Abstract
Primary and metastatic tumors of the central nervous system are a heterogeneous group of neoplasms with varied outcomes and management strategies. Recently, improved survival observed in 2 randomized clinical trials established combined chemotherapy and radiation as the new standard for treating patients with pure or mixed anaplastic oligodendroglioma harboring the 1p/19q codeletion. For metastatic disease, increasing evidence supports the efficacy of stereotactic radiosurgery in treating patients with multiple metastatic lesions but low overall tumor volume. These guidelines provide recommendations on the diagnosis and management of this group of diseases based on clinical evidence and panel consensus. This version includes expert advice on the management of low-grade infiltrative astrocytomas, oligodendrogliomas, anaplastic gliomas, glioblastomas, medulloblastomas, supratentorial primitive neuroectodermal tumors, and brain metastases. The full online version, available at NCCN.org, contains recommendations on additional subtypes.
Overview
In 2013, an estimated 23,130 people in the United States will be diagnosed with primary malignant brain and other central nervous system (CNS) neoplasms.1 These tumors will be responsible for approximately 14,080 deaths. The incidence of primary brain tumors has been increasing over the past 30 years, especially in elderly persons.2 Metastatic disease to the CNS occurs much more frequently, with an estimated incidence approximately 10 times that of primary brain tumors. An estimated 20% to 40% of patients with systemic cancer will develop brain metastases.3
Principles of Management
Primary and metastatic brain tumors are a heterogeneous group of neoplasms with varied outcomes and management strategies. Primary brain tumors range from pilocytic astrocytomas, which are very uncommon, noninvasive, and surgically curable, to glioblastoma multiforme, the most common intraparenchymal brain tumor in adults, which is highly invasive and virtually incurable. Likewise, patients with metastatic brain disease may have rapidly progressive systemic disease or no systemic cancer at all. These patients may have one or dozens of brain metastases, and they may have a malignancy that is highly responsive or, alternatively, highly resistant to radiation or chemotherapy. Because of this marked heterogeneity, the prognostic features and treatment options for brain tumors must be carefully reviewed on an individual basis and sensitively communicated to each patient. In addition, CNS tumors are associated with a range of symptoms and complications, such as edema, seizures, endocrinopathy, fatigue, psychiatric disorders, and venous thromboembolism, that can seriously impact quality of life. The involvement of an interdisciplinary team, including neurosurgeons, radiation therapists, oncologists, neurologists, and neuroradiologists, is a key factor in the appropriate management of these patients. For any subtype of malignant brain lesions, the NCCN CNS Panel encourages a thorough multidisciplinary review of each patient case once the pathology results are available.
Treatment Principles
Several important principles guide surgical and radiation therapy (RT) for adults with brain tumors. Regardless of tumor histology, neurosurgeons generally provide the best outcome for their patients if they remove as much tumor as possible (maximal safe resection), minimize surgical morbidity, and ensure an accurate diagnosis through providing sufficient representativ e tumor tissue. Decisions regarding aggressiveness of surgery for primary brain lesions are complex and depend on the 1) age and performance status (PS) of the patient; 2) proximity to “eloquent” areas of the brain; 3) feasibility of decreasing the mass effect with aggressive surgery; 4) resectability of the tumor (including the number and location of lesions); and 5) time since last surgery in patients with recurrent disease.4








Surgical options include stereotactic biopsy, open biopsy, subtotal resection, or complete resection (gross total resection). The pathologic diagnosis is critical and may be difficult to determine accurately without sufficient tumor tissue. Review by an experienced neuropathologist is highly recommended. In addition, a postoperative MRI scan, with and without contrast, should be obtained 24 to 72 hours after surgery to document the extent of disease after surgical intervention.
Radiation oncologists use several different treatment modalities in patients with primary brain tumors, including brachytherapy, stereotactic fractionated RT, and stereotactic radiosurgery (SRS). Standard fractionated external-beam RT (EBRT) is the most common approach, whereas hypofractionation is emerging as an option for select patients (eg, elderly and patients with compromised performance). RT for patients with primary brain tumors is administered within a limited field (tumor and surround), whereas whole-brain RT (WBRT) and SRS are used primarily for brain metastases.
Clinicians are advised to consult the algorithm sections, “Principles of Brain Tumor Imaging” (BRAIN-A, page 1129) and “Principles of Brain Tumor Surgery” (BRAIN-B, page 1129), for further discussion of these diagnostic and treatment modalities. The dose of radiation administered varies depending on the pathology results, as seen in “Principles of Brain Tumor Radiation Therapy” (BRAIN-C, page 1130). Appropriate chemotherapeutic and biologic regimens for each tumor subtype are listed under “Principles of Brain Tumor and Spinal Cord Systemic Therapy” (BRAIN-D, page 1131).
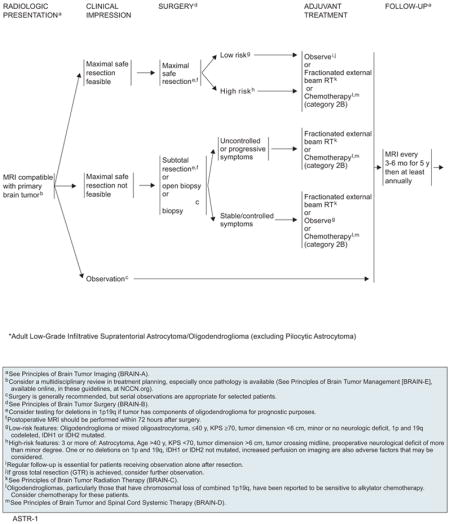
Low-Grade Infiltrative Astrocytomas and Oligodendrogliomas
Diffusely infiltrative low-grade gliomas (eg, astrocytomas, oligodendrogliomas, mixed oligoastrocytomas) are a diverse group of relatively uncommon malignancies classified as grade II under the WHO grading system.5 Multivariate analysis of 2 phase III trials conducted by the EORTC revealed that age of 40 years or older, astrocytoma histology, largest dimension of tumor as 6 cm or greater, tumor crossing midline, and presence of neurologic deficit before resection were unfavorable prognostic factors.6 In a separate validation study of 203 patients treated in a North American Intergroup trial, high-risk patients as defined by EORTC criteria (>2 risk factors) had a median overall survival of 3.9 years compared with 10.8 years in the low-risk group.7
Seizure is a common symptom (81%) of low-grade gliomas, and is more frequently associated with oligodendrogliomas.8 The median duration from onset of symptoms to diagnosis ranges from 6 to 17 months. These tumors typically are nonenhancing, low-attenuation/low-signal-intensity lesions on CT or MRI scans.
Diffuse astrocytomas are poorly circumscribed and invasive, and most gradually evolve into higher-grade astrocytomas. Although these were traditionally considered benign, they can behave aggressively and will undergo anaplastic transformation within 5 years in approximately half of patients.9,10 The most common noninfiltrative astrocytomas are pilocytic astrocytomas, which are circumscribed, often surgically resectable, and rarely transform; however, the NCCN algorithm does not encompass pilocytic astrocytomas because these tumors are curable with surgery alone.
Oligodendrogliomas are thought to arise from oligodendrocytes, whereas mixed oligoastrocytomas probably develop from a common glial stem cell. Radiographically, low-grade oligodendrogliomas appear well demarcated, occasionally contain calcifications, and do not enhance with contrast. The typical “fried egg” appearance of these tumors is evident in paraffin but not in frozen sections. More than half of oligodendrogliomas have specific molecular genetic alterations (allelic losses of chromosomes 1p and 19q) that can help distinguish them from other types of gliomas.11 Grade II oligodendrogliomas have a much better 5-year survival rate (70%) than mixed gliomas (56%) and astrocytomas (37%).12
Treatment Overview
Surgery
The best management strategy for infiltrative low-grade gliomas has yet to be defined.13 Surgery remains an important diagnostic and therapeutic modality. The primary surgical goal is to provide adequate tissue for a pathologic diagnosis and grading. Needle biopsies are often performed when lesions are in deep or critical regions of the brain. Biopsy results can be misleading, because gliomas often have varying degrees of cellularity, mitoses, or necrosis from one region to another; thus, small samples can provide a lower histologic grade.
The role of maximal tumor resection in low-grade astrocytomas remains unresolved. Because these tumors are relatively uncommon, published series generally include patients treated for decades, which introduces additional variables. For example, the completeness of surgical excision was based on the surgeon’s report in older studies. This approach is relatively unreliable when compared with assessment using modern postoperative imaging studies. Furthermore, most patients also received RT, and thus the net effect of the surgical procedure on outcome is difficult to evaluate. Most of the available retrospective biomedical literature suggests a survival benefit from aggressive surgical resection,14–17 although some data reported no difference.18 Maximal safe resection may also delay or prevent malignant progression19–21 and recurrence.22
Biological considerations also favor an attempt at a complete excision of an astrocytoma. First, the tumor may contain higher-grade foci, which may not be reflected in a small specimen. Second, complete excision may decrease the risk of future dedifferentiation to a more malignant astrocytoma.19 Third, a large tumor burden is removed, which also may enhance the effect of RT. As a result of these considerations, the general recommendation for treating an astrocytoma is to first attempt as complete an excision of tumor as possible (based on postsurgical MRI verification) without compromising function. Low-grade oligodendrogliomas are often amenable to total excision because of their location in the frontal lobes and distinct tumor margins. However, for tumors that involve eloquent areas, total removal may not be feasible and an aggressive approach could result in neurologic deficits.
Radiation Therapy
No consensus exists regarding the proper timing of postoperative EBRT for low-grade gliomas. Some oncologists advocate immediate fractionated EBRT, whereas others delay radiation until tumor progression is evident. In the EORTC 22845 randomized trial of early versus delayed RT in adult patients,23 those with low-grade gliomas were randomly assigned to either 54-Gy postoperative radiation or no immediate therapy. In an interim analysis, the 5-year disease-free survival was better with immediate postoperative radiation (44% vs 37%; P=.02). However, overall survival was similar, indicating that deferring postoperative therapy can be an option for a select group of patients. Long-term follow-up of these patients showed that overall survival was not increased in patients who had received early RT (7.4 vs 7.2 years); however, seizures were better controlled.24 Although delaying radiation in young, healthy patients without progressive neurologic decline can be controversial, there is a consensus to proceed with immediate postoperative radiation in older patients after a less-than-total resection, because their survival is as poor as patients with anaplastic astrocytoma. When radiation is deferred, regular follow-up is essential for patients receiving observation alone after resection. However, a consensus exists that high-risk patients with low-grade gliomas as defined by the EORTC experience benefit from early, upfront RT in terms of progression-free and overal survivals.
When radiation is given to patients with low-grade gliomas, it is administered with restricted margins. A T2-weighted and/or fluid-attenuated inversion recovery (FLAIR) MRI scan is the best means for evaluating tumor extent, because these tumors enhance weakly or not at all. The clinical target volume is defined by the FLAIR or T2-weighted tumor with a 1- to 2-cm margin. Every attempt should be made to decrease the radiation dose outside the target volume. This can be achieved with 3-dimensional planning or intensity-modulated RT. The standard radiation dose for low-grade astrocytomas is 45 to 54 Gy, delivered in 1.8- to 2.0-Gy fractions. The selection of 45 to 54 Gy as the standard dose range is based on its relative safety when applied to a limited volume of the brain and on the lack of evidence for increased efficacy with higher doses.25,26 In a randomized trial conducted by the EORTC in patients with low-grade astrocytomas, no survival difference was observed when 45.0 Gy was compared with 59.4 Gy.27 With a median follow-up of 6 years, the 5-year disease-free survival and overall survival were the same. A combined NCCTG (North Central Cancer Treatment Group), RTOG, and ECOG study randomized patients to receive either 50.4 Gy in 28 fractions or 64.8 Gy in 36 fractions.28 With a median follow-up of 6.3 years, the 5-year disease-free and overall survivals were again the same, indicating that lower doses of RT are probably as effective as higher doses of radiation for low-grade gliomas. Enthusiasm for SRS in low-grade gliomas has waned because of insufficient evidence for therapeutic advantage.29
Systemic Therapy
Chemotherapy is not a traditional mainstay of upfront treatment for low-grade gliomas. Some data support temozolomide as adjuvant therapy, and it is included as a category 2B recommendation based on nonuniform panel consensus. A phase II trial of temozolomide achieved a 61% objective response rate in 46 patients.30 Alternate protracted dosing schedules have produced response rates of 20% to 52%.31,32 RTOG conducted a clinical trial (RTOG 9802) that allowed observation alone for favorable patients (age <40 with gross total resection) and randomly assigned unfavorable patients (age ≥40 following any resection or younger patients who were subtotally resected) to postoperative radiation with or without combination PCV (lomustine [CCNU], procarbazine, and vincristine). Results have been presented in abstract form. In the favorable arm, the 5-year progression-free survival and overall survival rates were 50% and 94%, respectively.33 In the unfavorable arm, the addition of chemotherapy to radiation conferred a survival advantage beyond 2 years.34
In the absence of randomized trial data, several regimens are currently considered acceptable for recurrence or progressive disease, including temozolomide,31,35 nitrosourea, PCV, and platinum-based therapy.36–38
Patients with low-grade oligodendrogliomas, especially those with 1p/19q deletions, may represent favorable candidates for chemotherapy in light of good response rates reported in literature; however, this has never been prospectively determined.39–44
NCCN Recommendations
Primary and Adjuvant Treatment
When possible, maximal safe resection is recommended for low-grade infiltrative astrocytomas and oligodendrogliomas, and the actual extent of resection should be documented with a T2-weighted or FLAIR MRI scan within 72 hours after surgery. If the tumor is found to have components of oligodendroglioma, 1p/19q deletion testing should be considered because it is a favorable prognostic factor. Managing the disease through serial observation alone is appropriate for selected patients. The NCCN CNS Panel also discussed the role of the isocitrate dehydrogenase 1 or 2 (IDH1, IDH2) genes in low-grade gliomas. Mutations in the IDH genes are common and are reported to be a significant marker of positive prognosis.45 However, routine IDH testing as a recommendation is not included in the algorithm at this point because its impact on treatment is still unclear.
The following are considered low-risk features for low-grade gliomas: age of 40 years or younger; Karnofsky performance status (KPS) of 70 or greater; minor or no neurologic deficit; oligodendroglioma or mixed oligoastrocytoma; tumor dimension less than 6 cm; 1p and 19q codeletion; and IDH1 or IDH2 mutation. Patients are categorized as being high risk if they have 3 or more of the following: age older than 40 years, KPS less than 70, tumor larger than 6 cm, tumor crossing midline, or preoperative neurologic deficit of more than minor degree. Other adverse factors to consider include increased perfusion on imaging; one or no deletion on 1p and 19q; and wild-type IDH1 or IDH2. If gross total resection is achieved, most low-risk patients may be observed without adjuvant therapy. However, close follow-up is essential because more than half of these patients eventually experience disease progression.46 Low-grade gliomas can behave aggressively in high-risk patients, and adjuvant radiation or chemotherapy (category 2B for chemotherapy) is recommended for this group, although select patients may be observed.
Patients who only had a stereotactic biopsy, open biopsy, or subtotal excision should be treated with immediate fractionated EBRT or chemotherapy (category 2B), particularly if their symptoms are uncontrolled or progressive. Because of concerns about the neurotoxicity of RT,47 patients with asymptomatic residual tumors or stable symptoms may also be followed until their disease progresses. Patients should be followed using MRI every 3 to 6 months for 5 years, and then at least annually.
Recurrence
At the time of recurrence, surgery is recommended (if resectable) followed by chemotherapy in patients who previously underwent fractionated EBRT. At progression after chemotherapy, the options are to 1) consider another regimen; 2) consider reirradiation; and 3) provide palliative/best supportive care. Reirradiation is a good choice if the patient has been progression-free for more than 2 years after prior RT, the new lesion is outside the target of previous RT, or the recurrence is small and geometrically favorable. If the patient has not previously received radiation, they should first undergo surgery if the lesion is resectable. Patients may receive RT or chemotherapy after surgery (category 2B for chemotherapy).
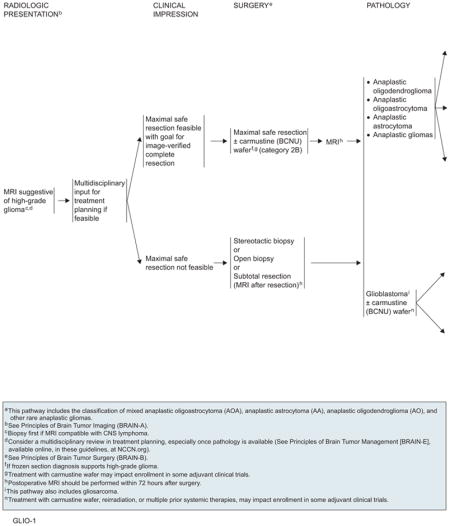
Anaplastic Gliomas and Glioblastomas
Anaplastic astrocytomas (grade III) and glioblastomas (grade IV astrocytomas) are the most common of the primary malignant brain tumors in adults, accounting for 7% and 54% of all gliomas, respectively.48 Glioblastoma is the most lethal brain tumor, with only a third of patients surviving for 1 year and fewer than 5% living beyond 5 years. The 5-year survival rate for anaplastic astrocytoma is 27%. The most important prognostic factors identified in an analysis of 1578 patients are histologic diagnosis, age, and PS.49
High-grade astrocytomas diffusely infiltrate surrounding tissues and frequently cross the midline to involve the contralateral brain. Patients with these neoplasms often present with symptoms of increased intracranial pressure, seizures, or focal neurologic findings related to the size and location of the tumor and to associated peritumoral edema. These tumors usually do not have associated hemorrhage or calcification, but produce considerable edema and mass effect and enhance after the administration of intravenous contrast (>65% of anaplastic gliomas and 96% of glioblastoma). Tumor cells have been found in the peritumoral edema, which corresponds to the T2-weighted MRI abnormalities. As a result, this volume is frequently used to define radiation treatment portals.
It is difficult to assess the results of therapy using CT or MRI scans, because the extent and distribution of contrast enhancement, edema, and mass effect are more a function of blood-brain barrier (BBB) integrity than of changes in the size of the tumor. Thus, other factors that exacerbate BBB dysfunction (eg, surgery, radiation, and tapering of corticosteroids) can mimic tumor progression through increasing contrast enhancement, T2-weighted abnormalities, and mass effect.
Anaplastic oligodendrogliomas are relatively rare; they are characterized by high cellularity, nuclear pleomorphism, frequent mitosis, endothelial proliferation, and necrosis. On histopathologic assessment, these tumors can be confused with glioblastoma multiforme; however, characteristic allelic losses of chromosomes 1p and 19q are present in anaplastic oligodendrogliomas.11 This distinct histologic subtype has a much better prognosis than anaplastic astrocytomas and glioblastomas because of its marked sensitivity to chemotherapy50; half of the patients are alive at 5 years.48
Treatment Overview
Surgery
The goals of surgery are to obtain a diagnosis, alleviate symptoms related to increased intracranial pressure or compression, increase survival, and decrease the need for corticosteroids. A prospective study in 565 patients with malignant glioma showed that aggressive surgery is a strong prognostic factor compared with biopsy alone (P<.0001).51 Retrospective analyses also suggest that gross total resection lengthens survival and is especially effective in patients with good PS.52–54 Unfortunately, the infiltrative nature of high-grade astrocytomas frequently renders gross total removal difficult. However, total resection is often possible for oligodendrogliomas, because most occur in the frontal lobes and the tumors are frequently well demarcated.
Unfortunately, nearly all glioblastomas recur. At recurrence, reoperation may improve the outcome for select patients.55 According to an analysis by Park et al,56 tumor involvement in specific critical brain areas, poor KPS, and large tumor volume were associated with unfavorable reresection outcomes.
Radiation Therapy
Fractionated EBRT after surgery is standard adjuvant therapy for patients with high-grade astrocytomas. Use of RT is based on 2 randomized trials conducted in the 1970s that showed extension in survival. Walker et al57 compared postoperative supportive care, carmustine (BCNU), RT, and RT plus BCNU in 303 patients, and reported median survivals of 14.0, 18.5, 35.0, and 34.5 weeks, respectively. Another trial of 118 patients also found a benefit in median survival with RT after surgery compared with no RT (10.8 vs 5.2 months).58 The typical dose is 60 Gy in 1.8- to 2.0-Gy fractions. Use of hypofractionated courses of radiation (total 40–50 Gy) has been shown to be efficacious in older patients with glioblastoma.59–61 Studies including a radiosurgery or brachytherapy boost to conventional RT did not show a survival benefit.62,63
A lack of prospective data exist for reirradiating recurrent gliomas. Based on retrospective patient series, repeat RT using modern high-precision techniques such as fractionated stereotactic RT may be a palliative option for select patients with good PS and small recurrent tumors.64,65
Chemotherapy/Systemic Therapy
Traditionally, chemotherapy was believed to be of marginal value in the treatment of newly diagnosed patients with high-grade gliomas, but this perception has shifted. In particular, combined chemoradiation has emerged as a new standard of care for patients with 1p/19q codeleted anaplastic oligodendroglioma or oligoastrocytoma and good PS nonelderly glioblastoma.
Most earlier trials studied nitrosourea-based chemotherapy regimens. The Medical Research Council reported results from the largest randomized trial of adjuvant chemotherapy in high-grade gliomas.66 In this study, 674 patients were randomly assigned to either radiation alone or radiation plus PCV. No survival benefit was seen with the addition of PCV, even in patients with anaplastic astrocytomas. In contrast, 2 meta-analyses reviewed data from randomized trials of patients with high-grade glioma, and both found a modest survival benefit when chemotherapy was added to postoperative radiation.67,68 Specifically, the Glioma Meta-Analysis Trialists Group reviewed 12 studies involving approximately 3000 patients and reported an absolute increase in 1-year survival rate from 40% to 46% and a 2-month increase in median survival when chemotherapy was added to postoperative radiation (hazard ratio [HR], 0.85; 95% CI, 0.78–0.91; P<.0001).67 An earlier analysis by Fine et al68 on 16 randomized trials also found a 10% and 9% increase in survival at 1 and 2 years, respectively.
Implanted Wafers
Other routes of chemotherapy drug delivery have been evaluated. Local administration of BCNU using a biodegradable polymer (wafer) placed intraoperatively in the surgical cavity has shown a statistically significant improvement in survival for patients with recurrent high-grade gliomas (31 vs 23 weeks; adjusted HR, 0.67; P=.006).69 As a result, the FDA approved the BCNU wafer for this indication. A phase III placebo-controlled study in 32 patients with malignant glioma showed a statistically significant prolongation of survival when BCNU polymer was used as initial therapy in combination with RT.70 A larger phase III trial in 240 newly diagnosed patients with malignant glioma also found a statistically significant improvement in median survival from 11.6 months in the placebo group to 13.9 months in the BCNU wafer–treated group.71 This benefit was maintained 2 and 3 years after implantation.72 Based on these studies, the FDA extended the approval of BCNU polymer wafers for use in malignant gliomas as initial therapy. However, clinicians and patients should be aware that BCNU can potentially interact with other agents, resulting in increased toxicity (see later discussion), and that implantation of the wafer may preclude future participation in clinical trials of adjuvant therapy.
Temozolomide
Temozolomide, an alkylating (methylating) agent, is now the standard of care in conjunction with postoperative RT for younger patients with glioblastoma with good PS. Stupp et al73 conducted a phase III randomized study that assessed the drug in 573 patients with glioblastoma aged 70 years and younger with a WHO PS of 2 or less. Patients received either daily temozolomide administered concomitantly with postoperative RT followed by 6 cycles of adjuvant temozolomide, or RT alone. Side effects for temozolomide include hair loss, nausea, vomiting, headaches, fatigue, and anorexia. Because of the risk of lymphocytopenia and subsequent opportunistic infection, prophylaxis against Pneumocystis carinii pneumonia (PCP) is required when the agent is administered with RT. The chemoradiation arm resulted in a statistically better median survival (14.6 vs 12.1 months) and 2-year survival rate (26.5% vs 10.4%) than RT. Final analysis confirmed the survival advantage at 5 years (10% vs 2%).74 However, the study design does not shed light on which is responsible for the improvement: temozolomide administered with radiation, following radiation, or both. The temozolomide dose used in this trial is 75 mg/m2/d concurrent with RT, then 150 to 200 mg/m2 post-RT on a 5-day schedule every 28 days. Alternate schedules such as a 21/28 dose-dense regimen or a 50-mg/m2 continuous daily schedule have been explored in a phase II trial for newly diagnosed glioblastoma.75 A comparison of the dose-dense 21/28 and standard 5/28 schedules has been completed with RTOG 0525 and the results showed no improvement with the post-RT dose-dense temozolomide arm when compared with the standard temozolomide arm.76
Wick et al77 performed a phase III trial of sequential radiochemotherapy in 318 patients with anaplastic gliomas. The 3 randomized arms were: 1) RT; 2) PCV; and 3) temozolomide. At progression, patients in arm 1 received PCV or temozolomide, whereas patients in arms 2 and 3 were irradiated. The 3 strategies resulted in comparable time-to-progression and survival. Another phase III study conducted by the same group (NOA-08) randomized 412 patients with anaplastic astrocytoma (11%) or glioblastoma (89%) who were older than 65 years and had a good performance score (KPS ≥60) to receive temozolomide alone or radiation alone.78 Results showed that temozolomide treatment was noninferior to RT in terms of survival.
The international Nordic trial randomized 291 patients with glioblastoma and good PS across 3 groups: temozolomide, hypofractionated RT, or standard RT.61 Patients older than 70 years had better survival with temozolomide or fractionated RT compared with standard radiation.
MGMT (O-6-methylguanine-DNA methyl-transferase) is a DNA repair enzyme that can cause resistance to DNA-alkylating drugs. Oligodendrogliomas frequently exhibit MGMT hypermethylation and low expression levels, which may explain the enhanced chemosensitivity.79 In the temozolomide arm of both the Nordic and German trials, patients with MGMT promoter methylation had longer survival than those without (9.7 vs 6.8 months; HR, 0.56; 95% CI, 0.34–0.93).61 This difference was not observed in the radiation groups.
No published data directly compare the benefit of temozolomide to nitrosourea for postoperative chemoradiation in patients with newly diagnosed anaplastic astrocytomas. This RTOG study (RTOG 9813) was prematurely discontinued because of lack of availability of BCNU.
Safety concerns exist regarding adjuvant use of temozolomide in patients with implanted BCNU wafers. However, temozolomide combined with RT after BCNU wafer placement seemed to be safe in multiple studies.80–82 For patients older than 70 years but with good performance, some evidence from small monocentric studies suggests the usefulness of temozolomide in addition to adjuvant radiation despite old age.83,84 For frail patients, temozolomide may be administered alone. A retrospective review of patients aged 70 years or older with mean KPS of 70 found no survival difference between those receiving radiation alone and those taking monthly temozolomide only.85 Given the susceptibility of elderly patients to radiation-induced neurotoxicity, especially when the PS is poor, chemotherapy alone seems to be a reasonable option.
Combination Chemoradiation
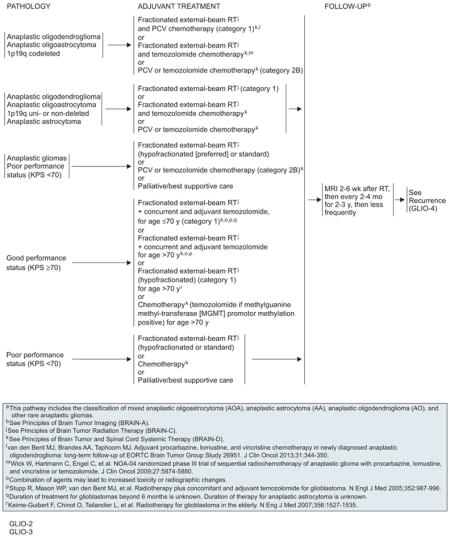
Improved survival observed in 2 randomized clinical trials established combined PCV chemotherapy and radiation as the new standard for treating patients with pure or mixed anaplastic oligodendroglioma harboring the 1p/19q codeletion. RTOG 9402 randomized 291 patients to PCV followed by immediate RT or RT alone.86 No difference was observed between the arms for the entire cohort. However, an unplanned analysis showed that patients with the codeletion lived longer than those without, and among patients with codeleted tumors, median survival was doubled when PCV was added to radiation (14.7 vs 7.3 years; HR, 0.59; 95% CI, 0.37–0.95; P=.03). This difference was not observed for patients without 1p/19q codeletion.
Similarly, EORTC 26951 randomly assigned 368 patients with pure or mixed anaplastic oligodendroglioma to RT or RT followed by PCV.87 At a median follow-up of 140 months, overall survival was longer in the combination arm than in the radiation arm (42.3 vs 30.6 months; HR, 0.75; 95% CI, 0.60–0.95). Median survival was not reached in patients with codeleted tumors who received PCV/RT compared with 112 months for those in the RT group. No survival advantage was found with the addition of PCV among patients without the codeletion.
Systemic Therapy for Recurrence
Unfortunately, currently available chemotherapy does not provide cures. Patients with malignant gliomas eventually experience disease recurrence or progression. In addition to temozolomide35,88,89 and nitrosoureas,69,90 regimens that are commonly used as second-line or salvage chemotherapy include combination PCV,91 cyclophosphamide (category 2B recommendation),92,93 and platinum-based regimens (category 2B recommendation).38 Anaplastic gliomas may also be treated with irinotecan94 or etoposide.95
Bevacizumab, an antiangiogenic agent, received accelerated approval in 2009 for recurrent glioblastoma based on 2 phase II studies. AVF 3708g randomized 167 patients to bevacizumab with or without irinotecan. MRI-defined objective response was achieved in 28% and 38% of patients, respectively.96 Median survival was around 9 months, similar to that of a previous phase II trial.97 A published report of the other pivotal study (NCI 06-C-0064E) recorded a median survival of 31 weeks in 48 heavily pretreated patients.98 Bevacizumab alone or in combination with chemotherapy has also shown activity in anaplastic gliomas.99–104 Although efficacious, bevacizumab is associated with potentially serious adverse events, including hypertension, impaired wound healing, colonic perforation, and thromboembolism.
Alternating Electric Field Therapy
In 2011, the FDA approved a portable medical device that generates low-intensity electric fields, termed tumor treating fields (TTF), for the treatment of recurrent glioblastoma. Approval was based on results of a clinical trial that randomized 237 patients to TTF or chemotherapy.105 Similar survival was observed in the arms, and TTF therapy was associated with lower toxicity and improved quality of life.
NCCN Recommendations
Primary Treatment
When a patient presents with a clinical and radiologic picture suggestive of high-grade glioma, neurosurgical input is needed regarding the feasibility of maximal safe tumor resection. Whenever possible, major tumor removal should be performed. One exception is when CNS lymphoma is suspected; a biopsy should be performed first and management should follow the corresponding pathway if the diagnosis is confirmed. If high-grade glioma is supported by intraoperative frozen section diagnosis, BCNU wafer placement is an option (category 2B). The extent of tumor debulking should be documented with a postoperative MRI scan within 72 hours after surgery, with and without contrast. If major tumor removal is deemed too risky, a stereotactic or open biopsy or subtotal resection should be performed to establish the diagnosis. Multidisciplinary consultation is encouraged once the pathology results are available.
Adjuvant Therapy
After surgical intervention, the choice of adjuvant therapy depends on the tumor pathology, status of the 1p/19q loci, and PS of the patient. For patients with 1p/19q codeleted anaplastic oligodendroglioma or oligoastrocytoma, fractionated EBRT plus PCV given before or after RT is a category 1 recommendation. Fractionated radiation plus concurrent temozolomide is an acceptable option, whereas PCV or temozolomide alone is designated category 2B. In the case of anaplastic astrocytoma, anaplastic oligodendroglioma, or oligoastrocytoma without 1p/19q codeletion, fractionated EBRT remains the standard (category 1). Other choices include fractionated radiation plus concurrent temozolomide, and PCV or temozolomide chemotherapy (deferred radiation). Patients with a poor KPS (<70) can be managed with radiation (hypofractionation is preferred over standard fractionation); PCV or temozolomide chemotherapy (category 2B); or palliative/best supportive care.
If glioblastoma is diagnosed, the adjuvant options mainly depend on the patient PS. Patients with good PS (KPS ≥70) are further stratified by age. Fractionated RT plus concurrent and adjuvant temozolomide is a category 1 recommendation for patients aged 70 years or younger. The panel noted that although data are focused on 6 cycles of post-RT temozolomide, 12 cycles are increasingly common, especially in recent clinical trial designs. Options for those older than 70 years include fractionated radiation plus concurrent and adjuvant temozolomide (category 2A for this group), hypofractionated RT (category 1), or chemotherapy with deferred RT. Patients opting for chemotherapy should receive temozolomide if they had MGMT methylation.
For patients with glioblastoma and with KPS lower than 70, options include fractionated EBRT, chemotherapy, or palliative/best supportive care. In the absence of data, panelists debated whether chemoradiation is appropriate for elderly patients with poor PS and ultimately agreed not to include this option.
The panel noted that given the complexity of symptoms and handicaps that can arise from malignant gliomas, PS score is a suboptimal measure of fitness for all patients. Similarly, a patient’s ability to tolerate toxic therapy does not necessarily correlate with chronologic age.106
Follow-Up and Recurrence
Patients should be followed closely with serial MRI scans (at 2–6 weeks post-RT, then every 2–4 months for 2–3 years, then less frequently) after the completion of RT. Because RT can produce additional BBB dysfunction, corticosteroid requirements may actually increase; therefore, scans may appear worse during the first 3 months after completion of RT, even though no actual tumor progression is present. Early MRI scans allow for appropriate titration of corticosteroid doses, depending on the extent of mass effect and brain edema. Later scans are used to identify tumor recurrence. Early detection of recurrence is warranted, because local and systemic treatment options are available for patients with recurrent disease. However, MR spectroscopy, MR perfusion, or PET can be considered to rule out radiation-induced necrosis or “pseudoprogression.”107,108
Management of recurrent tumors depends on the extent of disease and patient condition. For local recurrence, repeat resection, with or without wafer placement in the surgical bed, can be performed if possible. After reresection, or if the local recurrence is unresectable, patients with poor PS should undergo palliative/best supportive care without further active treatment. If PS is favorable, systemic chemotherapy may be administered (especially for anaplastic oligodendrogliomas); reirradiation is a category 2B option to consider if prior radiation produced a good/durable response. Patients with recurring glioblastoma may also consider alternating electric field therapy (category 2B). In the case of diffuse or multiple recurring lesions, the options are: 1) palliative/ best supportive care for patients with poor PS; 2) systemic chemotherapy; 3) surgery to relieve mass effect; or 4) consider alternating electric field therapy for glioblastomas (category 2B).
All patients should receive best supportive care.
Medulloblastoma and Supratentorial Primitive Neuroectodermal Tumors
Cranial primitive neuroectodermal tumors (PNETs) are embryonal neoplasms showing varying degrees of differentiation. They are described by their location as infratentorial (medulloblastomas) and supratentorial (cerebral neuroblastoma, pineoblastoma, or esthesioneuroblastoma). The WHO classification system further divided these tumors into histologic variants.5 CNS PNETs are infrequent in children and very rare in adults, with an overall incidence of 0.26 per 100,000 person-years reported by the Central Brain Tumor Registry of the United States.109 Overall, it represents only 1.8% of all brain tumors, although it is the most common type among pediatric brain malignancies.
Approximately half of the affected patients will present with elevated intracranial pressure. Headache, ataxia, and nausea are commonly observed symptoms.110 All PNETs of the brain are WHO grade IV, because they are invasive and rapidly growing. They also have the tendency to disseminate through the cerebrospinal fluid (CSF). Larger retrospective case series of adult patients reported a 10-year survival rate of 48% to 55%, with frequent recurrence beyond 5 years, commonly in the posterior fossa.111,112
Treatment Overview
Surgery
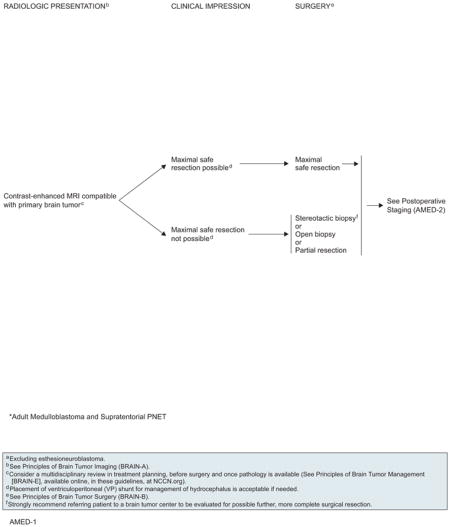
Evidence in adult patients is meager for this rare disease and no randomized trial data are available, but a general consensus exists that surgical resection should be the routine initial treatment to establish diagnosis, relieve symptoms, and maximize local control. Complete resection can be achieved in half of the patients110,113,114 and is associated with improved survival.113,115 In addition, surgical placement of a ventriculoperitoneal shunt can be used to treat hydrocephalus.
Radiation Therapy
Adjuvant radiation after surgery is the current standard of care, although most studies are based on the pediatric population. The conventional dose is 30 to 36 Gy of craniospinal irradiation and a boost to a total of 55.8 Gy to the primary brain site.113,115 A lower craniospinal dose of 23.4 Gy, combined with chemotherapy, has gained popularity for average-risk patients to lessen side effects while maintaining 55.8 Gy to the posterior fossa,111,116,117 although one randomized trial found an increased relapse risk with dose reduction.118 SRS demonstrated safety and efficacy in a small series of 12 adult patients with residual or recurrent disease.119
Systemic Therapy
The use of postirradiation chemotherapy to allow radiation dose reduction is becoming increasingly common especially for children,116,117 but optimal use of adjuvant chemotherapy is still unclear for adult patients.110–112,120,121 A phase III study that enrolled more than 400 patients between ages 3 and 21 years to receive postirradiation cisplatin-based chemotherapy regimens recorded an encouraging 86% 5-year survival.122
Several regimens are being used in the recurrence setting, most of which include etoposide.123–125 Temozolomide has also been used in this setting.126 High-dose chemotherapy in combination with autologous stem cell transplantation is a feasible strategy for patients who have had a good response with lower doses.125,127
NCCN Recommendations
Primary Treatment
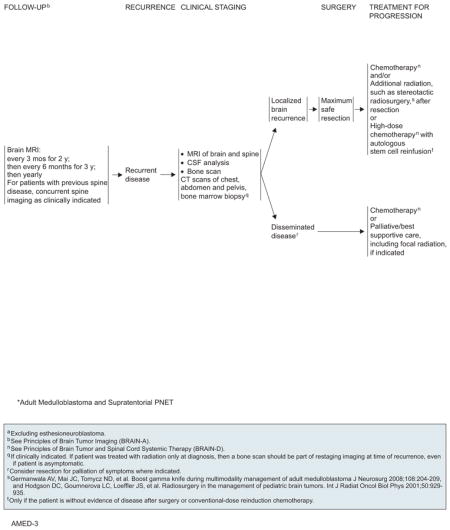
MRI scan is the gold standard for assessing and diagnosing PNET. The typical tumor shows enhancement and heterogeneity. Fourth ventricular floor infiltration is a common finding related to worse prognosis.111,112,121 Multi-disciplinary consultation before treatment initiation is advised. Maximal safe resection is recommended wherever possible. Contrast-enhanced brain MRI should be performed within 24 to 72 hours after surgery, but spinal MRI should be delayed by 2 to 3 weeks. Because of the propensity of PNET to CSF seeding, CSF sampling after spine imaging via lumbar puncture is also necessary for staging. Medulloblastoma should be staged according to the modified Chang system using information from both imaging and surgery.128,129
Adjuvant Therapy
Patients should be stratified according to recurrence risk for planning of adjuvant therapy (reviewed by Brandes et al130). The NCCN CNS Panel agrees that patients with large cell or anaplastic medulloblastoma, supratentorial PNET, disease dissemination, unresectable tumors, or residual tumors larger than 1.5 cm2 postsurgery are at heightened risk. These patients should undergo irradiation of the neuraxis followed by chemotherapy. Collection of stem cells before radiation should be considered for potential future autologous stem cell reinfusion at disease progression. For patients at average risk, craniospinal radiation alone or concurrent chemoradiation followed by chemotherapy are both viable options.
Recurrence and Progression
No robust data support an optimal follow-up schedule for PNETs. General guidelines include brain MRI every 3 months for the first 2 years, biannual brain MRI for the next 3 years, then yearly brain scans. If recurrent disease is detected on these scans, CSF sampling is also required. Concurrent spine imaging should be performed as clinically indicated for patients with previous spinal disease. Bone scans, CT scans, and bone marrow biopsies should be conducted as indicated.
Maximal safe resection should be attempted on recurrent brain tumors. High-dose chemotherapy with autologous stem cell rescue is also feasible for patients showing no evidence of disease after resection or conventional reinduction chemotherapy. On disease progression, options include chemotherapy alone, radiation alone (including SRS), and chemoradiation. Patients with metastases should be managed with chemotherapy or best supportive care, such as palliative radiation.
Brain Metastases
Metastases to the brain are the most common intracranial tumors in adults and may occur up to 10 times more frequently than primary brain tumors. Population-based data reported that 8% to 10% of cancer patients are affected by symptomatic metastatic tumors in the brain.131,132 A much higher incidence based on autopsy has been reported. As a result of advances in diagnosis and treatment, most patients improve with treatment and do not die of these metastatic lesions. Primary lung cancers are the most common source, accounting for half of intracranial metastases, although melanoma has been documented to have the highest predilection to spread to the brain. Diagnosis of CNS involvement is becoming more common in patients with breast cancer as therapy for metastatic disease is improving.133
Nearly 80% of brain metastases occur in the cerebral hemispheres, an additional 15% occur in the cerebellum, and 5% occur in the brainstem.134 These lesions typically follow a pattern of hematogenous spread to the gray-white junction where the relatively narrow caliber of the blood vessels tends to trap tumor emboli. Most cases have multiple brain metastases evident on MRI scans. The presenting signs and symptoms of metastatic brain lesions are similar to those of other mass lesions in the brain, such as headache, seizures, and neurologic impairment.
Treatment Overview
Surgery
Advances in surgical technique have rendered upfront resection followed by WBRT the standard of care for solitary brain metastases. A retrospective analysis of 13,685 patients admitted for resection of metastatic brain lesions showed a decline in in-hospital mortality from 4.6% in the period of 1988 through 1990 to 2.3% in the period of 1997 through 2000.135 High-volume hospitals and surgeons produced superior outcomes.
Patchell et al136 conducted a study that randomized 95 patients with single intracranial metastases to complete resection alone or surgery plus adjuvant WBRT. Postoperative radiation was associated with dramatic reduction in tumor recurrence (18% vs 70%; P<.001) and likelihood of neurologic deaths (14% vs 44%; P=.003). No difference in overall survival, a secondary end point, was seen between the arms. Comparison of surgery plus WBRT versus WBRT alone is discussed in the WBRT section, opposite page.
In the case of multiple lesions, the role of surgery is more restricted to obtaining biopsy samples or relieving mass effect from large symptomatic metastases. However, evidence from retrospective series suggested survival benefits from tumor resection for selected patients with good prognosis with up to 3 metastatic sites.137,138
Stereotactic Radiosurgery
The advent of SRS offered a minimally invasive alterantive to surgery. Patients undergoing SRS avoid the risk of surgery-related morbidity. Late side effects such as edema and radiation necrosis are uncommon.139
Accumulating retrospective evidence suggests that low disease volume instead of number of meta-static lesions is predictive of survival benefits.140,141 Hence, patients with multiple lesions but a low total disease volume may be amenable to SRS. Other predictors of longer survival with SRS include younger age, good PS, and primary tumor control.141–144
In a randomized Japanese study of 132 patients with 1 to 4 metastatic brain tumors smaller than 3 cm, addition of WBRT to SRS did not prolong median survival compared with SRS alone (7.5 vs 8.0 months, respectively).145 However, the 1-year brain recurrence rate was lowered in the WBRT plus SRS arm (47% vs 76%; P<.001). Another small randomized trial of 58 patients with 1 to 3 brain metastases was stopped early because of a significant decline in learning and memory function among the group receiving both SRS and WBRT compared with the SRS group (52% vs 24%).146 Analysis showed that SRS plus WBRT was associated with a better 1-year recurrence-free survival rate (73%) than SRS alone (27%). A third trial recruited 359 patients with 1 to 3 metastatic brain lesions who underwent surgery or SRS.147 They were randomized to either adjuvant WBRT or observation. Compared with the observation arm, intracranial relapse rates and neurologic mortality were lower in the WBRT arm, but overall survival and duration of functional independence were similar. A meta-analysis concluded no overall survival improvement with the addition of WBRT to SRS.148
Retrospective comparative studies showed that SRS plus WBRT resulted in equivalent if not better survival compared with surgery and WBRT.149–151 SRS also conferred a significant improvement in local control, especially for patients with radiosensitive tumors or solitary brain lesions. SRS alone compared with resection plus WBRT was evaluated in a randomized controlled trial by Muacevic et al.152 The study was stopped prematurely because of poor accrual. In the final analysis based on 64 patients with solitary brain metastases, radiosurgery alone was less invasive and resulted in equivalent survival and local control, but it was associated with a higher rate of distant relapse.
Small patient series have shown local control rates greater than 70% with SRS in the recurrence setting for patients with good PS and stable disease who have received prior WBRT.153–157
Whole-Brain Radiation Therapy
Historically, WBRT was the mainstay of treatment for metastatic lesions in the brain. It continues to play multiple roles in the modern era, such as primary intervention when surgery or SRS is not feasible (eg, polymetastatic brain metastases), as adjunctive therapy to prevent recurrence, and as treatment for recurrent disease.
Three randomized trials investigated the effectiveness of WBRT with or without surgery in patients with single brain metastases. In a study of 48 patients, Patchell et al158 showed that surgery followed by WBRT lengthened overall survival (40 vs 15 weeks in WBRT arm; P<.01) and functional dependence (38 vs 8 weeks; P<.005), and decreased recurrence (20% vs 52%; P<.02) compared with radiation alone. Similarly, combined treatment led to longer survival and functional independence in another randomized study by Vecht et al159 (n=63). The greatest difference was observed in patients with stable disease: median survival was 12 versus 7 months, and functional independence was 9 versus 4 months. A third study of 84 patients found no difference in survival between the strategies; however, patients with extensive systemic disease and lower performance level were included, which likely resulted in poorer outcomes in the surgical arm.160
The impact of SRS boost in addition to WBRT was evaluated in 2 published randomized controlled studies. A multi-institutional trial by RTOG (RTOG 9508) randomly assigned 333 patients with 1 to 3 brain metastases to WBRT plus SRS or WBRT only.161 Despite the inclusion of larger tumors (3–4 cm) that are not favorable to SRS, the authors found a significant survival benefit in the combined arm (6.5 vs 4.9 months; P=.04) when treating a single metastases; this benefit was not observed in patients with multiple (2 or 3) lesions. A much smaller trial of 27 patients with 2 to 4 lesions found no significant difference in survival, although SRS did extend time to local failure (36 vs 6 months; P=.0005).162
Taken together, WBRT in conjunction with surgery or SRS leads to better clinical outcomes than WBRT alone for good performance patients with solitary metastatic intracranial lesions. However, many patients are not candidates for resection because of the inaccessibility of the tumor, extensive systemic disease, or other factors. WBRT is the main choice of primary therapy for this patient group.
No randomized data are available in the recurrent setting, but case series reported 31% to 70% of symptom-relieving response to irradiation.163–165
Systemic Therapy
Systemic therapy is rarely used as primary therapy for brain metastases. In randomized studies, the addition of carboplatin or temozolomide to WBRT did not improve overall survival compared with radiation alone,166,167 although an increase in progression-free survival or radiologic response has been reported with temozolomide.167,168 Many tumors that metastasize to the brain are not very chemosensitive or have been already heavily pretreated with organ-specific effective agents. Poor penetration through the BBB is an additional concern. Therefore, chemotherapy is usually considered as a last line of therapy for recurrent disease when other options have been exhausted (ie, surgery, SRS, radiation). The choice of agent depends on the histology of the primary tumor. BCNU wafer implantation is a reasonable option at recurrence when resection is considered.169
Among various agents, temozolomide may be useful in some patients with previously untreated brain metastases from metastatic melanoma.170 Temozolomide given on a prolonged schedule in combination with thalidomide has been tested in a phase II study of patients with brain metastases, but the high toxicity and lack of response rendered the regimen inappropriate.171
A study of high-dose methotrexate in patients mostly with breast cancer achieved disease control in 56% of patients.172 Other agents shown to have activity in breast cancer include platinum plus etoposide173,174 and capecitabine with or without lapatinib.175–177
A phase I/II study of topotecan plus WBRT has shown a 72% response rate in 75 patients with brain metastases.178 Unfortunately, a follow-up phase III trial was closed early because of slow accrual.179
NCCN Recommendations
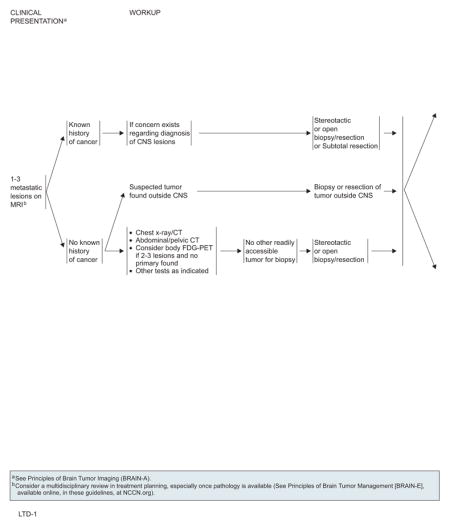
Workup
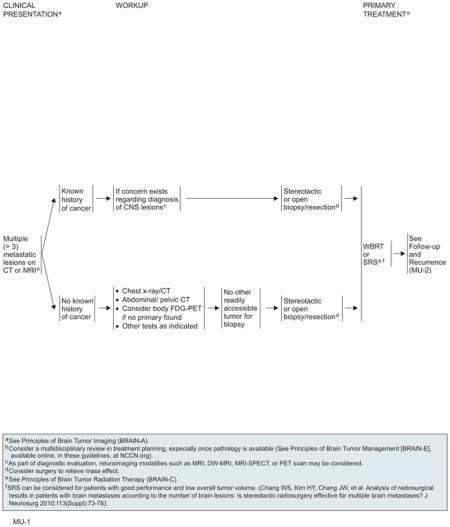
Patients who present with a single mass or multiple lesions on MRI or CT imaging suggestive of metastatic cancer to the brain, and who do not have a known primary, require a careful systemic workup with chest radiograph or CT, abdominal or pelvic CT, or other tests as indicated. FDG-PET can be considered if more than one brain lesion is present and no primary has yet been found. If no other readily accessible tumor is available for biopsy, a stereotactic or open biopsy resection is indicated to establish a diagnosis. Among patients with a known history of cancer and if concerns exist regarding the diagnosis of CNS lesions, a stereotactic or open biopsy resection or subtotal resection is also needed. Because brain metastases are often managed with multiple modalities, the NCCN CNS Panel encourages multidisciplinary consultation before treatment for optimal planning.
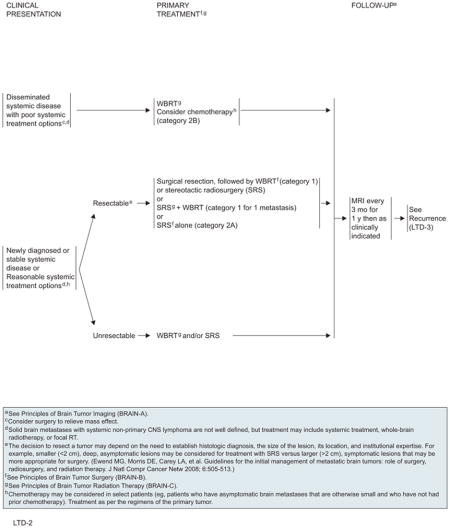
Treatment for Limited (1–3) Metastatic Lesions
For patients with limited systemic disease or for whom reasonable systemic treatment options exist, aggressive management should be strongly considered. For surgical candidates, high-level evidence supports category 1 recommendations for surgical resection plus postoperative WBRT and for SRS plus WBRT if only one brain lesion is involved. SRS alone or after resection are also reasonable options. Macroscopic total removal is the objective of surgery. The choice between open resection and SRS depends on multiple factors, such as tumor size and location. The best outcome for SRS is achieved for small, deep lesions at institutions with experienced staff. If the tumor is unresectable, WBRT and/or radiosurgery can be used.
Patients with progressive extracranial disease whose survival is less than 3 months should be treated with WBRT alone, but surgery may be considered for symptom relief. The panel did not reach a consensus on the value of chemotherapy (category 2B). It may be considered in select patients using regimens specific to the primary cancer. In patients with systemic cancers and druggable targets (eg, epidermal growth factor receptor mutations in non–small cell lung cancer and BRAF mutations in metastatic melanoma), targeted therapy in neurologically asymptomatic patients with brain metastases is considered reasonable before administration of RT.
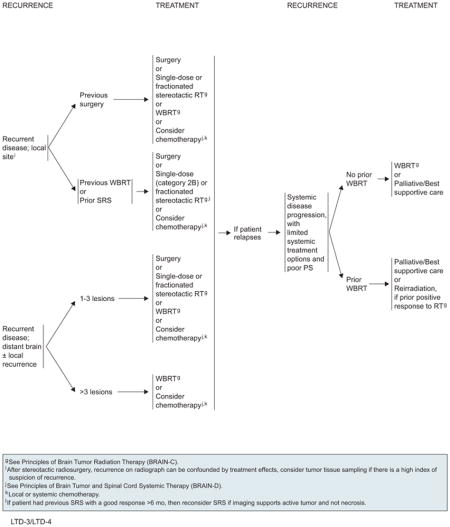
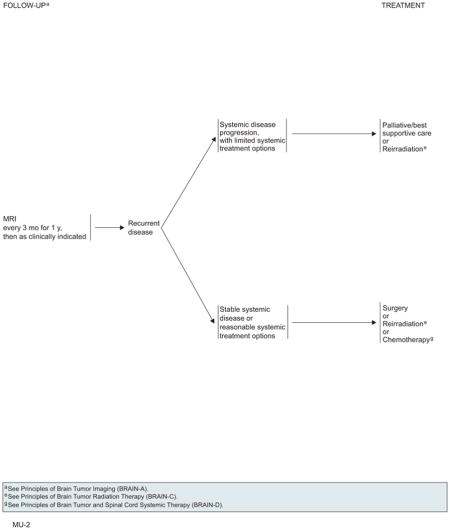
Patients should be followed with MRI every 3 months for 1 year and then as clinically indicated. Recurrence seen on radiograph can be confounded by the treatment effects of SRS. Consider tumor tissue sampling if there is a high index of suspicion of recurrence. On detection of recurrent disease, prior therapy clearly influences the choice of further therapies. Patients with recurrent CNS disease should be assessed for local versus systemic disease, because therapy will differ. For local recurrences, patients who were previously treated with surgery can receive the following options: 1) surgery; 2) single-dose or fractionated SRS; 3) WBRT; or 4) chemotherapy. However, patients who previously received WBRT probably should not undergo WBRT at recurrence because of concern regarding neurotoxicity. If the patient had previous SRS with a durable response for more than 6 months, reconsider SRS if imaging supports active tumor and not necrosis. Repeat SRS to a prior location is a category 2B recommendation. The algorithm for distant brain recurrences branches depending on whether patients have either 1 to 3 lesions or more than 3 lesions. In both cases, patients may receive WBRT or consider local/systemic chemotherapy, but patients with 1 to 3 recurrent tumors have the additional options of surgery or SRS.
WBRT should be used (30–45 Gy, given in 1.8- to 3.0-Gy fractions) depending on the patient’s PS, if this modality was not used for initial therapy. Local or systemic chemotherapy may be considered for select patients if the multiple lesions cannot be controlled by a combination of surgery and radiosurgery.180
If systemic CNS disease progression occurs in the setting of limited systemic treatment options and poor PS, WBRT should be administered for patients have not been previously irradiated. For patients who have received prior WBRT, reirradiation is an option only if they had a positive response to the first course of RT treatment. Palliative/best supportive care is also an option in either case.
Treatment for Multiple (>3) Metastatic Lesions
All patients diagnosed with more than 3 metastatic lesions should be treated with WBRT or SRS as primary therapy. The standard regimens for WBRT are 30.0 Gy in 10 fractions or 37.5 Gy in 15 fractions. For patients with poor neurologic performance, a more rapid course of RT can be considered (20.0 Gy, delivered in 5 fractions). SRS may be considered in patients with good PS and low overall tumor volume. Palliative neurosurgery should be considered if a lesion is causing a life-threatening mass effect, hemorrhage, or hydrocephalus.
After WBRT or SRS, patients should have a repeat contrast-enhanced MRI scan every 3 months for 1 year. If a recurrence is found, the algorithm branches depending on whether patients have 1) systemic disease progression with limited systemic treatment options, or 2) stable systemic disease or reasonable systemic treatment options. For patients with systemic disease progression, options include palliative/best supportive care or reirradiation. For patients with stable systemic disease, options include surgery, reirradiation, or chemotherapy.
Table 1.
Individual Disclosures for the NCCN Central Nervous System Cancer Panel
| Panel Member | Clinical Research Support | Advisory Boards, Speakers Bureau, Expert Witness, or Consultant | Patent, Equity, or Royalty | Other | Date Completed |
|---|---|---|---|---|---|
| Mario Ammirati, MD | None | None | None | None | 3/29/12 |
| Philip J. Bierman, MD | Otsuka Pharmaceuticals; and Seattle Genetics | None | None | None | 5/1/13 |
| Henry Brem, MD | None | None | None | None | 9/27/12 |
| Nicholas Butowski, MD | None | Roche Laboratories, Inc. | None | None | 8/17/13 |
| Marc C. Chamberlain, MD | NCCN-funded Bendamustine trial for recurrent high-grade gliomas | PharmacoKinesis Advisory Board | None | None | 8/9/12 |
| Lisa M. DeAngelis, MD | None | None | None | None | 8/20/12 |
| Robert A. Fenstermaker, MD | None | None | None | None | 8/28/13 |
| Allan Friedman, MD | None | None | None | None | 9/26/12 |
| Mark R. Gilbert, MD | RTOG Trial | Abbott Laboratories; Genentech, Inc.; Merck & Co., Inc.; and EMD Serono | None | None | 8/18/13 |
| Deneen Hesser, MSHSA, RN, OCN | None | None | None | None | 9/27/12 |
| Matthias Holdhoff, MD, PhD | None | None | None | None | 11/7/12 |
| Larry Junck, MD | None | None | None | None | 3/14/13 |
| Ronald Lawson, MD | None | None | None | None | 8/29/13 |
| Jay S. Loeffler, MD | None | None | None | None | 8/20/13 |
| Moshe H. Maor, MD | None | None | None | None | 8/24/12 |
| Paul L. Moots, MD | ECOG; and EMD Sereno | None | None | None | 10/9/12 |
| Tara Morrison, MD | None | Merck & Co., Inc. | None | None | 10/1/12 |
| Maciej M. Mrugala, MD, PhD, MPH | None | Enzon Pharmaceuticals; and Schering-Plough Corporation | None | None | 11/24/09 |
| Louis Burt Nabors, MD | AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim GmbH; Eli Lilly and Company; Genentech, Inc.; GlaxoSmithKline; ImClone Systems Incorporated; Merck KGaA; and Transmolecular | Merck KGaA | None | None | 3/3/13 |
| Herbert B. Newton, MD | None | Eisai Inc.; Genentech, Inc.; and Merck & Co., Inc. | None | None | 8/16/12 |
| Jana Portnow, MD | None | None | None | None | 11/28/12 |
| Jeffrey J. Raizer, MD | Eli Lilly and Company; Genentech, Inc.; MedImmune Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; Arno; Celldex; Diffusion; Geron; and Myriad | Enzon Pharmaceuticals; Genentech, Inc.; Merck & Co., Inc.; and Novartis Pharmaceuticals Corporation | None | None | 10/3/12 |
| Lawrence Recht, MD | Genentech, Inc.; and Celtic Pharmaceuticals | Genentech, Inc. | None | None | 2/27/13 |
| Dennis C. Shrieve, MD, PhD | None | None | None | None | 2/26/13 |
| Allen K. Sills Jr, MD | None | None | None | None | 8/19/13 |
| David Tran, MD, PhD | None | None | None | None | 9/26/12 |
| Nam Tran, MD, PhD | None | None | None | None | 10/8/12 |
| Frank D. Vrionis, MD, MPH, PhD | Globus; and Synthes | Florida Board of Medicine; and Southeastern Brain Tumor Association | None | Orthofix | 11/13/12 |
| Patrick Y. Wen, MD | None | Amgen Inc.; AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim GmbH; Eisai Inc.; Exelixis Inc.; Genentech, Inc.; MedImmune Inc.; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; EMD Serono; Geron; Vascular Biogenics; and sanofi-aventis U.S. | None | None | 6/3/13 |
The NCCN Guidelines Staff have no conflicts to disclose.
NCCN Central Nervous System Cancers Panel Members
*f,gLouis Burt Nabors, MD/ChairΨ
University of Alabama at Birmingham
Comprehensive Cancer Center
Mario Ammirati, MD, MBA¶
The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
cPhilip J. Bierman, MD†‡
UNMC Eppley Cancer Center at The Nebraska Medical Center
aHenry Brem, MD¶
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
Nicholas Butowski, MDΨ
UCSF Helen Diller Family Comprehensive Cancer Center
*a,b,e,gMarc C. Chamberlain, MDΨ
University of Washington/Seattle Cancer Care Alliance
a,cLisa M. DeAngelis, MDΨ
Memorial Sloan-Kettering Cancer Center
a,bRobert A. Fenstermaker, MD¶
Roswell Park Cancer Institute
Allan Friedman, MD¶
Duke Cancer Institute
Mark R. Gilbert, MDΨ
The University of Texas MD Anderson Cancer Center
a,iDeneen Hesser, MSHSA, RN, OCN¥
American Brain Tumor Association
gMatthias Holdhoff, MD, PhD†
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
a,f,g,hLarry Junck, MDΨ
University of Michigan Comprehensive Cancer Center
Ronald Lawson, MD
St. Jude Children’s Research Hospital/The University of Tennessee Health Science Center
a,hJay S. Loeffler, MD§
Massachusetts General Hospital Cancer Center
*b,hMoshe H. Maor, MD§
The University of Texas MD Anderson Cancer Center
aPaul L. Moots, MDΨ
Vanderbilt-Ingram Cancer Center
iTara Morrison, MDΨ
Fox Chase Cancer Center
*c,d,gMaciej Mrugala, MD, PhD, MPHΨ
University of Washington/Seattle Cancer Care Alliance
Herbert B. Newton, MDΨ
The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
*d,gJana Portnow, MD†Ψ
City of Hope Comprehensive Cancer Center
a,fJeffrey J. Raizer, MDΨ
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
Lawrence Recht, MDΨ
Stanford Cancer Institute
hDennis C. Shrieve, MD, PhD§
Huntsman Cancer Institute at the University of Utah
a,b,h,iAllen K. Sills Jr, MD¶
Vanderbilt-Ingram Cancer Center
David Tran, MD, PhD†
Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine
Nam Tran, MD, PhD¶
Moffitt Cancer Center
*b,eFrank D. Vrionis, MD, MPH, PhD¶
Moffitt Cancer Center
gPatrick Y. Wen, MDΨ
Dana-Farber/Brigham and Women’s Cancer Center
NCCN Staff: Maria Ho, PhD, and Nicole McMillian, MS
KEY:
*Writing Committee Member
Subcommittees: aMeningiomas; bMetastatic Spine Tumors; cPCNSL Review; dAdult Medulloblastoma; ePrimary Spinal Cord Tumors; fPrinciples of Imaging; gPrinciples of Systemic Therapy; hPrinciples of Radiation Therapy; iPrinciples of Brain Tumor Management (Note: Underlining denotes subcommittee lead)
Specialties: †Medical Oncology; ‡Hematology/Hematology Oncology; §Radiotherapy/Radiation Oncology; ΨNeurology/ Neuro-Oncology; ¶Surgery/Surgical Oncology; ¥Patient Advocacy
Footnotes
NCCN Categories of Evidence and Consensus
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way. The full NCCN Guidelines for Central Nervous System Cancers are not printed in this issue of JNCCN but can be accessed online at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2013, All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosures for the Central Nervous System Cancers Panel
At the beginning of each NCCN Guidelines panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Central Nervous System Cancers Panel members can be found on page 1151. (The most recent version of these guidelines and accompanying disclosures are available on the NCCN Web site at NCCN.org.)
These guidelines are also available on the Internet. For the latest update, visit NCCN.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Maher EA, McKee AC. Neoplasms of the central nervous system. In: Skarin AT, Canellos GP, editors. Atlas of Diagnostic Oncology. 3. London, United Kingdom: Elsevier Science Ltd; 2003. [Google Scholar]
- 3.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 4.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1055. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 5.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 7.Daniels TB, Brown PD, Felten SJ, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2011;81:218–224. doi: 10.1016/j.ijrobp.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 9.Piepmeier J, Christopher S, Spencer D, et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996;38:872–878. doi: 10.1097/00006123-199605000-00002. discussion 878–879. [DOI] [PubMed] [Google Scholar]
- 10.Afra D, Osztie E, Sipos L, Vitanovics D. Preoperative history and postoperative survival of supratentorial low-grade astrocytomas. Br J Neurosurg. 1999;13:299–305. doi: 10.1080/02688699943727. [DOI] [PubMed] [Google Scholar]
- 11.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 12.CBTRUS. Statistical report: Primary Brain Tumors in the United States, 1995–1999. Chicago: Central Brain Tumor Registry of the United States; 2002. [Google Scholar]
- 13.Lang FF, Gilbert MR. Diffusely infiltrative low-grade gliomas in adults. J Clin Oncol. 2006;24:1236–1245. doi: 10.1200/JCO.2005.05.2399. [DOI] [PubMed] [Google Scholar]
- 14.Lo SS, Cho KH, Hall WA, et al. Does the extent of surgery have an impact on the survival of patients who receive postoperative radiation therapy for supratentorial low-grade gliomas? Int J Cancer. 2001;96(Suppl):71–78. doi: 10.1002/ijc.10359. [DOI] [PubMed] [Google Scholar]
- 15.Philippon JH, Clemenceau SH, Fauchon FH, Foncin JF. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993;32:554–559. doi: 10.1227/00006123-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Soffietti R, Chio A, Giordana MT, et al. Prognostic factors in well-differentiated cerebral astrocytomas in the adult. Neurosurgery. 1989;24:686–692. doi: 10.1227/00006123-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 18.Shaw EG, Daumas-Duport C, Scheithauer BW, et al. Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg. 1989;70:853–861. doi: 10.3171/jns.1989.70.6.0853. [DOI] [PubMed] [Google Scholar]
- 19.Kilic T, Ozduman K, Elmaci I, et al. Effect of surgery on tumor progression and malignant degeneration in hemispheric diffuse low-grade astrocytomas. J Clin Neurosci. 2002;9:549–552. doi: 10.1054/jocn.2002.1136. [DOI] [PubMed] [Google Scholar]
- 20.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–707. doi: 10.1227/01.NEU.0000325729.41085.73. author reply 707–708. [DOI] [PubMed] [Google Scholar]
- 21.Chaichana KL, McGirt MJ, Laterra J, et al. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2010;112:10–17. doi: 10.3171/2008.10.JNS08608. [DOI] [PubMed] [Google Scholar]
- 22.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Karim AB, Afra D, Cornu P, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys. 2002;52:316–324. doi: 10.1016/s0360-3016(01)02692-x. [DOI] [PubMed] [Google Scholar]
- 24.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 25.Shaw EG, Tatter SB, Lesser GJ, et al. Current controversies in the radiotherapeutic management of adult low-grade glioma. Semin Oncol. 2004;31:653–658. doi: 10.1053/j.seminoncol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol. 2003;5:153–160. doi: 10.1215/S1152-8517-02-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 28.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/ Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 29.Roberge D, Souhami L, Olivier A, et al. Hypofractionated stereotactic radiotherapy for low grade glioma at McGill University: long-term follow-up. Technol Cancer Res Treat. 2006;5:1–8. doi: 10.1177/153303460600500101. [DOI] [PubMed] [Google Scholar]
- 30.Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21:646–651. doi: 10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15:330–337. doi: 10.1158/1078-0432.CCR-08-0888. [DOI] [PubMed] [Google Scholar]
- 32.Pouratian N, Gasco J, Sherman JH, et al. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol. 2007;82:281–288. doi: 10.1007/s11060-006-9280-4. [DOI] [PubMed] [Google Scholar]
- 33.Shaw EG, Berkey B, Coons SW, et al. Initial report of Radiation Therapy Oncology Group (RTOG) 9802: prospective studies in adult low-grade glioma (LGG) [abstract] J Clin Oncol. 2006;24(Suppl 18):Abstract 1500. [Google Scholar]
- 34.Shaw EG, Wang M, Coons S, et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG) [abstract] J Clin Oncol. 2008;26(Suppl 15):Abstract 2006. [Google Scholar]
- 35.Perry JR, Rizek P, Cashman R, et al. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer. 2008;113:2152–2157. doi: 10.1002/cncr.23813. [DOI] [PubMed] [Google Scholar]
- 36.Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100:65–71. doi: 10.1007/s11060-010-0136-6. [DOI] [PubMed] [Google Scholar]
- 37.Moghrabi A, Friedman HS, Ashley DM, et al. Phase II study of carboplatin (CBDCA) in progressive low-grade gliomas. Neurosurg Focus. 1998;4:e3. doi: 10.3171/foc.1998.4.4.6. [DOI] [PubMed] [Google Scholar]
- 38.Brandes AA, Basso U, Vastola F, et al. Carboplatin and teniposide as third-line chemotherapy in patients with recurrent oligodendroglioma or oligoastrocytoma: a phase II study. Ann Oncol. 2003;14:1727–1731. doi: 10.1093/annonc/mdg494. [DOI] [PubMed] [Google Scholar]
- 39.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 40.Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 41.Buckner JC, Gesme D, Jr, O’Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21:251–255. doi: 10.1200/JCO.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Cairncross G, Macdonald D, Ludwin S, et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994;12:2013–2021. doi: 10.1200/JCO.1994.12.10.2013. [DOI] [PubMed] [Google Scholar]
- 43.Ino Y, Betensky RA, Zlatescu MC, et al. Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- 44.van den Bent M, Chinot OL, Cairncross JG. Recent developments in the molecular characterization and treatment of oligodendroglial tumors. Neuro Oncol. 2003;5:128–138. doi: 10.1215/S1522-8517-02-00028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 46.Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109:835–841. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo SS, Hall WA, Cho KH, et al. Radiation dose response for supratentorial low-grade glioma—institutional experience and literature review. J Neurol Sci. 2003;214:43–48. doi: 10.1016/s0022-510x(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 48.Central Brain Tumor Registry of the United States. [Accessed January 17, 2013];CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. Available at: http://www.cbtrus.org/2010-NPCR-SEER/CBTRUS-WEBREPORT-Final-3-2-10.pdf.
- 49.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 50.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 51.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 52.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 53.Wood JR, Green SB, Shapiro WR. The prognostic importance of tumor size in malignant gliomas: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6:338–343. doi: 10.1200/JCO.1988.6.2.338. [DOI] [PubMed] [Google Scholar]
- 54.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 55.Barker FG, 2nd, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709–720. doi: 10.1097/00006123-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Park JK, Hodges T, Arko L, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–3843. doi: 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 58.Kristiansen K, Hagen S, Kollevold T, et al. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer. 1981;47:649–652. doi: 10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 59.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 60.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 61.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 62.Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005–1011. doi: 10.1016/s0360-3016(98)00159-x. [DOI] [PubMed] [Google Scholar]
- 63.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Nieder C, Astner ST, Mehta MP, et al. Improvement, clinical course, and quality of life after palliative radiotherapy for recurrent glioblastoma. Am J Clin Oncol. 2008;31:300–305. doi: 10.1097/COC.0b013e31815e3fdc. [DOI] [PubMed] [Google Scholar]
- 65.Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:167. doi: 10.1186/1471-2407-7-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 67.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 68.Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 69.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 70.Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–48. doi: 10.1097/00006123-199707000-00011. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 71.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148:269–275. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 73.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 74.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 75.Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–3867. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: A randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM) [abstract] J Clin Oncol. 2011;29(Suppl 15):Abstract 2006. [Google Scholar]
- 77.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 78.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 79.Mollemann M, Wolter M, Felsberg J, et al. Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer. 2005;113:379–385. doi: 10.1002/ijc.20575. [DOI] [PubMed] [Google Scholar]
- 80.Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel(R)) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25:459–469. doi: 10.3109/02688697.2010.550342. [DOI] [PubMed] [Google Scholar]
- 81.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salvati M, D’Elia A, Frati A, et al. Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high grade gliomas at first diagnosis. J Neurosurg Sci. 2011;55:1–6. [PubMed] [Google Scholar]
- 83.Brandes AA, Vastola F, Basso U, et al. A prospective study on glioblastoma in the elderly. Cancer. 2003;97:657–662. doi: 10.1002/cncr.11097. [DOI] [PubMed] [Google Scholar]
- 84.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88:97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 85.Glantz M, Chamberlain M, Liu Q, et al. Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer. 2003;97:2262–2266. doi: 10.1002/cncr.11323. [DOI] [PubMed] [Google Scholar]
- 86.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 88.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 89.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 90.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Triebels VH, Taphoorn MJ, Brandes AA, et al. Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology. 2004;63:904–906. doi: 10.1212/01.wnl.0000137049.65631.db. [DOI] [PubMed] [Google Scholar]
- 92.Chamberlain MC, Tsao-Wei DD. Salvage chemotherapy with cyclophosphamide for recurrent, temozolomide-refractory glioblastoma multiforme. Cancer. 2004;100:1213–1220. doi: 10.1002/cncr.20072. [DOI] [PubMed] [Google Scholar]
- 93.Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with cyclophosphamide for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer. 2006;106:172–179. doi: 10.1002/cncr.21582. [DOI] [PubMed] [Google Scholar]
- 94.Chamberlain MC, Wei-Tsao DD, Blumenthal DT, Glantz MJ. Salvage chemotherapy with CPT-11 for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer. 2008;112:2038–2045. doi: 10.1002/cncr.23404. [DOI] [PubMed] [Google Scholar]
- 95.Fulton D, Urtasun R, Forsyth P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. J Neurooncol. 1996;27:149–155. doi: 10.1007/BF00177478. [DOI] [PubMed] [Google Scholar]
- 96.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 97.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 98.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chamberlain MC, Johnston S. Bevacizumab for recurrent alkylator-refractory anaplastic oligodendroglioma. Cancer. 2009;115:1734–1743. doi: 10.1002/cncr.24179. [DOI] [PubMed] [Google Scholar]
- 100.Chamberlain MC, Johnston S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J Neurooncol. 2009;91:359–367. doi: 10.1007/s11060-008-9722-2. [DOI] [PubMed] [Google Scholar]
- 101.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 102.Soffietti R, Ruda R, Trevisan E, et al. Phase II study of bevacizumab and nitrosourea in patients with recurrent malignant glioma: a multicenter Italian study [abstract] J Clin Oncol. 2009;27(Suppl 15S):Abstract 2012. [Google Scholar]
- 103.Taillibert S, Vincent LA, Granger B, et al. Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology. 2009;72:1601–1606. doi: 10.1212/WNL.0b013e3181a413be. [DOI] [PubMed] [Google Scholar]
- 104.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 105.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 106.Yovino S, Grossman SA. Treatment of glioblastoma in “elderly” patients. Curr Treat Options Oncol. 2011;12:253–262. doi: 10.1007/s11864-011-0158-0. [DOI] [PubMed] [Google Scholar]
- 107.Tsien C, Galban CJ, Chenevert TL, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28:2293–2299. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12:240–252. doi: 10.1007/s11864-011-0157-1. [DOI] [PubMed] [Google Scholar]
- 109.Surawicz TS, McCarthy BJ, Kupelian V, et al. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kunschner LJ, Kuttesch J, Hess K, Yung WK. Survival and recurrence factors in adult medulloblastoma: the M.D. Anderson Cancer Center experience from 1978 to 1998. Neuro Oncol. 2001;3:167–173. doi: 10.1093/neuonc/3.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Padovani L, Sunyach MP, Perol D, et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68:433–440. doi: 10.1016/j.ijrobp.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 112.Carrie C, Lasset C, Alapetite C, et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74:2352–2360. doi: 10.1002/1097-0142(19941015)74:8<2352::aid-cncr2820740821>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 113.Chan AW, Tarbell NJ, Black PM, et al. Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery. 2000;47:623–631. doi: 10.1097/00006123-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 114.Frost PJ, Laperriere NJ, Wong CS, et al. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995;32:951–957. doi: 10.1016/0360-3016(94)00612-o. [DOI] [PubMed] [Google Scholar]
- 115.Chargari C, Feuvret L, Levy A, et al. Reappraisal of clinical outcome in adult medulloblastomas with emphasis on patterns of relapse. Br J Neurosurg. 2010;24:460–467. doi: 10.3109/02688691003739881. [DOI] [PubMed] [Google Scholar]
- 116.Douglas JG, Barker JL, Ellenbogen RG, Geyer JR. Concurrent chemotherapy and reduced-dose cranial spinal irradiation followed by conformal posterior fossa tumor bed boost for average-risk medulloblastoma: efficacy and patterns of failure. Int J Radiat Oncol Biol Phys. 2004;58:1161–1164. doi: 10.1016/j.ijrobp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 117.Merchant TE, Kun LE, Krasin MJ, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55. 8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70:782–787. doi: 10.1016/j.ijrobp.2007.07.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deutsch M, Thomas PR, Krischer J, et al. Results of a prospective randomized trial comparing standard dose neuraxis irradiation (3,600 cGy/20) with reduced neuraxis irradiation (2,340 cGy/13) in patients with low-stage medulloblastoma. A Combined Children’s Cancer Group-Pediatric Oncology Group Study. Pediatr Neurosurg. 1996;24:167–176. doi: 10.1159/000121042. [DOI] [PubMed] [Google Scholar]
- 119.Germanwala AV, Mai JC, Tomycz ND, et al. Boost Gamma Knife surgery during multimodality management of adult medulloblastoma. J Neurosurg. 2008;108:204–209. doi: 10.3171/JNS/2008/108/2/0204. [DOI] [PubMed] [Google Scholar]
- 120.Riffaud L, Saikali S, Leray E, et al. Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg. 2009;111:478–487. doi: 10.3171/2009.1.JNS081004. [DOI] [PubMed] [Google Scholar]
- 121.Herrlinger U, Steinbrecher A, Rieger J, et al. Adult medulloblastoma: prognostic factors and response to therapy at diagnosis and at relapse. J Neurol. 2005;252:291–299. doi: 10.1007/s00415-005-0560-2. [DOI] [PubMed] [Google Scholar]
- 122.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 123.Ashley DM, Meier L, Kerby T, et al. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14:1922–1927. doi: 10.1200/JCO.1996.14.6.1922. [DOI] [PubMed] [Google Scholar]
- 124.Chamberlain MC, Kormanik PA. Chronic oral VP-16 for recurrent medulloblastoma. Pediatr Neurol. 1997;17:230–234. doi: 10.1016/s0887-8994(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 125.Dunkel IJ, Gardner SL, Garvin JH, Jr, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12:297–303. doi: 10.1093/neuonc/nop031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 127.Gill P, Litzow M, Buckner J, et al. High-dose chemotherapy with autologous stem cell transplantation in adults with recurrent embryonal tumors of the central nervous system. Cancer. 2008;112:1805–1811. doi: 10.1002/cncr.23362. [DOI] [PubMed] [Google Scholar]
- 128.Cohen ME, Duffner P, editors. Brain Tumors in Children. 2. New York, NY: McGraw-Hill; 1994. [Google Scholar]
- 129.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 130.Brandes AA, Franceschi E, Tosoni A, et al. Adult neuroectodermal tumors of posterior fossa (medulloblastoma) and of supratentorial sites (stPNET) Crit Rev Oncol Hematol. 2009;71:165–179. doi: 10.1016/j.critrevonc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 131.Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 132.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 133.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 134.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 135.Barker FG., 2nd Craniotomy for the resection of metastatic brain tumors in the U.S. 1988–2000: decreasing mortality and the effect of provider caseload. Cancer. 2004;100:999–1007. doi: 10.1002/cncr.20058. [DOI] [PubMed] [Google Scholar]
- 136.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 137.Paek SH, Audu PB, Sperling MR, et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005;56:1021–1034. [PubMed] [Google Scholar]
- 138.Stark AM, Tscheslog H, Buhl R, et al. Surgical treatment for brain metastases: prognostic factors and survival in 177 patients. Neurosurg Rev. 2005;28:115–119. doi: 10.1007/s10143-004-0364-3. [DOI] [PubMed] [Google Scholar]
- 139.Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119–1127. doi: 10.1056/NEJMct0806951. [DOI] [PubMed] [Google Scholar]
- 140.Banfill KE, Bownes PJ, St Clair SE, et al. Stereotactic radiosurgery for the treatment of brain metastases: impact of cerebral disease burden on survival. Br J Neurosurg. 2012;26:674–678. doi: 10.3109/02688697.2012.690913. [DOI] [PubMed] [Google Scholar]
- 141.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 142.Kased N, Binder DK, McDermott MW, et al. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys. 2009;75:1132–1140. doi: 10.1016/j.ijrobp.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 143.Karlsson B, Hanssens P, Wolff R, et al. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. doi: 10.3171/2008.10.JNS08214. [DOI] [PubMed] [Google Scholar]
- 144.Hunter GK, Suh JH, Reuther AM, et al. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 145.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 146.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 147.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 149.O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 150.Rades D, Kueter JD, Veninga T, et al. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT+SRS) versus surgery plus whole brain radiotherapy (OP+WBRT) for 1–3 brain metastases: results of a matched pair analysis. Eur J Cancer. 2009;45:400–404. doi: 10.1016/j.ejca.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 151.Schoggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142:621–626. doi: 10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- 152.Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 153.Akyurek S, Chang EL, Mahajan A, et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007;30:310–314. doi: 10.1097/01.coc.0000258365.50975.f6. [DOI] [PubMed] [Google Scholar]
- 154.Loeffler JS, Kooy HM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol. 1990;8:576–582. doi: 10.1200/JCO.1990.8.4.576. [DOI] [PubMed] [Google Scholar]
- 155.Noel G, Medioni J, Valery CA, et al. Three irradiation treatment options including radiosurgery for brain metastases from primary lung cancer. Lung Cancer. 2003;41:333–343. doi: 10.1016/s0169-5002(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 156.Noel G, Proudhom MA, Valery CA, et al. Radiosurgery for reirradiation of brain metastasis: results in 54 patients. Radiother Oncol. 2001;60:61–67. doi: 10.1016/s0167-8140(01)00359-0. [DOI] [PubMed] [Google Scholar]
- 157.Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247–254. doi: 10.3171/jns.2005.102.s_supplement.0247. [DOI] [PubMed] [Google Scholar]
- 158.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 159.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 160.Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470–1476. doi: 10.1002/(sici)1097-0142(19961001)78:7<1470::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 161.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 162.Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 163.Cooper JS, Steinfeld AD, Lerch IA. Cerebral metastases: value of reirradiation in selected patients. Radiology. 1990;174:883–885. doi: 10.1148/radiology.174.3.2305074. [DOI] [PubMed] [Google Scholar]
- 164.Sadikov E, Bezjak A, Yi QL, et al. Value of whole brain reirradiation for brain metastases—single centre experience. Clin Oncol (R Coll Radiol) 2007;19:532–538. doi: 10.1016/j.clon.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 165.Wong WW, Schild SE, Sawyer TE, Shaw EG. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585–590. doi: 10.1016/0360-3016(95)02156-6. [DOI] [PubMed] [Google Scholar]
- 166.Guerrieri M, Wong K, Ryan G, et al. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer. 2004;46:107–111. doi: 10.1016/j.lungcan.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 167.Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 168.Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20:3644–3650. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 169.Ewend MG, Brem S, Gilbert M, et al. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin Cancer Res. 2007;13:3637–3641. doi: 10.1158/1078-0432.CCR-06-2095. [DOI] [PubMed] [Google Scholar]
- 170.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 171.Krown SE, Niedzwiecki D, Hwu WJ, et al. Phase II study of temozolomide and thalidomide in patients with metastatic melanoma in the brain: high rate of thromboembolic events (CALGB 500102) Cancer. 2006;107:1883–1890. doi: 10.1002/cncr.22239. [DOI] [PubMed] [Google Scholar]
- 172.Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78:255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 173.Cocconi G, Lottici R, Bisagni G, et al. Combination therapy with platinum and etoposide of brain metastases from breast carcinoma. Cancer Invest. 1990;8:327–334. doi: 10.3109/07357909009012049. [DOI] [PubMed] [Google Scholar]
- 174.Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599–1605. [PubMed] [Google Scholar]
- 175.Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107:1348–1354. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 176.Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22:625–630. doi: 10.1093/annonc/mdq434. [DOI] [PubMed] [Google Scholar]
- 177.Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases--the UK experience. Br J Cancer. 2010;102:995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hedde JP, Neuhaus T, Schuller H, et al. A phase I/II trial of topotecan and radiation therapy for brain metastases in patients with solid tumors. Int J Radiat Oncol Biol Phys. 2007;68:839–844. doi: 10.1016/j.ijrobp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 179.Neuhaus T, Ko Y, Muller RP, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer. 2009;100:291–297. doi: 10.1038/sj.bjc.6604835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lang FF, Chang EL, Abi-Said D. Metastatic brain tumors. In: Winn H, editor. Youman’s Neurological Surgery. 5. Philadelphia, PA: Saunders; 2004. pp. 1077–1097. [Google Scholar]


