Abstract
The kidney has the highest abundance of cytochrome P-450 of all extrahepatic organs. Within the kidney, the highest concentration of cytochrome P-450 is found in the proximal tubule. Whether 20- or 19(S)-hydroxyeicosatetraenoic acid (HETE), the major P-450 metabolites of arachidonic acid in the proximal tubule, affect transport in this segment has not been previously investigated. We examined the direct effects of 20- and 19(S)-HETE on volume absorption (Jv) in the rabbit proximal straight tubule (PST). Production of 20-HETE by rabbit PST was demonstrated by incubating microdissected tubules with [3H]arachidonic acid and separating the lipid extract by HPLC. There was significant conversion of [3H]arachidonic acid to 20-HETE in control tubules that was inhibited by 10−5MN-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS). Addition of exogenous 20-HETE had no effect on PST volume transport. However, inhibition of endogenous production of 20-HETE using DDMS stimulated transport. In the presence of DDMS, 20-HETE inhibited PST Jv. 19(S)-HETE in the bathing solution stimulated PST Jv alone and in the presence of DDMS. Thus ω- and ω-1-hydroxylase products of arachidonic acid have direct effects on PST transport. Endogenous production of 20-HETE may play a role in tonic suppression of transport and may therefore be an endogenous regulator of transport in the proximal tubule.
Keywords: in vitro microperfusion, cytochrome P-450, ω-hydroxylase, hypertension, sodium-potassium-adenosinetriphosphatase, hydroxyeicosatetraenoic acids
The mammalian renal cortex is an abundant source of cytochrome P-450 isozymes (21, 32). Within the kidney, the highest concentration of cytochrome P-450 is found in the proximal tubule (12). The isozymes present in the rat proximal tubule have recently been shown to be in the CYP4A class and metabolize arachidonic acid predominately by the ω-hydroxylase pathway (17). Microsomes from rabbit renal cortex have also been shown to metabolize arachidonic acid by the ω-hydroxylase pathway to 20- and 19(S)-hydroxyeicosatetraenoic (HETE) acids (23).
Metabolites of the renal cytochrome P-450 ω-hydroxylase system have multiple effects on renal function. Infusion of 20-HETE into rat renal arteries resulted in natriuresis without affecting glomerular filtration rate or systemic blood pressure (34). Inhibition of endogenous ω-hydroxylase activity by infusion of 17-octadecynoic acid into rat renal arteries also caused an increase in urine flow rate and sodium excretion (38). These results are difficult to interpret, because these compounds are known to alter renal hemodynamics and affect transport in multiple nephron segments (1, 3, 13, 14, 16, 22, 27, 31). 20-HETE constricts canine renal arcuate arteries (22) and rat renal arterioles (16) and increases renal vascular resistance (1). The vascular effects of 20-HETE also play a role in autoregulation and tubuloglomerular feedback (36, 37). 20-HETE has been shown to modulate thick ascending limb sodium chloride transport (3, 13, 14, 31). Cells from the thick ascending limb produce 20-HETE in culture, and production is stimulated by arginine vasopressin and calcitonin (13, 31). 20-HETE inhibits transport in the thick ascending limb by blocking the Na-K-2Cl cotransporter (3, 14). This inhibitory effect of 20-HETE on transport may be part of the signal transduction mechanism for the inhibition of transport by pharmacological doses of angiotensin II in this segment (4).
The proximal tubule is a major site of renal cytochrome P-450 (12, 17, 22). It has been estimated that the majority of 20-HETE produced by the kidney comes from the proximal tubule (20). The effects of 20-HETE on proximal tubule transport remain unknown. The purpose of the present study was to directly examine the effect of 20- and 19(S)-HETE on volume transport in the in vitro microperfused rabbit proximal straight tubule (PST).
METHODS
Production of 20-HETE
The production of 20-HETE from rabbit PST was measured using a modification of the method developed by Ito and Roman (18). Briefly, PST from rabbit kidneys were dissected as described below and transferred to a test tube in Hanks’ solution. The tubules were permeabilized with three freeze-thaw cycles using liquid nitrogen to snap freeze the tissue, followed by thawing in warm water. The tubules were then centrifuged at 4°C at 2,000 rpm for 5 min. The supernatant was aspirated, and the cells were resuspended in 1 ml of a buffer containing (in mM) 100 potassium phosphate (pH 7.4), 10 MgCl2, and 1 EDTA. [3H]arachidonic acid (4 μCi/ml; New England Nuclear, Boston, MA), 1 mM NADPH, 10 mM isocitrate, and 0.4 U/ml isocitrate dehydrogenase (as an NADPH generating system) were then added to each tube. The tubes were then incubated at 37°C for 60 min with 100% O2 blown over the tops. Three tubes were control with vehicle added, and three tubes were treated with 10−5MN-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS). One tube contained the solutions without tubules to assess the rate of nonenzymatic conversion to 20-HETE. The reaction was terminated by adding 250 μl of 1 M formic acid. The lipids were then extracted by adding 2 ml of chloroform, vortexing, centrifuging at 2,000 rpm for 5 min, and then drying under nitrogen. The samples were then resuspended in 20 μl of ethanol and separated by HPLC using a C18 column (150 × 2.1 mm, 3 μm, ODS, Hypersil; Thermo-Quest, San Jose, CA). The mobile phase was an acetonitrile: water:acetic acid (62.5:37.5:0.5) gradient to 100% acetonitrile in 20 min. Retention times for 20-HETE and arachidonic acid were determined by running standards under the same conditions. The fraction that came off at 7 min corresponded to 20-HETE, and the fraction at 19 min corresponded to arachidonic acid. Using this system, we found it was not possible to separate 19- from 20-HETE. The aqueous phase was saved to determine the protein concentration by bicinchoninic acid assay (BCA; Pierce Chemical, Rockford, IL).
Results were calculated by counting the 7- and 19-min fractions in a liquid scintillation counter. The counts were totaled, and the 20-HETE fraction was expressed as a fraction of the total, then factored for protein concentration. Results are expressed as the mean and standard error. Comparisons were made using unpaired t-test and significance was taken to be P < 0.05.
In vitro microperfusion
Superficial PST (S2 segment) from New Zealand White rabbits were perfused in vitro as previously described (11, 26). Briefly, PST were dissected in cooled (4°C) modified Hanks’ solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 Tris · HCl, 0.25 CaCl2, 2 glutamine, and 2 L-lactate. This solution was bubbled with 100% O2 and had a pH of 7.4. Tubules were then transferred to a 1.2-ml thermostatically controlled (37–38°C) bathing chamber and perfused with concentric glass pipettes. The perfusion solution simulated an ultrafiltrate of plasma and contained (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 sodium acetate, 1.8 CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine. The bathing solution was similar, but contained 6 g/dl of albumin. All solutions were bubbled with 95% O2 and 5% CO2 at 37°C and had a pH of 7.4. The osmolalities of the perfusion and bathing solutions were adjusted to 295 mosmol/kgH2O by the addition of water or NaCl. The bathing solution was exchanged at a rate of 0.5 ml/min to keep the osmolality and pH constant.
The control period began after a 60-min incubation period. Volume absorption (Jv; in nl · min−1 · mm−1) was measured as the difference between the perfusion and collection rates and normalized per millimeter of tubule length. The collection rate was determined by timed collections using a constant-volume pipette. Exhaustively dialyzed [methoxy-3H]inulin (New England Nuclear) was added to the perfusate at a concentration of 50 μCi/ml so that the perfusion rate could be calculated. The tubule length (L) was measured using an eyepiece micrometer.
The transepithelial potential difference (PD, mV) was measured by using the perfusion pipette as the bridge into the tubular lumen. The recording and reference calomel half-cells were connected to the perfusion and bathing solutions via agarose bridges containing 3.6 M KCl/0.9 M KNO3. This arrangement avoids direct contact between the solution bathing the tubule and the KCl/KNO3 agarose bridge. The recording and reference calomel half-cells were then connected to the high- and low-impedance sides, respectively, of an electrometer (model 602; Keithley Instruments, Cleveland, OH).
After five control measurements of Jv and PD were made, the eicosanoid to be studied was added to the bathing solution. After 15 min of incubation, five measurements of Jv and PD were made in the experimental period. Measurements of Jv and PD in each period were averaged for that period.
Results are expressed as the means and standard error of all tubules in each series. Comparisons were made using paired t-test and significance was taken to be P < 0.05.
RESULTS
20-HETE production
Rabbit proximal tubules were shown to metabolize arachidonic acid to 20-HETE. The control rate of conversion was 47.7 ± 3.4 (0.18 ± 0.02% · μg protein−1 · 60 min−1). DDMS significantly reduced this conversion rate to 17.7 ± 1.8% · tube−1 · 60 min−1 (0.05 ± 0.01% · μg protein−1 · 60 min−1; P < 0.01, n = 3). The nonenzymatic conversion of arachidonic acid to 20-HETE was minimal (9.5% · tube−1 · 60 min−1) and was approximately the same as the DDMS-treated tubules. Thus rabbit PST are capable of producing 20-HETE and this is inhibited by DDMS.
In vitro microperfusion
The first series of experiments was designed to determine whether 20-HETE had a direct effect on PST Jv. During the experimental period in these series, 20-HETE (10−6Mor 10−5 M) was added to the bathing solution. 20-HETE had no direct effect on Jv [control, 0.50 ± 0.10 vs. 10−6 M 20-HETE, 0.50 ± 0.12 nl · min−1 · mm−1; n = 4, P = not significant (NS); and control, 0.45 ± 0.12 vs. 10−5 M 20-HETE, 0.46 ± 0.14 nl · min−1 · mm−1; n = 6, P = NS]. There was also no change in the PD (control, −1.2 ± 0.3 vs. 10−6M 20-HETE, −1.2 ± 0.2 mV; and control, −2.2 ± 0.9 vs. 10−5M20-HETE, −2.3 ± 0.8 mV; P = NS).
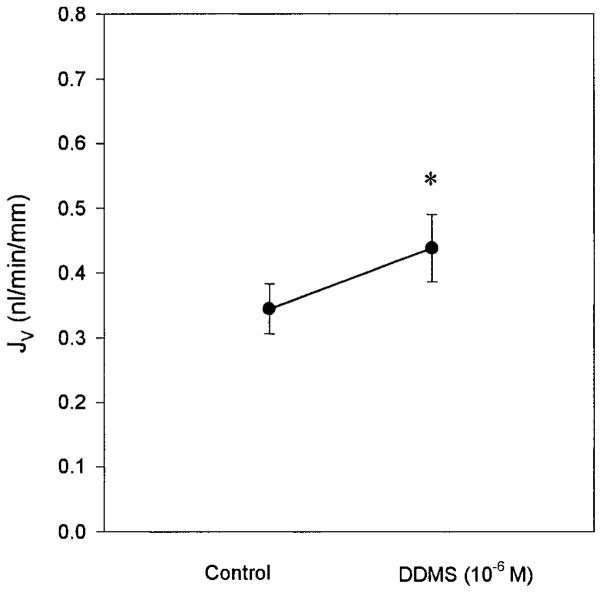
Since the major metabolite of arachidonic acid in rat proximal tubules is 20-HETE (24) and we had demonstrated that rabbit proximal tubules also produce 20-HETE, it is possible that the endogenous production rate is sufficiently high to mask the effect of any exogenously administered 20-HETE. To evaluate this possibility, endogenous production of 20-HETE was blocked using DDMS, an ω-hydroxylase inhibitor with little effect on epoxygenase activity (1). Figure 1 shows the effect of 10−6 M DDMS when added to the bath. This concentration stimulated Jv in the proximal tubule (control, 0.35 ± 0.04 vs. 10−6 M DDMS, 0.44 ± 0.05 nl · min−1 · mm−1; P < 0.05). There was no change in the PD (control, −2.0 ± 0.7 vs. 10−6 M DDMS, −2.0 ± 0.4 mV; P = NS). Thus inhibition of endogenous production of 20-HETE resulted in a 26% stimulation in proximal tubule transport.
Fig. 1.
The effect of inhibition of 20-hydroxyeicosatetraenoic acid (20-HETE) production on proximal straight tubule (PST) transport. During the experimental period, 10−6 M N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS) was added to the bath (n = 3). DDMS is a specific inhibitor of the ω-hydroxylase enzyme. There was a significant stimulation of transport. *P < 0.05 vs. control. Jv, volume absorption.
The next series of experiments examined the effect of addition of 20-HETE in the presence of DDMS. As seen in Fig. 2, when endogenous 20-HETE production is inhibited with 10−5 M DDMS, exogenous administration of 10−5 M 20-HETE has a direct effect to inhibit transport in the PST [control, 0.49 ± 0.05 vs. 10−5 M DDMS, 0.58 ± 0.06 nl · min−1 · mm−1 (P < 0.05) vs. 10−5 M DDMS and 10−5 M 20-HETE, 0.52 ± 0.05 nl · min−1 · mm−1 (P < 0.05)]. There was no effect of 20-HETE on the PD [control, −1.1 ± 0.3 vs. 10−5 M DDMS, −1.3 ± 0.3 mV (P = NS) vs. 10−5 M DDMS and 10−5M20-HETE, −1.4 ± 0.3 mV (P = NS)].
Fig. 2.
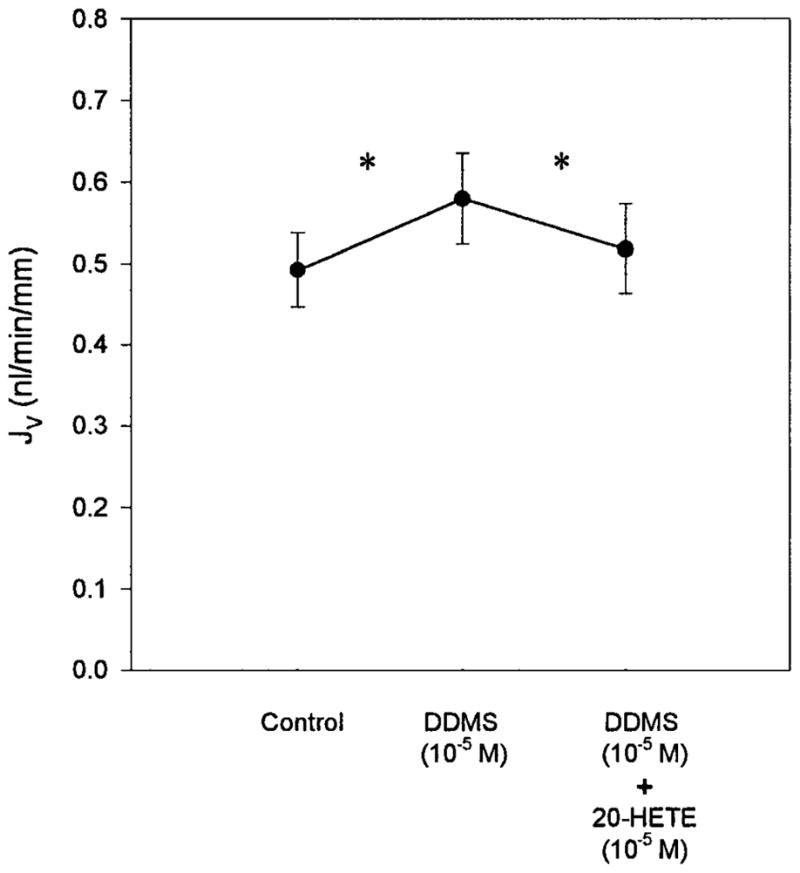
Effect of 20-HETE in the presence of ω-hydroxylase inhibition (n=7). Addition of 10−5 M DDMS to the bath significantly stimulated Jv. In the presence of 10−5 M DDMS, 20-HETE (10−5 M) inhibited PST Jv. *P < 0.05 vs. DDMS (10−5 M).
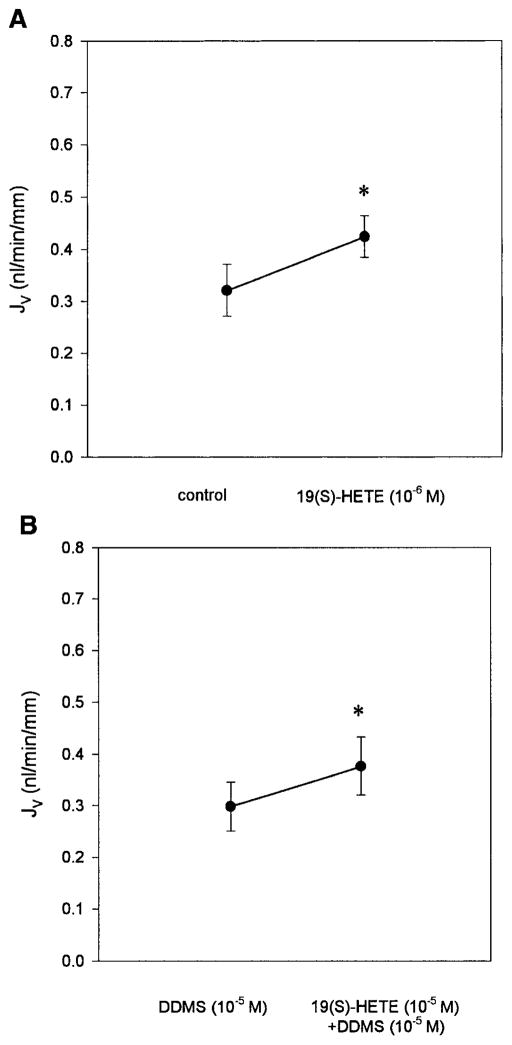
The effect of 19(S)-HETE was examined in the last series of experiments. The addition of 10−6 M 19(S)-HETE was shown to stimulate proximal tubule transport (Fig. 3A) [control, 0.32 ± 0.05 vs. 19(S)-HETE 10−6 M, 0.42 ± 0.04 nl · min−1 · mm−1; P < 0.05]. There was no change in the PD [control, −1.6 ± 0.5 vs. 10−6M 19(S)-HETE, −1.6 ± 0.3 mV; P = NS]. Because this stimulation could have occurred secondary to inhibition of 20-HETE production (2), the effects of 19(S)-HETE were examined in the presence of DDMS. As can be seen in Fig. 3B, 19(S)-HETE stimulated proximal tubule transport when endogenous 20-HETE production was inhibited [10−5 M DDMS, 0.30 ± 0.05 vs. 10−5 M DDMS and 10−5 M 19(S)-HETE, 0.38 ± 0.06 nl · min−1 · mm−1; P < 0.05]. Thus the effect to stimulate transport must be a direct effect and not due to any effect on 20-HETE production.
Fig. 3.
Effect of 19(S)-HETE on PST transport. The addition of 19(S)-HETE directly stimulated PST volume transport. A: 19(S)-HETE is added alone (10−6 M, n = 7). B: 19(S)-HETE (10−5 M, n = 6) is added in the presence of 10−5 M DDMS. Thus the effect of 19(S)-HETE on PST transport is not dependent on an interaction with 20-HETE. *P < 0.05 vs. control (A) or vs. 10−5 M DDMS (B).
DISCUSSION
The present study examined the direct effects of ω-hydroxylase products of arachidonic acid on proximal tubule volume transport. We demonstrated that rabbit PST are capable of converting arachidonic acid to 20-HETE. This conversion is inhibited by DDMS. Addition of exogenous 20-HETE, the major product of ω-hydroxylase, had no direct effect on PST volume transport. Inhibition of 20-HETE production with the ω-hydroxylase inhibitor, DDMS, stimulated volume transport in the PST. This implies that the high endogenous production of 20-HETE may play a role in suppressing transport rates. In the presence of DDMS, exogenous 20-HETE had a significant effect to inhibit transport. The ω-1 product of arachidonic acid, 19(S)-HETE, stimulated transport regardless of whether DDMS was present. This indicates that 19(S)-HETE directly stimulates transport without having to affect 20-HETE metabolism. Thus the ω-hydroxylase products of arachidonic acid play a role in the control of volume transport in the PST.
Although the effects of these compounds on proximal tubule transport have not been previously examined, their effects on Na-K-ATPase activity have been studied (15, 25, 27). 20-HETE inhibits Na-K-ATPase in this segment and is thought to play a role in the effect of parathyroid hormone and dopamine to inhibit transport in this segment (25, 27). 19(S)-HETE, the major ω-1 product of ω-hydroxylase, has been shown to stimulate rat renal Na-K-ATPase activity (15). Although Na-K-ATPase activity in the proximal tubule is a determinant of solute and volume transport, effects on the Na-K-ATPase do not always correlate with effects on Jv. Dopamine, for example, has been shown to inhibit proximal convoluted tubule Na-K-ATPase but has no direct effect on transport in this segment (5, 9, 10). Thus the relationship between regulating Na-K-ATPase activity and Jv rates is complex and indicates the importance of directly examining proximal tubule transport.
Changes in Jv rates in the present study were not associated with changes in PD. This is in contrast to previous studies in which increases in Jv rate correlated with increases in the PD (6) and decreases in transport correlated with decreases in the PD (8, 29). This suggests that the mechanism by which the Jv rates were increased may be due to changes in electroneutral transport and not due to direct changes in the Na-K-ATPase (7).
The proximal tubule reabsorbs between 60 and 70% of the glomerular ultrafiltrate (30). Thus small changes in the volume absorption rate in this nephron segment lead to large changes in overall fluid balance of the organism. Inhibiting ω-hydroxylase activity in this nephron segment led to an increase in transport by 16–28%, which could then cause volume overload and hypertension. This indicates that renal P-450 ω-hydroxylase may be involved in extracellular fluid volume and blood pressure regulation.
The role of the renal P-450 system in the development of hypertension has been complex. The Dahl salt-sensitive rat (SS) is a model of hypertension that has been extensively studied, and renal cytochrome P-450 abnormalities may be involved in the development of hypertension in this model (28). In vivo perfusion studies indicate that the SS rats reabsorb more sodium in the loop of Henle than the salt-resistant (SR) or Lewis rats, indicating that tubular transport is higher in these animals (33, 35). Addition of 20-HETE to the perfusate in these studies reduced the chloride transport rates (35). More recently, thick ascending limbs from SS animals were shown to have higher transport rates that were reduced with 20-HETE than SR animals (19). Thus 20-HETE is a key factor in regulating sodium and chloride transport and volume regulation.
The above in vivo and in vitro studies examined the role of 20-HETE on thick ascending limb of Henle transport (19, 33, 35). Since the highest concentration of renal P-450 is in the proximal tubule, the effect of 20-HETE production on transport in this segment was important to examine (12). The present study directly demonstrates that endogenous production of 20-HETE exerts an effect to inhibit transport in the PST. When the endogenous production was blocked, exogenous administration of 20-HETE inhibited transport. These findings are consistent with the above studies linking a low production rate of 20-HETE with the development of hypertension.
The present study demonstrated direct effects of 20- and 19(S)-HETE on proximal tubule transport. The addition of 20-HETE inhibited proximal tubule transport only when endogenous production was inhibited with DDMS. 19(S)-HETE was capable of stimulating volume transport in the absence and presence of DDMS. Thus the ω-hydroxylase products of arachidonic acid play a role in regulating proximal tubule transport.
Acknowledgments
We are grateful for the technical assistance provided by Amber Lisec and the secretarial assistance of Janell McQuinn.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-02232 (R. Quigley), DK-41612 (M. Baum), and DK-38226 (R. Quigley and J. R. Falck), and by the Robert A. Welch Foundation (J. R. Falck).
References
- 1.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Galicia M, Falck JR, Harder DR, Roman RJ. Structural determinants of the renal vascular response to 20-hydroxyeicosatetraenoic acid (20-HETE) FASEB J. 1997;11:A244. [Google Scholar]
- 3.Amlal H, Legoff C, Vernimmen C, Paillard M, Bichara M. cotransport in medullary thick ascending limb: control by PKA, PKC, and 20-HETE. Am J Physiol Cell Physiol. 1996;271:C455–C463. doi: 10.1152/ajpcell.1996.271.2.C455. [DOI] [PubMed] [Google Scholar]
- 4.Amlal H, Legoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. ANG II controls cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol Cell Physiol. 1998;274:C1047–C1056. doi: 10.1152/ajpcell.1998.274.4.C1047. [DOI] [PubMed] [Google Scholar]
- 5.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted segments. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;252:F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- 6.Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. doi: 10.1172/JCI112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum M, Berry CA. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit convoluted tubule. J Clin Invest. 1984;74:205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum M, Hays SR. Phorbol myristate acetate and dioctanoylglycerol inhibit transport in rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;254:F9–F14. doi: 10.1152/ajprenal.1988.254.1.F9. [DOI] [PubMed] [Google Scholar]
- 9.Bello-Reuss E, Higashi Y, Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1982;242:F634–F640. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- 10.Bertorello A, Aperia A. Inhibition of proximal tubule Na+-K+-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;259:F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- 11.Burg MB, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966;210:1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- 12.Endou H. Cytochrome P-450 monooxygenase system in the rabbit kidney: its intranephron localization and its induction. Jpn J Pharmacol. 1983;33:423–433. doi: 10.1254/jjp.33.423. [DOI] [PubMed] [Google Scholar]
- 13.Escalante B, Erlij D, Falck JR, McGiff JC. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 14.Escalante B, Erlij D, Falck JR, McGiff JC. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol Cell Physiol. 1994;266:C1775–C1782. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- 15.Escalante B, Falck JR, Yadagiri P, Sun L, Laniado-Schwartzman M. 19(S)-Hydroxyeicosatetraenoic acid is a potent stimulator of renal Na+-K+-ATPase. Biochem Biophys Res Commun. 1988;152:1269–1274. doi: 10.1016/s0006-291x(88)80422-4. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Zou AP, Stec DE, Harder DR, Falck JR, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol Regulatory Integrative Comp Physiol. 1996;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 17.Ito O, Alonso-Galicia M, Hopp KA, Roman RJ. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol Renal Physiol. 1998;274:F395–F404. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- 18.Ito O, Roman RJ. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol Regulatory Integrative Comp Physiol. 1999;276:R1749–R1757. doi: 10.1152/ajpregu.1999.276.6.R1749. [DOI] [PubMed] [Google Scholar]
- 19.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33:419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 20.Kroetz DL, Huse LM, Thuresson A, Grillo MP. Developmentally regulated expression of the CYP4Agenes in the spontaneously hypertensive rat kidney. Mol Pharmacol. 1997;52:362–372. doi: 10.1124/mol.52.3.362. [DOI] [PubMed] [Google Scholar]
- 21.Laniado-Schwartzman M, Abraham NG. The renal cytochrome P-450 arachidonic acid system. Pediatr Nephrol. 1992;6:490–498. doi: 10.1007/BF00874022. [DOI] [PubMed] [Google Scholar]
- 22.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 23.Morrison AR, Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc Natl Acad Sci USA. 1981;78:7375–7378. doi: 10.1073/pnas.78.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omata K, Abraham NG, Schwartzman ML. Renal cytochrome P-450 arachidonic acid metabolism: localization and hormonal regulation in SHR. Am J Physiol Renal Fluid Electrolyte Physiol. 1992;262:F591–F599. doi: 10.1152/ajprenal.1992.262.4.F591. [DOI] [PubMed] [Google Scholar]
- 25.Ominato M, Satoh T, Katz AI. Regulation of Na-K-ATPase activity in the proximal tubule: role of the protein kinase C pathway and of eicosanoids. J Membr Biol. 1996;152:235–243. doi: 10.1007/s002329900101. [DOI] [PubMed] [Google Scholar]
- 26.Quigley R, Baum M. Effects of growth hormone and insulin-like growth factor I on rabbit proximal convoluted tubule transport. J Clin Invest. 1991;88:368–374. doi: 10.1172/JCI115312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro CMP, Dubay GR, Falck JR, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through a cytochrome P-450 pathway. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F497–F505. doi: 10.1152/ajprenal.1994.266.3.F497. [DOI] [PubMed] [Google Scholar]
- 28.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens. 1997;10:63S–67S. [PubMed] [Google Scholar]
- 29.Salmon RF, Baum M. Intracellular cystine loading inhibits transport in the rabbit proximal convoluted tubule. J Clin Invest. 1990;85:340–344. doi: 10.1172/JCI114443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sands JM, Kokko JP, Jacobson HR. Intrarenal heterogeneity: vascular and tubular. In: Seldin DW, editor. The Kidney: Physiology and Pathophysiology. NewYork: Raven; 1992. pp. 1087–1155. [Google Scholar]
- 31.Schwartzman M, Ferreri NR, Carroll MA, Songu-Mize E, McGiff JC. Renal cytochrome P450-related arachidonate metabolite inhibits (Na++K+)ATPase. Nature. 1985;314:620–622. doi: 10.1038/314620a0. [DOI] [PubMed] [Google Scholar]
- 32.Schwartzman ML, McGiff JC. Renal cytochrome P450. J Lipid Mediat Cell Signal. 1995;12:229–242. doi: 10.1016/0929-7855(95)00021-h. [DOI] [PubMed] [Google Scholar]
- 33.Stec DE, Mattson DL, Roman RJ. Inhibition of renal outer medullary 20-HETE production produces hypertension in Lewis rats. Hypertension. 1997;29:315–319. doi: 10.1161/01.hyp.29.1.315. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Capdevila J, Karara A, Falck JR, Jacobson HR, Badr KF. Cytochrome P-450 arachidonate metabolites in rat kidney: characterization and hemodynamic response. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;258:F781–F789. doi: 10.1152/ajprenal.1990.258.4.F781. [DOI] [PubMed] [Google Scholar]
- 35.Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension. 1996;27:631–635. doi: 10.1161/01.hyp.27.3.631. [DOI] [PubMed] [Google Scholar]
- 36.Zou AP, Imig JD, De Montellano PRO, Sui Z, Falck JR, Roman RJ. Effect of P-450 ω-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F934–F941. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 37.Zou AP, Imig JD, Kaldunski M, De Montellano PRO, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F275–F282. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 38.Zou AP, Ma YH, Sui ZH, De Montellano PRO, Clark JE, Masters BS, Roman RJ. Effects of 17-octadecynoic acid, a suicide-substrate inhibitor of cytochrome P450 fatty acid w-hydroxylase, on renal function in rats. J Pharmacol Exp Ther. 1994;268:474–481. [PubMed] [Google Scholar]