Summary
Astrocytes are critically important for neural circuit assembly and function. Mammalian protoplasmic astrocytes develop a dense ramified meshwork of cellular processes to form intimate contacts with neuronal cell bodies, neurites and synapses. This close neuron-glia morphological relationship is essential for astrocyte function, but it remains unclear how astrocytes establish their intricate morphology, organize spatial domains, and associate with neurons and synapses in vivo. Here we characterize a Drosophila glial subtype that shows striking morphological and functional similarities to mammalian astrocytes. We demonstrate the Fibroblast growth factor (FGF) receptor Heartless autonomously controls astrocyte membrane growth, and the FGFs Pyramus and Thisbe direct astrocyte processes to ramify specifically in CNS synaptic regions. We further show the shape and size of individual astrocytes are dynamically sculpted through inhibitory or competitive astrocyte-astrocyte interactions and Heartless FGF signaling. Our data identify FGF signaling through Heartless as a key regulator of astrocyte morphological elaboration in vivo.
Introduction
Astrocytes are among the most abundant cell types in the mammalian central nervous system (CNS) and fulfill diverse functions in brain development and physiology. In the mature brain astrocytes buffer ions and pH, metabolically support neurons, and clear neurotransmitters (Kimelberg and Nedergaard, 2010). Astrocytes can sense neuronal activity, react with transient increases of intracellular calcium ion concentration and in turn modulate neuronal activity (Perea and Araque, 2010). The diverse homeostatic and modulatory roles for astrocytes are essential for neuronal function, and evidence is mounting that this tight physiological relationship between astrocytes and neurons is highly regulated, and provides astrocytes with the capacity to exert powerful and dynamic control over neuronal circuits.
Astrocytic functions are critically dependent on the intimate spatial relationship between astrocytes and neurons, and accordingly astrocytes exhibit a highly ramified morphology. Primary cellular extensions radiate from the soma of grey matter astrocytes, which then branch into hundreds of increasingly finer cellular processes, ultimately forming a dense meshwork in the brain that associates closely with synapses, neuronal cell bodies and the brain vasculature. Intriguingly, individual mature mammalian astrocytes occupy unique spatial domains within the brain, apparently “tiling” through a mechanism akin to dendritic tiling, such that the processes of neighboring astrocytes exhibit very limited overlap (Bushong et al., 2004; Bushong et al., 2002; Ogata and Kosaka, 2002). Whether these unique spatial domains are functionally important remains a point of speculation.
Despite recent advances in understanding the molecular basis of astrocyte fate specification, control of synapse formation and neuronal signaling, pathways regulating astrocyte morphogenesis in vivo remain poorly understood (Molofsky et al., 2012). While there appears to be a spatial restriction of astrocyte subtypes to particular regions of the vertebrate CNS (Hochstim et al., 2008; Tsai et al., 2012), it is not clear whether astrocytes selectively associate with predetermined subsets of neurons. The morphology of individual mammalian astrocytes is quite variable, suggesting sculpting of their morphology may be stochastic and shaped by cell-cell interactions (Bushong et al., 2004; Bushong et al., 2002).
In this study we characterize a glial cell type in Drosophila remarkably similar to mammalian protoplasmic astrocytes. We show that Drosophila astrocytes dynamically and progressively invade the synaptic neuropil late in embryonic development, for a dense meshwork and associate closely with synapses throughout the CNS, and tile with one another to establish unique spatial domains. We identify the Heartless FGF receptor signaling pathway as a key mediator of astrocyte outgrowth into synaptic regions and the size of individual astrocytes. Through ablation studies we demonstrate that individual astrocytes have a remarkable potential for growth, and the establishment of astrocyte spatial domains is mediated by astrocyte-astrocyte inhibitory and/or competitive interactions. Our work provides new insights into cell-cell interactions governing astrocyte growth in vivo, and demonstrates the requirement for astrocytes is an ancient feature of the nervous system of complex metazoans.
Results
Drosophila astrocytes densely infiltrate the neuropil, are highly polarized, and exhibit tiling behavior
We, and others (Awasaki et al., 2008), recently described a novel astrocyte-like subtype in the synapse-rich neuropil of the adult Drosophila brain (Doherty et al., 2009). To explore these cells in greater detail we turned to the larval ventral nerve cord (VNC). Astrocytes were labeled by the alrm-Gal4 driver, expressed the glial marker Repo (not shown) and were organized in a semi-stereotyped pattern in the 3rd instar larval (L3) VNC. We found 5–6 Alrm+ glia per hemisegment that were typically organized into: a dorso-medial group (3 cells), a dorso-lateral group (2 cells) and a single uniquely identifiable ventrally-positioned cell (Figure 1A, B). Expression of UAS-CD8-GFP using alrm-Gal4 revealed that Alrm+ glia densely infiltrate the entire synaptic neuropil, while their cellular processes are absent from the surrounding cell cortex, which houses neuronal cell bodies (Figure 1C).
Figure 1. Drosophila astrocytes are morphologically similar to mammalian protoplasmic astrocytes.
(A–I) Confocal analysis of L3 larval central nervous systems. (A, B) Colabeling of astrocytic nuclei using alrm-Gal4 UAS-lacZ-NLS (green) together with α-HRP for neuronal membranes (red) and α-Brp for the synaptic neuropil (blue). Projection of a confocal stack (A) and mid VNC orthogonal cross section (B) showing the nuclear positions of astrocytes around the neuropil. (C) alrm-Gal4 UAS-CD8-GFP expression (green) labels the dense meshwork of fine processes of astrocytes in the synaptic neuropil. Single confocal section in the VNC (C′) and mid VNC orthogonal cross section (C). (D–F) Z-projections of confocal stacks of CD8-GFP marked MARCM clones labeling astrocytes (green). All astrocytes are labeled with α-Gat antibody (red). Arrowhead in (F) shows a contralateral projection of an astrocyte. (G-I) Two color flip out strategy using alrm-Gal4 UAS-CD8>GFP>RFP repoFLP labeling astrocytes with GFP (green) or RFP (red) reveals their tiling behavior. (G′–I′) show higher magnification views of the boxed area in (G–H). Scale bars represent 50μm in (A) and 25μm in (B–I). See also Figure S1.
Analysis of single cell MARCM (Mosaic analysis with a repressible cell marker) clones (Lee and Luo, 1999) in L3 animals revealed that astrocytes have remarkable morphological similarities to mammalian protoplasmic astrocytes (Bushong et al., 2002; Ogata and Kosaka, 2002). Several main branches emanated from the cell body and then branched into a dense, ramified meshwork of processes (Figure 1D–F). Individual Alrm+ glia generally occupied regions of the neuropil close to their cell bodies but the size and shape of these domains varied quite strikingly (Figures 1D–F and S1).
To analyze the cytoskeletal organization of the astrocytes, we co-expressed the membrane marker mCD8-Cherry together with GFP tagged cytoskeletal markers using alrm-Gal4. Actin42A-GFP was distributed with mCD8-Cherry into fine processes (Figure S1C, D). Tau-GFP, a microtubule binding protein, preferentially labeled cell bodies and main astrocytic branches (Figure S1E). The microtubule (MT) minus end marker Nod-GFP was largely restricted to the cell body and proximal main branches (Figure S1G), while the plus end directed MT motor Khc-GFP accumulated in fine processes (Figure S1F). These data suggest astrocyte fine processes are actin-rich, and the MT cytoskeleton of astrocytes is oriented with a bias of plus-ends away from cell bodies.
To analyze the interface between adjacent astrocytes we labeled astrocytes with either GFP or RFP using a FLP out strategy (see Figure S4A). We found individual astrocytes occupied largely separate territories with only limited overlap with neighbors. While occasionally single astroglial branches of up to 20–30μm extended into neighboring territories, the core zone of overlap at cell-cell borders was typically small (0–5μm, Figure 1G–I).
Drosophila astrocytes associate closely with synapses and are critical regulators of GABA signaling
We sought to determine whether fly astrocytes were in close proximity to synapses and might play a role in the clearance of γ-aminobutyric acid (GABA), as is the case with mammalian protoplasmic astrocytes (Danbolt, 2001; Schousboe et al., 2004). CG1732 (Gat), encodes the sole SLC6-family GABA transporter encoded in the Drosophila genome (Neckameyer and Cooper, 1998; Thimgan et al., 2006). We generated an antibody directed against a C-terminal peptide of Gat and found Gat was exclusively expressed in astrocytes in embryos, larvae, pupae, and adults, with anti-Gat antibody labeling the entire membrane surface of astrocytes (e.g. Figures, 2A, B, 3B–J and data not shown). We confirmed the specificity to Gat in gat null homozygous embryos and larvae expressing astrocyte-specific gatRNAi (see below).
To explore the astrocyte-synapse relationship we first immuno-labeled L3 ventral nerve cords with anti-Gat antibody and the presynaptic active zone marker anti-Bruchpilot (Brp, Figure 2A, B). Gat+ glial processes were found in close apposition to synaptic profiles (Figure 2A, B). We next performed transmission electron microscopy (TEM) on the L3 ventral nerve cord. We tentatively identified astrocyte processes by their electron-dense cytoplasm and determined their average occupancy of the neuropil and the distance from the closest synapse (Figure 2C, D). Drosophila astrocytes covered 4.61±1.47% ±SD of the total area in each field of view (Figure S2B), and the average synapse-glial process distance was 0.88 ±0.66μm ±SD, and was somewhat variable (Figure 2D). Individual synapses did not seem to be wrapped by glial membranes, and we did not detect an obvious correlation of synaptic density and glial coverage (Figure S2A–C).
Figure 2. Astrocytes closely associate with synapses.
(A, B, E, F) Confocal analysis of L3 larval VNCs. (A, B) Astrocytic membranes are labeled with α-Gat antibody (green), synaptic profiles with α-Brp (red). Higher magnification view of the boxed area in (A–A″) is shown in (B–B″), showing close apposition of astrocytic membranes and Brp+ synaptic puncta. (C, C′) TEM image of a section of the neuropil in the ventral nerve cord in a L3 larva. (C) Tracings of synapses (green) and putative astrocyte processes (red) of the original image (C′). (D) Quantification of the distance from a synapse to the next glial process. Scatterplot with mean and SD representing error bars are shown (N=498). (E) α-Gat (blue) colabels alrm-Gal4 UAS-CD8-GFP (green) positive astrocytes and the synaptic neuropil is labeled by α-Brp (red). (F) Knockdown of Gat in astrocytes with alrm-Gal4 UAS-CD8-GFP UAS-gatRNAi shows the specificity of the α-Gat antibody. (G) Western blot analysis of Gat knockdown with alrm-Gal4 by RNAi in L3 larval CNS extracts. GD: UAS-gat-RNAiGD13359, KK: UAS-gat-RNAiKK106638, JF: UAS-gat-RNAiJF03358 (H) Quantification of Gat levels in western blots normalized to LaminC signal and to the control lane (N=3). (I) Quantification of locomotion defects in alrm-Gal4 UAS-CD8-GFP UAS-gatRNAi animals compared to control animals (alrm-Gal4 UAS-CD8-GFP/+). control: N=11, gatRNAi: GD: N=20, KK: N=10, JF: N=10, ***p<0.0001. Scale bars represent 25μm in (A, E), 2μm in (B) and 1μm in (C). See also Figure S2, Movies 1–4, and Movie 8.
To determine whether astrocytes were positioned to detect synaptically released neurotransmitters we used the fluorescent extracellular glutamate reporter iGluSnFR (Marvin et al., 2013). In intact L1 larvae expressing UAS-iGluSnFR in neurons using elav-Gal4, we observed spontaneous, local bursts of fluorescence in the neuropil, and coordinated waves of activity running along the anterio-posterior axis associated with larval crawling (Movie 1). Expression of iGluSnFR on astrocyte membranes using alrm-Gal4 revealed very similar patterns of activation in the neuropil (Movie 2). Thus Drosophila astroglial membranes are sufficiently close to glutamatergic synapses to detect dynamic changes in glutamate release with iGluSnFR, and are positioned to clear neurotransmitters from synapses.
To explore Gat function we generated a deficiency deleting ~64kb of genomic DNA including the gat gene (Df(4)gatΔ64kb, Figure S2D). Animals homozygous for Df(4)gatΔ64kb died as embryos, with grossly normal CNS and astrocyte morphology (Figure S3A–D), and anti-Gat immunoreactivity was lost from astrocytes (Figure S3B′, D, F). Embryonic lethality of Df(4)gatΔ64kb was rescued to larval lethality by resupplying two genomic constructs that only had the gat gene in common (Figure S2D, G). We next generated a gat mutant using a transcription activator-like effector nuclease (TALEN) approach. We recovered gat22-1, which deletes 496bp including part of exon1 and the first exon-intron junction of the gat gene (Figure S2E, F), exhibits severely reduced α-Gat immunoreactivity when crossed to Df(4)gatΔ64kb (Figure S3E, F), and did not complement Df(4)gatΔ64kb (Figure S2G). gat22-1/Df(4)gatΔ64kb transheterzygotes and homozygous gat22-1 mutants died as late embryos, but were rescued to adulthood by genomic gat P[acman] clones or astrocyte expression of a UAS-gat construct (Figure S2G). Taken together these data indicate gat22-1 is a null or strong hypomorphic allele of gat, loss of gat causes embryonic lethality, and that Gat functions primarily in astrocytes. The gross morphology of gat22-1 mutant embryos was normal with respect to FasII+ axonal morphology (Figure S3G, H) and Engrailed+ neuron numbers (Figure S3I–K), suggesting the lethality in gat22-1 embryos highlights a key physiological role for Gat in CNS function rather than morphogenesis.
To assay for roles for Gat in behavior we used three independent UAS-gatRNAi constructs to deplete Gat from astrocytes. We found that astrocytic gatRNAi resulted in severely reduced Gat levels in immunostains (Figure 2E, F) and western blots (Figure 2G, H) of L3 animals. Interestingly, astrocytic gatRNAi larvae showed severe behavioral defects: gatRNAi animals were uncoordinated, exhibited slowed waves of muscular contraction, and were often found crawling on their sides or upside–down a behavior never observed in controls (Movie 3). gatRNAi animals showed a strong reduction in crawling velocity compared to control animals (Figure 2I), and locomotion defects were also observed in adult gatRNAi animals (Movie 4). Thus astrocytic Gat activity is critical for normal motor function in Drosophila, likely through GABA clearance.
Astrocytes infiltrate the neuropil during late embryonic stages in a stepwise fashion
Drosophila astrocytes are derived from the longitudinal glioblast (LGB), an embryonic neural stem cell that generates 9 Repo+ longitudinal glia per hemisegment (Beckervordersandforth et al., 2008; Jacobs et al., 1989; Schmidt et al., 1997). Six of these 9 longitudinal glia are genetically distinct (Beckervordersandforth et al., 2008; Thomas and van Meyel, 2007), activate expression of Gat, and ultimately differentiate into astrocytes (see below). We followed the development of astrocytes by live imaging of arlm-Gal4/UAS-CD8-GFP (Movies 5–7). However, alrm-Gal4 is initially expressed in all longitudinal glia before resolving into primarily astrocytes (data not shown), so we also assayed astrocyte CNS infiltration using anti-Gat immunoreactivity in fixed preparations (Figure 3B–E). By late stage 16, six Gat+, ellipsoidal, immature astrocytes were found positioned on the dorsal surface of the neuropil in each hemisegment. Glial processes next extended along the surface of the neuropil—at this time one of the six Gat+ cell bodies began to migrate ventrally, and astroglial processes eventually surrounded the neuropil (Movie 5; Figure 3B). Astroglial projections next invaded the neuropil, branched to form an increasingly dense meshwork, and ultimately covered the entire neuropil space (Movies 6, 7; Figure 3C–E).
Figure 3. Htl FGF signaling controls infiltration of astrocyte processes into the neuropil.
(A) Schematic view of the embryonic CNS showing the approximate positions of confocal images shown in (B-J) with longitudinal plane of section shown in (A, B–J, B′–J′) and a cross section in (A′, B″–J″, B‴–J‴). (B–J) Astrocytes are labeled by α-Gat antibody (red) and the neuropil by α-Brp (green). (B–E) Wild type progression of astrocyte infiltration of the neuropil during the last hours of embryogenesis. Wild type embryos hatch at ~21h of development. Control (F), htlAB42 (G), Df(2R)BSC25 (H), pyrS0439/ pyrS3547 (I) and Df(2R)ths238 mutant embryos at the end of embryogenesis. Scale bars represent 10μm. (K) Quantification of the infiltration phenotype using an infiltration score (IF) from 0–5. (L) Quantification of the number of ventral astrocytes (VC) per segment. control: N=25, htlAB42: N=36, Df(2R)BSC25: N=34, pyrS0439/ pyrS3547: N=13, pyr18: N=13, ths759: N=13 and Df(2R)ths238: N=19. See also Movies 5–7.
The Heartless FGF-receptor signaling pathway is required for astroglial infiltration
The Drosophila FGF receptor Heartless (Htl) is expressed in embryonic glia, including astrocytes (Shishido et al., 1997). To determine whether Htl might regulate astrocyte morphogenesis we examined astrocyte morphology in htlAB42 null mutants. At late embryonic stages we found ~6 Gat+ astrocytes per hemisegment located on the dorsal surface of the neuropil in htlAB42 mutant animals, similar to controls, indicating that Htl is not required for specification of astrocytes (Figure S7J). Astroglial processes surrounded the neuropil in htlAB42 mutants with the exception of the ventral-most neuropil region (Figure 3G–G‴)—in htlAB42 animals the ventrally located astrocytes failed to migrate to their normal positions (Figure 3L). Most strikingly, htlAB42 mutants exhibited a severe loss of astrocytic infiltration into the neuropil (Figure 3F, G, K). We scored infiltration phenotypes on a scale from 0–no or minimal infiltrating processes– to 5 full infiltration at end of embryogenesis based on density of infiltration, evenness of infiltration and thickness of processes (Figure 3K).
Pyramus (Pyr) and Thisbe (Ths) are the only known FGFs activating Htl in Drosophila (Kadam et al., 2009; Klingseisen et al., 2009; Stathopoulos et al., 2004). Interestingly, Df(2R)BSC25, which deletes pyr and ths, phenocopied the htlAB42 mutant phenotype: glial infiltration was severely impaired, and the ventral-most astrocytic glial cell did not migrate to its normal position (Figure 3H, K, L). We analyzed pyr and ths single mutants to determine the contribution of each of the ligands to astrocyte development. pyrS0439/pyrS3547 (a strong hypomorphic combination) or the null mutant pyr18 showed no or only a very mild infiltration phenotype and a mild defect in the migration of ventral astrocytes (Figure 3I, K, L). In contrast, ths alleles such as Df(2R)ths238 and ths759 exhibited weak, but clear defects in infiltration (Figure 3J, K) and ventral cell migration (Figure 3L). These observations argue the FGFs Pyr and Ths function redundantly in promoting astrocyte growth, with Ths likely playing a more prominent role.
While htlAB42 mutants showed a severe reduction of astroglial processes infiltrating the neuropil, cell bodies and remaining glial processes surrounding the neuropil did not show obvious morphological signs of apoptosis by the end of embryogenesis (data not shown). Moreover, expressing UAS-P35 (a potent inhibitor of apoptosis) in astrocytes in the htlAB42 mutant background did not rescue astroglial infiltration nor was the general appearance of astrocytes changed, supporting the notion that the observed defects do not result from apoptotic cell death (Figure 4A, B, E). Together these data reveal that Pyr/Ths-dependent signaling through the FGF receptor Htl controls astrocyte morphogenesis, and is critical for the elaboration of astrocytic processes in the synaptic neuropil.
Figure 4. The FGFR Htl acts cell autonomously in astrocytes to control infiltration behavior and domain size.
(A–D) confocal images of late embryonic VNCs in single longitudinal sections (A–D, A′–D′) and orthogonal cross sections (A″–D″, A‴–D‴) with astrocytes labeled with α-Gat antibody (red) and the neuropil with α-Brp (green). (B) Expression P35 in astrocytes does not rescue astrocyte infiltration defects in htlAB42 mutant embryos. Expression of wild type Htl (C) or constitutive active λ-Htl (D) in an htlAB42 mutant background is able to rescue astrocytic infiltration. Scale bar represents 10μm (A–D). (E) Quantification of infiltration scores (IF). (F) Quantification of the number of ventral astrocytic cells (VC) per segment. control: N=25, htlAB42: N=36, htlAB42 alrm-Gal4 UAS-P35: N=16, htlAB42 alrm-Gal4 UAS-htl: N=13, alrm-Gal4 UAS-λ-htl: N=35. ***p<0.001, ns: not significant with p>0.05.
(G–I) MARCM analysis of htl and dof function in the L3 larval VNC. Cross sectional 3D projections are shown. Clones are labeled with CD8-GFP (green) and all astrocytes with α-Gat antibody (red). While control clones show normal infiltration behavior (G) Astrocytes mutant for htlAB42 (H) or the FGFR adapter dof1 (I) show severely reduced infiltration and cell domain size. Scale bar represents 25μm (G–I). (J) Quantification of infiltration/ cell size defects shown in (G–I). control: N=84, htlAB42: N=24, dof1: N=12, ***p<0.001. Clones in thoracic and abdominal segments of the VNC were analyzed. (K) Quantification of astrocyte cell domain volume after clonal manipulation of Htl signaling strength using the alrm>QF>Gal4 repoFLP system driving clonal expression of either UAS-htlRNAi or UAS-λ-htl. control: N=20, htlRNAi: N=22, λ-htl: N=27, ***p<0.001. See also Figure S4. Note that in (K) only clones in abdominal segments were analyzed and that average domain size in wild type is smaller in abdominal segments compared to thoracic segments (Figure S1B).
The FGF receptor Htl acts cell autonomously in astrocytes
We expressed UAS-htl using alrm-Gal4 in the htlAB42 mutant background (Figure 4A, C) and observed a strong restoration of astroglial infiltration in the neuropil (Figure 4C, E). However, proper migration of ventral-most astrocytes was not restored (Figure 4F). This may indicate that alrm-Gal4 does not drive expression early enough to rescue cell migration defects. We next generated htlAB42 mutant clones in single astrocytes using MARCM. Compared to control MARCM clones, we found the processes of htl mutant clones in the L3 VNC were largely restricted to the surface of the neuropil (Figure 4G, H), and astrocytic domain size was severely reduced (Figure 4J). Similar results were found when we generated MARCM clones for downstream of FGF receptor/Stumps (dof), an adaptor protein essential for FGF receptor signaling (Figure 4I)(Imam et al., 1999; Michelson et al., 1998; Vincent et al., 1998). These data support the notion that Htl signaling is required cell autonomously in astrocytes for their morphogenesis.
The intensity of Htl signaling and cell-cell interactions define astrocyte domain size
Based on the reduced volume of htl mutant MARCM clones we speculated the intensity of FGF signaling via Htl might dictate astrocyte size. To directly test this idea we manipulated Htl signaling strength in single cells (see Figure S4B). Clonal expression of htlRNAi in astrocytes reduced cell body size and infiltration volume compared to controls (htl-RNAi: 3261±1036 μm3 ±SD; ctrl: 5499±2081 μm3 ±SD; Figures 4K and S4G–I). Reciprocally, clonal over-activation of the Htl signaling pathway by expression of the constitutively active λ-Htl protein resulted in a cell autonomous increase in astrocyte domain volume (λ-htl: 8287±2366 μm3 ±SD; Figures 4K and S4J–L). Therefore the intensity of FGF signaling can directly modulate astrocyte size.
The stochastic nature of astrocyte morphology suggests dynamic cell-cell interactions might sculpt astrocyte domains. To test this possibility we performed ablations of astrocytes by expressing the pro-apoptotic factors Head involution defective (Hid) or Reaper. Expression of Hid led to embryonic to early larval lethality (data not shown), arguing that Drosophila astrocytes are essential for animal survival. Expression of Reaper resulted in only a partial ablation of astrocytes. These animals survived to late larval stages and to a lesser degree even to adulthood, with typically less than 2–4% of the ~170 Alrm+ astrocytes in the entire L3 larval CNS surviving. Such larvae were severely uncoordinated and exhibited a pronounced reduction in larval motility (Figure 5F). It therefore appears that even a very small number of astrocytes is sufficient for larval survival, although normal behavioral output requires significant coverage of the CNS neuropil by astrocytes.
Figure 5. Interactions among astrocytes shape their domain size.<.

br>(A–D) Image projections of confocal stacks of L3 VNCs labeled with α-Brp (blue), α-Gat (red) and/or CD8-GFP (green). (A) Wild type and (B) control MARCM analysis animals show the full complement of Gat+ astrocytes (red). GFP+ MARCM clones (green) in (B) demonstrate the wild type morphology of astrocytes. Astrocytic Reaper expression (alrm-Gal4 UAS-CD8GFP UAS-reaper) induces widespread ablation of astrocytes (C, D). Surviving astrocytes can exhibit striking cell growth (C) while some survivors show less growth and clearly aberrant morphology potentially due to effects of Reaper still being expressed (D). Note that low level expression of alrm-Gal4 UAS-CD8-GFP in ensheathing and nerve root glia seems unaffected (asterisks (C, D)). Scale bar represents 25μm (A–D). (E) Quantification of projected area of astrocytes of control MARCM clones and surviving cells in alrm-Gal4 UAS-CD8-GFP UAS-reaper ablations. control: N=54, reaper N=29, ***p<0.001 and the associated locomotion defects in L3 larvae (F), control: N=22, reaper: N=33, ***p<0.001. See also Figure S5.
Many surviving isolated astrocytes demonstrated a remarkable capacity for growth: individual cells generated a meshwork of processes over a much greater area of the CNS (1623.0±814.6 μm2 ±SD) compared to controls clones (843.8±411.4 μm2 ±SD, Figure 5E), often spanning several segments (Figure 5A–D). When we performed genetic ablations and labeled individual cells using the repoFLP UAS-CD8>GFP>RFP approach, we observed normal tiling behavior whenever two astrocytes came in contact with one another (Figure S5A, N=13), although they exhibited similar levels of overgrowth compared to astrocytes growing in isolation (see Figure 5C, D).
Finally, to exclude the possibility that the expression of proapoptotic factors was causing overgrowth we used an alternative genetic strategy which led to the elimination of Gal4 in surviving cells. Briefly, we generated an alrm>Gal4>lexA::GAD construct (Figure S4C). Without Flippase activity Gal4 is expressed under the control of the alrm promoter, but upon Flippase activity the Gal4 coding sequence is excised and the lexA::GAD transcriptional activator (Lai and Lee, 2006) is expressed. In combination with a heatshock inducible Flippase this approach allowed us to control expression of the apoptosis inducing factor Hid in most of the astrocytes but “rescue” cells by the early excision of Gal4. Using this approach we were able to accomplish ablation of 73.4±7.7% ±SD (N=4) of all astrocytes in the VNC. Surviving cells (non-Hid expressing cells) were able to compensate to a high degree: while few regions in the neuropil were not or only poorly covered (data not shown), astroglial processes were generally found throughout the neuropil (Figure S5B).
Astrocytes thus have a striking intrinsic growth potential that is normally terminated by inhibitory interactions with neighboring astrocytes or competition among astrocytes for growth factors within the neuropil. We propose this compensatory growth behavior underlies the ability of astrocytic processes to reliably cover the entire synaptic neuropil in wild type animals.
Pyr and Ths can provide spatial cues to direct process outgrowth of astrocytes
To determine the source of the FGF ligands Pyr and Ths during astrocytes morphogenesis we performed fluorescent RNA in situ hybridizations with pyr and ths antisense probes. The ths probe labeled specific subsets of a small number of Repo-negative (i.e. non-glial) cells from embryonic stage 13 onwards (Figure S6A–E), becoming broader and less specific in older embryos (Figure S6E). The pyr probe exhibited broad and less specific signal in the CNS (data not shown). Consistent with a neuronal source of FGF ligands rescue experiments using the pan-neuronal driver elav-Gal4 or glutamatergic driver OK371-Gal4 in the Df(2R)BSC25 mutant background (Figure 6A–D), resulted in a clear but partial rescue of astroglial infiltration, however migration of ventral astrocytes was not restored (Figure 6E).
Figure 6. FGFs Pyr and Ths are likely secreted from neurons to control astrocytes infiltration.
(A–C) Confocal images of late embryonic VNCs in single longitudinal sections (A–C, A′–C′, F–G) and cross sections (A″–C″, A‴–C‴). Gal4 expression patterns are highlighted by GFP expression (green), in (A) and (F–I), astrocytes are labeled with α-Gat (red) and the neuropil with α-Brp in green (B, C) or in blue (F, G). (A) Expression pattern of OK371-Gal4 (glutamateric neurons) in a Df(2R)BSC25 mutant background (green). (B, C) Expression of either Pyr or Ths in glutamateric neurons is able to rescue astrocytic infiltration in Df(2R)BSC25 mutants. (D) Quantification of infiltration phenotypes. (E) Quantification of the number of ventral astrocytes per segment. Control: N=25, Df(2R)BSC25: N=34, Df(2R)BSC25 elav-Gal4 UAS-pyr: N=13, Df(2R)BSC25 elav-Gal4 UAS-ths: N=10, Df(2R)BSC25 Ok371-Gal4 UAS-pyr: N=12, Df(2R)BSC25 Ok371-Gal4 UAS-ths: N=19, ***p<0.001, ns: not significant with p>0.05. (F–I) Expression of Pyr or Ths in an extremely small number of neurons using the clonal RN2-FLP system in a Df(2R)BSC25 mutant background (green). In (F, G) single section confocal images are shown for the red (Gat) and blue (Brp) channels while the green channel (RN2-FLP neurons) shows z-projections of the image stacks to demonstrate the position of expressing neurons. (H, I) cross sectional 3D projections. Scale bars represent 10μm. See also Figure S6.
To assay for differences in the signaling abilities of Pyr or Ths we limited their expression to single neurons using a RN2-FLP, Tub84B-FRT-CD2-FRT-GAL4, UAS-CD8-GFP chromosome (Ou et al., 2008) in a Df(2R)BSC25 mutant background. Briefly, this mosaic approach allows us to resupply Pyr or Ths in single cells in a background lacking both ligands, and assay astroglial growth with respect to GFP-labeled neurons. Pyr expression from a single neuron failed to rescue astroglial process outgrowth (0% rescue of Pyr expressing cells, N=142 cells, Figure 6F, H). However, expression of Ths in single neurons resulted in robust rescue of astrocyte infiltration even some distance beyond the volume covered by Ths+ neurites (Figure 6G, I). Thus, under these conditions Pyr and Ths differ in their abilities to stimulate astrocytic outgrowth.
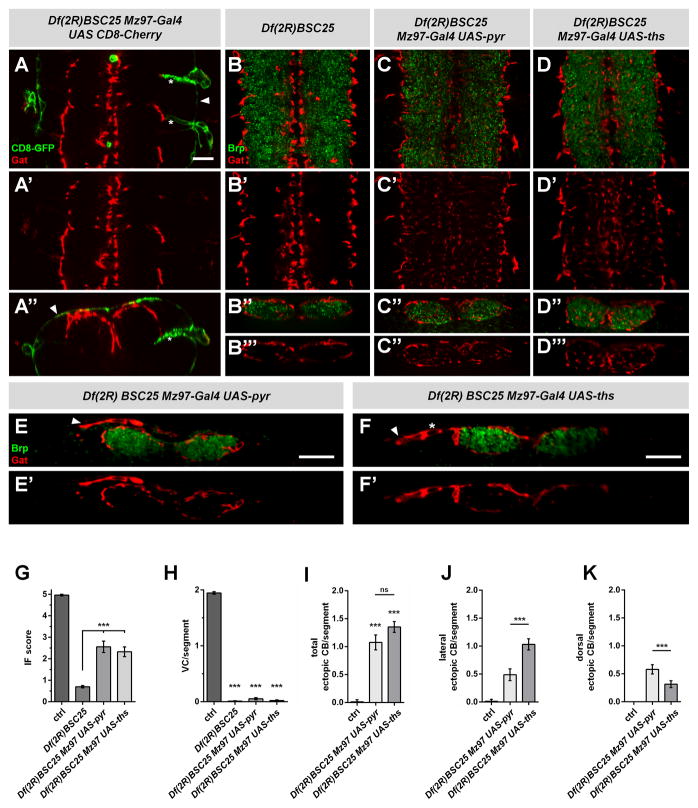
Does Htl signaling provide directional cues for astrocyte processes, or is it permissive? We expressed λ-Htl, a ligand-independent activated version of Htl in astrocytes in a htlAB42 mutant background. We found λ-Htl partially rescued astroglial infiltration defects (Figure 4D), although not as strongly as with wild type Htl (Figure 4C, D, E). This observation argued that Htl signaling is largely permissive. Nevertheless, we then ectopically expressed Pyr or Ths in subperineurial and nerve root glia that line the surface of the CNS and nerve root (but do not extend processes into the neuropil) using Mz97-Gal4 in the Df(2R)BSC25 mutant background and assayed astrocyte growth (Figure 7A). Consistent with a permissive role for the Htl signaling pathway, ectopic expression of these ligands led to partial rescue of astroglial infiltration of the neuropil (Figure 7C, D, G). However, we also observed ectopic astroglial projections along the dorsal CNS surface and nerves (Figure 7E, F), and astroglial cell bodies displaced to dorsal and lateral positions (Figure 7E, F, I–K). Based on the above data we conclude that Pyr and Ths are able to act at a distance to signal to developing astrocytes, as directional chemoattractants for both the migration of cell bodies and outgrowth of astroglial processes, and that the two FGFs differ in their properties in how they do so.
Figure 7. Ectopic expression of Pyr and Ths<.
br>(A–F) Confocal images of late embryonic VNCs in single longitudinal sections (A–D, A′–D′) and cross sections (A″–D″, B‴-D‴, E, F). Expression pattern of Mz97-Gal4 is highlighted by GFP expression (green in (A)), astrocytes are labeled with α-Gat (red) and the neuropil with α-Brp (green in B–F). (A) Expression of Mz97-Gal4 UAS-actin5c-GFP (green) in a Df(2R)BSC25 mutant background in subperineurial (arrowheads) and nerve root glia (asterisks). Ectopic expression of Pyr and Ths with Mz97-Gal4 can partially rescue astrocyte infiltration into the neuropil in Df(2R)BSC25 mutants (C, D, G); however, ectopic recruitment of astrocyte processes (arrowheads) or lateral displacements of cell bodies (asterisks) were also observed (E, F). Scale bars represent 10μm. (G) Quantification of infiltration phenotypes. (H) Quantification of the number of ventral astrocytes per segment. Control: N=25, Df(2R)BSC25: N=34, Df(2R)BSC25 Mz97-Gal4 UAS-pyr: N=18, Df(2R)BSC25 Mz97-Gal4 UAS-ths: N=17. Quantification of the number of all ectopic cell bodies (I) or the number of laterally (J) or dorsally (K) displaced cell bodies per segment upon ectopic expression of FGFs. Control: N=25, Df(2R)BSC25 Mz97-Gal4 UAS-pyr: N=13, Df(2R)BSC25 Mz97-Gal4 UAS-ths: N=17. ***p<0.001, ns: not significant with p>0.05. See also Figure S7.
How do Pyr or Ths act at a distance to drive astrocyte processes into the neuropil? Heparan sulfate proteoglycans (HSPGs) are critically important for concentrating FGF ligands for efficient signaling through FGFRs. The Drosophila HSPG Syndecan (Sdc) is highly enriched in the neuropil (Figure S7G)(Johnson et al., 2004). We ectopically expressed UAS-sdc-GFP with Mz97-Gal4 in a wild type or a Df(2R)BSC25 heterozygous background (to partially reduce levels of FGF ligands) and analyzed L3 VNCs. In both genotypes we observed consistent ectopic recruitment of Gat+ astrocyte processes to the dorsal surface of the CNS and at least one patch of ectopic processes per VNC (N=19, Figure S7F′), this was similar to the effect of ectopic Ths, although weaker (N=3, Figure S7D, arrowheads). Late embryonic sdc2639 null mutants also exhibited mild infiltration defects (Figure S7H) and defects in the migration of ventral astrocytes (Figure S7I), and in L3 sdc2639 or sdc23 mutants ventral cell counts were reduced compared to wild type (Figure S7L), also similar to ths759 mutants (Figure 3K, S7L). These data support the notion that Syndecan plays a modulatory role in Htl FGF-dependent development of astrocytes, perhaps by concentrating FGFs in the neuropil.
Discussion
Drosophila astrocytes are morphologically and functionally similar to their mammalian counterparts
Astrocytes remain a poorly understood cell type in the brain. Their precise in vivo functions in neural circuits are only beginning to be unraveled and their evolutionary origins remain unclear (Hartline, 2011). The discovery of an astrocyte-like cell type in Drosophila indicates that astrocyte-like cell types are an essential component of even comparatively simple nervous systems. Broader studies of astrocytes in diverse taxa will be required to determine whether Drosophila astrocytes are homologous to mammalian astrocytes in evolutionary terms, or represent a remarkable case of convergent evolution.
Drosophila astrocytes form a highly ramified and dense meshwork of processes that infiltrate the entire neuropil and associate closely with synapses. This close spatial relationship is reminiscent of the mammalian “tripartite synapse,” thought to be critical for neurotransmitter clearance and the modulation of synaptic activity during complex behaviors (Araque et al., 1999). In the L3 VNC the majority of synapses were in close proximity to astroglial processes, although not directly ensheathed (Figure 2C, D). Nevertheless, using the iGluSnFR reporter we demonstrated local increases in extracellular glutamate readily reached astrocyte membranes, indicating they are within the functional range of synapses.
Functional roles of Drosophila astrocytes also appear well conserved when compared to mammals. The glutamate transporter EAAT1 is expressed in Drosophila astrocytes and is essential for coordinated locomotor activity in larvae and prevention of excitotoxicity in the adult (Rival et al., 2004; Stacey et al., 2010). Here we have demonstrated astrocyte-specific expression the GABA transporter Gat and partial loss of Gat impeded larval locomotion. GABA transporter inhibitors also impair larval coordinated locomotion (Leal et al., 2004; Leal and Neckameyer, 2002; Neckameyer and Cooper, 1998), and Manduca and Trichoplusia Gat homologs are high affinity GABA transporters (Gao et al., 1999; Mbungu et al., 1995), supporting the notion that gat-depleted animals experience disruption of GABA neurotransmitter clearance. Despite apparently normal CNS morphology, gat null animals die as late embryos. Astrocytic Gat is therefore essential for viability, and we propose Gat plays a central role for astrocyte-mediated GABA clearance even before animal hatching.
Ca2+ microdomain signaling in mammalian astrocytes is emerging as a key mechanism by which astrocytes respond to, and regulate neuronal activity (Panatier et al., 2011; Shigetomi et al., 2011). Drosophila cortex glia, cells closely associated with neuronal cell bodies, also exhibit microdomain Ca2+ oscillations (Melom and Littleton, 2013) and glial Ca2+ signaling events can modulate fly circadian behavior (Ng et al., 2011) and seizure activity (Melom and Littleton, 2013). Interestingly, we found Drosophila astrocytes exhibit spontaneous, local Ca2+ transients in vivo (Z. Ma,. T. Stork and M.R. Freeman, unpublished observations), and seem to be coupled with respect to Ca2+ signaling: laser induced injury of a single astrocyte in the larva induced an increase in intracellular calcium in the injured cell which subsequently spread into neighboring astrocytes (Movie 8).
Our data taken together argue strongly that Drosophila astrocytes will prove an excellent in vivo system in which to study many fundamental aspects of astrocyte biology and astrocyte-neuron interactions.
Astrocytes are essential for animal survival
We have shown Drosophila astrocytes are critically important for animal survival. Partial ablation of mouse astrocytes during development also led to death at birth (Tsai et al., 2012). Interestingly, astrocyte depletion by ~30% in selected spinal cord domains led to atrophy and loss of neuropil and synapses. In Drosophila larvae lacking the majority of astrocytes, gross CNS morphology was surprisingly normal. Therefore fly astrocytes may not be strictly required for neuronal survival, although earlier ablation or ablations in the adult could yield different results. Alternatively, other subtypes of CNS glia (e.g. ensheathing or cortex glia) might functionally substitute for astrocytes and promote neuronal survival.
Depletion of astrocytes from large regions of the mammalian CNS did not lead to a repopulation of these zones by astrocytes from neighboring domains, suggesting that astrocytes possess a high regional specificity and low invasive behavior (Tsai et al., 2012). However, while dramatic movement of populations of astrocytes was not observed, it is less clear whether astrocytes at the border of astrocyte-depleted regions react more locally with increased growth. Regional astrocyte ablation studies in mammals followed by the use of markers that highlight single cell astrocyte morphology will be essential to definitively resolve these questions.
Domain morphogenesis of astrocytes is stochastic, and involves cell-cell competition for neuropil space and tiling behavior
It has been proposed that astrocyte domain organization and association with specific subsets of neurons has an important role in the proper function of neuronal networks (Bushong et al., 2002; Nedergaard et al., 2003). While Drosophila astrocytes are quite stereotyped in cell number and cell body position, the domains of the neuropil covered by astrocyte processes show variability in size and shape. It therefore seems unlikely that individual astrocytes are genetically programmed to associate with particular regions of the brain, or specific synapses.
Astrocytes appear to harbor a massive growth potential, but exert a strong growth-inhibiting effect on one another. First, when adjacent cells are ablated, astrocytes expand their territories, while tiling where they are in contact with other astrocytes. Second, when we generated htl or dof mutant clones that failed to infiltrate the neuropil, the space adjacent to these clones was efficiently infiltrated by other astrocytes. Finally, while enhancing Htl signaling increased domain size, neighboring cells still “tiled” and the overlap of astrocytic domains did not increase noticeably. How tiling of astrocytes occurs remains unclear, but could be accomplished through contact dependent growth inhibition or competition for neuropil growth factors. Nevertheless, based on the multiple lines of evidence presented here, we propose that astrocyte morphology is shaped dynamically during development by neuron-astrocyte and astrocyte-astrocyte interactions.
Finally, while the relative overlap of neighboring astrocytes appears to be higher in Drosophila compared to mammalian astrocytes (Bushong et al., 2004; Bushong et al., 2002; Ogata and Kosaka, 2002), it is important to note from a mechanistic perspective that the size of a Drosophila astrocyte is smaller compared to mouse and that the absolute overlap of astrocyte processes in mouse and fly seem comparable. Our discovery of tiling behavior in Drosophila suggests that fly and mammalian astrocytes may share common molecular mechanisms by which neighboring cells define their territories.
The Htl signaling pathway is a key regulator of astrocyte outgrowth and cell size
Loss of the FGF receptor Htl, its ligands Pyr and Ths, or the downstream signaling molecule Dof/Stumps blocked the infiltration of astrocyte processes into the neuropil, demonstrating that the Htl signaling pathway is critical for effective astrocytic growth into the synapse-rich neuropil. The level of Htl signaling is also critically involved in the regulation cell and domain size of astrocytes, with increased Htl signaling leading to increased astrocyte volume. Our expression data, clonal analysis, and astrocyte-specific rescue experiments all indicate that Htl and Dof function autonomously in glia. Precisely where the FGF ligands Pyr and Ths are generated during development was more difficult to determine. However, based on its expression pattern and the ability to rescue astrocyte outgrowth when expressed in neurons, we propose that at least Ths is primarily derived from neurons.
Ectopic expression of Pyr or Ths away from the neuropil, or astrocytic expression of a constitutively active form of Htl, are both able to partially restore astrocyte infiltration. These observations suggest a permissive role for the Htl signaling pathway in astroglial growth. However, expression of Pyr or Ths is also able to promote the outgrowth of ectopic astroglial branches outside of the neuropil, indicating these ligands can provide directional cues for astrocyte outgrowth. Pyr and Ths appear different in their signaling abilities: single neuron expression revealed Pyr was unable to promote extension of astrocyte processes, while Ths drove robust astrocytic process outgrowth, suggesting that the promotion of outgrowth by Ths can act at a short range.
How can Pyr and Ths direct astrocyte process growth into the neuropil even when ectopically expressed? FGF signaling is critically dependent on heparan sulfate proteoglycans (HSPGs) in vivo (Lin et al., 1999). Two of the four HSPGs in Drosophila, Dally-like and Syndecan have been reported to be prominently enriched in the embryonic neuropil where they have been shown to act in Slit dependent axon guidance (Johnson et al., 2004; Steigemann et al., 2004). We have confirmed expression of Sdc in the neuropil, found that ectopic Sdc expression was sufficient to redirect astrocyte membranes outside of the neuropil, and that loss of Sdc led to a defect in the ventral migration of astrocyte cell bodies and—to a lesser extent—problems in early neuropil infiltration. Based on these observations we speculate that Sdc plays a modulatory role in the development of astrocytes by concentrating the FGFs Pyr and Ths in the neuropil to drive directional infiltration even when the ligands are provided ectopically. Finally, Pyr and Ths might act redundantly with additional unidentified neuropil-restricted factors that can provide directional information for astrocytic process outgrowth.
ths null mutants showed a slight decrease in the number of astrocytes in late embryos and L3 larvae (Figure S7J, K) while embryonic htlAB42 mutants did not show a reduction in cell counts (Figure S7J). sdc mutants also showed a similar slight reduction in total cell number in L3 larvae (Figure S7K). These data suggest that astrocytes are generated in the embryo at normal numbers in FGF-pathway mutants but that individual cells might be outcompeted by neighbors during process outgrowth resulting in death of individual cells. Since we are not able to uniquely identify the presumptive ventral cell among the dorsally located cells, it is not clear if the non-migrating presumptive ventral cells preferentially die or if cell death is stochastic among all astrocytes. While the mechanism of such adjustment of cell numbers through cellular competition remains poorly understood, it might be based on competition for trophic factors or a more active form of cell killing by “winning” neighbors (Amoyel and Bach, 2014). Our data deepen our understanding of the diverse roles FGF signaling plays in insect glial development, where FGFs have been shown to also regulate glial proliferation, survival, migration and ensheathment of axons (Avet-Rochex et al., 2012; Franzdottir et al., 2009; Gibson et al., 2012; Shishido et al., 1997), and glial wrapping of FGF2-coated beads in grasshopper (Condron, 1999).
FGF signaling has also been implicated in mammalian astrocyte development. Mammalian FGFs can act as mitogens for glial precursors, and potentiate the ability of secreted factors CNTF and LIF to promote astroglial fate in neural progenitors (Kang and Song, 2010; Song and Ghosh, 2004; Vaccarino et al., 1999). In addition, FGF application can induce maturation of astroglia in cell culture by controlling morphological stellation in two dimensions and the expression of GFAP and glutamine synthetase (Perraud et al., 1988a; Perraud et al., 1988b; Reilly et al., 1998). FGF receptors 1–3 are expressed in astrocytes and their precursors (Cahoy et al., 2008; Ohkubo et al., 2004; Pringle et al., 2003; Reilly and Kumari, 1996). In particular FGFR3 is highly enriched in the radial precursor cells in the ventricular zone and immature and mature astrocytes (Cahoy et al., 2008; Pringle et al., 2003) and in FGFR3 and other FGF pathway mutants GFAP expression in astrocytes is perturbed in vivo (Irmady et al., 2011; Pringle et al., 2003; Reuss et al., 2003). Furthermore FGFR1/2 mutants show a reduction in GFAP positive astrocytes in the cortex and impaired Bergmann glia morphology in the cerebellum (Muller Smith et al., 2006; Muller Smith et al., 2012). The exact roles of mammalian FGFRs and their ligands in astrocyte ramification, association with neurons and synapses and establishment of astrocytic domain size, however, remain to be tested. Our observations of an essential requirement for FGF signaling in astrocyte development in vivo in Drosophila suggests that a detailed analysis of FGF signaling pathways in mammalian astrocyte development should prove fruitful. FGF signaling is known to be perturbed in glioma (Allerstorfer et al., 2008; Mohanan et al., 2012; Yamada et al., 2002) and our observations of the key role for FGFs in astrocyte process outgrowth may ultimately provide insight into the highly invasive nature of glioma cells in the brain.
Experimental procedures
Immunohistochemistry and confocal analysis
Larval or late stage embryonic central nervous systems were dissected and fixed for 25 min in PBS with 4% formaldehyde at room temperature and subsequently immuno-labeled following standard procedures. The following primary antibodies were used: rabbit α-β-Gal (Promega, 1:3000), goat α-HRP conjugated to Cy3 or Cy5 (Jackson ImmunoResearch Laboratories, 1:400), mouse α-Bruchpilot (nc82, 1:20), mouse α-Repo (8D12, 1:10) mouse-α-Prospero (MR1A, 1:150), mouse α-FasII (1D4, 1:10) all Developmental Studies Hybridoma Bank (DSHB), rabbit or mouse α-GFP (Molecular Probes) 1:400, rabbit α-RFP (Clontech) 1:200, rabbit α-Gat (this study, 1:6000), rat α-mouse-CD8 (eBioscience) 1:200). Primary antibodies were detected with the appropriate goat secondary antibodies conjugated to FITC, Dylight488, Cy3, Cy5 or Dylight649 (Jackson ImmunoResearch). Samples were mounted in Vectashield antifade reagent (Vector Laboratories) and confocal images were obtained on an Innovative Imaging Innovations (3I) spinning disc confocal microscope equipped with a Yokogawa CSX-X1 scan head or on a Zeiss LSM 5 Pascal.
Live imaging
Homozygous alrm-Gal4 UAS-CD8-GFP embryos were prepared for live imaging essentially as described (Parton et al., 2010) and imaged on a 3I spinning disc confocal microscope or on a Zeiss LSM 7 MP microscope. We genetically immobilized older animals for imaging using a myosin heavy chain mutation (mhc1/mhc1;alrm-Gal4, UAS-CD8-GFP).
L1 larvae were immobilized by gently squeezing them under a cover glass in halocarbon oil. For laser injury experiments L3 larvae were dissected as a filet preparation under HL-3 or HL-6 solution (1.5mM Ca2+), injured with a Micropoint laser ablation system (430nm), mounted on a 3I spinning disc confocal microscope.
Gat antibody generation
α-Gat antibodies were generated by co-injecting N-terminal (KHESIEMSKELGHTC) and C-terminal (CLREAYAKEIEFNSL) peptides into rabbits using standard techniques (New England Peptide). Sera were subsequently immunoaffinity purified using C-and N-terminal peptides on separate columns.
Measurements of larval crawling velocity
L3 larvae were grown at 25 °C, briefly washed in water and starved for 10–20 minutes. Individual larvae were then transferred at room temperature to a 24 × 24-cm 2% agar plate and allowed to recover for 30 seconds before larval tracks were recorded by manually tracing the larva on a transparency film on the plate lid. Tracks were scanned and track length was measured using Fiji.
In situ hybridization
DIG-labeled RNA antisense probes were generated by in vitro transcription (Roche) from linearized vectors pBS-ths and pJet1.2-pyrProbe2. The ths cDNA was PCR amplified from a reverse transcriptase reaction using the primers GATCGATCGCGGCCGCTTAGCCAGCGCGTTATCAC and GATCGATCGCGGCCGCCTACGCAAATCTCTGATGAGTG and cloned into pBS using the Not I site. A 1.7 kb fragment of pyr was amplified from genomic DNA using the primers AGCTCCAAGCTTTAGCGCCTACAATCCAGTGCTG and AGCTATCTCGAGGTTTGTCAGCAGACCACCATCG and subsequently cloned into pJet1.2 according to the manufacturer’s instructions (Fermentas). In situ hybridizations were performed largely as described (Broadus and Doe, 1995) and probe detection was performed according to the manufacturer’s instructions using anti-DIG-POD coupled antibodies (Roche) and the Perkin Elmer TSA-plus Fluorescein detection kit.
Generation of transgenes
All transgenic flies were generated at BestGene or Rainbow Transgenics and a detailed description of the generation of the transgenes can be found in the supplementary material.
Drosophila genetics
Flies were kept on a standard cornmeal agar supplemented with dry yeast at 25°C unless stated otherwise. Detailed information of the fly stocks used in this study can be found in the supplemental material.
Generation of Df(4)gatΔ64kb and gat22-1
The Df(4)gatΔ64kb deletion chromosome was generated by FLP-FRT recombination as described (Parks et al., 2004) using the insertions PBac[WH]f02782 and PBac[WH]f08075. The gat22-1 allele was generated by a TALEN approach. TALEN target sites were TTGTCGAAATGTACACAAACT and TACACACATCTCCTCCTCCGT. More detailed information can be found in the supplementary material.
Generation of repoFLP6–2
To isolate a more efficient repoFLP source to be used in MARCM experiments, the initial P-element insertion repoFLP5 was mobilized using Δ2–3 transposase and repoFLP6–2 was selected based on its strong ability to induce glial expression when crossed to an act5C>CD2>Gal4, UAS-CD8-Cherry stock.
MARCM analysis and generation of FLP-out clones
FLP out clones were generated by using the following recombinant chromosomes: alrm-Gal4 UAS-CD8>GFP>RFP repoFLP5, alrm>Gal4>lexA::GAD repoFLP5 and alrm>QF>Gal4 QUAS-CD8-GFP UAS-CD8-Cherry repoFLP5. Crosses using alrm>QF>Gal4 QUAS-CD8-GFP UAS-CD8-Cherry repoFLP5 were conducted at 29°C to increase expression strength. MARCM clones were generated with alrm-Gal4 UAS-CD8-GFP repoFLP6-2; FRT82B tub-Gal80 crossed to FRT82B, FRT82B htlAB42 or FRT82B dof1 chromosomes.
Quantification of astrocytic occupied volume and covered area
Confocal stacks of marked astrocytes clones were segmented by manually thresholding the image in 3D and subsequently measuring the volume using Slidebook software (Innovative Imaging Innovations). Average cell volume was determined by dividing the measured volume by the number of cells in the clone. Area covered was estimated by manually outlining the area occupied by astrocytes in a maximum intensity projection along the Z-axis. The area was measured and divided by the number of cells in the clone.
Transmission electron microscopy
TEM on L3 central nervous systems was conducted at the University of Massachusetts Medical School Electron Microscopy core facility as described in (Tasdemir-Yilmaz and Freeman, 2014).
Statistical analysis
Values in the text are given as average ±standard deviation (SD). Error bars in graphs indicate standard error of the mean (SEM) unless otherwise stated. Statistical significance was calculated with Graphpad Prism software using either two-tailed Student’s t-test for pairwise comparisons or one-way ANOVA with either Dunnett’s or Tukey’s posthoc test as appropriate for comparisons of more than two groups. All embryonic infiltration scores and ventral cell counts were analyzed in a common ANOVA with Tukey’s posthoc test and results are displayed in separate graphs for clarity.
Supplementary Material
Acknowledgments
We are grateful to Christian Klämbt, Angelike Stathopoulos, Arno Müller, Ingolf Reim, Matthias Landgraf, Hermann Aberle, Marion Silies, Melissa Rolls, Tzumin Lee, Motojiro Yoshihara, Joachim Schulz, Gerd Vorbrüggen, Roger Jacobs as well as the Vienna Drosophila RNAi Center, the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) and the Bloomington Stock Center for generously providing fly stocks. The monoclonal antibodies mAb 1D4, mAb 8D12, mab MR1A and mAb nc82 developed by C Goodman, CQ Doe and E Buchner respectively were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242 USA. Plasmids containing QF, codon optimized Gal4 and iGluSnFR were obtained from Addgene. Roger Tsien generously provided a plasmid containing mCherry sequence and Tzumin Lee a plasmid containing LexA::GAD. We thank Looren Looger for generously sharing a GCaMP5a construct prior to publication. We further thank Michael Brodsky and the University of Massachusetts Medical School Mutagenesis Core for assistance with TALEN-mediated mutagenesis, the University of Massachusetts Medical School Electron Microscopy core facility for assistance with TEM experiments (supported by award number S10RR027897 from the National Center For Research Resources), Harleen Saini for help with laser injuries of astrocytes and Johnna Doherty for generating a ths cDNA. T.S. was supported by a postdoctoral fellowship by the Deutsche Forschungsgemeinschaft (DFG). This work was supported by NINDS grant R01NS053538 (to MRF). MRF is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, Czech T, Marosi C, Buchroithner J, Pichler J, et al. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–4190. doi: 10.1038/onc.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Bach EA. Cell competition: how to eliminate your neighbours. Development. 2014;141:988–1000. doi: 10.1242/dev.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Avet-Rochex A, Kaul AK, Gatt AP, McNeill H, Bateman JM. Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain. Development. 2012;139:2763–2772. doi: 10.1242/dev.074179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM. Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev. 2008;125:542–557. doi: 10.1016/j.mod.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Broadus J, Doe CQ. Evolution of neuroblast identity: seven-up and prospero expression reveal homologous and divergent neuroblast fates in Drosophila and Schistocerca. Development. 1995;121:3989–3996. doi: 10.1242/dev.121.12.3989. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron B. Spatially discrete FGF-mediated signalling directs glial morphogenesis. Development. 1999;126:4635–4641. doi: 10.1242/dev.126.20.4635. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Gao X, McLean H, Caveney S, Donly C. Molecular cloning and functional characterization of a GABA transporter from the CNS of the cabbage looper, Trichoplusia ni. Insect Biochem Mol Biol. 1999;29:609–623. doi: 10.1016/s0965-1748(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Gibson NJ, Tolbert LP, Oland LA. Activation of glial FGFRs is essential in glial migration, proliferation, and survival and in glia-neuron signaling during olfactory system development. PLoS One. 2012;7:e33828. doi: 10.1371/journal.pone.0033828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK. The evolutionary origins of glia. Glia. 2011;59:1215–1236. doi: 10.1002/glia.21149. [DOI] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam F, Sutherland D, Huang W, Krasnow MA. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics. 1999;152:307–318. doi: 10.1093/genetics/152.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmady K, Zechel S, Unsicker K. Fibroblast growth factor 2 regulates astrocyte differentiation in a region-specific manner in the hindbrain. Glia. 2011;59:708–719. doi: 10.1002/glia.21141. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Hiromi Y, Patel NH, Goodman CS. Lineage, migration, and morphogenesis of longitudinal glia in the Drosophila CNS as revealed by a molecular lineage marker. Neuron. 1989;2:1625–1631. doi: 10.1016/0896-6273(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–747. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Song MR. Diverse FGF receptor signaling controls astrocyte specification and proliferation. Biochem Biophys Res Commun. 2010;395:324–329. doi: 10.1016/j.bbrc.2010.03.174. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingseisen A, Clark IB, Gryzik T, Muller HA. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development. 2009;136:2393–2402. doi: 10.1242/dev.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- Leal SM, Kumar N, Neckameyer WS. GABAergic modulation of motor-driven behaviors in juvenile Drosophila and evidence for a nonbehavioral role for GABA transport. J Neurobiol. 2004;61:189–208. doi: 10.1002/neu.20061. [DOI] [PubMed] [Google Scholar]
- Leal SM, Neckameyer WS. Pharmacological evidence for GABAergic regulation of specific behaviors in Drosophila melanogaster. J Neurobiol. 2002;50:245–261. doi: 10.1002/neu.10030. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen TW, Bargmann CI, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbungu D, Ross LS, Gill SS. Cloning, functional expression, and pharmacology of a GABA transporter from Manduca sexta. Arch Biochem Biophys. 1995;318:489–497. doi: 10.1006/abbi.1995.1258. [DOI] [PubMed] [Google Scholar]
- Melom JE, Littleton JT. Mutation of a NCKX Eliminates Glial Microdomain Calcium Oscillations and Enhances Seizure Susceptibility. J Neurosci. 2013;33:1169–1178. doi: 10.1523/JNEUROSCI.3920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson AM, Gisselbrecht S, Buff E, Skeath JB. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development. 1998;125:4379–4389. doi: 10.1242/dev.125.22.4379. [DOI] [PubMed] [Google Scholar]
- Mohanan V, Temburni MK, Kappes JC, Galileo DS. L1CAM stimulates glioma cell motility and proliferation through the fibroblast growth factor receptor. Clin Exp Metastasis. 2012 doi: 10.1007/s10585-012-9555-4. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Smith K, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci. 2006;9:787–797. doi: 10.1038/nn1705. [DOI] [PubMed] [Google Scholar]
- Muller Smith K, Williamson TL, Schwartz ML, Vaccarino FM. Impaired motor coordination and disrupted cerebellar architecture in Fgfr1 and Fgfr2 double knockout mice. Brain Res. 2012;1460:12–24. doi: 10.1016/j.brainres.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS, Cooper RL. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert Neurosci. 1998;3:279–294. doi: 10.1007/BF02577688. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci. 2004;24:6057–6069. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Chwalla B, Landgraf M, van Meyel DJ. Identification of genes influencing dendrite morphogenesis in developing peripheral sensory and central motor neurons. Neural Dev. 2008;3:16. doi: 10.1186/1749-8104-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Parton RM, Valles AM, Dobbie IM, Davis I. Collection and mounting of Drosophila embryos for imaging. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5403. pdb prot5403. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. GLIA modulates synaptic transmission. Brain Res Rev. 2010;63:93–102. doi: 10.1016/j.brainresrev.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Perraud F, Besnard F, Pettmann B, Sensenbrenner M, Labourdette G. Effects of acidic and basic fibroblast growth factors (aFGF and bFGF) on the proliferation and the glutamine synthetase expression of rat astroblasts in culture. Glia. 1988a;1:124–131. doi: 10.1002/glia.440010204. [DOI] [PubMed] [Google Scholar]
- Perraud F, Labourdette G, Miehe M, Loret C, Sensenbrenner M. Comparison of the morphological effects of acidic and basic fibroblast growth factors on rat astroblasts in culture. J Neurosci Res. 1988b;20:1–11. doi: 10.1002/jnr.490200102. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Howell M, Colvin JS, Ornitz DM, Richardson WD. Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development. 2003;130:93–102. doi: 10.1242/dev.00184. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Kumari VG. Alterations in fibroblast growth factor receptor expression following brain injury. Exp Neurol. 1996;140:139–150. doi: 10.1006/exnr.1996.0124. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Maher PA, Kumari VG. Regulation of astrocyte GFAP expression by TGF-beta1 and FGF-2. Glia. 1998;22:202–210. doi: 10.1002/(sici)1098-1136(199802)22:2<202::aid-glia11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Reuss B, Dono R, Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. J Neurosci. 2003;23:6404–6412. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iche M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido E, Ono N, Kojima T, Saigo K. Requirements of DFR1/Heartless, a mesoderm specific Drosophila FGF receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–2128. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci. 2004;7:229–235. doi: 10.1038/nn1192. [DOI] [PubMed] [Google Scholar]
- Stacey SM, Muraro NI, Peco E, Labbe A, Thomas GB, Baines RA, van Meyel DJ. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J Neurosci. 2010;30:14446–14457. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–699. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol. 2006;209:3383–3404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Thomas GB, van Meyel DJ. The glycosyltransferase Fringe promotes Delta-Notch signaling between neurons and glia, and is required for subtype-specific glial gene expression. Development. 2007;134:591–600. doi: 10.1242/dev.02754. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vincent S, Wilson R, Coelho C, Affolter M, Leptin M. The Drosophila protein Dof is specifically required for FGF signaling. Mol Cell. 1998;2:515–525. doi: 10.1016/s1097-2765(00)80151-3. [DOI] [PubMed] [Google Scholar]
- Yamada SM, Yamada S, Hayashi Y, Takahashi H, Teramoto A, Matsumoto K. Fibroblast growth factor receptor (FGFR) 4 correlated with the malignancy of human astrocytomas. Neurol Res. 2002;24:244–248. doi: 10.1179/016164102101199864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.