cortex, representing the output of the decision process. The results show that actions are chosen on the basis of P values, that right dorsolateral prefrontal cortex (dlPFC) activity tracks these values and also modulates motor cortex activity in an action-specific manner. The relative advantage of an action appears, therefore, to be an important input into the decision-making process enabling action-selection.
Results
Behavioural choices and causal ratings track the best action
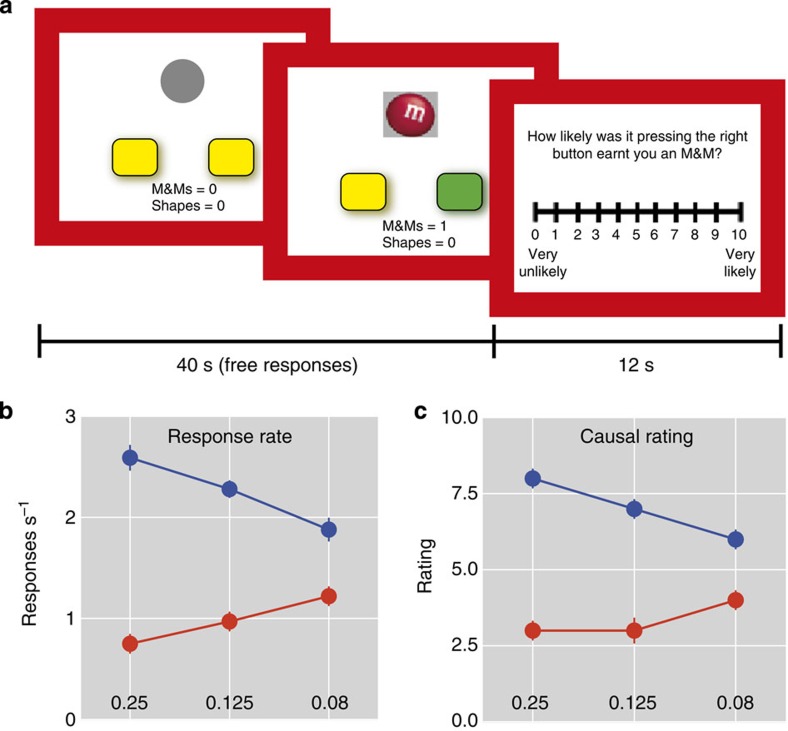
Participants freely chose between two actions (left or right button presses) for a snack food reward (M&M chocolates or BBQ-flavoured crackers) in 40-s interval blocks (Fig. 1a). One action–outcome contingency (action value) was always higher than the other action; however, the identity of the high-value action varied across blocks so participants had to learn anew which action led to more rewards. The difference between action values also varied from large to small across blocks so the task difficulty ranged from easy (large) to difficult (small) conditions. We measured response rates on each action, as well as subjective causal ratings (0–10) for each action after each block. Across conditions, each participant selected the higher-value action more often than the low-value action (Fig. 1b; main effect of action contingency F=34.62, P<0.001). Causal judgments also closely reflected the differences in action value of each block (Fig. 1c; main effect of action contingency F=42.26, P<0.001.
Figure 1. Experimental stimuli, behavioural choices and causal ratings.
(a) Before the choice, no stimuli indicated which button was more likely to lead to reward. When the participant made a choice, the button chosen was highlighted (green) and on rewarded trials the reward stimulus was presented for 1,000 ms duration. After each block of trials, the participant rated how causal each button was. (b) Mean response rate (responses per second) was higher for the high-contingency action (blue) over low-contingency action (red) in each condition. (c) Causal ratings were higher for the high-contingency action (blue) over low-contingency action (red) in each condition. Response rate and causal rating significantly varied with contingency, P<0.001. Vertical bars represent s.e.m.
The relative advantage and the Q difference guides choice
We fit a Bayesian learning model, based on the relative advantage, to each subjects’ choice responses, which allowed us to generate P, that is, which action was more likely to result in reward. We also fit a Q-learning model to each individual subject using the maximum likelihood estimation method to generate D, that is, the difference between action–outcome contingencies (QLeft and QRight). In addition, we generated a hybrid model in which choices are guided by both Q-learning and the relative advantage model (see Supplementary Fig. 1 for the negative log likelihoods). The results of a likelihood ratio test indicated that the hybrid model provided a better fit to participant choices than Q-learning, after taking into account the difference in number of parameters (Table 1). This shows the relative advantage model accounted for unique variance in the subject choices over Q-learning alone. Individual model fit statistics and parameters are provided in Supplementary Table 1.
Table 1. Model comparisons between the hybrid model and its special cases.
| Hybrid | Q-learning | Relative advantage | |
|---|---|---|---|
| Negative log likelihood | 5421 | 5506 | 5558 |
| Aggregate LRT favouring hybrid | — | X240=170*** | X220=274*** |
| No. of favouring hybrids | — | 13 | 8 |
| Pseudo R2 | 0.608 | 0.602 | 0.597 |
Shown for each model: negative log likelihood; test statistic and P-value for a likelihood ratio test against the hybrid (full) model, aggregated across subjects; the number of subjects favoring the hybrid model on a likelihood ratio test (P<0.05); and the degree to which the model explained the choice data averaged over the individual fits (pseudo R2). ***P<1E-16.
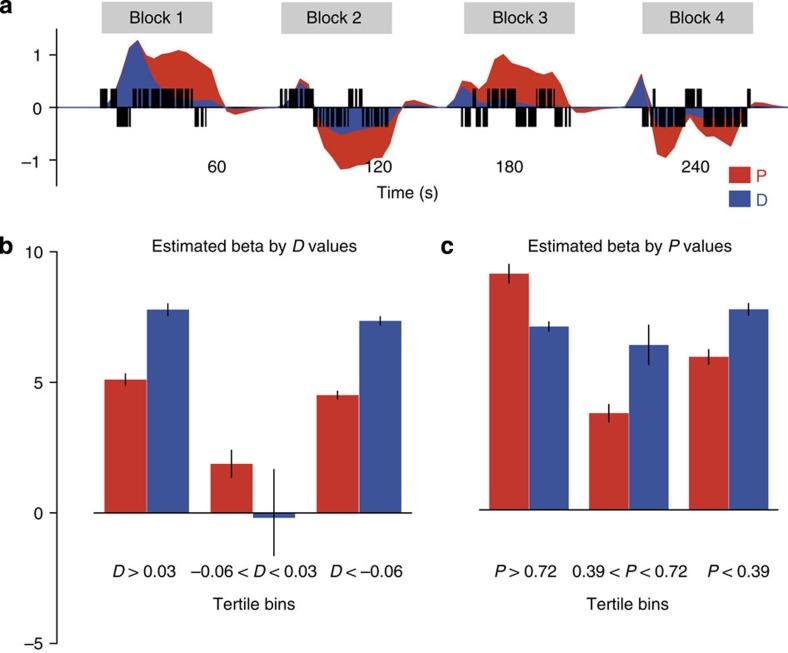
Inspection of the time course of P and D values across the session revealed they both discriminated the best action (Fig. 2a). However, the D signal quickly decayed towards the programmed difference in contingency in each block, which was usually small (that is, <0.2), whereas the relative advantage of the best action (P) was sustained across the block. To determine whether P was more predictive of choice when the difference in action values was small (that is, at intermediate values of D near or equal to zero), we compared the predictive value of P and D over choice at different levels of P and D in a logistic regression. Figure 2b shows we were able successfully to identify conditions under which P and D are differentiated: at small differences in action values (the middle tertile of D values), P was a significant predictor, whereas D was not. Conversely, Fig. 2c shows that P and D were significant predictors across all tertiles of P values (ps<0.001). This result confirms that when choices were made in the presence of small differences in action value, P values better discriminated the best action.
Figure 2. Model values P and D predict choices.
(a) Trial-by-trial example of the actual choices made by Subject 7 (black vertical bars: left actions upward, right actions downwards), and the model-predicted values in arbitrary units for P (red) and D (blue) across the first four blocks (from easy to hard). Notice P represents a sustained advantage across each block, while D decays towards the experimental contingency in each block. (b) The regression weights of P (red) and D (blue) values across tertile bins of D values showing that as the difference in QLeft and QRight approaches zero (middle tertile of D values) only P values significantly predict choice. (c) Regression weights of P and D across tertile bins of P values showing that P and D are both significant predictors of choice across all tertiles of P.
Dorsolateral prefrontal cortex tracks the relative advantage
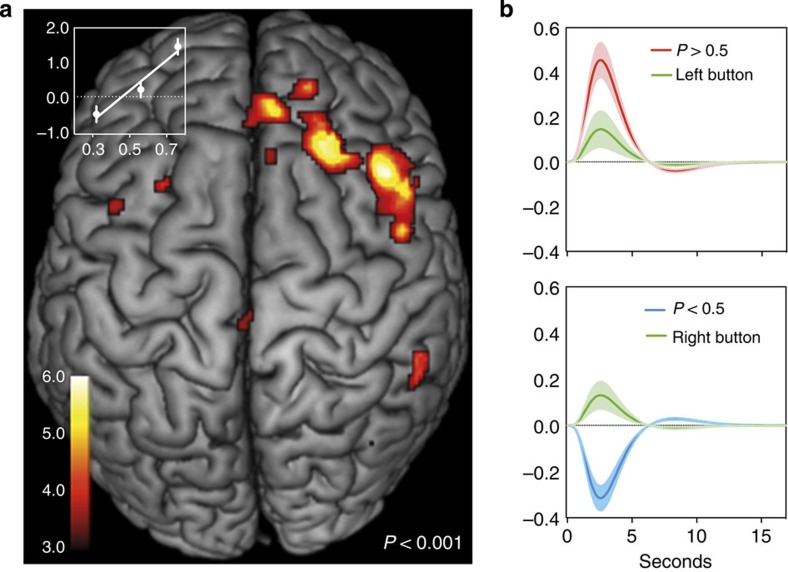
To identify the neural regions involved in the computation of the relative advantage values that guided choice, we defined a stick function for each response and parametrically modulated this by P in a response-by-response fashion for each participant. As we used a free-response task and the interval between choices was not systematically jittered, we cannot determine whether the model variables had separate effects at the time of each choice (or between choice and feedback). We can only determine whether neural activity was related to the time course of the model variables across the 40-s block as subjects tried to learn the best action (for example, Fig. 2a). An SPM one-sample t-test with the parametric regressor representing P revealed neural activity positively related to P in a single large cluster in the right middle frontal gyrus, with the majority of voxels overlapping BA9 (dlPFC22,23; peak voxel: 44, 25, 37; t=5.98, family-wise cluster (FWEc) P=0.012). Figure 2a shows the cortical regions where the BOLD response covaried with the P values of each response, implicating these regions in encoding the relative likelihood that the left action is best (QLeft>QRight).
Figure 3a inset shows that the per cent signal change at the peak voxel in the right dlPFC cluster was linearly related to the magnitude and direction of P, after splitting the P values into three separate and equal-sized bins (high, medium and low tertiles) and calculating the mean local per cent signal change in each bin using rfxplot24. Figure 3b shows the right dlPFC distinguished when the relative advantage of the left action was greater than the right (P>0.5) and when the right action was greater than the left (P<0.5), alongside the BOLD response when the left and right button press occurred. Comparison of the fitted response with the high and low P values relative to button presses clearly showed that the right dlPFC activity did not simply reflect the motor response (button press), because the direction of the BOLD signal discriminated between high and low P values, but not action choices.
Figure 3. Right dlPFC tracked the relative advantage signal.
(a) Cortical regions correlated with the relative advantage signal (P). Only the right dlPFC (BA9) was significant FWEc P<.05. Inset, per cent signal change in the right dlPFC was linearly related to P. (b) Fitted responses in arbitrary units showing action-specific modulation of brain activity (red and blue) by P, as well as non-specific activity due to left and right actions (button presses) in the right dlPFC.
Differentiating action contingencies and action policies
We tested for regions representing the difference between action values (D) in a similar but separate GLM. As P and D were highly correlated for some subjects (for example, Pearson r=0.86 for Subject 01; see Supplementary Table 2 for a complete list), a separate GLM was used to avoid the orthogonal transformation of parametric modulators in SPM and preserve the integrity of the signal. In the same manner as described above for P values, we defined a stick function for each response and parametrically modulated this by D in a response-by-response fashion for each participant. An SPM one-sample t-test of this modulator revealed that no clusters met our conservative correction for multiple comparisons (FWEc<0.05). The peak voxel occurred in a marginally non-significant cluster in the right inferior parietal lobe (BA40: 38, -38, 34; t=5.58, FWEc=0.066). The effect of the D signal in the right dlPFC at the same coordinates identified for P (44, 25, 37) was t=2.99, FWEc=0.236. The failure to find an action-specific delta signal in the brain is consistent with at least one other study that also reported no spatially coherent effect of delta signal13.
The Q-learning model also contains a policy function that maps value differences (D) to choice, π. Policy (π) represents the probability of taking each action on the basis of the size of the difference between actions, and so it may characterize an alternative to the relative advantage signal. For this reason we also tested for brain activity correlating with π in a separate GLM. An SPM one-sample t-test of this modulator revealed that no clusters exceeded our cluster-level correction (FWEc=0.37). The absence of a D or policy signal in prefrontal regions does not support the results of our behavioural modelling, which suggested that under large contingency differences (that is, large D values) subject’s choices were predicted by D. Our behavioural modelling also showed that large D values were rare in our task, so there may not have been sufficient power to detect fMRI-related changes in the current test.
To formally determine which of the variables (P, D or π) provided the best account of neural activity in the right dlPFC, we performed a Bayesian model selection analysis25,26. Specifically we used the first-level Bayesian estimation procedure in SPM8 to compute the log evidence for both signals in every subject in a 5-mm sphere centred on the right dlPFC (44, 25, 37). Subsequently, to model inference at the group level, we applied a random effects approach to construct the exceedance posterior probability (that is, how likely a specific model generated the data of a random subject) for each signal in the right dlPFC. The results found the P signal provided a better account of neural activity in the right dlPFC than the D or π signal (exceedance posterior probabilities 0.84, 0.12, 0.04, respectively). Thus, the weight of evidence suggests that right dlPFC activity represents the likelihood of the best action, rather than the difference in action–outcome contingencies or a policy based on that difference.
Chosen action values and the ventromedial prefrontal cortex
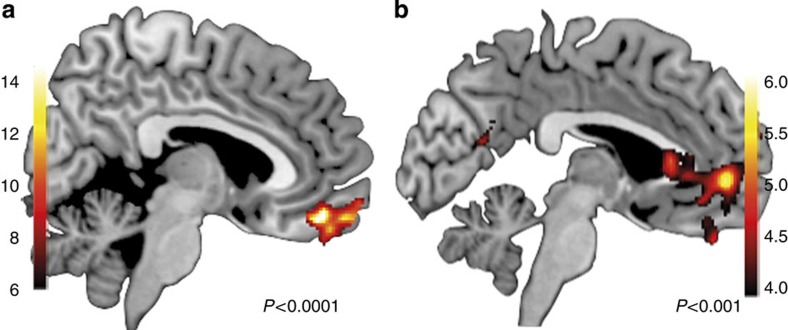
We also tested whether the contingency of the chosen action could be distinguished in separate brain regions (QChosen). This test represents an important (positive) control since chosen action values, or expected reward values, have been widely reported in the ventromedial prefrontal cortex. However, chosen values are not the focus of the present study as they can only be established post-decision and so cannot serve as an input into the decision process. The peak voxel corresponding to the chosen value in the whole-brain occurred in a single cluster in the medial frontal gyrus in the orbitofrontal cortex (OFC: −11, 47, −11; t=23.73, FWEc P<0.0001). Figure 4a shows the extent of the cluster extending rostrally to the ventromedial prefrontal cortex. No other regions were significant (FWEc P>0.05). To further explore the effect of post-decision values, we tested the contingency of the unchosen action. Figure 4b shows a cluster slightly dorsal to the effect of chosen action in the anterior cingulate (AC: 3, 50, −2; t=5.76, FWEc P=0.001). The fact that chosen action values occurred in a cortical area regionally distinct from the action-value comparisons we found in the right dlPFC indicates we were able to successfully distinguish pre-choice and post-choice values. The finding of chosen action values in the ventromedial prefrontal cortex replicates a number of other findings12,14,19,27,28,29,30,31,32,33, and is consistent with the suggestion that the output of the decision process is passed to ventral cortical regions for the purpose of updating action values, perhaps via reinforcement learning.
Figure 4. Ventromedial PFC tracked post-choice values.
(a) Peak voxel in the medial orbitofrontal cortex region correlated with the chosen action value (expected reward). (b) Peak voxel in the ventromedial prefrontal cortex correlated with the unchosen action value.
Action control of motor cortex is modulated by right dlPFC
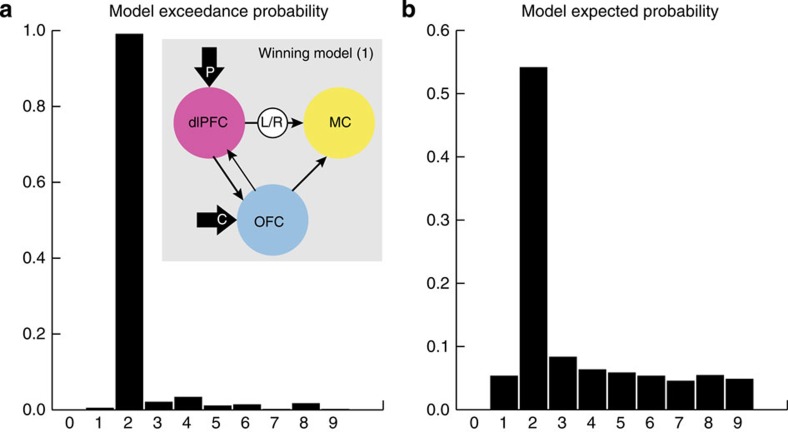
To compare the role of the regions indicated by the GLM analyses of action-value comparisons (right dlPFC) and chosen values (OFC) on the control of actions in the motor cortex, we compared competing dynamic causal models using DCM10 (ref. 34) and tested inferences on model space using Bayesian model selection. The goal was to determine whether motor cortex activity, representing the output of the decision process, was better explained by action-specific modulation from the right dlPFC or the OFC. We extracted activation time courses from each individual’s peak voxel in the right dlPFC, OFC and motor cortex, and constructed eight different models of potential connectivity between each area (Supplementary Fig. 2), as well as a null model with no modulation (model 0). Each model varied the location of action-specific modulation of motor cortex activity, as well as the driving inputs to the dlPFC and OFC. The results of the Bayesian model selection (Fig. 5) established that the winning model was model 1 (Fig. 5a inset), with an exceedance probability of 99.02 per cent (Fig. 5a). Only this model specified action-specific modulation of the motor cortex from the right dlPFC in combination with P values as the driving input. The expected probability, that is, how likely a specific model generated the data of a random subject, for each model is shown in Fig. 5b. The expected probability for the winning model (model 1) was 54.15 per cent, meaning that evidence for model 1 is likely to be obtained in the majority of any randomly selected subjects, and indicates the generalizability of these findings. Overall, the results of the DCM analysis provided clear evidence that the action executed by the motor cortex is guided by the action-value comparisons computed by the right dlPFC likely via the caudate. Indeed, ROI analysis of caudate activity in the current study confirmed that, as described previously, the anterior caudate covaried with the experienced correlation between response rate and reward32 (peak voxel in ROI: 16,18,4; t=6.74, P=0.002 svc—see Supplementary Fig. 3).
Figure 5. Right dLPFC modulated motor cortex.
(a) Probability that one model is more likely than any other model. Inset, winning model with dlPFC modulating motor cortex activity in an action-specific manner (b) How likely a specific model generated the data of a random subject.
Discussion
A critical question in decision neuroscience is how and where in the brain actions are compared to guide choice12. The present results provide evidence that actions are compared on the basis of their relative advantage (P) in a two-armed bandit task, that is, the probability that an action is more likely to lead to reward than another action, and this comparison is utilized by the right dlPFC to control choice behaviour. Activity in the right dlPFC tracked the relative advantage (P) over other comparison signals (for example, the relative strength of the best action, D), which also could be used to predict choice. Furthermore, activity in this region was not differentially modulated by post-choice values, such as the chosen action contingency, or the actions taken (for example, a right or left button press). Effective connectivity analysis showed the right dlPFC-modulated activity in the motor cortex, the major output pathway for choice behaviour, in an action-specific manner. As a consequence, this directional signal may represent an important input into the decision-making process, enabling the subject to choose the course of action more likely to lead to reward.
The dlPFC is also connected with the orbitofrontal cortex, which represents important value signals such as the expected reward value29. In particular, we found that activity in the OFC tracked the chosen action contingency, which is equivalent to the expected reward value in this task. A number of studies have found expected reward signals in this region14,27,28,29,30,31,32,33, as well as the medial prefrontal cortex32,35 and amygdala36. Some studies have also found that the reward signal in the OFC precedes the dlPFC response37, which implies that reward value information is relayed from the OFC to the dlPFC. Our DCM analysis did not indicate this direction of effect (albeit, the parameters of our task did not provide sufficient temporal resolution to distinguish the order of effect). However, expected reward values are necessary to compute a prediction error in model-free reinforcement learning to update action values before the next trial10. As such, they are quite distinct from action values and cannot serve as inputs to the comparison process because they reflect the value of actions already selected in the decision, that is, expected reward values reflect decision output rather than input, which was the focus of the present study.
We also tested the relative roles of the right dlPFC and the OFC in action selection by comparing DCMs with relevant variations in action-specific modulation between regions. The most likely models, given our data, indicated the dlPFC modulated motor cortex activity in an action-specific manner. We failed to find any substantive evidence for models in which the right dlPFC modulated OFC activity, or the OFC modulated motor cortex activity. It is worth noting that effective connectivity does not reflect or require direct connections between regions, as the effective connectivity can be mediated polysynaptically38. We speculate the effect of the right dlPFC on motor cortex is mediated via connections with the caudate, given the evidence of anatomical connections between these regions39,40, and the caudate’s established role in goal-directed choice41. We did not, however, observe action-specific value signals in the dorsal striatum, as has been reported in single-unit studies in primates18,20,42. Nevertheless, it is likely that connections between the dlPFC, the OFC and the striatum32,35 participate in a circuit to compare action values, select actions and update action values once a choice has been made.
Single-unit studies in primates have found that a number of neurons in the dlPFC are predictive of an animal’s decision in choices between discriminative cues37,43,44, and stimulation of dlPFC neurons can bias a decision45,46. A prevalent idea about the functional specialization of the prefrontal cortex is that the OFC processes information about reward value31,47,48, whereas the dlPFC functions in the selection of goals and actions44,49,50. The dlPFC is heavily interconnected with areas responsible for motor control51,52,53 and so may represent an area where information about reward value and action converges to allow action comparisons to take place; however, there are other regions that could integrate reward information and motor action, such as anterior cingulate54 and the parietal cortex5. Overall, our results extend the established role of the dlPFC in the selection of goals and actions to include the computational comparison of action values. Furthermore, the dlPFC determines that this comparison occurs in terms of relative likelihood of the best action rather than the relative strength of the best action.
Evidence that action-value comparisons occur in the human brain has been scarce12. Wunderlich et al.21 identified action-specific values in the supplementary motor cortex and premotor area using very distinct motor actions in order to discriminate between choices (for example, hand versus eye movements). Wunderlich et al.21 also found that post-choice values (unchosen over chosen values) were compared in the anterior cingulate cortex, where we found unchosen action values were tracked. Although such results clearly distinguish separate action-specific value signals in different regions of the motor cortex, the regressors tested involved post-choice values and so were not precursors to choice. Evidence of a neural signal representing the difference between Q values and that could act as an input into a decision comparator has been provided by another study using magnetoencephalography14. This study reported that the direction of comparison was contralateral to the hemisphere of the delta signal (that is, QContralateral−QIpsilateral); however, whether this comparison reflected action values or stimulus values is uncertain due to the discriminative cues provided by the task. Even so, it is worth noting that the comparison we found (that is, QLeft>QRight) in the right prefrontal cortex is consistent with evidence that decision values occur in the contralateral hemisphere14,19. We did not find the inverse direction in the left hemisphere (that is, QRight>QLeft), presumably because our participants only used their right hand to respond; however, there are many differences in the task and temporal dynamics of the image data that may account for this. Ultimately, a single bidirectional signal is sufficient to guide choice, so the unilateral effect we found may reflect an innate bias in right-handed actions or right-handed subjects seeking neural efficiency.
Our modelling of choice performance implied people used more than one strategy for selecting between actions—both P and D were predictive of choice; however, when the difference between action values (D) was small, participants used the relative advantage (P) to select the best action (Fig. 2b). We cannot determine from our data alone whether the use of relative advantage (P) occurs generally or only when D is difficult to compute or uncertain. Likewise, we cannot determine whether the right dlPFC computes the best action on the basis of each response or whether P is computed over a set of responses. However, as discussed by others (and above)3,55,56, when action outcomes are uncertain, a good heuristic solution in a multi-armed bandit problem is to restrict estimation of each action contingency until the values indicate a likely winner rather than to continue estimating each action contingency after an advantage is known. This strategy is represented by the relative advantage comparison, which has also been shown to scale well when the number of choices increases above two (for example, 10-arm bandit3). Thus, the fact that the neural signal in the dlPFC reflected P, even under conditions in which D was predictive (Fig. 2c), represents a dissociation consistent with a unique role for this neural region in this task. To our knowledge, this is the first demonstration of such a computational comparison in humans or other animals.
Finally, our results have implications for neural models of decision-making. We used a model-based form of Bayesian learning that directly estimates the action contingencies (state transition probabilities) from the conditional probabilities of reward, rather than a model-free approach that uses prediction errors to estimate action values. The model-based method was chosen on the basis of prior evidence that the cortical regions of interest are sensitive to contingency changes32,35. Although our modelling was not able to determine conclusively whether or not people adopted a model-free or model-based strategy, the subjective causal ratings of each action corresponded closely with the action contingencies, demonstrating participants were aware of the contingencies in each block. Under such conditions, people may be more likely to adopt a model-based strategy, rather than an implicit model-free strategy. Recent model-based accounts of decision-making assume uncertainty around each action/stimulus value determines how quickly the value is updated (that is, the learning rate)57,58. In such models, uncertainty is represented separately in the decision process, as well as the brain58,59. By contrast, the relative advantage signal we found summarizes the difference between action values as well as the uncertainty around them in a single value. The implication for models of decision-making is that action values and uncertainty are not always represented separately at the decision-point, but instead are combined to indicate the best action.
In conclusion, the present report provides direct evidence of an action-specific comparison signal in the human cortex. It is striking that existing studies of action-specific values using human fMRI have not previously succeeded in revealing a comparison signal in the cortex that is regionally homogenous. As such, these results may also suggest that the comparison process revealed here is a unique feature of goal-directed decision-making and may not reflect a more general action-value comparison strategy based, for example, on predictive stimuli.
Methods
Subjects
Twenty-three right-handed subjects (11 females), age range 17–32 years, were recruited for the study. Three participants were removed due to excessive head movement (> 2 mm). Thus, n=20, and all participants were unmedicated, free of neurological or psychiatric disease and consented to participate. The study was approved by the Human Research Ethics Committee at Sydney University (HREC no. 12812). After scanning, all participants were reimbursed $45 in shopping vouchers, in addition to the snack foods that they earned during the test session.
Stimuli and task
The instrumental learning task (Fig. 1a) involved choosing between two action, left and right button presses, for a snack food reward (M&M or BBQ shape) and was conducted in a single replication. Participants were instructed to press the left or right button with their right hand, and try to earn as many snacks as they can. Actions were taken by pressing separate buttons on a Lumina MRI-compatible two-button response pad. The session was arranged in 12 blocks of 40-s duration, and in each block the participant responded freely for reward32,35. Reward was indicated by the presentation of a visual stimulus depicting the outcome (for example, M&M) for 1,000 ms and the visual tally of the total number of rewards was increased. No feedback was provided in the absence of a win. At the end of each block, participants were given 12 s to rate how causal each action was with respect to the outcome on a visual analogue scale from 1 to 10.
Blocks differed according to their outcome contingencies on the left and right actions but only one outcome was available in each block. Thus, there were six pairs of action contingencies [0.25, 0.05], [0.05, 0.25], [0.05, 0.125], [0.125, 0.05], [0.08, 0.05] and [0.05, 0.08], and each was repeated twice, once for each outcome (M&M and BBQ shapes). Importantly, no cue indicated which action was the high contingency action at the time of the decision. As the contingencies changed between blocks and the beginning of each block was cued, participants had to learn anew within each block which action led to more rewards. At the end of the session, participants received the total number of snack foods they had earned.
Bayesian learning model
To estimate the action contingencies on the basis of experience for each participant, we used a Bayesian learning method. This method treats the contingency as a random variable, and calculates its probability distribution.
We assumed the probability of receiving reward by executing each action is a binomial distribution with parameters pLeft and pRight for left and right actions, respectively. These probabilities were then represented with Beta distributions:
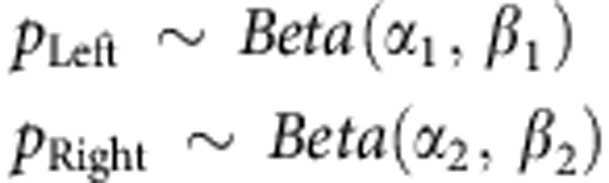 |
We assumed uninformative priors over the parameters that roll off at boundaries, (α1, α2, β1, β2=1.1). After executing each action i (i=Left, Right) and receiving the outcome, the underlying distributions update according to Bayes rule:
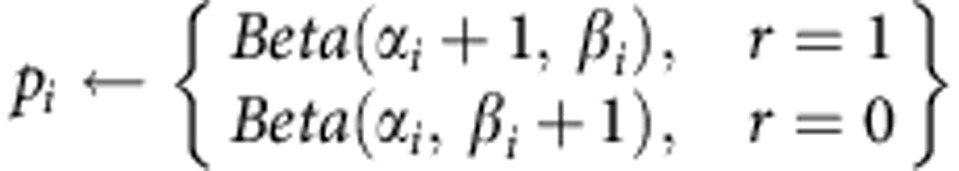 |
Where r=1 is reward, and r=0 is non-reward. Finally, we define delta as Δ=pLeft−pRight. By denoting:
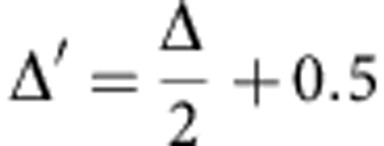 |
We will have:
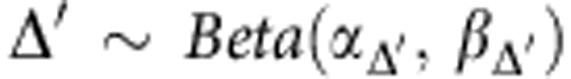 |
Where
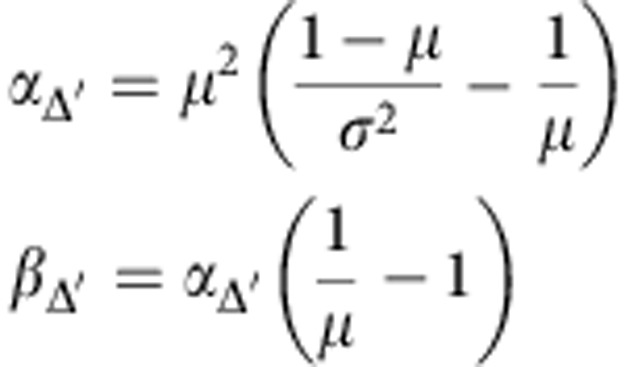 |
Where μ and σ2 are mean and variance of Δ′, respectively, and can be calculated in a straightforward manner. Based on this, the relative advantage is equivalent to:
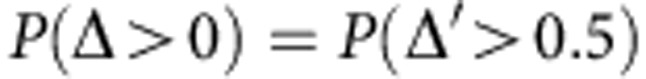 |
Hereafter, we will represent the relative advantage P(Δ>0) as P.
In this manner, we modelled the action-specific comparisons that allow the decision-maker to make choices without perfect knowledge of the contingencies. In fact, the relative advantage will change as the certainty around each contingency estimate changes, as well as the distance between the most likely estimates of each contingency changes. The relative advantage also reflects the assumption that once an action is estimated to be more likely to lead to reward than the other action with absolute certainty, that is, P=1, the advantage does not further increase with increases in contingency.
Q-learning model
As an alternative to the Bayesian learning model, we used a Q-learning method, which estimates a value for each action. After executing each action i (i=left, right) and receiving outcome, the value of each action updates according to the temporal-difference rule:
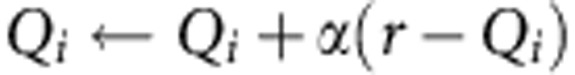 |
where α is the learning rate. If the action is rewarded r=1, otherwise r=0. We defined the difference between action values as follows:
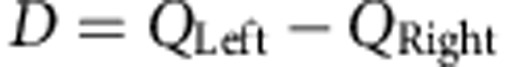 |
The values for QLeft and QRight were initially set to zero.
Action selection
To model individual choices according to experience, we assumed that the probability of taking each action is proportional to its values, and its relative advantage over the other action. Using the softmax rule, the probability of taking the left action, π(left) will be:
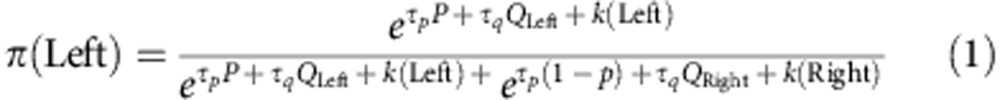 |
where τp and τq are the ‘inverse temperature’ parameters, and controls exploration–exploration balance. τp and τq control the contribution of the P and Q values to the choice probabilities, respectively. k(A) is the action preservation parameter and captures the general tendency of taking the same action as the previous trial60,61. k(A) is equal to k when the chosen action in the previous trial is the same as A, and otherwise it is equal to zero.
We generated the model described in equation (1) as well as two nested models by setting τp=0 and τq=0, and fitting them to the subject’s behaviour individually, using the maximum-likelihood estimate. For optimization we used the Ipopt software package62. We compared models using the likelihood ratio test and measured the overall goodness of fit by computing pseudo R2 using the best fit model for each subject. Pseudo R2 was defined as (R–L)/R for each subject, where L and R are the negative log likelihoods of the hybrid model (1) and a null model of random choices, respectively28.
For the purpose of generating model-predicted time series for fMRI regression analysis, D and π values for each individual were generated using the restricted model τp=0 with parameters (τq, α and k) set to the maximum-likelihood estimate over the whole group63, similar to other work in this field64. Simulations determined these Q-learning parameters could be accurately recovered from choice data (Supplementary Table 3). We also tested D values using the hybrid model but since it made no difference to the final result, only the test of the nested model values are provided here. P values were generated using the restricted model τq=0 and are independent of model parameters (note: P values generated from the hybrid model and nested model did not differ). Each individual’s P and D values were entered as a parametric modulator of responses in the fMRI analysis below to identify brain areas where the value comparison computation might be carried out.
fMRI data acquisition
Gradient-echo T2*-weighted echo-planar images (EPI) were acquired on a Discovery MR750 3.0T (GE Healthcare, UK) with a resolution of 1.88 × 1.88 × 2.0 mm. Fifty-two slices were acquired (echo time 20 ms; repetition time 3.0 s; 0.2 mm gap) in an interleaved acquisition order. The acceleration factor (ASSET) was 2, which allowed data acquisition from a whole-brain volume with 240 mm field of view angled 15° from AC-PC in each subject to reduce signal loss. In each session 260 images were collected (~13 min each).
Image analysis
Preprocessing and statistical analysis were performed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK; www.fil.ion.ucl.ac.uk/spm). The first four images were automatically discarded to allow for T1 equilibrium effects, then images were slice-time-corrected to the middle slice and realigned with the first volume. The mean whole-brain image was then normalized to MNI space and the resulting normalization parameters applied to the remaining images. Images were then smoothed with a Gaussian kernel of 8-mm FWHM.
Based on our behavioural analysis, we estimated several general linear models (GLM) for each individual. Block duration, rating periods, responses and rewards were included as separate subject-specific regressors in each GLM. Responses were parametrically modulated by the relative advantage value P in the first GLM. Separate GLMs modulated responses by D (the expected value of the difference between action contingencies), which replicates methods used in other reports13. We also tested the chosen action contingency as this represents the expected reward value of the chosen action and serves as a useful comparison to other reports of expected values in the prefrontal cortex30,65. The chosen action contingency was calculated as the experienced contingency between the current action and its accumulated rewards since the beginning of the block. The resulting stimulus functions were convolved with the canonical hemodynamic response function. Regression was performed using standard maximum likelihood in SPM. Low-frequency fluctuations were removed using a high-pass filter (cutoff 128 s) and remaining temporal autocorrelations were modelled with a two-parameter auto-regression model.
To enable inference at the group level, we calculated second-level group contrasts using a one-sample t-test in SPM. Regions exceeding a voxel-wise threshold P<0.001, along with an FWEc threshold P<0.05 to correct for multiple comparisons are reported. As P and D are action-specific values, that is, a comparison of one action over another action, the values must provide a direction of comparison in order to ultimately guide action selection (for example, QLeft>QRight or QRight<QLeft). Determining the direction of comparison each subject employed a priori was not possible, so we assumed a single direction of comparison for all subjects in a unidirectional t-test (SPM default) and then determined the direction of comparison by examining the eigenvariate of each subject at the group peak voxel. The neural responses from only three subjects had an inverse relationship with P and D relative to the rest of the group and reversing their direction did not change the imaging results, so we report here the results of our initial analysis, assuming the same direction for all subjects.
Dynamic causal modelling
To compare the role of the regions associated with action comparisons and choice on the control of choice behaviour in the motor cortex, we specified seven competing models of functional architecture using DCM10 (ref. 34) and tested inferences on model space using Bayesian model selection. The goal was to determine whether motor cortex activity, representing the output of the decision process, was better explained by action-specific changes in effective connectivity from the right dlPFC or the OFC, since both these regions were indicated in the GLM analysis of action comparisons and chosen action contingencies described above.
The analysis was carried out in several steps66. First, activation time courses were extracted from each individual’s peak voxel within 5 mm of the global peak voxel coordinates of the group in each of three analyses: action-specific comparisons using the relative advantage values, peak MNI coordinates [+44, 25, 37]; the chosen action contingency, peak MNI coordinates [−11, 47, −11] and button presses (responses), peak MNI coordinates [−4, 25, 70]. Second, we specified eight different models of potential connectivity between each area, with varying locations of action-specific modulation of motor cortex activity (Supplementary Fig. 2, models 1–8), as well as a null model with no modulation (model 0). For each model tested, two driving inputs were included: (1) an input representing the relative advantage values to the right dlPFC and (2) an input representing the chosen action contingency to the OFC. As we wished to explain activity in the motor cortex in terms of connectivity, no driving input was included for the motor cortex. In addition, action-specific changes in coupling strength were modelled by specifying left and right button presses separately. Note, only models 1 and 2 (Supplementary Fig. 2) included action-specific coupling between the right dlPFC and motor cortex. We then identified the best model using Bayesian model selection67. Briefly, this technique treats the models as random variables and computes a probability distribution for all models under consideration. This procedure permits the computation of the exceedance probabilities for each model, which represents the probability that each model is the most likely one to be correct. The exceedance probabilities add to one over the comparison set, and thus generally decrease as the number of models considered increases.
Author contributions
R.W.M. analysed the data, interpreted the results and wrote the paper. A.D. modelled the data, interpreted the results and wrote the paper. K.R.G. designed the experiment, collected the data and wrote the paper. B.W.B. designed the experiment, interpreted the results and wrote the paper. This research was supported by a Laureate Fellowship from the Australian Research Council to BWB (ARC FL0992409).
Additional information
How to cite this article: Morris R. W. et al. Action-value comparisons in the dorsolateral prefrontal cortex control choice between goal-directed actions. Nat. Commun. 5:4390 doi: 10.1038/ncomms5390 (2014).
Supplementary Material
Supplementary Figures 1-3, Supplementary Tables 1-3
Acknowledgments
This study was supported by a Laureate Fellowship from the Australian Research Council #FL0992409 to B.W.B.
References
- Gittins J. C. Bandit processes and dynamic allocation indices. J. R. Statist. Soc. B 148–177 (1979). [Google Scholar]
- Monica B. & Tze Leung L. Incomplete learning from endogenous data in dynamic allocation. Econometrica 68, 1511–1516 (2000). [Google Scholar]
- Scott S. L. A modern Bayesian look at the multi-armed bandit. Appl. Stochastic Models Bus. Ind. 26, 639–658 (2010). [Google Scholar]
- Dickinson A. & Balleine B. Motivational control of instrumental action. Curr. Dir. Psychol. Sci. 4, 162–167 (1995). [Google Scholar]
- Platt M. & Glimcher P. Neural correlates of decision variables in parietal cortex. Nature 400, 233–238 (1999). [DOI] [PubMed] [Google Scholar]
- Rangel A., Camerer C. & Montague P. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 9, 545–556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A. & Kahneman D. The framing of decisions and the psychology of choice. Science 211, 453–458 (1981). [DOI] [PubMed] [Google Scholar]
- Von Neumann J. & Morgenstern O. The Theory of Games and Economic Behavior Princeton University Press (1947). [Google Scholar]
- Dayan P. & Abbott L. F. Theoretical Neuroscience MIT Press (2001). [Google Scholar]
- Sutton R. S. & Barto A. G. Reinforcement Learning MIT (1998). [Google Scholar]
- Watkins C. J. C. H. & Dayan P. Q-Learning. Machine Learning 8, 279–292 (1992). [Google Scholar]
- Rangel A. & Hare T. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 20, 262–270 (2010). [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. H., Friston K. J. & Dolan R. J. Action-specific value signals in reward-related regions of the human brain. J. Neurosci. 32, 16417–16423 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. T., Woolrich M. W., Rushworth M. F. S., Behrens T. E. J. & Diedrichsen J. Trial-type dependent frames of reference for value comparison. PLoS Comput. Biol. 9, e1003225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. R. On the likelihood that one unknown probability exceeds another in view of the evidence of two samples. Biometrika 25, 285–294 (1933). [Google Scholar]
- Thompson W. R. On the theory of apportionment. Am. J. Math. 57, 450–456 (1935). [Google Scholar]
- Granmo O.-C. Solving two-armed bernoulli bandit problems using a bayesian learning automaton. Int. J. Intell. Comput. Cybernet. 3, 207–234 (2010). [Google Scholar]
- Lau B. & Glimcher P. W. Value representations in the primate striatum during matching behavior. Neuron 58, 451–463 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri S., Boraud T., Lafargue G., Dubois B. & Pessiglione M. Brain hemispheres selectively track the expected value of contralateral options. J. Neurosci. 29, 13465–13472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K., Ueda Y., Doya K. & Kimura M. Representation of action-specific reward values in the striatum. Science 310, 1337–1340 (2005). [DOI] [PubMed] [Google Scholar]
- Wunderlich K., Rangel A. & O’Doherty J. P. Neural computations underlying action-based decision making in the human brain. Proc. Natl Acad. Sci. USA 106, 17199–17204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J. K., Paxinos G. & Voss T. Atlas of the Human Brain 3rd edn Elsevier (2008). [Google Scholar]
- Maldjian J. A., Laurienti P. J., Kraft R. A. & Burdette J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- Glascher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics 7, 73–82 (2009). [DOI] [PubMed] [Google Scholar]
- Penny W. D., Trujillo-Barreto N. J. & Friston K. J. Bayesian fMRI time series analysis with spatial priors. Neuroimage 24, 350–362 (2005). [DOI] [PubMed] [Google Scholar]
- Rosa M.J., Bestmann S., Harrison L. & Penny W. Bayesian model selection maps for group studies. Neuroimage 49, 217–224 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman E., Behrens T., Woolrich M. & Rushworth M. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron 62, 733–743 (2009). [DOI] [PubMed] [Google Scholar]
- Daw N. D., O’Doherty J. P., Dayan P., Seymour B. & Dolan R. J. Cortical substrates for exploratory decisions in humans. Nature 441, 876–879 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J., Hampton A. N. & O’Doherty J. P. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb. Cortex 19, 483–495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton A. N., Bossaerts P. & O’Doherty J. P. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. J. Neurosci. 26, 8360–8367 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. & Assad J.A. Neurons in the orbitofrontal cortex encode economic value. Nature 441, 223–226 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S. C., Balleine B.W. & O'Doherty J. P. Calculating consequences: brain systems that encode the causal effects of actions. J. Neurosci. 28, 6750–6755 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin V. V., Dickinson A. & O’Doherty J. P. Determining the neural substrates of goal-directed learning in the human brain. J. Neurosci. 27, 4019–4026 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros A. C., Kiebel S. J. & Friston K. J. Dynamic causal modelling for fMRI: a two-state model. Neuroimage 39, 269–278 (2008). [DOI] [PubMed] [Google Scholar]
- Liljeholm M., Tricomi E., O'Doherty J. P. & Balleine B. W. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J. Neurosci. 31, 2474–2480 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J. A., O'Doherty J. & Dolan R. J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301, 1104–1107 (2003). [DOI] [PubMed] [Google Scholar]
- Wallis J. & Miller E. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur. J. Neurosci. 18, 2069–2081 (2003). [DOI] [PubMed] [Google Scholar]
- Friston K. J. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78 (1994). [Google Scholar]
- Haber S. N., Kim K. S., Mailly P. & Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 26, 8368–8376 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L. D. & Goldman-Rakic P. S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci. 5, 776–794 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. H., Ostlund S. B., Knowlton B. J. & Balleine B. W. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 22, 513–523 (2005). [DOI] [PubMed] [Google Scholar]
- Seo M., Lee E. & Averbeck B. B. Action selection and action value in frontal-striatal circuits. Neuron 74, 947–960 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. & Shadlen M. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci. 2, 176–185 (1999). [DOI] [PubMed] [Google Scholar]
- Tsujimoto S., Genovesio A. & Wise S. Comparison of strategy signals in the dorsolateral and orbital prefrontal cortex. J. Neurosci. 31, 4583–4592 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I., Barborica A. & Ferrera V. Microstimulation of the dorsolateral prefrontal cortex biases saccade target selection. J. Cognit. Neurosci. 17, 893–904 (2005). [DOI] [PubMed] [Google Scholar]
- Camus M. et al. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex decreases valuations during food choices. Eur. J. Neurosci. 30, 1980–1988 (2009). [DOI] [PubMed] [Google Scholar]
- Kennerley S. & Wallis J. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur. J. Neurosci. 29, 2061–2073 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 30, 31–56 (2007). [DOI] [PubMed] [Google Scholar]
- Genovesio A., Brasted P. & Wise S. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. J. Neurosci. 26, 7305–7316 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J. & Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 88, 37–57 (2008). [DOI] [PubMed] [Google Scholar]
- Lu M., Preston J. & Strick P. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J. Comparat. Neurol. 341, 375–392 (1994). [DOI] [PubMed] [Google Scholar]
- Preuss T. & Goldman-Rakic P. Connections of the ventral granular frontal cortex of macaques with perisylvian premotor and somatosensory areas: anatomical evidence for somatic representation in primate frontal association cortex. J. Comparat. Neurol. 282, 293–316 (1989). [DOI] [PubMed] [Google Scholar]
- Takada M. et al. Organization of prefrontal outflow toward frontal motor-related areas in macaque monkeys. Eur. J. Neurosci. 19, 3328–3342 (2004). [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2, 417–424 (2001). [DOI] [PubMed] [Google Scholar]
- Keramati M., Dezfouli A. & Piray P. Speed/accuracy trade-off between the habitual and the goal-directed processes. PLoS Comput. Biol. 7, e1002055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. A. & Daw N. Environmental statistics and the trade-off between model-based and TD learning in humans. Neural Inf. Process. Syst. 24, 1–9 (2011). [Google Scholar]
- Dayan P., Kakade S. & Montague P. Learning and selective attention. Nat. Neurosci. 3, (Suppl):1218–1223 (2000). [DOI] [PubMed] [Google Scholar]
- Bach D. & Dolan R. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nat. Rev. Neurosci. 13, 572–586 (2012). [DOI] [PubMed] [Google Scholar]
- Behrens T., Woolrich M., Walton M. & Rushworth M. Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221 (2007). [DOI] [PubMed] [Google Scholar]
- Ito M. & Doya K. Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J. Neurosci. 29, 9861–9874 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B. & Glimcher P. W. Dynamic response-by-response models of matching behaviour in rhesus monkeys. J. Exp. Anal. Behav. 84, 555–579 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter A. & Biegler L. T. On the implementation of a primal-dual interior point filter line search algorithm for large scale nonlinear programming. Math. Program. 106, 25–57 (2006). [Google Scholar]
- Daw N. D. inAttention and Performance XXIII: Decision Making, Affect, and Learning (eds Delgado M. R., Phelps E. A., Robbins T. W. 3–38Oxford University. Press (2011). [Google Scholar]
- Glascher J., Daw N., Dayan P. & O'Doherty J. P. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66, 585–595 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T. A., O'Doherty J., Camerer C. F., Schultz W. & Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 28, 5623–5630 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K. E. et al. Ten simple rules for dynamic causal modeling. Neuroimage 49, 3099–3109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K. E., Penny W. D., Daunizeau J., Moran R. J. & Friston K. J. Bayesian model selection for group studies. Neuroimage 46, 1004–1017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-3, Supplementary Tables 1-3