Abstract
The roles that astrocytes play in the evolution of abnormal network excitability in chronic neurological disorders involving brain injury, such as acquired epilepsy, are receiving renewed attention due to improved understanding of the molecular events underpinning the physiological functions of astrocytes. In epileptic tissue, evidence is pointing to enhanced chemical signaling and disrupted linkage between water and potassium balance by reactive astrocytes, which together conspire to enhance local synchrony in hippocampal microcircuits. Reactive astrocytes in epileptic tissue both promote and oppose seizure development through a variety of specific mechanisms; the new findings suggest several novel astrocyte-related targets for drug development.
Introduction
The first thing you have to consider is that these are not your father’s astrocytes, those flaccid cells that lay at the bottom of the dish, unable even to generate a spike. Today, astrocytes are recognized as more than simple bit players on the brain’s stage. For example, in the healthy brain, thrombospondins and other proteins secreted by immature astrocytes control synaptogenesis (Christopherson et al., 2005), and individual astrocytes cradle and shepherd newborn dentate granule cells to their final destinations (Shapiro et al., 2005).
Whether astrocytes play a role in information processing normally or in certain pathological conditions such as epilepsy is an intriguing notion receiving increased attention. Epilepsy—a family of neurological disorders characterized by the unpredictable but recurrent occurrence of seizures—is one of the most common disorders of the brain. Approximately 3% of us who live to the age of 80 will have developed epilepsy. Epileptic seizures are uncontrolled sudden attacks of a convulsive or nonconvulsive nature associated with unusually intense neuronal firing. Neuronal firing patterns during a seizure typically include periods of asynchronous activity and periods of synchronous bursts. A spatially restricted seizure focus in the brain can often be identified for epilepsies acquired after head trauma, tumors, or other severe focal insults to the brain. Other epilepsies by contrast typically involve seizures that spread throughout the brain so rapidly that the focus cannot be localized. The epileptic brain exists in two functional states: the ictal state during a seizure and the longer interictal state between seizures. The interictal, or “epileptiform,” burst consists of a brief (50–200 ms) neuronal discharge that is synchronous over several millimeters of tissue. Development of an epileptic seizure requires physiological changes that lead to both heightened neuronal excitability and abnormal synchronization of discharge within the affected neuronal network. The evident part that astrocytes play in this neurocentric disorder is the subject of growing interest.
The functions of astrocytes in the healthy adult brain are traditionally considered to involve potassium buffering, interstitial volume control, and maintenance of a low interstitial glutamate concentration. Astrocytic processes present sheet-like extensions that enwrap neurons and blood vessels in the brain. Accumulating evidence points to functional heterogeneity within the astrocyte population of the healthy adult brain (Ridet et al., 1997; Walz, 2000; Seifert et al., 2006). Reactive gliosis, a component of neuroinflammation that involves structural and metabolic changes in astrocytes and microglia, is often a prominent feature of mesial temporal lobe human epilepsy and most animal models of recurrent seizures. Three major questions related to the potential roles of reactive astrocytes in either seizure generation or epileptogenesis are receiving much attention: Has astrocyte-neuron glutamate signaling gone awry? Is water or potassium balance impaired? And, does reactive gliosis cause, exacerbate, or combat seizures? We will consider these issues and the evidence for each in turn. A better understanding of the pro- and anticonvulsant actions of reactive astrocytes could lead to novel strategies for epilepsy. Several recent reviews complement the discussion that follows (Amiry-Moghaddam and Ottersen, 2003; Kofuji and Newman, 2004; Volterra and Meldolesi, 2005; Seifert et al., 2006; Halassa et al., 2007).
Excessive Astrocyte-Neuron Chemical Signaling
One of the most captivating changes in our view of astrocyte functions is the gradual acceptance of the notion that astrocytes can respond to neurotransmitters, hormones, and other stimuli via increases in intracellular Ca2+, which in turn can promote release of chemical mediators, including D-serine, ATP, and glutamate (reviewed in Haydon and Carmignoto, 2006). The major issues driving this field today are (1) exploring the cellular pathways that regulate this stimulus-release process, (2) identifying the conditions under which it occurs, and (3) determining whether and when such “gliotransmission” influences the excitability of nearby neurons. A most intriguing question is whether exuberant neuron-astrocyte-neuron transmitter-mediated signaling promotes abnormal neuronal synchronization in epilepsy.
Glutamate Release from Astrocytes
Glutamate release from pure astrocyte cultures was demonstrated by HPLC or enzymatic assays following treatment with agonists of Gq-coupled GPCRs (Parpura et al., 1994; Jeftinija et al., 1997; Bezzi et al., 1998). When glutamate is released from astrocytes, what is the route? Several routes appear to operate, including reverse transport (Rossi et al., 2000), cystine-glutamate xc- antiporter (Cavelier and Attwell, 2005), volume-sensitive anion channels (Mongin and Kimelberg, 2005; Abdullaev et al., 2006; Liu et al., 2006; Ramos-Mandujano et al., 2007), gap junction hemichannels (Ye et al., 2003), and vesicular exocytosis (Jeftinija et al., 1997; Bezzi et al., 2004; Bowser and Khakh, 2007; Xu et al., 2007). For example, Ramos-Mandujano et al. (2007) showed that hypo-osmotic medium caused glutamate release from astrocytes in culture, a process that was prevented by several volume-sensitive anion channel blockers. They concluded that cell swelling such as occurs during ischemia or seizures can cause astrocytic glutamate release.
Of the various routes for astrocytic glutamate release, perhaps the most complete evidence is for Ca2+-dependent exocytosis of vesicular glutamate from astrocytes. The evidence consists of three parts. First, astrocytes, like many other non-neuronal cells (Rothman, 2002; Bonifacino and Glick, 2004), express proteins that mediate fusion of vesicles with cellular membranes (Bezzi et al., 2004; Montana et al., 2006). These include the SNARE proteins cellubrevin and synaptobrevin 2, the vesicular glutamate transporters VGLUT1/2, and synaptotagmin IV, homologs of which are required for Ca2+-mediated vesicle fusion in neurons. These vesicle release proteins are, however, typically expressed at much lower levels than their homologs in neurons. Second, glutamate release from cultured astrocytes in response to mechanical stimulation could be prevented by omission of Ca2+ in the medium or by selective proteolysis of SNARE proteins by intracellular infusion of botulinum toxin (Araque et al., 2000). Finally, burst-like release from DHPG-treated astrocytes of acridine orange that had been concentrated within VGLUT-EGFP-labeled vesicles was revealed by the appearance of fluorescent flashes, consistent with exocytotic release of vesicle contents (Bezzi et al., 2004).
Modulation of Neuronal Excitability via Astrocytic Release of Glutamate
Numerous studies have appeared reporting that astrocytic Ca2+ waves induce local glutamate release that acts on nearby neurons to produce depolarization or neuronal Ca2+ transients (Pasti et al., 1997; Parri et al., 2001; Angulo et al., 2004; Fellin et al., 2004; Fiacco and McCarthy, 2004). The experimental design has been progressively improved to rule out neuronal sources of glutamate, and two recent studies now provide compelling evidence that astrocytic glutamate release can influence the strength of synaptic transmission at nearby synapses. Jourdain et al. (2007) showed that strong repetitive depolarization of an astrocyte by current pulses through a patch pipette increases the frequency of spontaneous AMPA receptor-mediated miniature EPSCs (mEPSC) recorded in nearby dentate granule cells. Importantly, this effect was not seen following pulses delivered extracellularly to the neuropil and was insensitive to tetrodotoxin, but was prevented by direct astrocytic infusion of the active light chain of the tetanus toxin protease (TeNTLC), which had been shown previously to prevent exocytosis by destroying a component of the SNARE complex, and by NMDA receptor blockade. These and other supporting results suggest that Ca2+-evoked glutamate release from a strongly depolarized astrocyte acts on presynaptic NMDA receptors to increase the probability of transmitter release. They went on to show a role for the Gq-coupled P2Y1 receptor, which is robustly expressed by dentate astrocytes. Their key experiment was to demonstrate that introduction of the Ca2+ chelator BAPTA into astrocyte cytoplasm was sufficient to abolish the P2Y1 receptor-mediated increase in mEPSC frequency in nearby neurons. Repetitive stimulation of the perforant path elicited Ca2+ signals in molecular layer astrocytes that could be reduced in amplitude by antagonists of both metabotropic glutamate or P2Y1 receptors. Altogether, this study builds a strong case for dentate gyrus astrocytes responding to intense perforant path activation by releasing a substance that activates NMDA receptors on presynaptic perforant path terminals, which in turn potentiates synaptic transmission by increasing the probability of transmitter release (Figure 1).
Figure 1. Integrated Control of Synaptic Release Probability by Astrocytes in Dentate Molecular Layer.
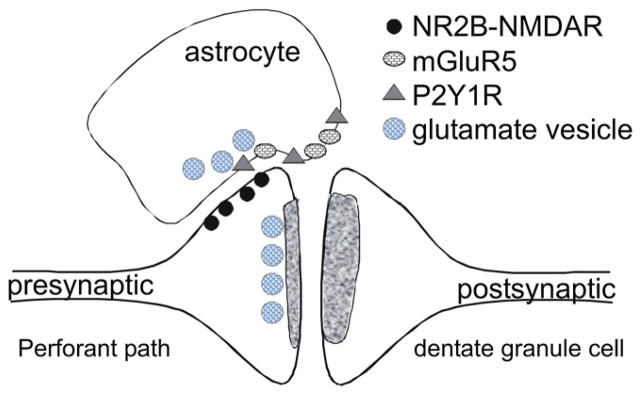
Immunoelectron microscopy suggests the existence of NR2B-containing NMDA receptors on perforant path terminals opposing astrocytic membranes that contain vesicles close to the astrocytic plasma membrane (Jourdain et al., 2007). Cell-derived ATP and glutamate act on two astrocytic Gq-coupled GPCRs, mGluR5 and P2Y1R, to increase [Ca2+]i and promote astrocytic release of glutamate into the extrasynaptic space. The resulting activation of presynaptic NMDA receptors appears to contribute to frequency facilitation at perforant path synapses.
The second study by Perea and Araque (2007) showed that an astrocytic Ca2+ transient induced by photostimulation of astrocytes loaded with caged Ca2+ was associated with increased EPSC amplitude in nearby CA1 pyramidal cell synapses, which was in turn caused by increased probability of transmitter release rather than a postsynaptic modulation. Important controls confirmed that light flashes did not modify synaptic transmission if (1) the pipette used to deliver the caged Ca2+ was placed in the neuropil rather than an astrocyte, (2) the cage was omitted, or (3) the delivery pipette also contained the light chain of TeNT. Interestingly, although the astrocytic Ca2+ surge was virtually immediate after photostimulation, synaptic efficacy rose gradually over about 10 min. The intervening steps within the latent period are unknown.
In both studies discussed above, intense astrocytic Ca2+ elevations were followed by increased local presynaptic transmitter release, but only in about one-third of tested synapses. Fiacco et al. (2007) took a different approach to restrict Ca2+ transients to astrocytes. They created a transgenic mouse that expresses a foreign (non-brain) Gq-coupled receptor (MrgA1) selectively in astrocytes. Activation of the MrgA1 receptor by the FLRFa peptide elicited robust Ca2+ signals in most (80%–90%) astrocytes in hippocampal slices, but these Ca2+ transients restricted to astrocytes were not associated with measurable changes in mEPSC amplitude or frequency in nearby CA1 pyramidal cells (13 neurons from 13 slices were studied). Although no effects were observed on spontaneous synaptic currents, to understand the potential role of astrocytic Ca2+ signaling in epilepsy, it will be important to explore synaptic plasticity in MgrA1 mice. Fiacco et al. (2007) concluded from this study that Ca2+ elevation, per se, is not a sufficient stimulus for astrocytic glutamate release.
Given these disparate findings, one wonders whether an un-identified variable determines whether an astrocytic Ca2+ transient will influence local synaptic transmission. For example, Ca2+ elevation might act in concert with other signaling pathways (e.g., MAPK pathways) to trigger glutamate release but might not be sufficient by itself. Whereas P2Y1 and mGluR5 receptors are known to engage multiple signaling pathways (Peavy et al., 2002; Fam et al., 2003; Neary et al., 2003; Edling et al., 2007), to date, MrgA1 is only known to signal via Gq/11 (Han et al., 2002). In sum, it is clear that Ca2+-dependent glutamate release from astrocytes can, under certain conditions, influence the strength of nearby excitatory synapses in the hippocampus. This exciting development begs the next question.
Does Astrocytic Glutamate Release Synchronize Neuronal Activity?
Astrocytic glutamate release can thus enhance nearby excitatory synaptic transmission, but the specific role of this process in epilepsy has not been defined. It was recently proposed that slow inward currents (SICs) recorded in hippocampal neurons are caused by astrocytic glutamate release (Angulo et al., 2004; Fellin et al., 2004; Tian et al., 2005) and, moreover, that SICs represent the “paroxysmal depolarizing shift” (PDS) underlying an interictal burst (Tian et al., 2005). Indeed, many of the in vitro hippocampal models of synchronous epileptiform bursting or electrographic seizures that have been studied for decades are associated with the appearance of SICs in hippocampal pyramidal neurons (Tian et al., 2005). Although the SIC itself has a similar time course as the PDS, it almost certainly reflects an entirely distinct event. That is, the hallmarks of SICs are (1) resistance to TTX, (2) sensitivity to blockade of NMDA receptors, and (3) synchrony over short distances (<100 μm). By contrast, PDS differ from SICs in being (1) blocked by TTX, (2) truncated but not eliminated by NMDA receptor blockers, and (3) synchronous over many millimeters of brain tissue (Korn et al., 1987; Fellin et al., 2006). With respect to the sensitivity to NMDA receptor blockers in particular, PDS, interictal-like burst firing, and more prolonged seizure-like events can be produced by a large number of conditions, which are variably sensitive to NMDAR blockers. For example, D-APV truncates but does not suppress PDS caused by GABAA receptor blockade (Dingledine et al., 1986), whereas PDS in low Ca2+ are insensitive to D-APV (Heinemann et al., 1985). Taken together, these findings support the conclusion that the PDS and SIC are distinct events.
Although astrocyte-induced SICs in nearby pyramidal neurons are not epileptiform bursts per se, they represent a very interesting, newly recognized synchronizing mechanism that appears to be particularly prominent in juveniles. Using paired field potentials or cellular Ca2+ recordings, three groups (Angulo et al., 2004; Fellin et al., 2004; Tian et al., 2005) showed that SICs are synchronous only over short distances of ~100 μm. This is a key finding and recalls the observation that individual astrocytes have spatial exclusivity over ~60 μm, within which other astrocytes are virtually excluded (Bushong et al., 2002). The sphere of influence of a modest cluster of two to three astrocytes is even smaller than that of microcircuits (Grillner et al., 2005) but seems well suited to mediate highly local neuron synchrony. Ca2+ uncaging in a single astrocyte can be followed by SICs in nearby neurons with a latency as low as 400 ms (Tian et al., 2005), although more characteristically the latency is several seconds. Given their long and variable latency, SICs seem better suited to elevating the general excitability of a local neuron population rather than being a component of widespread synchronous firing.
SICs were proposed to be caused by astrocytic release of glutamate (Angulo et al., 2004; Fellin et al., 2004; Tian et al., 2005). Albeit a remote possibility, neuronal alternatives to an astrocytic source of glutamate, such as release of glutamate from nonsynaptic (dendrites or soma) sites (cf. Zaidi and Matthews, 1999) or release from injured neurons, have not been entirely ruled out experimentally. Hippocampal slices often require some type of conditioning before SICs appear, for example multiple stimulus trains (Fellin et al., 2004), multiple puffs of glutamate (Xu et al., 2007), or prolonged exposure to DHPG or altered ionic conditions. Such conditioning treatments invariably induce astrocyte swelling, and the possibility that swelling or the consequent shrinkage in extracellular space is a prerequisite for SICs must be considered. Hippocampal slices are typically incubated in a holding chamber for approximately an hour before use. This might be long enough to initiate reactive gliosis in response to injury during the cutting process. Astrocyte swelling elicits a homeostatic volume-control mechanism that involves the release of glutamate, chloride, and other anions through volume-sensitive organic anion channels (Anderson and Swanson, 2000). This process might contribute to the variability in observation of SICs in different laboratories (cf. Fiacco et al., 2007). Indeed, Fiacco et al. (2007) reported that hypo-osmotic medium reliably elicited SICs, even in mice that lacked the IP3R2 receptor, which is required for intracellular Ca2+ release in astrocytes. Bafilomycin, which blocks vesicular transmitter release in neurons, did not prevent SICs in the IP3R2 knockout mice. Collectively, these results demonstrate that SICs can be produced in pathologic conditions involving cell swelling and do not necessarily require vesicular neurotransmitter release from neurons or IP3 receptor-dependent Ca2+ elevation in astrocytes.
In slices bathed in Mg-free, picrotoxin-containing ACSF, SICs were observed many seconds after an LTP-like stimulus train delivered to the Schaffer collateral input or after perfusion of hippocampal slices with the group I mGluR agonist DHPG (Angulo et al., 2004; Fellin et al., 2004). DHPG-evoked SICs were reduced in amplitude by the NR2B-selective NMDA receptor antagonist ifenprodil and were resistant to tetrodotoxin and to a tetanus toxin fragment that blocks excitatory synaptic transmission. A similar phenomenon, which is TTX resistant and variably blocked by NMDA receptor antagonists, can be triggered in CA1 pyramidal cells by the broad-spectrum potassium channel blocker 4-aminopyridine, by medium lacking Mg2+, by the GABA receptor blockers penicillin and bicuculline, and by Ca2+-free medium (Tian et al., 2005).
To summarize, the basic finding is that a substance, presumably glutamate, aspartate, or D-serine, via an action potential-independent process, can be released from astrocytes in sufficient quantities to elicit NMDA receptor-mediated short-range synchronous depolarizations of CA1 pyramidal neurons. The role of SICs in epilepsy is uncertain, although the most intriguing feature of this astrocyte-driven phenomenon is the induction of highly local, microsynchronous firing among populations of two to four adjacent neurons. Thus, whereas SICs do not themselves represent interictal bursts for reasons discussed above, they are well poised to produce the “grandfather neuron,” which initiates such bursts in epileptic tissue that is already prone to excitability.
Glial Glutamate Transporters in Epilepsy
Five subtypes of glutamate transporter have been cloned: EAAT1 (known as GLAST in rodents), EAAT2 (GLT-1 in rodents), EAAT3 (or EAAC-1), EAAT4, and EAAT5. Two glial-specific transporters are expressed in astrocytes, EAAT1/GLAST and EAAT2/GLT-1, which are largely responsible for glutamate clearance from the extracellular space (Rothstein et al., 1994; Lehre et al., 1995). The glial transporters appear to account for the majority of glutamate uptake in the brain, as judged by studies employing mice with genetic deletion (Tanaka et al., 1997) or antisense oligonucleotide-mediated inhibition (Rothstein et al., 1996) of GLT-1. Glutamate clearance by transporters undoubtedly plays a critical role in limiting extrasynaptic glutamate receptor activation.
Several experiments in knockout mice suggest that impaired glutamate uptake by astrocytes has the potential to contribute to the development of seizures. Mice with genetic deletions of the astroglial transporter GLT-1/EAAT2 develop spontaneous seizures, which suggests that GLT-1 is critical for normal glutamatergic transmission in the CNS (Tanaka et al., 1997). Yet, the same study showed that the knockdown of GLT-1 with anti-sense oligonucleotides does not result in the development of seizures. Additional experiments with conditional transporter knockouts are needed to gain a better understanding of how the regulation of extracellular glutamate contributes to seizures and epilepsy.
Whether glutamate transporters are altered in human epilepsy is controversial. Tessler et al. (1999) and Eid et al. (2004) found no change in transporter expression in resected tissue from temporal lobe epilepsy patients. This stands in contrast to the work of Mathern et al. (1999), who demonstrated regionally specific changes in EAAT1-3 expression in the hippocampus in temporal lobe epilepsy. Proper et al. (2002) examined transporter expression in sclerotic and nonsclerotic tissue and found that EAAT3, a glutamate transporter with glial and neuronal distribution, was increased in both groups. There are several possible explanations for the different observations among researchers, including differences in tissue processing between groups, in disease severity, or epileptogenesis development. It is unknown whether changes in transporters represent causative or compensatory changes during epileptogenesis.
Functionally Linked Water and Potassium Homeostasis
Changes in ECS volume or extracellular potassium level powerfully regulate the excitability of brain tissue. As with any closed system, when neuronal and/or glial cells of the CNS swell, extracellular space inevitably shrinks. A reduction in ECS volume produces hyperexcitability and enhanced epileptiform activity (Dudek et al., 1990). Increasing ECS volume, on the other hand, has the opposite effect, attenuating hyperexcitability in hippocampal slices exposed to high physiological levels of extracellular potassium or zero-calcium bathing medium (Traynelis and Dingledine, 1989; Dudek et al., 1990). Moreover, electrolyte imbalances are often associated with seizures. This is especially true in hypo-osmolar situations such as hyponatremia (Saly and Andrew, 1993) or overhydration, which causes a rapid drop in extracellular osmolarity (Manley et al., 2004). We focus attention here on the fact that glial volume largely regulates the size of the ECS (Sykova, 2005) and thereby controls both the concentration and diffusion rate of extracellular transmitters and ions as well as the strength of ephaptic (electric field) communication among neurons. Decreasing ECS volume magnifies the effects of transient changes in extracellular ion and/or transmitter concentrations. When the ECS volume shrinks, ephaptic interactions among tightly packed neurons would increase due to an increase in the ohmic resistance of the ECS. Release of transmitters from astrocytes via volume-sensitive anion channels could also increase as these cells swell.
Aquaporin 4 and Kir4.1
The underlying mechanisms by which changes in ECS osmolarity affect brain excitability were not obvious from initial studies. Most cells, including glia, have effective regulatory mechanisms to move ions and water between extracellular and cytoplasmic compartments (Olson and Kimelberg, 1995; Holthoff and Witte, 1996). Recent attention has pointed to aquaporins and certain potassium channels as key mediators of ECS volume. The aquaporins are a family of at least 11 integral membrane proteins in mammals that mediate constitutive and regulated transport of water across cellular membranes in many organs, including the brain (Amiry-Moghaddam and Ottersen, 2003). In the brain, the water channel aquaporin 4 (AQP4) is predominantly, if not exclusively, expressed in glia (Nielsen et al., 1997). AQP4 protein in astrocytes faces the neuropil but is especially concentrated at peri-vascular endfeet (Nielsen et al., 1997; Nagelhus et al., 2004) (Figure 2, left). This localization pattern makes AQP4 well suited to mediate the bidirectional flow of water between brain extracellular space and blood and consequently to regulate the osmolarity of the interstitial fluid surrounding neurons in the brain. Indeed, mice lacking AQP4 have decreased levels of brain water following water intoxication and focal cerebral ischemia (Manley et al., 2000) and impaired clearance of water in models of vasogenic edema (Papadopoulos et al., 2004). These observations demonstrate an important role for AQP4 in brain water transport under pathological conditions. However, other than sensorineural deafness, AQP4 knockout mice have no obvious CNS abnormalities under normal conditions (Manley et al., 2004).
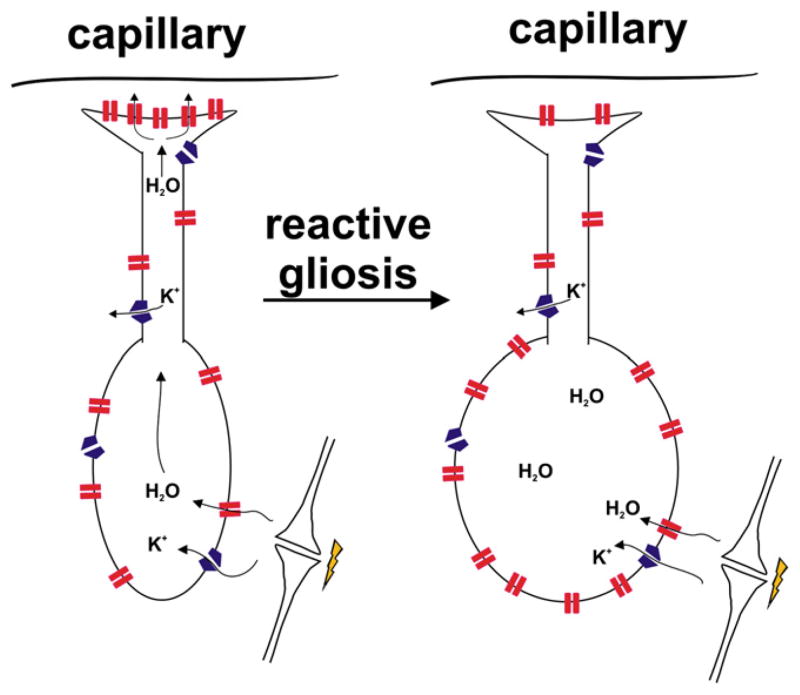
Figure 2. Hypothesis for Role of Astrocytes in Water Balance Dysfunction in Epilepsy.
The left panel depicts a typical bipolar brain astrocyte with processes in the neuropil (bottom) and endfeet near the perivascular space (top). The distribution of aquaporin 4 water channels (red) and Kir4.1 potassium channels (blue) is shown. Following neuronal activity, the potassium taken up into astrocytes via Kir4.1 is accompanied by water entry through AQP4 to maintain osmotic balance. Excess water may be dumped into the perivascular space by AQP4. In the epileptic state (right panel), there is a partial redistribution of AQP4 away from perivascular endfeet to the neuropil. The predicted consequences of this redistribution include enhanced entry of water into the neuropil but impaired egress of water into perivascular space, leading to astrocytic swelling and reduced interstitial space volume and thus enhanced ephaptic interactions among neurons.
The inwardly rectifying K+ channel, Kir4.1, is abundantly expressed in cortical astrocytes, where it directly or indirectly helps set a very negative resting potential (Kucheryavykh et al., 2007; Djukic et al., 2007). In the brain, Kir4.1 protein and transcripts are found in ~50% of astrocytes (Higashi et al., 2001; Schroder et al., 2002). Like AQP4, Kir4.1 is also polarized in brain astrocytes, being concentrated at astrocytic endfeet near the perivascular space (Higashi et al., 2001) and subpial surface on one end and neuropil on the other (Figure 2, left). Several key observations support the hypothesis that AQP4 and Kir4.1 work together to regulate potassium and water levels in the ECS. First, AQP4 and Kir4.1 coimmunoprecipitate and colocalize to the same subcellular regions (Nagelhus et al., 2004). Second, AQP4 null mice have slower K+ clearance but no difference in peak extracellular potassium concentration following electrical stimulation of the cortex (Binder et al., 2006). Correspondingly, these animals also showed a significant increase in the duration of stimulus-evoked seizures. These data support the hypothesis that AQP4 and Kir4.1 cooperate in the clearance of K+ and water after neural activity. Because Kir4.1 faces capillaries and [K+]o is higher in blood than CSF, Kir4.1 might function to import K+ from the blood into the brain’s astrocyte syncytium. In this view, the density of AQP4 and Kir4.1 channels on perivascular endfeet and the rate of potassium import into the brain from blood would be primary determinants of the ECS. Furthermore, blood vessel endothelial cells are not very permeable to potassium, which helps protect the brain. Unlike vitreous fluid in the eye (Newman et al., 1984), blood is probably not a major sink for distributing excess potassium in the brain. Potassium clearance in hippocampal CA1 is delayed in slices from AQP4-deficient mice (Amiry-Moghaddam et al., 2003), which reinforces the idea that water flux through glial endfeet is necessary for efficient potassium buffering. For these reasons, a primary function of Kir4.1 may be to act in concert with AQP4 to regulate ECS volume.
Although it has been known for quite some time that neuronal excitability is sensitive to both osmolarity and the size of the extracellular space, emerging insights into the molecular mechanisms by which extracellular volume is controlled by astrocytes give rise to an intriguing and novel hypothesis for the development of hyperexcitability after status epilepticus or traumatic brain injury (Figure 2). During normal periods of activity, much of the potassium released into the ECS by spiking neurons is taken up by astrocytes and neurons. The notion is that normally potassium uptake is accompanied by water influx via AQP4 into astrocytes in the perisynaptic space and then excess water is dumped via AQP4 at perivascular endfeet. By this means, focal glial swelling and thus ECS shrinkage during hyperactivity could normally be minimized. During seizures, there is focal swelling in the area of the seizure focus (Traynelis and Dingledine, 1989; Hattori et al., 2003; Binder et al., 2006). In sclerotic human hippocampi, AQP4 is redistributed from perivascular to perisynaptic space (Eid et al., 2005), with an overall increase in AQP4 expression (Lee et al., 2004). The effect of this redistribution would plausibly be to both enhance water entry near synapses and slow water egress distantly (Figure 2, right), leading to local astrocyte swelling with accompanying restriction in neuropil ECS.
Gap Junctions
Gap junctions consist of aggregates of connexin proteins that form functional channels between adjacent cells (Unger et al., 1999). Astrocytic processes are coupled through gap junctions to form large intercellular networks, which allows astrocytes to disperse small molecules such as K+ or glutamate and therefore prevent their extracellular accumulation during neuronal firing. However, a recent study weighs against the old notion that K+ buffering is primarily mediated by redistribution of K+ through gap junctions in the glial syncytium (Wallraff et al., 2006). Connexins 30 and 43 are required structural proteins of astrocyte gap junctions (Nagy et al., 2004). Comparison of wild-type mice with connexin-43 and connexin-30 double knockouts shows that under most conditions the absence of gap junctions had little effect on the spatial decay of K+ in the CA1 stratum radiatum following antidromic spikes; only maximal stimulation led to increased extracellular K+ levels in knockout compared to wild-type mice. These results strongly suggest that gap junctions in the glia syncytium play a negligible role in the spatial redistribution of potassium. However, hippocampal slices from mice lacking gap-junction-coupled astrocytes exhibited spontaneous interictal bursts in area CA1 (Wallraff et al., 2006), which suggests that the loss of gap-junction-connected astrocytes does indeed result in neuronal hyperexcitability. Whether hyper-excitability is due to the inability to redistribute K+ through gap junctions remains to be seen. It is not known whether other properties of glial cells are changed by knockout of the gap junction proteins and what effects this might have on neuronal-astrocyte signaling and neurotransmission.
Do Reactive Astrocytes Oppose or Exacerbate Seizure Activity?
Almost all insults to the brain, including prolonged seizures, result in reactive gliosis, which is characterized by severe morphological and biochemical changes of pre-existing astrocytes (Binder and Steinhäuser, 2006; Ravizza et al., 2008) as well as the generation of new astrocytes from stem cells (Borges et al., 2006). There are no reports of the global impact of selectively preventing reactive gliosis in epilepsy, but studies of ischemic or traumatic brain injury could be relevant. For example, Myer et al. (2006) used a conditional transgenic mouse in which reactive, proliferating astrocytes were ablated by gangciclovir; they measured neurodegeneration after moderate or severe controlled cortical impact as a model of traumatic brain injury (TBI). The ablation of reactive astrocytes carried out over the week following moderate TBI markedly exacerbated neural damage and increased leukocyte infiltration into the affected cortical area. The consequence of gradually ablating proliferating astrocytes was similar after mild stab injury to the spinal cord (Faulkner et al., 2004) or cortex (Bush et al., 1999). These studies demonstrate a protective function of reactive astrocytes during the week after mild injury, but do not address the acute effects shortly after injury or the responsible mechanisms.
The entirety of changes that occur in astrocytes during epilepsy, as well as the cellular and molecular processes responsible for astrogliosis, are still poorly understood. Astrocytes in the region of brain inflammation undergo hypertrophy of cellular processes, especially near the soma, which accentuates their stellate morphology and is associated with upregulation of the intermediate filament proteins vimentin, nestin, and glial fibrillary acidic protein. Many other proteins are upregulated in reactive astrocytes, including mGluR3, mGluR5, mGluR8 (Aronica et al., 2000; Tang et al., 2001; Tang and Lee, 2001; Notenboom et al., 2006), the proinflammatory proteins COX2 and CXCR4 (Lee et al., 2007), nerve growth factor and its receptors (Sofroniew et al., 2001), estrogen receptor α (Tokuhara et al., 2005), a variety of cytokines, including transforming growth factor β, tumor necrosis factor α, interleukins 1, 4, 6, and 10, and enzymes including iNOS and cathepsins D and G (Ridet et al., 1997; Ledeboer et al., 2000; Hulshof et al., 2002). Glutamine synthase is prominently downregulated in reactive astrocytes (Eid et al., 2004). Do the functional changes in reactive astrocytes expected from these expression changes modify seizure susceptibility?
Pro- and Anticonvulsant Roles of Cytokines Released by Reactive Astrocytes
Astrocytes have dynamic functions and can produce many pro-and anti-inflammatory molecules. The production of TNFα and IL-1β by astrocytes can lead to beneficial or detrimental outcomes depending on which receptors are activated and the timing of expression (Wilde et al., 2000; Bernardino et al., 2005; Vezzani and Granata, 2005). Work by Vezzani et al. (2000) demonstrated that transgenic overexpression in astrocytes of IL-1Ra, a natural antagonist of IL-1β, significantly delays the onset and reduces the duration of generalized seizures in mice challenged with bicuculline; these effects of IL-1Ra were absent in the IL-1R1 knockout mouse, implying that activation of IL-1R1 by IL-1β can reduce seizure threshold. Seizures rapidly induce production of both IL-1β and IL-1Ra in astrocytes (De Simoni et al., 2000). These findings together point to an important role of astrocytic IL-1β in regulating seizure susceptibility. Likewise, the cytokines IL-10, IL-4, and transforming growth factor β, which are all produced by astrocytes after various insults (Ledeboer et al., 2000; Rasley et al., 2006), reduce expression of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor α (TNFα), as well as nitric oxide (Ledeboer et al., 2000).
TNFα, a pleiotropic cytokine produced by both astrocytes and activated microglia, is implicated in diverse biological events from inflammation to proliferation depending on the cell target, exposure duration, and receptor activated. TNFα appears to cause cell damage through activation of TNFR1, which contains an intracellular “death domain.” Activation of TNFR2, on the other hand, reduces the intensity of acute kainate-evoked seizures (Balosso et al., 2005), suggesting that the balance between activation of TNFR1 and TNFR2 will determine whether the net effect of TNFα release is pro- or anticonvulsant. TNFα also appears to promote neuronal excitability, as described next.
Potentiation of Astrocytic Glutamate Signaling in Reactive Astrocytes
There is no question that astrocytes can release glutamate and that under certain pathological conditions (e.g., Fiacco et al., 2007) this phenomenon is more noticeable. Curiously, 20–40 s of continuous astrocyte stimulation appears to be necessary to increase spontaneous mEPSC frequency in granule cells, even though the astrocytic Ca2+ signal peaks in 10 s or less (Jourdain et al., 2007). A similarly long latency between astrocyte Ca2+ signals and CA1 pyramidal cell SICs has typically been reported (Angulo et al., 2004; Fellin et al., 2004; Tian et al., 2005), suggesting that a series of steps after Ca2+ elevation may intervene before productive glutamate release. What could these intervening steps be? Clues can be drawn from studies of glutamate release from cultured astrocytes.
Studies by Bezzi and colleagues (Bezzi et al., 1998, 2001) identified TNFα as a prominent glutamate release modulator in cultured astrocytes. TNFα released into the medium by reactive astrocytes and activated microglia activates astrocytic TNFR1 receptors, which leads to production of a prostaglandin; the formed prostaglandin in turn strongly increases glutamate release from astrocytes in response to activation of Gq-coupled GPCRs in astrocytes. A similar situation obtains for synergy between TNFR1 activation and activation by released ATP of Gq-coupled P2Y1 receptors on astrocytes (Domercq et al., 2006). In this study, it was further shown that glutamate release evoked by P2Y1 receptor activation was largely suppressed in astrocytes isolated from TNFR1 null mice or in astrocytes treated with cyclooxygenase inhibitors or the Ca2+ chelator BAPTA-AM. TNFα release was preserved in each of the above conditions but could, however, be reduced by inhibitors of ERK-type MAP kinase, suggesting that this MAP kinase pathway is upstream of TNFα release. These findings together cement the roles of TNFα and a prostanoid (possibly PGE2 or PGD2) as key boosters of astrocytic glutamate release. The induction of mGluR5 in reactive astrocytes could synergize with this amplification system. The overall picture is that reactive astrocytes or activated microglia, via release of TNFα and subsequently PGE2, amplify astrocytic glutamate release in response to astrocytic Gq-coupled GPCR activation. This “two-hit” hypothesis (Figure 3) for priming of astrocytic glutamate release by activated microglia and reactive astrocytes presents a sinister feature of sclerotic brain tissue that could, by enhancing SIC frequency, depolarize neurons in the affected regions and thus promote seizure initiation.
Figure 3. Hypothesis for Enhanced Ability of Reactive Astrocytes to Release Glutamate.
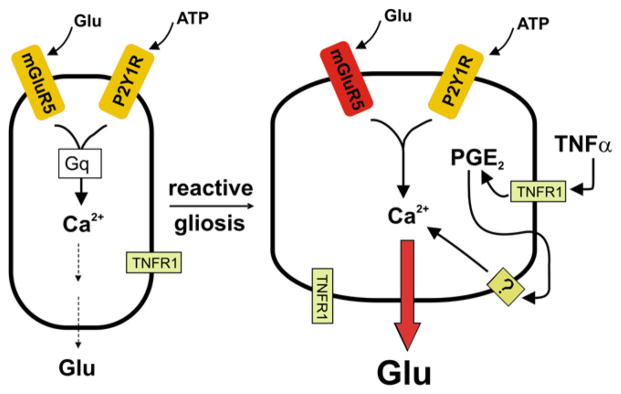
(Left) Scheme for control of astrocytic glutamate release by two G protein-coupled receptors. (Right) In the epileptic brain, mGluR5 is upregulated, and activated microglia as well as reactive astrocytes release TNFα, which acts on TNFR1 receptors in a pathway that promotes prostaglandin formation. Prostaglandin in turn activates a Gq-coupled prostanoid receptor that boosts intra-astrocyte Ca2+ release and thus astrocytic glutamate release. Hypothesis adapted from work reported by Bezzi et al. (1998, 2001) and Domercq et al. (2006).
Glutamine Synthetase Downregulation in Sclerotic Brain Could Promote Seizures
Sclerotic brain tissue removed during epilepsy surgery is characterized by downregulation of glutamine synthetase in astrocytes (Eid et al., 2004). In the brain, glutamine synthetase is expressed nearly exclusively by astrocytes and oligodendroglia (Martinez-Hernandez et al., 1977). Following its synaptic release from the terminals of glutamatergic neurons, glutamate is transported into astrocytes where it is converted by glutamine synthetase into glutamine; glutamine in turn is exported and taken up by neurons, where it is converted back to glutamate by mitochondrial glutaminase. This glutamine cycle normally serves as a major mechanism for ammonia detoxification in the brain and also as a buffered reservoir of a precursor (glutamine) for glutamate and GABA synthesis (Hassel and Dingledine, 2006). Systemic administration of a glutamine synthetase inhibitor causes severe seizures in laboratory animals (Folbergrova et al., 1969; Szegedy, 1978), which supports the idea that impaired glutamate to glutamine cycling could contribute to seizures in the epileptic brain. Indeed, glutamine-glutamate cycling appears to be slowed in sclerotic human hippocampus removed during epilepsy surgery (Petroff et al., 2002).
There are several potential mechanisms by which reduced glutamine synthetase could promote seizures. First, downregulation of glutamine synthetase could result in the accumulation of glutamate in astrocytes and thus its buildup in the extracellular space, because rapid metabolism of intracellular glutamate appears to be needed for glutamate uptake into astrocytes (Otis and Jahr, 1998). Second, because vesicular GABA levels are in equilibrium with intraterminal glutamate concentration, which in turn is partially dependent upon glutamine transport into inhibitory neurons, downregulation of glutamine synthetase could partially deplete inhibitory synaptic terminals of GABA and thus impair GABAergic inhibition. Evidence for this notion was recently provided by Liang et al. (2006), who showed that inhibition of glutamine synthesis by methionine sulfoximine reduced the amplitude of IPSCs in CA1 pyramidal neurons during repetitive stimulus trains, an effect that was traced to reduced quantal content rather than lower release probability. Importantly, the effect of the inhibitor could be overcome by replacing the lost glutamine with bath perfusion. A similar effect of methionine sulfoximine was not seen on evoked EPSCs (Kam and Nicoll, 2007), although in this study the synapses were not subjected to stimulus trains. Moving forward, it will be important to determine whether synaptic inhibition is reduced in the vicinity of reactive astrocytes and whether reversal of this effect occurs when GS is selectively reintroduced into reactive astrocytes.
Impaired Water and Potassium Balance in Reactive Astrocytes
Comparison of hippocampal slices obtained from patients with or without mesial temporal sclerosis shows that K+ buffering is impaired in sclerotic tissue (Heinemann et al., 2000). In the pilocarpine epilepsy model, the ability of Ba2+ (a blocker of all known Kir) to increase baseline [K+]o was dramatically lower (Gabriel et al., 1998), which suggests that Kir currents in astrocytes are reduced several days after status epilepticus. This fits well with a patch-clamp study that provided direct evidence for smaller Kir currents in astrocytes from epileptic hippocampus (Schroder et al., 1999; Hinterkeuser et al., 2000). Together, these data associate impaired K+ buffering with reduced expression of functional Kir in the sclerotic condition but do not demonstrate that Kir themselves are the major K+ clearance route. Impaired astrocytic K+ buffering would be expected to result in slower K+ clearance, which in turn could lower seizure threshold and thereby contribute to seizure generation. The prediction is that in epileptic tissue redistribution of glial AQP4 away from perivascular membranes perturbs water flux and thus impairs K+ buffering. Under these conditions, a normally unremarkable level of neuronal activity could exacerbate glial swelling and thus ECS shrinkage, which would increase the likelihood of seizures. Whereas the data are consistent with the idea that AQP4 and Kir4.1 participate in clearance of K+, additional studies are required to clarify the expression and regulation of both AQP4 and Kir4.1 in the hippocampus and their changes during epileptogenesis. In sum, an impaired ability of reactive glia to redistribute water and thereby maintain osmotic balance throughout brain microdomains could contribute to the hyperexcitable epileptic state.
Summary and Open Issues
Astrocytes in the healthy brain mediate glutamate reuptake, regulate the ionic environment and interstitial volume, and serve as a component of the neurovascular unit that controls blood-brain barrier permeability (Abbott et al., 2006). Although reactive astrocytes in the epileptic focus are well known to undergo extensive morphological and physiological changes that modify these three overarching functions, the implications for epilepsy are just emerging. Moreover, we have only a primitive understanding of astrocyte heterogeneity and its consequences.
In models of ischemic or traumatic brain injury, conditional ablation of proliferating astrocytes increases neuronal injury and increases infiltration of the damaged region by leukocytes, which strongly point to a protective function of proliferating astrocytes in these two conditions. However, not all reactive astrocytes have undergone recent cell division. Additionally, environmental cues for astrocytes in the epileptic brain could well differ from those in the ischemic brain, so generalizing results from ischemia to epilepsy may not be prudent. At least two lines of evidence suggest that reactive astrocytes might contribute to the hyperex-citable condition. First, the redistribution of AQP4 in the epileptic state, away from perivascular membranes toward neuropil, should create a situation allowing rapid astrocytic swelling in neuropil with consequent shrinkage in ECS and enhanced ephaptic interactions among tightly packed neurons (Figure 2). Second, neuroinflammatory signaling pathways engaged in reactive glia in epileptic foci may allow astrocytic glutamate signaling to become prominent enough to contribute to the hyperexcitable state (Figure 3). Although reactive astrocytes develop both pro- and antiepileptic properties, the preponderance of current evidence favors the former. Going forward, it would be valuable to determine whether ablation of proliferating astrocytes in status epilepticus models of epilepsy exacerbates or blunts neurodegeneration, changes in synaptic plasticity, leucocyte infiltration, cognitive impairment, and other features of the latent period that precede the appearance of spontaneous seizures.
Over the past few years, the consequences of Ca2+ signaling in astrocytes have received considerable attention. Astrocytes, particularly in pathological situations involving neuroinflammation, may be important active members of many synapses, their roles extending well beyond structural support or extracellular ion scavengers. The existence of astrocytic glutamate release, most convincingly demonstrated in tissue culture, is challenging the old view of astrocytes as simple support structures. In a context-dependent manner, release of glutamate from a single astrocyte can depolarize two to four adjacent neurons; such micro-synchrony undoubtedly elevates excitability of the involved circuits. Determining whether interictal bursts can be triggered in the epileptic brain by microsynchronous depolarizations will require devising ways to isolate the consequences of astrocytic glutamate release with normal synaptic transmission intact.
The act of preparing hippocampal brain slices necessarily injures the tissue and might therefore cause a variable degree of reactive astrogliosis. Given the clear demonstration that TNFα, which is prominently secreted from reactive astrocytes and activated microglia, can increase astrocytic glutamate release substantially (Bezzi et al., 2001; Domercq et al., 2006), the possibility that astrogliosis is essential for SICs and other manifestations of gliotransmission must be considered. This possibility can be explored simply by determining whether inhibitors of cyclooxygenase or TNFR1 prevent these phenomena in brain slices.
Insights gleaned from careful studies of the properties of reactive astrocytes suggest several novel targets for drug development, including allosteric potentiators of glutamine synthetase, regulators of AQP4 trafficking, interleukin 1 antagonists, and agonists or allosteric potentiators of TNFR2. Unraveling the specific pathologic and protective pathways in reactive astrocytes has begun, and much work remains, but today it is clear that astrocytes play prominent roles in information processing in the epileptic brain. These are, indeed, not your father’s astrocytes.
Acknowledgments
Supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award #U01NS058158) and an American Epilepsy Foundation Fellowship (J.W.). We thank Drs. Asheebo Rojas and James McNamara for comments.
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization, phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci USA. 2003;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport, review of properties, regulation, physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, Vezzani A, Malva JO, Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–6744. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha, amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhäuser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Borges K, McDermott D, Irier H, Smith Y, Dingledine R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Exp Neurol. 2006;201:416–427. doi: 10.1016/j.expneurol.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hynes M, King GL. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes, control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Obenaus A, Tasker JG. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat, importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci Lett. 1990;120:267–270. doi: 10.1016/0304-3940(90)90056-f. [DOI] [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–340. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus, possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Lee TS, Thomas MJ, Amiry-Moghaddam M, Bjornsen LP, Spencer DD, Agre P, Ottersen OP, de Lanerolle NC. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci USA. 2005;102:1193–1198. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Kalia LV, Salter MW. Differential frequency dependence of P2Y1- and P2Y2- mediated Ca 2+ signaling in astrocytes. J Neurosci. 2003;23:4437–4444. doi: 10.1523/JNEUROSCI.23-11-04437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006;26:9312–9322. doi: 10.1523/JNEUROSCI.2836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci. 2004;24:722–732. doi: 10.1523/JNEUROSCI.2859-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Passonneau JV, Lowry OH, Schulz DW. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969;16:191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Eilers A, Kivi A, Kovacs R, Schulze K, Lehmann TN, Heinemann U. Effects of barium on stimulus induced changes in extracellular potassium concentration in area CA1 of hippocampal slices from normal and pilocarpine-treated epileptic rats. Neurosci Lett. 1998;242:9–12. doi: 10.1016/s0304-3940(98)00012-3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Markram H, De Schutter E, Silberberg G, LeBeau FE. Microcircuits in action—from CPGs to neocortex. Trends Neurosci. 2005;28:525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Dingledine R. Glutamate. In: Siegel GJ, Albers RW, Brady ST, Price DL, editors. Basic Neurochemistry. 7. Burlington, MA: Elsevier; 2006. pp. 267–290. [Google Scholar]
- Hattori H, Matsuoka O, Ishida H, Hisatsune S, Yamano T. Magnetic resonance imaging in occipital lobe epilepsy with frequent seizures. Pediatr Neurol. 2003;28:216–218. doi: 10.1016/s0887-8994(02)00615-x. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Franceschetti S, Hamon B, Konnerth A, Yaari Y. Effects of anticonvulsants on spontaneous epileptiform activity which develops in the absence of chemical synaptic transmission in hippocampal slices. Brain Res. 1985;325:349–352. doi: 10.1016/0006-8993(85)90338-5. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Gabriel S, Jauch R, Schulze K, Kivi A, Eilers A, Kovacs R, Lehmann TN. Alterations of glial cell function in temporal lobe epilepsy. Epilepsia. 2000;41(Suppl 6):S185–S189. doi: 10.1111/j.1528-1157.2000.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol. 2001;281:C922–C931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhauser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Witte OW. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J Neurosci. 1996;16:2740–2749. doi: 10.1523/JNEUROSCI.16-08-02740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshof S, Montagne L, De Groot CJ, Van Der Valk P. Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia. 2002;38:24–35. doi: 10.1002/glia.10050. [DOI] [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G. Cultured astrocytes express proteins involved in vesicular glutamate release. Brain Res. 1997;750:41–47. doi: 10.1016/s0006-8993(96)00610-5. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Brevé JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lee TS, Eid T, Mane S, Kim JH, Spencer DD, Ottersen OP, de Lanerolle NC. Aquaporin-4 is increased in the sclerotic hippocampus in human temporal lobe epilepsy. Acta Neuropathol. 2004;108:493–502. doi: 10.1007/s00401-004-0910-7. [DOI] [PubMed] [Google Scholar]
- Lee TS, Mane S, Eid T, Zhao H, Lin A, Guan Z, Kim JH, Schweitzer J, King-Stevens D, Weber P, et al. Gene expression in temporal lobe epilepsy is consistent with increased release of glutamate by astrocytes. Mol Med. 2007;13:1–13. doi: 10.2119/2006-00079.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain, quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia. 2006;54:343–357. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manley GT, Binder DK, Papadopoulos MC, Verkman AS. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience. 2004;129:983–991. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase, glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system, cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–913. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells, high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notenboom RG, Hampson DR, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, de Graan PN. Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain. 2006;129:96–107. doi: 10.1093/brain/awh673. [DOI] [PubMed] [Google Scholar]
- Olson JE, Kimelberg HK. Hypoosmotic volume regulation and osmolyte transport in astrocytes is blocked by an anion transport inhibitor, L-644,711. Brain Res. 1995;682:197–202. doi: 10.1016/0006-8993(95)00368-z. [DOI] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Peavy RD, Sorensen SD, Conn PJ. Differential regulation of metabotropic glutamate receptor 5-mediated phosphoinositide hydrolysis and extracellular signal-regulated kinase responses by protein kinase C in cultured astrocytes. J Neurochem. 2002;83:110–118. doi: 10.1046/j.1471-4159.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Ramos-Mandujano G, Vazquez-Juarez E, Hernandez-Benitez R, Pasantes-Morales H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia. 2007;55:917–925. doi: 10.1002/glia.20513. [DOI] [PubMed] [Google Scholar]
- Rasley A, Tranguch SL, Rati DM, Marriott I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Lasker Basic Medical Research Award. The machinery and principles of vesicle transport in the cell. Nat Med. 2002;8:1059–1062. doi: 10.1038/nm770. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Saly V, Andrew RD. CA3 neuron excitation and epileptiform discharge are sensitive to osmolality. J Neurophysiol. 1993;69:2200–2208. doi: 10.1152/jn.1993.69.6.2200. [DOI] [PubMed] [Google Scholar]
- Schroder W, Hager G, Kouprijanova E, Weber M, Schmitt AB, Seifert G, Steinhauser C. Lesion-induced changes of electrophysiological properties in astrocytes of the rat dentate gyrus. Glia. 1999;28:166–174. [PubMed] [Google Scholar]
- Schroder W, Seifert G, Huttmann K, Hinterkeuser S, Steinhauser C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol Cell Neurosci. 2002;19:447–458. doi: 10.1006/mcne.2001.1080. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Sykova E. Glia and volume transmission during physiological and pathological states. J Neural Transm. 2005;112:137–147. doi: 10.1007/s00702-004-0120-4. [DOI] [PubMed] [Google Scholar]
- Szegedy L. Enzyme histochemical studies in the rat hippocampus, cerebellar cortex and brainstem motor nuclei during methionine sulphoximine convulsions. Cell Mol Biol Incl Cyto Enzymol. 1978;23:43–53. [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tang FR, Lee WL. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol. 2001;30:137–143. doi: 10.1023/a:1011939223872. [DOI] [PubMed] [Google Scholar]
- Tang FR, Lee WL, Yang J, Sim MK, Ling EA. Metabotropic glutamate receptor 8 in the rat hippocampus after pilocarpine induced status epilepticus. Neurosci Lett. 2001;300:137–140. doi: 10.1016/s0304-3940(01)01579-8. [DOI] [PubMed] [Google Scholar]
- Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience. 1999;88:1083–1091. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhara D, Yokoi T, Nakajima R, Hattori H, Matsuoka O, Yamano T. Time course changes of estrogen receptor alpha expression in the adult rat hippocampus after kainic acid-induced status epilepticus. Acta Neuropathol. 2005;110:411–416. doi: 10.1007/s00401-005-1071-z. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Role of extracellular space in hyper-osmotic suppression of potassium-induced electrographic seizures. J Neurophysiol. 1989;61:927–938. doi: 10.1152/jn.1989.61.5.927. [DOI] [PubMed] [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wilde GJ, Pringle AK, Sundstrom LE, Mann DA, Iannotti F. Attenuation and augmentation of ischaemia-related neuronal death by tumour necrosis factor-alpha in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–24197. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes, a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi ZF, Matthews MR. Stimulant-induced exocytosis from neuronal somata, dendrites, and newly formed synaptic nerve terminals in chronically decentralized sympathetic ganglia of the rat. J Comp Neurol. 1999;415:121–143. doi: 10.1002/(sici)1096-9861(19991206)415:1<121::aid-cne9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]