Abstract
Importance
It is estimated that more than half of those with serious mental illness smoke tobacco regularly. Standard courses of pharmacotherapeutic cessation aids improve short-term abstinence, but most who attain abstinence relapse rapidly after discontinuation of pharmacotherapy.
Objective
To determine whether smokers diagnosed with schizophrenia and bipolar disease have higher rates of prolonged tobacco abstinence with maintenance pharmacotherapy than with standard treatment.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled, parallel-group, relapse-prevention clinical trial conducted in 10 community mental-health centers. Of 247 smokers with schizophrenia or bipolar disease recruited from March 2008-April 2012, 203 received 12-weeks' open-label varenicline and cognitive behavioral therapy and 87 met abstinence criteria to enter the relapse prevention intervention.
Interventions
Participants who had 2 weeks or more of continuous abstinence at week 12 of open treatment were randomly assigned to receive cognitive behavioral therapy and double-blind varenicline (1 mg, 2 per day) or placebo from weeks 12 to 52. Participants then discontinued study treatment and were followed up to week 76.
Main Outcomes and Measures
Seven-day rate of continuous abstinence at study week 52, the end of the relapse-prevention phase, confirmed by exhaled carbon monoxide. Secondary outcomes were continuous abstinence rates for weeks 12 through 64 based on biochemically verified abstinence and weeks 12 through 76, based on self-reported smoking behavior.
Results
Sixty-one participants completed the relapse-prevention phase; 26 discontinued participation (7 varenicline, 19 placebo) and were considered to have relapsed for the analyses; 18 of these had relapsed prior to dropout. At week 52, point-prevalence abstinence rates were 60% in the varenicline group (24 of 40) vs 19% (9 of 47) in the placebo group (odds ratio [OR], 6.2; 95% CI, 2.2-19.2; P < .001). From weeks 12 through 64,45% (18 of 40) among those in the varenicline group vs 15% (7 of 47) in the placebo group were continuously abstinent (OR, 4.6; 95% CI, 1.5-15.7; P = .004), and from weeks 12 through 76,30% (12 of 40) in the varenicline group vs 11% (5 of 47) in the placebo group were continuously abstinent (OR, 3.4; 95% CI, 1.02-13.6; P = .03). There were no significant treatment effects on psychiatric symptom ratings or psychiatric adverse events.
Conclusions and Relevance
Among smokers with serious mental illness who attained initial abstinence with standard treatment, maintenance pharmacotherapy with varenicline and cognitive behavioral therapy improved prolonged tobacco abstinence rates compared with cognitive behavioral therapy alone after 1 year of treatment and at 6 months after treatment discontinuation.
Although tobacco smoking among adults has declined by 55% in the United States since 1965,1 smoking prevalence among adults with serious mental illness remains higher now than it was in the general population in 1965.2 Six million of the 11.4 million adults (53%) with serious mental illness smoke tobacco.3 Relatively small trials have reported pharmacologic cessation aids–including bupropion alone or combined with nicotine replacement therapy and varenicline–increase initial abstinence rates over behavioral treatment alone for smokers with schizophrenia and schizoaffective disorder, with mean abstinence rates of 24% at the end of 8 to 12 weeks of treatment vs 5% with placebo plus behavioral therapy, suggesting behavioral treatment alone is ineffective for smoking cessation in this population.4-11 Abstinence rates in pharmacotherapy treatment groups declined from 24% to 12% 3 months after discontinuing pharmacotherapy,12 with some trials reporting the majority of relapses occurring within 2 weeks of stopping the medication,8 suggesting a possible need for longer-term pharmacotherapy for prolonged tobacco abstinence in this population.
In a recent trial, 12 weeks of maintenance treatment with varenicline improved abstinence rates at 1 year in smokers without psychiatric illness who attained initial abstinence with varenicline.13 An open-label trial involving recently abstinent smokers with serious mental illness found that maintenance therapy with bupropion, dual nicotine replacement therapy, and cognitive behavioral therapy (CBT) for 1 year resulted in a relapse rate of 35%,14 less than half the 77% relapse rate reported 12 months after discontinuation of a similar 12-week intervention,8 suggesting this approach may be feasible and effective in smokers with serious mental illness. The purpose of this study was to evaluate the efficacy of 40 weeks of maintenance varenicline and CBT in smokers with serious mental illness who achieved abstinence with 12 weeks of open-label varenicline and CBT.
Methods
The study was approved and monitored by the institutional review boards at Massachusetts General Hospital and 9 other study sites and by an independent data and safety monitoring board. All participants provided written informed consent.
Study Patients
Participants were enrolled from March 2008 to April 2012 from 10 community mental health centers in Massachusetts, Michigan, New Hampshire, Indiana, Alabama, and Minnesota. Participants were outpatients, aged 18 through 70 years, with schizophrenia, schizoaffective disorder, or bipolar disorder who reported smoking 10 or more cigarettes per day for at least the prior year, had expired carbon monoxide levels higher than 9 ppm at screening, expressed willingness to take varenicline, agreed to attempt smoking cessation by setting a quit date within 4 weeks of enrollment, and were taking a stable clinically determined dose of antipsychotic (schizophrenia spectrum) or mood stabilizing (bipolar disorder) medication for 30 days or more before enrollment. Participants were required to continue an antipsychotic or mood stabilizing medication throughout the trial at a dose that could be adjusted by their prescribing physician. Exclusion criteria included current suicidal or homicidal ideation, hospitalization for suicidality in the prior 12 months, other active substance use disorder, or major depressive episode in the prior 6 months. The initial trial design included those with schizophrenia and schizoaffective disorder and was amended in 2010 to include those with bipolar disorder in order to broaden the participant group.
Design and Study Interventions
Open-Label, Smoking Cessation Phase
Participants were provided 0.5 mg of varenicline per day for 3 days, 0.5 mg twice a day for 4 days, then 1.0 mg twice a day for 11 weeks and were asked to attend 12 weekly, 1-hour, manualized group CBT sessions,14,15 and were asked to set a quit date between study weeks 4 and 5. The protocol for the open-label phase has been described previously.16
Double-Blind, Placebo-Controlled, Relapse-Prevention Phase
Participants in the open phase who met criteria for biochemically verified, 7-day, point-prevalence abstinence at weeks 11 and 12 were considered to be continuously abstinent for at least 14 days and were randomized to the relapse prevention intervention: to continue varenicline, 1.0 mg twice a day, or switch to identical-appearing placebo for weeks 12 through 52 in conjunction with a tapering schedule of relapse prevention–focused CBT. Seven-day point-prevalence abstinence was defined as self-report of smoking no cigarettes in the past 7 days, confirmed biochemically by expired carbon monoxide of less than 9 ppm. Randomization was conducted via centralized computer-generated random sequence performed by Massachusetts General Hospital research pharmacy staff members, who were not otherwise involved in the trial, in double-blind fashion, in blocks of 4, stratified by study site and by a single categorical predictor that was a combination of psychiatric disorder and type of antipsychotic medication (eMethods 1 in the Supplement), using a permuted block design with 1:1 ratio.
All participants in the relapse-prevention phase received the same tapering schedule of CBT that focused on relapse-prevention skills. Sessions were held weekly for the first month (4 sessions), biweekly for 2 months (3 sessions), and monthly (8 sessions), for a total of 15 sessions over the 40-week relapse-prevention period. The curriculum focused on understanding relapse, learning and applying cognitive and behavioral skills (eg, refusal skills, problem-solving, identifying and responding to permission-giving beliefs to smoke), and implementing personalized relapse-prevention plans for high-risk situations. Groups were supervised by licensed clinicians. Group therapist training included didactic presentations, role-play, rating of videotaped CBT sessions, and observed mock sessions. Therapist certification required a score of 4 to 5 (very good or excellent) on a 5-point single-item overall session quality rating of a mock CBT session. To ensure ongoing fidelity to the manualized treatment, tapes of group sessions conducted by each therapist were reviewed via biweekly group telephone supervision using a fidelity scale that assessed protocol adherence and therapist competence.
Follow-up Phase
At week 52, smoking cessation treatment was discontinued, and participants were followed up for biochemically verified abstinence and safety outcomes under double-blind conditions through week 64. Telephone follow-up at week 76 for self-report of smoking behavior from those who had achieved continuous abstinence from weeks 12 through 64 was added to the protocol after trial commencement to better evaluate the duration of continuous abstinence after discontinuation of maintenance treatment.
Assessments
Baseline assessments included chart review and clinical interviews for psychiatric diagnosis, smoking, and medical history. The Fagerstrom Test for Nicotine Dependence (FTND)17 scale was administered to assess severity of nicotine dependence, urine was collected for pregnancy status, cotinine levels, and drug screens.
Assessments conducted at every study visit, including each CBT visit and follow-up visit, included self-report of smoking behavior since the previous visit, expired carbon monoxide, and assessment of nicotine withdrawal symptoms with the Wisconsin Smoking Withdrawal Scale,18 depressive symptoms with either the Calgary Depression Scale for Schizophrenia19 for those with schizophrenia or the Montgomery-Asberg Depression Rating Scale20 for those with bipolar disorder, and the Young Mania Rating Scale21 for those with bipolar disorder. Adverse events were ascertained by general inquiry and by specific query about headache, nausea, vomiting, tachycardia, excitement, agitation, anxiety, insomnia, and irritability. Additional assessments were conducted at baseline; at study weeks 12, 18, 26, 38, 52, and 64; or at early termination, evaluated psychiatric symptomatology with the Brief Psychiatric Rating Scale22 and Schedule for the Assessment of Negative Symptoms.23 Assessments for health-related quality of life (12-Item Short Form Health Survey [SF-12]) were conducted at baseline and weeks 12, 52, and 64 and weight was measured at baseline and weeks 12, 18, 26, 38, 52, 56, and 64. A final assessment at week 76 included only self-report of smoking behavior. Assessors were trained to administer structured rating scales and interrater reliability was monitored throughout the trial (eMethods 2 in the Supplement).
Primary and Secondary Outcomes
The primary outcome was the 7-day point-prevalence abstinence rate at week 52. Secondary smoking-related outcomes were continuous abstinence rates during the randomized and follow-up phases, point-prevalence abstinence rates at week 64, and median time to first smoking relapse. Point-prevalence abstinence at each visit was defined as self-report of smoking no cigarettes since the last visit and an expired carbon monoxide level of less than 9 ppm. Participants were considered continuously abstinent at any study visit if they demonstrated point-prevalence abstinence at that and at all preceding visits since randomization (at week 12). Participants who discontinued the study prior to week 52 and for whom no further information on smoking behavior was available were considered to have relapsed at the time of dropout.24 Secondary outcomes were effect of varenicline on psychiatric symptoms (Calgary Depression Scale for Schizophrenia, Brief Psychiatric Rating Scale, Schedule for Assessment of Negative Symptoms), nicotine withdrawal symptoms (Wisconsin Smoking Withdrawal Scale), health-related quality of life (SF-12), body mass index, and adverse events.
Statistical Analysis
The study was powered to show differences between varenicline and placebo for point-prevalence abstinence at week 52. Assuming a 35% to 40% relapse in the varenicline group and a 75% to 80% relapse in the placebo group, estimates based on trials of bupropion involving smokers with schizophrenia,8,14,25 we estimated that 48 participants per study group would provide 91% to 99% power and 40 patients per study group would provide 85% to 98% power to detect a treatment effect using a 2-group Fisher exact test with a .05, 2-sided significance level. Analyses were conducted in SAS, version 9.3 (SAS Institute Inc).
The original analysis plan specified a stratified analysis by study site and psychiatric treatment type. Due to sparsity of data in many strata, we used an exact logistic regression to calculate relative risks, confidence intervals, and P values, with main effects for site and antipsychotic medication. Various sensitivity analyses, including multiple imputation, were conducted and are reported in the Supplement. The time to the first smoking relapse was assessed with the Kaplan-Meyer product-limit estimator, and the hazard ratio of relapse was estimated via a Cox proportional hazards regression model. Secondary analyses were conducted to evaluate the effect of varenicline on psychiatric symptoms, health-related quality of life, nicotine withdrawal symptoms, and adverse events. These continuous variables were analyzed using repeated measures analysis of variance, adjusted for site, diagnosis, and antipsychotic type. The mean of the differences at each time point during the randomized treatment phase was the contrast used to test for a treatment effect. The Calgary Depression Scale for Schizophrenia score totals were modeled similarly except that a negative binomial distribution was used (PROC GENMOD).
Results
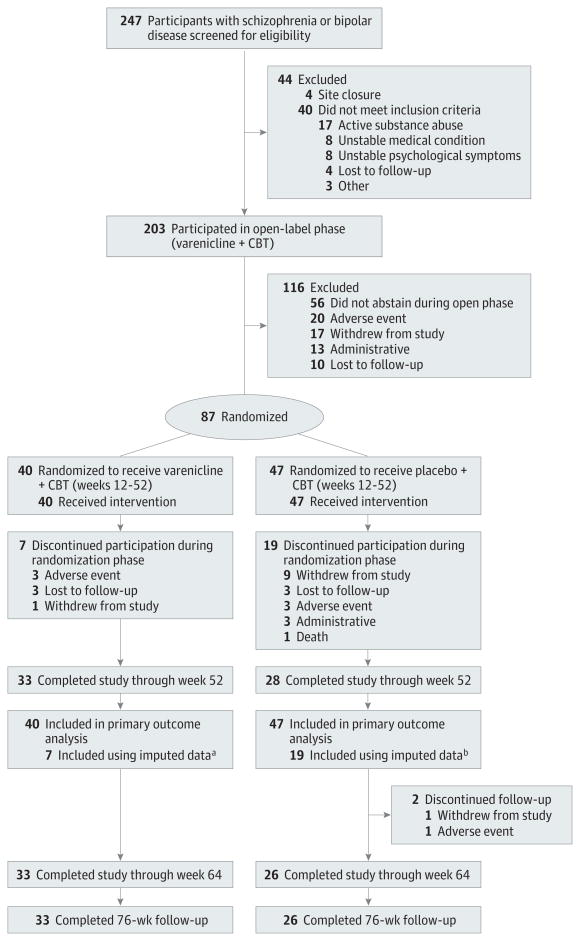
Four-hundred-twenty-one people contacted study staff and were provided information about the study, 247 provided written informed consent and were screened for eligibility for the trial; 203 met inclusion criteria and entered open-label treatment (Figure 1). Of those who entered the open-label treatment, 185 (91%) had schizophrenia spectrum disorder and 18 (9%) had bipolar disorder. Approximately 60% of the sample had severe nicotine dependence and smoked more than 20 cigarettes per day. A majority had made prior cessation attempts.
Figure 1. Study Flow Chart.
The reasons patients withdrew include that they did not want to attend or take placebo, they moved, or no reason was provided. The reasons for administrative withdrawal include poor medication adherence, poor attendance, medical instability, or site closure. Four participants who were considered withdrawn for adverse-events during the open-phase segment gave more than 1 reason for withdrawing.
a Data from 7 participants, 1 of whom had relapsed and 6 of whom had been continuously abstinent, were included in the primary analysis measured by imputation as relapsed at the time they dropped out.
bData from these 19 participants, 17 of whom had relapsed and 2 of whom had been continuously abstinent, were included in the primary analysis by imputation as relapsed at the time of dropout.
Forty-three percent (87 of 203) had at least 14 days of continuous abstinence at the end of the 12-week open phase and were randomized to receive varenicline or identical placebo and a tapering schedule of CBT for weeks 12 through 52 (Figure 1). Of those randomized, 77 (88.5%) had schizophrenia spectrum and 10 (11.5%) had bipolar disorder.
Treatment groups differed only on age, with participants assigned to varenicline being older than those assigned to placebo (Table 1). Eighty-two percent (33 of 40) of those assigned to varenicline and 60% (28 of 47) of those receiving placebo remained in the study from weeks 12 through 52. The 26 participants, 7 in the varenicline group and 19 in the placebo group, who prematurely discontinued study procedures during the randomized phase and were lost to follow-up were analyzed as relapsed at the time of dropout. Of these, 6 in the varenicline group and 2 in the placebo group had been continuously abstinent prior to dropout. The remainder of those who dropped out had relapsed prior to dropping out.
Table 1. Baseline Characteristics.
| Variable | Total Enrolled (n = 203) |
Nonrandomized (n=116) |
Placebo (n = 47) |
Varenicline (n = 40) |
|---|---|---|---|---|
| Age, ya | ||||
| Mean (SD) | 47.5 (10.2) | 46.9 (10.0) | 45.7 (10.3) | 51.4 (9.6) |
| Range | 22-68 | 22-68 | 23-66 | 23-65 |
| Women, No. (%) | 80 (39) | 48 (41) | 16 (34) | 16 (40) |
| Race/ethnicity, No. (%) | ||||
| White | 151 (74) | 87 (75) | 34 (72) | 30 (75) |
| Black | 35 (17) | 20 (17) | 6 (13) | 9 (23) |
| Other | 17 (8) | 9 (8) | 7 (15) | 1 (3) |
| Hispanic | 8 (4) | 6 (5) | 0 | 2 (5) |
| Not a high school graduate, No. (%) | 49 (24) | 30 (26) | 10 (22) | 9 (23) |
| Never married, No. (%) | 132 (65) | 80 (69) | 31 (66) | 21 (53) |
| Disabled, No. (%) | 132 (65) | 80 (69) | 29 (62) | 23 (58) |
| Cigarettes smoked per d, average lifetime | ||||
| Mean (SD) | 25.9 (14.2) | 28.0 (16.5) | 22.1 (9.6) | 24.2 (9.4) |
| Range | 4-100 | 4-100 | 7-40 | 10-50 |
| Expired carbon monoxide, ppm | ||||
| Mean (SD) | 23.1 (14.9) | 24.4 (15.5) | 22.6 (13.2) | 20.1 (15.1) |
| Range | 2-88 | 2-88 | 3-60 | 4-69 |
| Age at initiation of regular smoking, y | ||||
| Mean (SD) | 17.6 (5.7) | 18.0 (6.0) | 17.1 (5.1) | 17.4 (5.7) |
| Range | 6-45 | 7-45 | 6-39 | 8-32 |
| FTND score | ||||
| Mean SD | 6.1 (1.8) | 6.3 (1.8) | 5.9 (1.7) | 5.9 (2.0) |
| ≥6, No. (%) | 127 (64) | 76 (67) | 28 (61) | 23 (58) |
| Prior cessation attempts, No. (%) | ||||
| None | 25 (12) | 20 (17) | 3 (6) | 2 (5) |
| ≥5 | 70 (34) | 39 (34) | 16 (34) | 15 (38) |
| Psychiatric symptom ratings | ||||
| SANS composite score | ||||
| Mean (SD) | 35.0 (14.7) | 35.7 (14.8) | 32.7 (15.6) | 36.0 (13.3) |
| Range | 4-73 | 4-73 | 6-69 | 12-72 |
| BPRS total score | ||||
| Mean (SD) | 51.1 (13.1) | 50.0 (13.2) | 50.3 (11.7) | 55.6 (13.8) |
| Range | 29-98 | 29-89 | 33-72 | 30-98 |
| CDSS total score | ||||
| Mean (SD) | 4.1 (3.3) | 3.7 (3.1) | 4.7 (3.4) | 4.6 (3.9) |
| Range | 0-15 | 0-14 | 0-12 | 0-15 |
| Psychiatric diagnosis, No. (%) | ||||
| Schizophrenia spectrum | 185 (91) | 108 (93) | 41 (87) | 36 (90) |
| Bipolar disorder | 18 (9) | 8 (7) | 6 (13) | 4 (10) |
| Prior substance use disorder | 94 (48) | 50 (44) | 22 (48) | 22 (58) |
| Antipsychotic medication, No. (%) | ||||
| Conventional antipsychotics only | 24 (13) | 16 (15) | 4 (10) | 4 (11) |
| Atypical other than clozapine | 119 (65) | 67 (63) | 28 (68) | 24 (67) |
| Clozapine | 41 (22) | 24 (22) | 9 (22) | 8 (22) |
| No. of psychotropic medications | ||||
| Mean (SD) | 1.1 (0.9) | 1.1 (1.0) | 1.2 (1.0) | 1.1 (0.9) |
| Range | 0-4 | 0-3 | 0-4 | 0-3 |
| BMI | ||||
| Mean (SD) | 31.7 (6.7) | 31.5 (6.9) | 31.9 (6.3) | 32.0 (6.7) |
| Range | 19-58 | 20-58 | 20-48 | 19-49 |
Abbreviations: BPRS, Brief Psychiatric Rating Scale, for which higher scores in a range of 24 to 168, indicate more severe symptoms; BMI, body mass index, weight in kilograms divided by the square of the height in meters, for which 30 or higher indicates obesity; CDSS, Calgary Depression Scale for Schizophrenia, for which higher scores in a range of 0 to 27 indicate more severe symptoms; FTND, Fagerstrom Test for Nicotine Dependence, for which scores of 6 or higher in a range of 0 to 10 indicate severe dependence; SANS, Schedule for Assessment of Negative Symptoms, for which higher scores in a range of 0 to 85 indicate more severe symptoms.
There were no significant between-group differences in baseline characteristics in randomized groups except for age. P values based on 2-sample t tests or χ2 tests (P = .01; 2-sample t test) and a trend, P = .06, for BPRS scores to be higher at baseline in those assigned to varenicline.
Smoking Abstinence
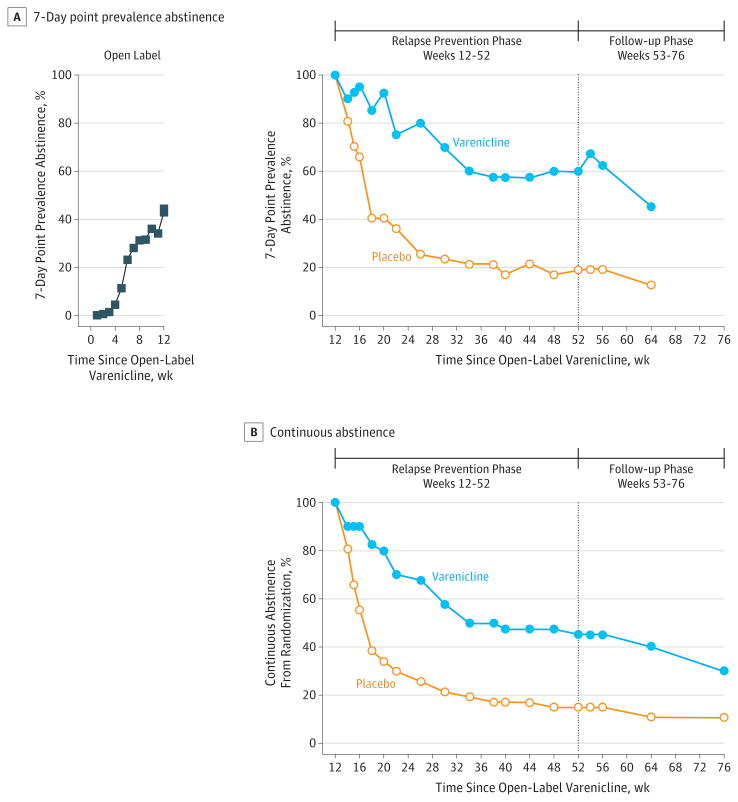
Twenty-four of 40 patients (60%) in the extended-duration varenicline group achieved biochemically verified, 7-day, point-prevalence abstinence at week 52 vs 9 of 47 patients (19%) in the placebo group (odds ratio [OR], 6.2; 95% CI, 2.2-19.2; P <.001). At week 64, 18 of 40 (45%) in the varenicline group vs 6 of 47 patients (13%) in the placebo group achieved 7-day, point-prevalence abstinence (OR, 5.1; 95% CI, 1.7-18.0; P = .002; Figure 2A). From weeks 12 through 52, 18 of 40 patients (45%) achieved continuous abstinence in the varenicline group vs 7 of 47 patients (15%) in the placebo group (OR, 4.6; 95% CI, 1.5-15.7; P = .004). After treatment discontinuation, from weeks 12 through 64, 16 of 40 patients (40%) in the varenicline group vs 5 of 47 patients (11%) in the placebo group were continuously abstinent (OR, 5.2; 95% CI, 1.6-20.4; P = .003). By week 76, 12 of 40 patients (30%) in the varenicline group vs 5 of 47 (11%) in the placebo group had been continuously abstinent since randomizations at week 12 (for a total of 16 months) (OR, 3.4; 95% CI, 1.02-13.6; P = .03; Figure 2B). The primary abstinence outcomes are also presented as prevalence ratios (eResults 1 in the Supplement). Abstinence rates at week 52 were also significantly higher among participants with schizophrenia spectrum and bipolar disorder who were taking varenicline when the groups were analyzed separately by psychiatric diagnosis (eResults 2 and eFigure 1 in the Supplement).
Figure 2. Point-Prevalence and Continuous Abstinence Rates During Study Treatment and Follow-up Phases.
At week 16, cognitive behavioral therapy sessions were tapered to twice a month; at week 20, to once a month. P values are based on Fisher exact tests. Seven-day point-prevalence was higher for those assigned to varenicline at week 52 (P < .001) and at week 64 (P < .01). Continuous abstinence was higher for those assigned to the varenicline group from weeks 12 through 52 (P < .01), weeks 12 through 64 (P < .01), and weeks 12 through 76 (P < .05). There were 40 participants in the varenicline and 47 in the placebo group throughout the relapse-prevention and follow-up phases, and there were 203 participants in the open-label phase.
Those in the varenicline group had a longer time to relapse, defined as time from randomization to self-report of smoking a single cigarette; having an expired carbon monoxide measurement higher than 9 ppm;or dropping out, whichever occurred sooner, with median time to relapse 358 days for those in the varenicline group and 35 days for those in the placebo group (P < .001; eResults 3 and eFigure 2 in the Supplement).
In accordance with the protocol, the 26 randomized participants (7 in the varenicline group and 19 in the placebo group) who prematurely discontinued study procedures during the randomized phase and were lost to follow-up were analyzed as having relapsed at the time they dropped out. However, 6 patients taking varenicline and 2 taking placebo had been continuously abstinent prior to that point. We conducted various sensitivity analyses, including multiple imputation, in which treatment effects on abstinence rates remained significant (eResults 4 in the Supplement).
Intervention Participation
Those in the placebo group attended a median of 24 of the 27 CBT group sessions (interquartile range [IQR], 21-26); those in the varenicline group, a median of 26 (IQR, 22-27) sessions. Forty-six patients (98%) in the placebo group and 37 (93%) in the varenicline group attended more than 75% of the group sessions while active in the study.
Assessments of Psychiatric Symptoms and Safety
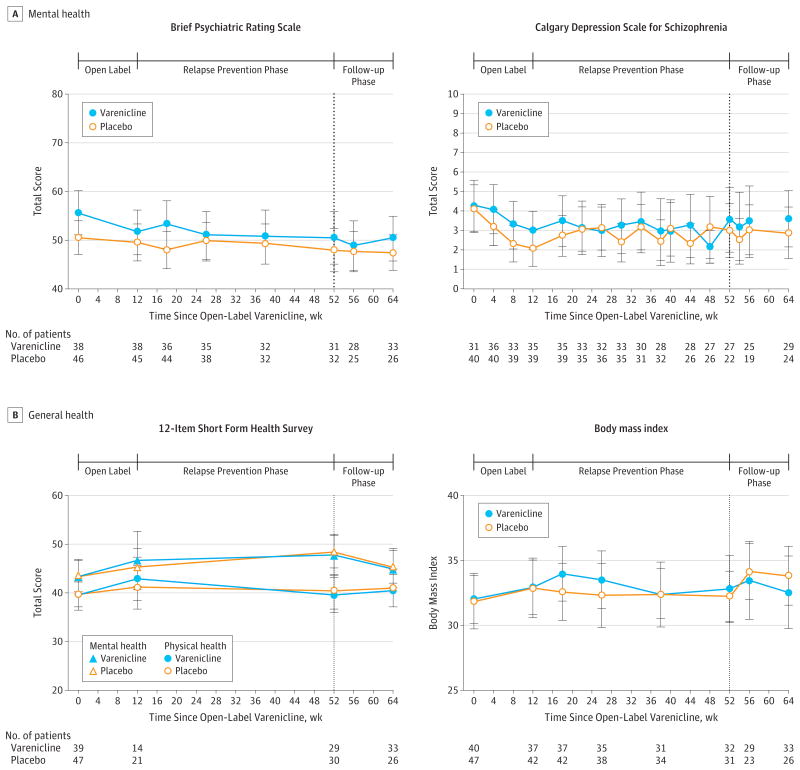
There was no effect of treatment assignment on severity of psychiatric symptoms, on self-report of overall health, body mass index (Figure 3), or on nicotine withdrawal symptoms (eFigure 2 in the Supplement). Adverse events are presented in Table 2. Among patients treated with placebo 25 of 40 correctly guessed their treatment assignment, which was not significantly different from chance (P = .15). Among those receiving varenicline treatment 25 of 33 correctly guessed their treatment assignment (P = .005).
Figure 3. Clinical Outcomes During the Open-Label, Randomized, Follow-up Phases.
At week 16, cognitive behavior therapy sessions were tapered to twice a month; at week 20, to once a month. Data presented are raw means. Error bars represent 95% confidence intervals. The 12-Item Short Form Health Survey is scored via a standard algorithm, with higher scores indicating better patient self perception of health, with a mean score of 50 and a standard deviation of 10 in a representative sample of the US population. See the legend in Table 1 for score definitions for Brief Psychiatric Rating Scale, and the Calgary Depression Scale for Schizophrenia.
Table 2. Adverse Events.
| Adverse Eventa | No. (%) of Participantsb | |||
|---|---|---|---|---|
| Open-Label Varenicline Phase | Maintenance Treatment Phase | |||
| Before Quite Date, wk 1-4 (n = 188) |
After Quit Date, wk 5-12 (n = 158) |
Placebo n = 47) |
Varenicline (n = 40) |
|
| Nausea | 84 (45) | 40 (25) | 10 (22) | 15 (38) |
| Anxiety | 55 (29) | 47 (30) | 12 (26) | 11 (28) |
| Irritability | 54 (29) | 43 (27) | 13 (28) | 7 (18) |
| Headache | 49 (26) | 48 (30) | 11 (24) | 17 (44)c |
| Agitation | 49 (26) | 51 (32) | 17 (37) | 5 (13)c |
| Excitement | 45 (24) | 45 (28) | 17 (37) | 7 (18)c |
| Insomnia | 45 (24) | 43 (27) | 11 (24) | 11 (28) |
| Vomiting | 40 (21) | 36 (23) | 6 (13) | 10 (26) |
| Tachycardia | 21 (11) | 22 (13) | 12 (26) | 7 (18) |
| Abnormal dreams | 21 (11) | 3 (2) | 2 (4) | 1 (3) |
| Suicidal ideationd | 1 (<1) | 5 (4) | 2 (5) | 2 (6) |
Adverse events that occurred in 10% of more of the participants at any time point, with additional inclusion of suicidal ideation.
Numbers are presented as the total number of participants providing data in at least 1 study visit in that period.
P < .05 Fisher exact test.
A rating of 2 or higher in item 8 of the Calgary Depression Scale for Schizophrenia suicide rating indicates active suicidal ideation (there were no ratings higher than 2 and no suicide attempts).
Eleven patients were hospitalized during the randomized phase: 4 for medical and 7 for psychiatric events. Eight of the 11 participants who had been hospitalized continued with the study during and after hospitalization. There were 2 medical hospitalizations among those taking placebo, 1 for sepsis associated with complications of diabetes that resulted in death and 1 for myocardial infarction, and 2 medical hospitalizations among those taking varenicline, 1 for pancreatitis and 1 for hyperglycemia. There were 5 psychiatric hospitalizations among participants taking placebo and 2 among those taking varenicline (eResults 5 in the Supplement). The risk ratio for psychiatric hospitalization was 0.45, but the confidence intervals are wide (95% CI, 0.04-2.9) given the relatively small size of the study.
Discussion
To our knowledge, this is the first randomized, controlled trial of maintenance pharmacotherapy for the prevention of relapse to smoking in persons with serious mental illness. Point-prevalence abstinence at 1 year was 3 times higher among those assigned to maintenance varenicline treatment (60%) than among those assigned to placebo (19%). The validity of this finding is supported by a similar treatment effect across a range of related, secondary smoking-related outcomes. Participants assigned to maintenance varenicline had higher point-prevalence and continuous-abstinence rates at every postrandomization visit during the 40 weeks of relapse-prevention treatment. The treatment effect on the primary abstinence outcomes remained significant when patients with schizophrenia-spectrum and bipolar disorder diagnoses were analyzed separately.
Consistent with prior studies reporting rapid relapse after discontinuation of pharmacotherapy,8 time to 50% relapse was only 35 days after discontinuation of active pharmacotherapy in the placebo group despite continuing weekly CBT during this period, indicating that weekly relapse-prevention focused CBT alone was not adequate to aid participants to maintain abstinence that they had achieved during 12 weeks of varenicline treatment and CBT. Time to relapse was significantly longer in the varenicline group; however, approximately one-third of the extended-duration varenicline group relapsed after CBT was tapered from weekly to monthly sessions, suggesting a possible synergistic effect of varenicline and weekly or bi-weekly CBT for sustained abstinence.
That relapse rates in the placebo group were more than 80%, whereas 60% of those randomized to extended-duration pharmacotherapy had point-prevalence abstinence at week 52, and 45% were continuously abstinent, supports consideration of tobacco dependence as a chronic condition for patients with serious mental illness, even after initial abstinence. Six-months after treatment discontinuation, the self-reported continuous abstinence rate in the varenicline group was greater than that of the placebo group. A longer duration study is needed to determine the optimal duration of maintenance pharmacotherapy.
Varenicline was well-tolerated over the 52-week study in this sample of treatment-stable outpatients with schizophrenia, schizoaffective disorder, or bipolar disorder, based on frequent, standard, clinician-administered ratings of psychiatric symptoms and spontaneous adverse-event reporting. This is consistent with reports of shorter controlled trials of varenicline in stable outpatients with schizophrenia,10,11,26,27 and other psychiatric illness.28-32 Although the study was not powered to detect changes in psychiatric symptoms, we detected no signal for varenicline to be associated with new or worsening neuropsychiatric symptoms. The most common adverse effects with varenicline in the randomized phase in this trial were headache and nausea.
Although this is one of the largest smoking cessation trials involving patients with serious mental illness and, to our knowledge, the first randomized, placebo-controlled, relapse-prevention trial involving this population, the primary limitation of this trial is the relatively small sample of 203 participants entering open-label treatment and 87 participants randomized to the relapse-prevention intervention. Additionally, 26 randomized participants discontinued study participation before the end of the relapse prevention phase and were considered for the analyses to have relapsed from the time they dropped out. Despite these limitations, this trial demonstrated a beneficial effect of maintenance pharmacotherapy on every smoking outcome measure. Although varenicline was well tolerated, because of the small sample size, it is not possible to accurately estimate the risk of serious adverse effects or to make claims regarding safety. Participants were recruited from community mental health centers so that the results should be generalizable to the large majority of outpatient smokers with serious mental illness who are treated in this type of setting.
Conclusion
Among smokers with serious mental illness who attain initial abstinence with a standard 12-week course of varenicline and CBT, 40 additional weeks of maintenance treatment with the pharmacologic smoking cessation aid, varenicline, administered concurrently with relapse-prevention-focused group CBT results in improved prolonged abstinence rates compared with placebo plus CBT. Such maintenance treatment may reduce the high prevalence of tobacco dependence and reduce the heavy burden of smoking-related morbidity and mortality in those with serious mental illness.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by grants R01 DA021245 by National Institute on Drug Abuse with supplemental financial and material support from an investigator-initiated award from Pfizer for study medications and funding, and by 05B1MACMHS to the Massachusetts Department of Mental Health from the Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Treatment Strategies for Smoking Cessation in Patients with Schizophrenia to the North Suffolk Mental Health Association (Dr Evins). Pfizer provided study medication and supplemental support through an investigator-initiated award after the protocol was approved by the institutional review board and the data and safety monitoring board.
Role of the Sponsor: The external funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, or approval of the manuscript; and decision to submit the manuscript for publication. At the time of submission and solely as a courtesy, a copy of the manuscript was given to Pfizer, which offered neither edits nor approval to publish.
Footnotes
Author Contributions: Dr Evins had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Evins, Cather, Goff, Schoenfeld.
Acquisition of data: Evins, Cather, Pratt, Pachas, Achtyes, Ayer.
Analysis and interpretation of data: Evins, Cather, Pachas, Hoeppner, Goff, Ayer, Schoenfeld.
Drafting of the manuscript: Evins, Cather, Schoenfeld.
Critical revision of the manuscript for important intellectual content: Evins, Cather, Pratt, Pachas, Hoeppner, Goff, Achtyes, Ayer, Schoenfeld.
Statistical analysis: Hoeppner, Schoenfeld.
Obtained funding: Evins.
Administrative, technical, or material support: Evins, Cather, Pratt, Pachas, Achtyes, Ayer.
Study supervision: Evins, Cather, Pratt, Pachas, Achtyes, Ayer.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Evins reports receiving product and financial support to her institution from EnVivo Pharmaceuticals for a NIDA funded trial of an alpha-7 nicotinic agonist for smoking cessation in non-psychiatrically ill smokers and from GSK to conduct Phase II trials of novel compounds for a NIDA funded Cooperative Drug Discovery Group for Nicotine Dependence and consulting for the Pfizer advisory board. Dr Cather reports consulting to Prophase. Dr Goff reports receiving financial support from Pfizer as the principal investigator of an investigator-initiated trial of high-dose ziprasidone to treat schizophrenia. Dr Achtyes reports consulting for Publicis Healthcare Communications Group to complete a survey and receiving salary paid to him by his full-time employer, Pine Rest Christian Mental Health Services, financial support to his institution from Cherry Street Health Services, where he is a consulting psychiatrist, and financial support to his institution from Michigan State University. Dr Achtyes reports that the following grants to his institution are pending from Janssen, Otsuka, North Shore Long Island Jewish Health System, Dartmouth College, Pine Rest Foundation, University of Chicago, AssurEx, and Eli Lilly. Dr Shoenfeld reports receiving financial support from the National Institutes of Health and receiving compensation for consultation to Pfizer Inc. No other disclosures were reported.
Publisher's Disclaimer: Disclaimer: Dr Goff, an associate editor of JAMA, was not involved in the evaluation or decision to publish this article.
Additional Contributions: We thank the following contributors, who received no compensation: Nancy Rigotti, MD (Massachusetts General Hospital and Harvard Medical School), Harry Lando, PhD (University of Minnesota), and Kim Mueser, PhD (Dartmouth Medical School and Boston University) for their thoughtful input at various stages of the project on study planning and design and protocol implementation; Anne Thorndike, MD (Massachusetts General Hospital and Harvard Medical School), chair of the data and safety monitoring board for her thoughtful and helpful input, and Ivan Montoya, MD, MPH (National Institute on Drug Abuse) for his unflagging support for this project. We also thank Johanna Nino, MD (Massachusetts General Hospital), who led the training and monitoring at all study sites for the first 2 years of the study and Erika Weisz, BS (Massachusetts General Hospital), who led the training and monitoring at all study sites and led the data collection at the Massachusetts General Hospital for the latter 2 years of the study; Sarah Carlini, BA (Massachusetts General Hospital), Megan Santos, LICSW (Dartmouth Medical School), and Heather Mayle, MA (Cherry Street Health Services) who led the data collection and monitoring at their respective institutions; and Max Curran, BA (Massachusetts General Hospital), who created all the graphical displays of the data.
Contributor Information
A. Eden Evins, Massachusetts General Hospital and Harvard Medical School, Boston.
Corinne Cather, Massachusetts General Hospital and Harvard Medical School, Boston.
Sarah A. Pratt, Geisel School of Medicine, Dartmouth College, Hanover, New Hampshire.
Gladys N. Pachas, Massachusetts General Hospital and Harvard Medical School, Boston.
Susanne S. Hoeppner, Massachusetts General Hospital and Harvard Medical School, Boston.
Donald C. Goff, New York University Langone Medical Center, New York.
Eric D. Achtyes, Cherry Street Health Services and Michigan State University College of Human Medicine, Grand Rapids.
David Ayer, Centerstone Research Institute and Indiana University, Bloomington.
David A. Schoenfeld, Massachusetts General Hospital and Harvard Medical School, Boston.
References
- 1.Garrett BE, Dube SR, Trosclair A, Caraballo RS, Pechacek TF Centers for Disease Control and Prevention (CDC) Cigarette smoking–United States, 1965-2008. MMWR Surveill Summ. 2011;60(suppl):109–113. [PubMed] [Google Scholar]
- 2.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100(12):2464–2472. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Results From the 2010 National Survey on Drug Use and Health: Mental Health Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- 4.Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained-release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. Am J Psychiatry. 2001;158(4):635–637. doi: 10.1176/appi.ajp.158.4.635. [DOI] [PubMed] [Google Scholar]
- 5.Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob Res. 2001;3(4):397–403. doi: 10.1080/14622200110073920. [DOI] [PubMed] [Google Scholar]
- 6.George TP, Vessicchio JC, Termine A, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52(1):53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- 7.Evins AE, Cather C, Deckersbach T, et al. A double-blind placebo-controlled trial of bupropion sustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol. 2005;25(3):218–225. doi: 10.1097/01.jcp.0000162802.54076.18. [DOI] [PubMed] [Google Scholar]
- 8.Evins AE, Cather C, Culhane MA, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- 9.George TP, Vessicchio JC, Sacco KA, et al. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry. 2008;63(11):1092–1096. doi: 10.1016/j.biopsych.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(5):654–660. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 11.Weiner E, Buchholz A, Coffay A, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res. 2011;129(1):94–95. doi: 10.1016/j.schres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253. doi: 10.1002/14651858.CD007253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Cather C, Dyer MA, Burrell HA, Hoeppner B, Goff DC, Evins AE. An open trial of relapse prevention therapy for smokers with schizophrenia. J Dual Diagn. 2013;9(1):87–93. doi: 10.1080/15504263.2012.749559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evins AE, Deckersbach T, Cather C, et al. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. J Clin Psychiatry. 2005;66(9):1184–1190. doi: 10.4088/jcp.v66n0915. [DOI] [PubMed] [Google Scholar]
- 16.Pachas GN, Cather C, Pratt SA, et al. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12-week, open-label trial. J Dual Diagn. 2012;8(2):117–125. doi: 10.1080/15504263.2012.663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 19.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;(22):39–44. [PubMed] [Google Scholar]
- 20.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 21.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS) Psychol Rep. 1962;10:799–812. [Google Scholar]
- 23.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;1989(7):49–58. [PubMed] [Google Scholar]
- 24.Hajek P, Stead LF, West R, Jarvis M, Hartmann-Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD003999. doi: 10.1002/14651858.CD003999.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Dale Horst W, Klein MW, Williams D, Werder SF. Extended use of nicotine replacement therapy to maintain smoking cessation in persons with schizophrenia. Neuropsychiatr Dis Treat. 2005;1(4):349–355. [PMC free article] [PubMed] [Google Scholar]
- 26.Shim JC, Jung DU, Jung SS, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37(3):660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong LE, Thaker GK, McMahon RP, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011;68(12):1195–1206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthenelli RM, Morris C, Ramey TS, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med. 2013;159(6):390–400. doi: 10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. Am J Psychiatry. 2013;170(12):1460–1467. doi: 10.1176/appi.ajp.2013.12121599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evins AE. Reassessing the safety of varenicline. Am J Psychiatry. 2013;170(12):1385–1387. doi: 10.1176/appi.ajp.2013.13091257. [DOI] [PubMed] [Google Scholar]
- 31.Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ. 2009;339:b3805. doi: 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103(1):146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.