Abstract
Among both civilian and veteran populations, substance use disorders (SUDs) and anxiety disorders frequently co-occur. One of the most common comorbid anxiety disorder is posttraumatic stress disorder (PTSD), a condition which may develop after exposure to traumatic events, such as military combat. In comparison with the general population, rates of both SUDs and PTSD are elevated among veterans. Recent data show that soldiers returning from Iraq and Afghanistan demonstrate high rates of co-occurring SUDs, PTSD, and traumatic brain injury. Careful assessment of these conditions is critical and may be complicated by symptom overlap. More research targeting integrated interventions for these conditions is needed to establish optimal treatments.
Keywords: posttraumatic stress disorder, PTSD, substance use disorders, combat, TBI
INTRODUCTION
Posttraumatic stress disorder (PTSD) is one of the most common anxiety disorders in the individuals with substance use disorders (SUDs). Initial reports focused on Vietnam veterans with PTSD, in which 64% to 84% met lifetime criteria for an alcohol use disorder and 40% to 44% met lifetime criteria for a drug use disorder, including nicotine dependence.1,2 In civilian populations with PTSD, estimates of lifetime prevalence of SUDs in epidemiologic samples range from 22% to 43%3,4 far higher than the estimates of SUDs in the general population.5 Reports from treatment-seeking samples of individuals with SUDs indicate a lifetime prevalence of PTSD between 36% and 50% and current prevalence between 25% and 42%.6 The need for greater information concerning the treatment of co-occurring SUD and PTSD has been made more acute by the high rates of PTSD in soldiers returning from Iraq and Afghanistan. A recent report from the Institute of Medicine (IOM) commissioned by the Department of Veteran’s Affairs in 2008 strongly recommended more research on the treatment of PTSD in veteran populations. The high comorbidity of PTSD with SUDs, depression and traumatic brain injury (TBI), and the lack of treatment research focused on individuals with co-occurring disorders were noted.7
In this review, the prevalence of PTSD, SUDs, and TBI will be covered with special emphasis on data from soldiers returning from Iraq and Afghanistan. Etiologic relationships and general principles of diagnosis and assessment will be discussed. Evidence concerning psychotherapeutic and pharmacotherapeutic treatment for co-occurring PTSD and SUDs will also be reviewed.
BASIC CONCEPTS
Posttraumatic Stress Disorder
PTSD involves exposure to a traumatic event that may be followed by the development of 3 clusters of symptoms: re-experiencing, avoidance, and hyperarousal. To receive a diagnosis of PTSD, the symptoms must last more than 1 month in duration and must cause clinically significant distress or impairment.
Traumatic Brain Injury
According to the Defense and Veterans Brain Injury Center, TBI is a traumatically induced physiologic disruption of brain functioning as evidenced by (a) loss of consciousness, (b) altered mental state, such as feeling dazed or “seeing stars,” (c) loss of memory for events immediately preceding or after the injury, and (d) focal neurologic deficits.8 Symptoms of TBI vary depending on the severity of the injury, but may include headaches, sleep impairment, heightened sensitivity to light and noise, cognitive alterations (eg, disturbances in memory, attention, language, or problem solving speed), and behavioral changes (eg, depression, anxiety, and impulsiveness). Importantly, some of the symptoms that follow blast exposures, such as irritability, fatigue, sleep problems, and cognitive deficits, are not exclusively attributable to brain trauma and are also associated with PTSD and depression; accordingly, careful assessment is an essential aspect of effective clinical care.9
PREVALENCE
Substance Use Disorders
Rates of SUDs are elevated in veterans.10,11 Data from the 2004 to 2006 National Survey on Drug Use and Health indicate that approximately 7.1% of veterans meet criteria for a current (ie, past 12 months) SUD.10 This rate is almost twice as high as data from the National Comorbidity Survey Replication (N = 9282), which estimated rates of past 12 months SUDs to be 3.8% in the general population.12 A recent longitudinal study of more than 88,000 US soldiers (91% male) returning from Iraq found that 12% endorsed alcohol misuse.13 Past year rates of alcohol abuse in the general population have been estimated at 3.0% to 4.7%.12,14,15 Finally, rates of daily past-month cigarette use have also been shown to be significantly higher among veterans when compared with nonveterans (18.8% vs 14.3%, respectively).11 In sum, veterans have substantially higher rates of alcohol misuse and tobacco exposure than civilians, and these higher prevalence rates undoubtedly translate into greater alcohol- and cigarette-related morbidity and mortality.
Posttraumatic Stress Disorder
Exposure to military combat is associated with an increased risk for PTSD.8,16,17 Among the US general population, current (ie, past 12 months) rates of PTSD are estimated to be approximately 3.5%.12 In comparison, recent, large-scale investigation of Milliken et al 13 of more than 88,000 veterans returned from the Iraq war found that, among active duty soldiers, 11.8% had symptoms of PTSD soon after deployment and 16.7% had symptoms of PTSD at 6 months postdeployment. These estimates are similar to results from other recent, independent large-scale studies (Rand Center for Military Health Policy, 2008; Office of The Surgeon General United States Army Medical Command, Mental Health Advisory Team V, 2008).7 Over the past few years, the need for PTSD services for veterans has been steadily increasing. An investigation of Veterans Administration Medical Center (VA) special mental health service delivery from 1997 to 2005 found that the number of veterans treated for PTSD doubled, from 139,062 to 279,256.17 Smith et al 16 reported from the Millennium Cohort Study almost half (45.7%) of soldiers with PTSD remained symptomatic 3 years later. It has been demonstrated that military deployment devoid of combat exposure does not necessarily raise the risk of PTSD.16 Deployed soldiers with combat exposure (eg, witnessing a person’s death due to war, prolonged threat of death or injury, and experiencing physical torture) have higher incidence rates for new cases of PTSD compared with deployed soldiers without combat exposure and nondeployed soldiers. In 1 study, PTSD incidence was 3 times as high in combat-exposed when compared with noncombat exposed deployed soldiers.16
Traumatic Brain Injury
TBI is much more common among soldiers compared with that of the general population. Hoge and coworker assessed more than 2500 Army infantry soldiers after they returned home from deployment in Iraq and found that approximately 15% reported mild TBI, defined as an injury involving loss of consciousness or altered mental state (ie, concussion). Similar rates have been reported by other recent investigations.18 By comparison, the incidence of brain injury-related visits to US hospital emergency departments (and not resulting in hospital admission) was approximately 0.4% in 1995-1996.19 Although these data are assumed to be an underestimate due to many civilian cases of mild TBI not presenting to emergency departments; nevertheless, the difference remains quite large.
The source of TBI for a majority of soldiers assessed in recent studies is a blast incident. Blasts can result in brain injury because of (a) the rapid pressure shifts they may cause, (b) air emboli that can form in the blood vessels and result in cerebral infarcts in the brain, or (c) blast waves and wind that can propel fragments that can cause brain injury on impact.18 Because of improved protective body armor and helmets, many soldiers in the Iraq war have been able to enter combat zones or situations and survive blasts and other injuries, which previously would have been fatal resulting in high rates of TBI.19 Risk factors for TBI exposure in recent combat include male gender and younger age.8
Relationships Between PTSD, TBI, and SUDs
SUDs, TBI, and PTSD often occur simultaneously, particularly in military populations.20 The relationship between these disorders may be a direct one with a shared neurobiology and behavioral alterations such as impulsivity. Alternatively, the neurobiologic deficits of one disorder may enhance vulnerability to the development of the other disorders, providing a different pathway to comorbidity.
PTSD and TBI
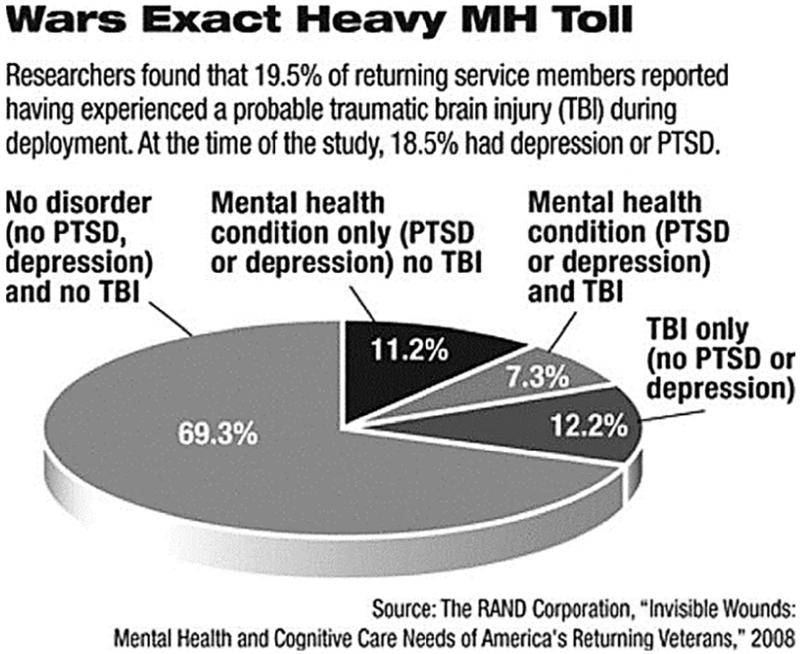
A strikingly large percentage (43.9%) of soldiers with TBI also meet criteria for PTSD.8 The severity of TBI, as judged by self-report, is associated with a greater incidence of PTSD. Approximately 44% of individuals, who lost consciousness, met criteria for PTSD when compared with 27% with altered mental status, 16% with other injuries, and 9% who were uninjured.8 Another recent study based on a survey of 1965 returning members of the military between August 2007 and January 2008 found that approximately 18.5% reported PTSD, 19.5% reported experiencing TBI, and 7.3% reported PTSD or depression plus TBI.7
Although the relationship between TBI and PTSD is not fully understood, neurobiologic models provide some insight. Bryant suggests that damage to the medial prefrontal cortex, which is necessary for the inhibition of conditioned fear reactions during injury, may lead to deficits in the ability to process anxiety. Thus, an individual with TBI damage to the prefrontal cortex may experience deficits in the ability to regulate fear reactions, which may increase risk for the development and maintenance of PTSD.9 A cognitive model might posit that TBI impairs cognitive resources leaving an individual less able to engage in coping skills that might prevent the development of PTSD and complicating the treatment of PTSD in individuals with TBI. Alternatively, pretrauma cognitive deficits may predispose individuals to the development and maintenance of PTSD, and influence TBI. In any case, both PTSD and TBI can adversely impact the neuroanatomical, neurobiological, and cognitive underpinnings of behavioral inhibition, thereby resulting in increased risk for the development of SUDs (Fig. 1).
Figure 1.
Wars Exact Heavy MH Toll
SUDs and PTSD
There are a number of connecting pathways between SUDs and PTSD. Intoxication may heighten the likelihood of exposure to trauma, hence the likelihood of developing PTSD. Furthermore, chronic substance use and withdrawal may increase anxiety or arousal states, making it more likely that individuals with SUDs will develop PTSD after trauma exposure. PTSD may also increase the risk of developing a SUD because individuals may use substances in an attempt to relieve symptoms of PTSD. However, SUDs can actually exacerbate symptoms and prolong the course of PTSD by preventing habituation to traumatic memories. These pathways are not mutually exclusive, and new evidence is emerging concerning the neurobiological underpinnings of potential causal pathways. In one recent study,3 individuals who had experienced any trauma and developed PTSD had an increased risk for the development of drug dependence, particularly nicotine dependence but not alcohol dependence.
The hypothalamic pituitary adrenal axis, the extrahypothalamic corticotrophin releasing factor (CRF), and the noradrenergic system are all intimately involved in the stress response, PTSD, and the pathophysiology of SUDs. Evidence is accumulating to support a role for CRF in mediating the effects of stress in increasing self-administration of drugs. Studies in rats have also demonstrated that withdrawal from chronic cocaine 21 or alcohol administration 22 in rats is associated with increases in CRF in the hypothalamus, amygdala, and basal forebrain. Elevated CRF has been found in humans during alcohol withdrawal 23 and in individuals with PTSD compared with healthy subjects.24,25 This finding is of particular interest because elevated brain CRF levels, especially in the amygdala, potentiate fear-related behavioral responses.26 As such, elevated levels of CRF may mediate symptoms of hyperarousal and increased risk for SUDs in PTSD. Increased CRF may enhance the reinforcing properties of some drugs, worsen the severity of withdrawal symptoms, and exacerbate symptoms of PTSD.
There are also abnormalities in noradrenergic systems in both PTSD and SUDs. Individuals with PTSD have elevated urinary excretion of both norepinephrine (NE) and epinephrine and elevated plasma levels of NE.27 Markers of noradrenergic activity are increased in both alcohol and opioid withdrawal.28-30 There are interconnections between brain CRF and noradrenergic systems. For example, stress increases CRF in the locus ceruleus,31 and intraventricular administration of CRF increases NE turnover in the hypothalamus, hippocampus, and prefrontal cortex.32 In the amygdala, NE stimulates the release of CRF.33 Koob and Kreek 34 hypothesized that interactions between CRF and the noradrenergic systems can function as a “feed-forward” system with progressive augmentation of the stress response with repeated stress exposure. This interaction could explain the worsening of PTSD symptoms during substance withdrawal and the increased vulnerability to the development of PTSD in individuals with SUDs who experience trauma.
SUDs and TBI
As with PTSD and TBI, SUDs and TBI have a number of potential links.35,36 Substance abusers constitute a disproportionate percentage of individuals who suffer from TBI, and a history of substance abuse predicts greater disability and poorer prognosis after TBI.37 A significant portion of TBI cases involve damage to the frontal lobes through acceleration and deceleration-related bruising and shearing.38 Because executive functions are concentrated in the frontal lobes, this type of TBI increases the likelihood of reduced inhibitory control and impaired decision making. Similarly, drug use is associated with dysregulation of several prefrontal regions of the brain associated with inhibitory control, reward, and memory, such as the orbitofrontal cortex, prefrontal cortex, and cingulate gyrus.39,40 In one study investigating the alcohol use disorders and mood disorders in 158 individuals with TBI,36 those with a history of alcohol use disorders had significantly reduced frontal gray matter volume and were significantly more likely to develop mood disorders when compared with those without an alcohol use disorder. Relapse to alcohol use after TBI resulted in a greater frequency of focal brain lesions preferentially involving the prefrontal cortices and anterior temporal lobes. This neural circuitry mediates critical aspects of addiction such as inhibitory control and reward expectation. These individuals also exhibited poorer performance on tests of executive functioning.36 The authors speculate that the neurotoxic effects of alcohol interact with the impact of TBI to produce greater disruption of the neural circuitry modulating reward, mood, and executive function.
Of interest, most studies show a decrease in substance use post-TBI, which may be a result of changes in lifestyle and functional status.41 However, a subpopulation of TBI patients display continued or even new-onset SUDs,37 and there is a negative correlation between functional status and continued substance abuse, which suggests that less severe TBI may be associated with subtle changes in insight.
DIAGNOSTIC ISSUES
A number of issues can make the diagnosis of PTSD challenging in individuals with SUDs. There is considerable symptom overlap between PTSD and drug or alcohol intoxication and withdrawal. PTSD symptoms in the hyperarousal cluster, such as sleep disturbance, irritability, and difficulty concentrating, can all be caused by drug intoxication or withdrawal. Symptoms in the avoidance cluster, such as lack of interest, social withdrawal, and feeling detached from others, can also be caused by substance abuse. Symptoms in the re-experiencing cluster are relatively specific to the diagnosis of PTSD, because they generally involve recollections and reactions to reminders of the traumatic event. For symptoms common to both PTSD and SUDs, it is important to ask about the chronological and functional relationship between the traumatic event, PTSD symptoms, and substance use. For example, if sleep disturbance and poor concentration began shortly after the traumatic experience and are tied closely to re-experiencing symptoms, they are likely the result of PTSD. An individual may not meet the necessary criteria for all 3 PTSD symptom clusters, but still exhibit a clinically significant syndrome, that is, in many ways indistinguishable from PTSD. This is generally referred to as partial or subthreshold PTSD.42,43 Subthreshold PTSD should be considered carefully and monitored as it could also influence substance use and be associated with deficits in functioning.44 It is important to note that in assessing for PTSD, symptoms in any individual may vary over time as a result of the transient nature of the disorder. Symptoms may worsen when individuals experience life events that remind them of their index trauma or around anniversary dates of traumatic events.
SCREENING AND ASSESSMENT
Given the high rates of co-occurring PTSD and SUDs in traumatized individuals, it is important to screen individuals at risk for PTSD (eg, combat exposed veterans, and psychiatric inpatients), so that the mental health costs to affected individuals, their families, and society can be minimized.45 Although numerous interviewer-rated and self-report assessments of PTSD are available,45-47 the multitude of options can be both an asset and a liability when deciding which screening device to use. There are occasions when a brief assessment method will prove adequate for diagnostic purposes. A recent study found that a brief, 10-item screening measure of reactions to traumatic events can be as effective as longer, more complicated interview assessments at predicting PTSD.46 Thus, it seems that the PTSD assessment of high-risk individuals can be performed without draining limited clinical resources (ie, staff/clinician time). Although brief screening methods are useful in many clinical situations and settings, there may be occasions when more detailed assessments (eg, thorough assessment of trauma history, determination of symptom severity) would substantially advance the quality of treatment planning and clinical care.
Several of the most widely used and psychometrically sound clinical interviews and self-report instruments for assessing PTSD are described later. Table 1 provides information on potentially useful instruments for assessing traumatic event exposure and PTSD. Although space limitations preclude an exhaustive review, the instruments selected for consideration here are particularly useful for assessing PTSD in high-risk populations such as those who have been exposed to combat, sexual assault, and natural disasters. Importantly, several instruments have been evaluated for use in persons with SUDs.
Table 1.
Additional Trauma and PTSD-Related Assessment and Screening Instruments
| Instrument (by Category) |
Aim or Instrument |
Instrument format |
Time to Complete | Obtain Instrument at |
|---|---|---|---|---|
| Trauma exposure assessment | ||||
| Combat exposure scale | Assesses wartime stressors | S-R | 5-min | http://www.ncptsd.va.gov/ncmain/ncdocs/assmnts/combat_exposure_scale_ces.html |
| Traumatic stress schedule | Multi-item assessment of 10 types of stressors |
I | 5–30 min | J Appl Soc Psychol. 1990;20:1704–1718 |
| Potential stressful events interview |
Assesses low- and high-magnitude stressors |
I | 90–120 min | Contact National Crime Victims Research and Treatment Center at 843-792-8209 |
| Brief PTSD symptom assessment |
||||
| SPAN | 4-item screen of anger and one symptom from each of the 3 PTSD symptom clusters |
S-R | <5-min | Contact Multi-Health Systems Inc. at customerservice@mhs.com |
| Short screening scale for PTSD |
7-item screen of PTSD-related avoidance and arousal |
S-R | <5-min | Contact Naomi Breslau at breslau@epi.msu.edu |
| Trauma screening questionnaire |
10-item screen of PTSD-rclatcd re-experiencing and arousal |
S-R | <5-min | Contact Chris Brcwin at c.brewin@ucl.ac.uk |
| Specialty assessment | ||||
| Deployment risk and resilience inventory |
Multicomponent instrument measuring psychosocial risk and resilience factors for military personnel and veterans |
S-R | >60 min | Contact Dawn Vogt at dawnnc.vogt@va.gov |
S-R, self report; I, interview; PTSD, posttraumatic stress disorder.
Clinician-Administered PTSD Scale
The Clinician-administered PTSD Scale (CAPS) is considered by many to be the “gold standard,” and it is one of the most comprehensive and widely used interviewer-rated assessments for diagnosing and measuring the severity of PTSD.48-50 This 30-item structured interview was developed at the National Center for PTSD and is designed for use by clinicians and nonclinicians. A checklist of potentially traumatic events is included to assess lifetime trauma exposure. Seventeen items assess the frequency and intensity of diagnostic PTSD symptoms (eg, re-experiencing, avoidance, and hyperarousal). In addition, associated features of PTSD (eg, survivor guilt, homicidality, and hopelessness), social and occupational functioning, and global PTSD severity are also rated. Various versions of the CAPS are available, including versions that assess past-week, past-month, and lifetime symptoms. The CAPS has been used with a variety of traumatized populations, including veterans, and it requires approximately 1 hour to administer (30 minutes if assessing only PTSD symptoms).49
Potential Stressful Events Interview and the National Women’s Study PTSD Module
The potential stressful events interview is a 62-item, multicomponent clinical interview for assessing trauma exposure history and PTSD symptomatology (via the National Women’s Study PTSD module).51 By using behaviorally specific questions, the potential stressful events interview trauma exposure module assesses crime-related (eg, sexual and physical assault), noncrime-related (eg, natural disaster, serious accident), and combat-related traumatic events. Age of onset for first and last occurrence of an event is also determined.
The National Women’s Study PTSD module is a 20-item structured clinical interview that is designed for use by lay interviewers.52 It was derived from the diagnostic interview schedule that was used in the National Vietnam Veterans Readjustment Study. Like the CAPS, this instrument has been shown to have strong psychometric properties and can be used to assess PTSD symptomatology in approximately 30 minutes.
Mississippi Scale for Combat-Related PTSD
The Mississippi Scale is a widely used 35-item, self-report instrument developed by Keane et al 53 to assess combat-related PTSD symptoms. Respondents use Likert scales to rate the severity of symptoms since the occurrence of the traumatic event(s). The Mississippi Scale has been translated into several languages and has strong psychometric properties, and a civilian version of the instrument has been developed.54
PTSD Checklist Military Version
The PCL is a 17-item self-report measure of PTSD symptoms based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition criteria.55 PCL scores range from 17 to 85 and higher scores reflect greater PTSD severity. The instrument is highly correlated with the CAPS (r = 0.93), and it has good diagnostic efficiency (>0.70) and robust psychometric properties.53
Impact of Events Scale-Revised
The Impact of Event Scale-Revised (IES-R) is a brief and popular 22-item scale for assessing PTSD symptoms.56 Respondents use Likert scales to rate “how distressed or bothered,” they were by each specified symptom over the previous week. The IES-R has been translated into several different languages and has been used with a variety of trauma populations.57-59 Recent research suggesting that the IES-R is a reliable, valid, and clinically sensitive measure of PTSD symptomatology when used with traumatized SUD and SUD-PTSD comorbid civilians.60
Modified PTSD Symptom Scale
The MPSS-SR is a 17-item self-report instrument that has been validated with clinical and nonclinical samples of trauma-exposed individuals.61 It has been shown to have good psychometric properties with respect to the assessment of PTSD symptoms in SUD samples, and so it can be used to screen PTSD in PTSD/SUD comorbid individuals.62,63
TREATMENT
Pharmacotherapy of PTSD
Although the treatment of PTSD is generally multimodal, pharmacotherapy is playing an increasingly important role. Recently, the IOM conducted a systematic search of the treatment literature for individuals with PTSD.7 More than 2700 studies were identified, 90 of which met stringent inclusion criteria for being randomized and controlled trials (IOM, 2007). Of the 90 studies reviewed, 53 investigated psychologic interventions and 37 investigated pharmacotherapy interventions. These 37 pharmacotherapy trials investigated antidepressants, antipsychotics, benzodiazepines, and [alpha]-adrenergic agents. The report concluded that there was inadequate evidence to support the use of any of these agents in the treatment of PTSD. The authors emphasized the fact that this did not mean that all of these agents were not efficacious, simply that further studies were needed before conclusive statements about efficacy could be made.
Literature on the use of the selective serotonin reuptake inhibitors (SSRIs) in the treatment of PTSD is the most extensive. In fact, a number of SSRIs (sertraline, paroxetine, and fluoxetine) have received Food and Drug Administration (FDA) approval for the treatment of PTSD. Of 14 SSRI trials reviewed in the recent IOM report (2008), 7 demonstrated a positive response. It has been suggested that individuals with combat-related PTSD respond to treatment differently than those with civilian PTSD; so, 7 studies with the fewest design and drop-out limitations were divided by study population and trauma type. Two studies conducted in veteran populations were negative,64,65 3 of 4 studies conducted in civilian populations were positive 66-68 and one negative, and one study in a mixed population was positive.69 The study by Friedman et al 65 is the most recent, largest, and most methodologically rigorous trial in predominantly male combat veterans. Although this study demonstrated no differential impact of sertraline on PTSD outcomes, there was a high differential dropout between treatment and control conditions. Unfortunately, this study does not include veterans of recent combat in Iraq and Afghanistan, and it had only a small percentage of female participants, so generalizability to recently returning soldiers is unclear. There has been speculation that the reason for differential response to SSRIs in combat studies is related to a better response to SSRIs in women when compared with men. Because the percentage of women involved in military combat has increased over the past 10 years, further investigation of SSRIs in combat-related PTSD is necessary. Another limitation of the studies cited earlier is the failure to include individuals with co-occurring SUDs or TBI. There is one, double-blind, placebo-controlled study of sertraline in the treatment of PTSD in men and women with comorbid alcohol dependence.70 Sertraline treatment was more efficacious than placebo in the treatment of PTSD symptoms, but there were no overall differences between groups in alcohol consumption. However, post hoc analysis revealed that treatment with sertraline significantly decreased alcohol consumption and in a subgroup of individuals with early onset PTSD (before 18 years), and later onset, less severe alcohol dependence.
Several other classes of compounds are of particular interest in the treatment of co-occurring PTSD and SUDs. A number of case series, open label, and small placebo-controlled trials suggest that anticonvulsant agents may be useful in the treatment of PTSD.71 Of particular interest, topirimate demonstrated efficacy in secondary measures of PTSD symptoms in one small placebo-controlled trial.72 Topirimate has also demonstrated efficacy in one placebo-controlled trial and one open-label study.73 Positive results have been reported in case series and open label studies for both gabapentin and vigabatrin in the treatment of PTSD.71 These agents also show promise in alcohol and cocaine dependence.74 Also, there is an evidence that adjunctive treatment with atypical antipsychotic agents, including risperidone and quetiapine, can provide therapeutic benefit in the treatment of PTSD.75,76 A recent preliminary study (N = 61) demonstrated decreased alcohol consumption in a subgroup of individuals treated with quetiapine when compared with placebo.77 Because of potential efficacy in treating both PTSD and SUDs, select anticonvulsant and antipsychotic agents warrant further exploration in the treatment of individuals with co-occurring PTSD/SUDs. Of interest, some of these agents have also been explored in the management of behavioral disorders associated with TBI.78 In general, pharmacotherapy studies focused on the treatment of TBI have been small and inconclusive; however, there are some preliminary data to support the use of antidepressants (particularly SSRIs) and anticonvulsants in the treatment of agitation and aggression associated with TBI.
Treatment of sleep disturbance is a particularly important issue in individuals with co-occurring PTSD and SUDs. Sleep disturbance is a prominent and troubling feature of both disorders and agents often used to treat sleep disturbance (ie, benzodiazepines) have abuse potential. Prazosin, an [alpha]-1adrenergic receptor antagonist, is an FDA-approved medication (for hypertension), which has been shown to improve sleep and decrease nightmares in individuals with PTSD,79 presumably through reduction of the excessive central noradrenergic overdrive characteristic of PTSD. Several preclinical studies 32,80-83 suggest that prazosin may decrease amphetamine, cocaine, heroin, alcohol, and nicotine self-administration in animal models, most likely by blockade of alpha-1mediated dopamine release. A recent piloted clinical trial conducted with 24 alcohol-dependent participants found improvement in alcohol-related outcomes in the prazosin-treated group when compared with the placebo group.84 As such, this is another agent that warrants further investigation in the treatment of co-occurring PTSD and SUDs.
Pharmacotherapy of Substance Use Disorders
A number of agents for the treatment of SUDs, including alcohol, nicotine, and opiate dependence, are FDA-approved. Unfortunately, there has been little investigation of the use of these agents in individuals with co-occurring psychiatric disorders. In one study of 254 outpatients with alcohol dependence and a variety of comorbid psychiatric disorders, including 42% with PTSD, Petrakis et al 85 investigated the efficacy of disulfiram, naltrexone, or their combination in a 12-week randomized trial. Participants treated with naltrexone or disulfiram, when compared with placebo, had significantly more consecutive weeks of abstinence and fewer drinking days per week. In comparison with naltrexone-treated participants, disulfiram-treated participants reported less craving from pretreatment to posttreatment. The differential effects of the medications across comorbid psychiatric disorder were not discussed, but active medication was associated with greater symptom improvement (eg, less anxiety). No clear advantage of combining medications was observed.
Opiate abuse or dependence is also an important issue for veterans with PTSD. Veterans with combat-related injuries may receive prescription opiates to treat pain syndromes. If these individuals also have PTSD, there may be some risk of misusing prescription opiates to manage PTSD symptoms. There are a number of pharmacotherapy treatments for opiate dependence that might be considered in cases where there is clear evidence of illicit opiate dependence or where there is a chronic pain syndrome and evidence of misuse of short-acting prescription opiates. Methadone is a long-acting opiate receptor agonist that has long been used as a maintenance strategy for individuals dependent on illicit opiates.86 However, prescription-monitoring requirements have limited the use of methadone in the treatment of chronic pain. Buprenorphine is a long-acting mixed opiate agonist antagonist approved for the treatment of pain syndromes and as a detoxification or maintenance strategy in the treatment of opiate dependence. When compared with methadone, buprenorphine is easier to use, because it can be prescribed in an office-based practice and a better safety profile because the mixed agonist or antagonist properties limit toxicity in overdose. More recently, a combination tablet (buprenorphine/naloxone in a 4:1 ratio) has been developed with the goal of decreasing diversion and abuse. Controlled studies with buprenorphine solution, buprenorphine monotablet, and buprenorphine/naloxone combination tablet have uniformly demonstrated the effectiveness of buprenorphine for opioid dependence treatment, and the combination tablet seems to decrease (but not eliminate) abuse potential.87 Further investigation of the use of buprenorphine in individuals with PTSD and opiate dependence with or without chronic pain syndromes is needed.
Also of interest, a recent trial compared the rate of smoking cessation in veterans with PTSD by integrating treatment, including pharmacotherapy (nicotine replacement or varenicline), into mental health care as opposed to delivering smoking cessation treatment separately by smoking cessation specialists.88 The investigators found that subjects assigned to integrated care were 5 times more likely to quit smoking at all assessments time points across a 9-month follow-up period. This study emphasizes the importance of integrated care for individuals with co-occurring disorders.
Psychosocial Treatment
There are a number of psychosocial treatments designed specifically to address PTSD or SUDs independently; however, few treatments that address both PTSD and SUDs simultaneously or sequentially have been developed. Two relatively small studies with female civilians and male veterans indicate that most patients with both conditions receive treatment for SUDs and may or may not receive later treatment to directly address PTSD symptoms.89,90 This is despite the fact that patients often report that their PTSD and SUDs are interrelated,91,92 and they would prefer treatment that addresses both disorders 90
Treatment models that address SUDs then address PTSD after a period of sobriety are typically called “sequential” models. They rely on the assumption that substance use should be stabilized and a sustained period of abstinence established before directly addressing trauma-related material. Two related assumptions are that addressing trauma-related issues before a sustained period of abstinence will aggravate substance use problems and that continued substance use would interfere with progress in PTSD-related treatment interventions. A number of studies have demonstrated the efficacy of a variety of cognitive behavioral therapy approaches to the treatment of PTSD (IOM, 2007). Exposure-based treatments are widely considered the gold standard psychosocial treatments for PTSD, although most forms of exposure treatment are delivered in combination with other treatment components, such as coping skills enhancement and cognitive restructuring. To date, there are more than 20 published randomized clinical trials on exposure-based treatments, with at least 7 meeting most or all of the stringent guidelines set forth by the IOM. All 7 studies resulted in statistically and clinically significant improvement in PSTD symptoms. Despite the empirical support for exposure-based treatments, many clinicians hesitate to use exposure in cases of comorbid PTSD and SUDs. The mechanism of action for exposure is thought to be repeated activation and gradual extinction of maladaptive fear responses, replacing negative conditioned associations with new more adaptive ones.93 Consequently, initial patient reactions to exposure are often characterized by intense affect, which some clinicians fear could lead to SUD relapse, despite the lack of empirical data to support these concerns.
To date, several cognitive behavioral therapy (CBT) integrated PTSD or SUD interventions have been examined empirically. Seeking safety (SS) has been the most widely studied integrated treatment.94,95 It is a present-focused treatment that can be implemented in a group or individual format focused on psychoeducation, coping skills training, and building self-efficacy. In a 12-week, randomized controlled trial (n = 107 women), SS and Relapse prevention (RP), a manualized CBT treatment for SUDs, were compared with treatment as usual (TAU).96 SS and RP both produced reductions in substance use, PTSD, and other psychiatric symptoms, whereas TAU participants worsened over time. SS and RP participants also sustained improvement in symptoms of both PTSD and substance use at 6- and 9-month follow-up.96 No significant differences between SS and RP were observed. Although SS was originally designed for adult women, a recent pilot study in civilian men (n = 5) examined SS combined with modified exposure treatment 97 with promising results. There is also a promising pilot study of SS in male veterans on methadone maintenance.98
There are 2 other PTSD/SUD integrated treatment models that use exposure-based treatment: substance dependence posttraumatic stress disorder therapy 99,100 and concurrent treatment with prolonged exposure (COPE).101,102 A pilot study (N = 19) of male and female civilians maintained on methadone maintenance indicated that substance dependence posttraumatic stress disorder therapy outcomes did not differ from those of Twelve-Step Facilitation.100 However, cocaine-dependent individuals treated with COPE in a pilot study (N = 39) demonstrated significant improvement in PSTD and substance use symptoms at 6 month posttreatment follow-up.101 Preliminary data (N = 20) from an ongoing randomized clinical trial shows substantial reductions in both PTSD symptoms and substance use severity in subjects receiving COPE versus TAU for substance use.103
Within the Veterans Health Administration system, there are 9 programs that offer integrated outpatient care for PTSD and SUDs. These substance use PTSD treatment programs were established in 1991. Although the 1999 Veterans Health Administration Program Guide (Mental Health Program Guidelines for the New Veterans Health Administration, 1999, p. 63)104 suggested that, “it appears preferable to establish SUD treatment capability within all specialized PTSD programs,” substance use PTSD treatment programs have not been established in VA medical centers across the country. There is one published outcome study on a VA-based treatment called “Transcend,” which combines PTSD and substance use in a partial hospitalization program.105 The 12-week manualized treatment consists of 10 hours of group therapy, 3 relaxation-training sessions, and several independent sessions of physical exercise per week. The treatment incorporates an eclectic blend of interventions from different theoretical orientations, divided into an initial skill-building stage followed by a trauma processing stage. In the published efficacy study (N = 46),105 there were statistically significant decreases on CAPS scores and measures of alcohol and other substance use. Study limitations include the fact that participants were required to be abstinent from active substance abuse for at least 30 days before treatment enrollment, and participants who did not complete both the 6- and 12-month follow-up assessments were excluded from analyses. This intervention, nevertheless, shows promise.
In conclusion, the findings of research conducted thus far, although limited to small studies, suggest that concerns about exacerbation of substance use by PTSD-relevant treatments are not supported by empirical data. Instead, integrated treatments offer patient-centered approaches that validate patients’ own sense that there is a connection between these disorders. This may be a key for improving retention in treatment for patients with co-occurring disorders, a group at risk for higher attrition from treatment.
Questions about the best treatment approach for individuals with co-occurring TBI and PTSD or SUD have been underexplored. Symptoms of TBI can interfere with the treatment of both PTSD and SUDs. In delivering CBT to individuals with TBI, adjustments may need to be made for patients who have impaired cognitive function. In a case study exploring the treatment of PTSD in an individual with TBI, investigators report promising results with an intervention combining CBT for PTSD with neurorehabilitation, defined as remediation of information processing and assistance with organization and problem-solving skills.106 The clinical management of individuals with TBI and coexisting SUDs can also present unique challenges. In both TBI and SUDs, impulsiveness and an inability to delay reinforcement and consider long-term consequences can be an issue. In one recent trial, financial incentives were used successfully to promote retention in the treatment for individuals with TBI and SUDs.20 Concrete incentives may be particularly important in helping individuals with TBI engage in treatment.
Recommendations for Clinical Care
There is still much work to be done in explicating the best treatment for co-occurring PTSD and SUDs. Studies to inform the treatment of individuals with co-occurring PTSD/SUD complicated by TBI are virtually nonexistent. However, evidence is accumulating to suggest that integrated treatment of PTSD and SUD is optimal. In particular, the strategy of postponing treatment of PTSD until abstinence achieved does not seem to be supported by the evidence and is not appealing to patients. There are a number of potential pharmacotherapeutic treatment options, but much of the data are inconclusive. However, some agents show promise in treating both PTSD symptoms and decreasing substance use, so that a targeted trial may be indicated. In treating individuals with SUDs, it is always important to consider the abuse potential of the agent being used and any potential interactions with substances of abuse should relapse occur. It is also important to consider the use of pharmacotherapeutic agents specifically targeting substance use. This is a growing area of medication development. Whatever the treatment choice, objective measurement of both PTSD and SUD symptom change over time is critical to guide on-going treatment decisions and avoid “therapeutic inertia.”
CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, PTSD, SUDs, and TBI commonly co-occur, and the relationship is complex. There is symptom overlap between PTSD, SUDs, and TBI, as well as common neurobiology, which may help to explain worsening of PTSD symptoms during early substance withdrawal and increased vulnerability to the development of PTSD in individuals with SUDs who experience trauma. TBI complicates the treatment of both PTSD and SUDs, and there is little empirical evidence to guide treatment. In particular, studies focused on the returning soldiers from the Iraq and Afghanistan wars, in which TBI is often an issue, are sorely needed.
Although a great deal of progress has been made in exploring the treatment of co-occurring PTSD and SUDs, there is still much work to be done. The bulk of evidence supports integrated treatment and the use of CBT therapy; however, the type of CBT and the best strategies for combining trauma-focused and SUD treatment warrants further exploration. When TBI complicates either or both of these disorders, the complexity of the treatment conundrum increases tremendously. A systematic program of research focused on innovative approaches to individuals with TBI and various commonly co-occurring disorders is needed to guide treatment development.
Pharmacotherapy trials have shown promise in subpopulations of individuals with co-occurring disorders, but clearly there is much work to be done in defining optimal treatment for individuals with complex comorbidity. In particular, the exploration of agents that may treat more than one disorder or symptoms that occur across disorders is critically important.
Acknowledgments
Supported by the National Institutes of Health/National Institute on Drug Abuse, Bethesda, MA, Drug Abuse Research Training Program (R25 DA020537), and Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women’s Health (P50 DA016511).
REFERENCES
- 1.Kulka R, Schlenger W, Fairbank J, et al. Description, Current Status, and Initial PTSD Prevalence Estimates. Final Report. Veterans Administration; Washington: 1988. [Google Scholar]
- 2.Keane T, Kaloupek D. Comorbid psychiatric disorders in PTSD. Ann N Y Acad Sci. 1998:24–32. doi: 10.1111/j.1749-6632.1997.tb48266.x. 360 Link. [DOI] [PubMed] [Google Scholar]
- 3.Breslau N, Davis GC, Andreski P, et al. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 7.Mental Health Advisory Team (MHAT)V . Operation Iraqi Freedom 06-08: Iraq, Operation Enduring Freedom 8: Afghanistan. Department of Defense Office of the Surgeon General Multi-National Force Iraq and Office of the Surgeon General United States Army Medical Command; 2008. [Google Scholar]
- 8.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 9.Bryant RA. Disentangling mild traumatic brain injury and stress reactions. N Engl J Med. 2008;358:525–527. doi: 10.1056/NEJMe078235. [DOI] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration Office of Applied Services . The NSDUH report: Serious psychological distress and substance use among veterans. Substance Abuse and Mental Health Services Administration Office of Applied Services; Rockville, MD: 2007. [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration Office of Applied Services . Alcohol Use and Alcohol-related Risk Behaviors among Veterans. Substance Abuse and Mental Health Services Administration Office of Applied Services; Rockville, MD: 2005. [Google Scholar]
- 12.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298:2141–2148. doi: 10.1001/jama.298.18.2141. [DOI] [PubMed] [Google Scholar]
- 14.Grant BF, Stinson FS, Dawson DA, et al. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- 15.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 16.Smith TC, Ryan MA, Wingard DL, et al. New onset and persistent symptoms of post-traumatic stress disorder self-reported after deployment and combat exposures: Prospective population based US military cohort study. BMJ. 2008;336:366–371. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenheck RA, Fontana AF. Recent trends in VA treatment of post-traumatic stress disorder and other mental disorders. Health Aff (Millwood) 2007;26:1720–1727. doi: 10.1377/hlthaff.26.6.1720. 360 Link. [DOI] [PubMed] [Google Scholar]
- 18.Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero JL, Thurman DJ, Sniezek JE. Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Inj. 2000;14:181–186. [PubMed] [Google Scholar]
- 20.Corrigan JD, Cole TB. Substance use disorders and clinical management of traumatic brain injury and posttraumatic stress disorder. JAMA. 2008;300:720–721. doi: 10.1001/jama.300.6.720. [DOI] [PubMed] [Google Scholar]
- 21.Sarnyai Z, Biro E, Gardi J, et al. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- 22.Merlo Pich E, Lorang M, Yeganeh M, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adinoff B, Martin PR, Bone GH, et al. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- 24.Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 25.Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41) Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- 27.Southwick SM, Bremner JD, Rasmusson A, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 28.Charney DS, Redmond DE, Jr, Galloway MP, et al. Naltrexone precipitated opiate withdrawal in methadone addicted human subjects: Evidence for noradrenergic hyperactivity. Life Sci. 1984;35:1263–1272. doi: 10.1016/0024-3205(84)90097-3. [DOI] [PubMed] [Google Scholar]
- 29.Hawley RJ, Major LF, Schulman EA, et al. Cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol and norepinephrine levels in alcohol withdrawal. Correlations with clinical signs. Arch Gen Psychiatry. 1985;42:1056–1062. doi: 10.1001/archpsyc.1985.01790340034005. [DOI] [PubMed] [Google Scholar]
- 30.Smith AJ, Brent PJ, Henry DA, et al. Plasma noradrenaline, platelet alpha 2-adrenoceptors, and functional scores during ethanol withdrawal. Alcohol Clin Exp Res. 1990;14:497–502. doi: 10.1111/j.1530-0277.1990.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 31.Chappell PB, Smith MA, Kilts CD, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang JJ, Swiergiel AH, Palamarchouk VS, et al. Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltammetry. Brain Res Bull. 1998;47:277–284. doi: 10.1016/s0361-9230(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 33.Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 34.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorge RE, Starkstein SE, Arndt S, et al. Alcohol misuse and mood disorders following traumatic brain injury. Arch Gen Psychiatry. 2005;62:742–749. doi: 10.1001/archpsyc.62.7.742. [DOI] [PubMed] [Google Scholar]
- 36.Jorge RE. Neuropsychiatric consequences of traumatic brain injury: A review of recent findings. Curr Opin Psychiatry. 2005;18:289–299. doi: 10.1097/01.yco.0000165600.90928.92. Ovid Full Text Bibliographic Links. [DOI] [PubMed] [Google Scholar]
- 37.Graham DP, Cardon AL. An update on substance use and treatment following traumatic brain injury. Ann NY Acad Sci. 2008;1141:148–162. doi: 10.1196/annals.1441.029. [DOI] [PubMed] [Google Scholar]
- 38.Lux WE. A neuropsychiatric perspective on traumatic brain injury. J Rehabil Res Dev. 2007;44:951–962. doi: 10.1682/jrrd.2007.01.0009. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parry-Jones BL, Vaughan FL, Miles Cox W. Traumatic brain injury and substance misuse: A systematic review of prevalence and outcomes research (1994-2004) Neuropsychol Rehabil. 2006;16:537–560. doi: 10.1080/09602010500231875. [DOI] [PubMed] [Google Scholar]
- 42.Schutzwohl M, Maercker A. Effects of varying diagnostic criteria for posttraumatic stress disorder are endorsing the concept of partial PTSD. J Trauma Stress. 1999;12:155–165. doi: 10.1023/A:1024706702133. [DOI] [PubMed] [Google Scholar]
- 43.Marshall RD, Beebe KL, Oldham M, et al. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 44.Matthews LR, Chinnery D. Prediction of work functioning following accidental injury: The contribution of PTSD symptom severity and other established risk factors. Int J Psychol. 2005;40:339–348. [Google Scholar]
- 45.Anthony MM, Orsillo SM, Roemer L. Practitioner’s Guide to Empirically Based Measures of Anxiety. Kluwer Academic/Plenum Publishers; New York: 2001. [Google Scholar]
- 46.Brewin CR. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18:53–62. doi: 10.1002/jts.20007. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JP, Keane TM. Assessing Psychological Trauma and PTSD. Guilford Press; New York: 1997. [Google Scholar]
- 48.Blake DD, Weathers FW, Nagy LM, et al. The Clinician Administered PTSD Scale-IV. National Center for Post-traumatic Stress Disorder, Behavioral Science Division—Boston, VA; Boston, MA: 1990. [Google Scholar]
- 49.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 50.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick D, Resnick H, Freedy J. The Potential Stressful Events Interview. Medical University of South Carolina; Charleston: In press. [Google Scholar]
- 52.Kilpatrick D, Resnick H, Saunders B, Best C. The National Women’s Study PTSD Module. Medical University of South Carolina; Charleston: In press. [Google Scholar]
- 53.Keane TM, Caddell JM, Taylor KL. Mississippi scale for combat-related posttraumatic stress disorder: Three studies in reliability and validity. J Consult Clin Psychol. 1998;56:85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- 54.McFall ME, Smith DE, Roszell DK, et al. Convergent validity of measures of PTSD in Vietnam combat veterans. Am J Psychiatry. 1990;147:645–648. doi: 10.1176/ajp.147.5.645. [DOI] [PubMed] [Google Scholar]
- 55.Weathers FW, Hushka J, Keane TM. The PTSD Checklist Military Version (PCL-M) National Center for PTSD; Boston: 1991. [Google Scholar]
- 56.Weiss DS, Marmar CR. The impact of event scale-revised. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD. Guilford Press; New York: 1997. pp. 399–411. [Google Scholar]
- 57.Baguena MJ, Villarroya E, Belena A, et al. Psychometric properties of the Spanish version of the Impact of Event Scale-Revised (IES-R) Analyisis y Modificacion de Conducta. 2001;27:581–604. [Google Scholar]
- 58.Asukai N, Kato H, Kawamura N, et al. Reliability and validity of the Japanese-language version of the impact of event scale-revised (IES-R-J): four studies of different traumatic events. J Nerv Mental Disease. 2002;190:175–182. doi: 10.1097/00005053-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Kazlauskas E, Gailiene D, Domanskaite-Gota V, et al. Psychometric properties of the lithuanian version of the impact of event scale-revised (IES-R) Psichologija. 2006;33:22–30. 360 Link. [Google Scholar]
- 60.Rash CJ, Coffey SF, Baschnagel JS, et al. Psychometric properties of the IES-R in traumatized substance dependent individuals with and without PTSD. Addict Behav. 2008;33:1039–1047. doi: 10.1016/j.addbeh.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falsetti SA. The modified PTSD symptom scale: A brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–162. [Google Scholar]
- 62.Dansky BS, Saladin ME, Coffey SF, et al. Use of self-report measures of crime-related posttraumatic stress disorder with substance use disordered patients. J Subst Abuse Treat. 1997;14:431–437. doi: 10.1016/s0740-5472(97)00120-7. [DOI] [PubMed] [Google Scholar]
- 63.Coffey SF, Dansky BS, Falsetti SA, et al. Screening for PTSD in a substance abuse sample: Psychometric properties of a modified version of the PTSD Symptom Scale Self-Report. Posttraumatic stress disorder. J Trauma Stress. 1998;11:393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- 64.Zohar J, Amital D, Miodownik C, et al. Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:190–195. doi: 10.1097/00004714-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711–720. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- 66.Tucker P, Potter-Kimball R, Wyatt DB, et al. Can physiologic assessment and side effects tease out differences in PTSD trials? A double-blind comparison of citalopram, sertraline, and placebo. Psychopharmacol Bull. 2003;37:135–149. [PubMed] [Google Scholar]
- 67.Davidson J, Pearlstein T, Londborg P, et al. Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: Results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1974–1981. doi: 10.1176/appi.ajp.158.12.1974. [DOI] [PubMed] [Google Scholar]
- 68.Brady K, Pearlstein T, Asnis G, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 69.Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry. 2002;63:199–206. doi: 10.4088/jcp.v63n0305. [DOI] [PubMed] [Google Scholar]
- 70.Brady KT, Sonne S, Anton RF, et al. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- 71.Van Ameringen M, Mancini C, Pipe B, et al. Antiepileptic drugs in the treatment of anxiety disorders: Role in therapy. Drugs. 2004;64:2199–2220. doi: 10.2165/00003495-200464190-00004. [DOI] [PubMed] [Google Scholar]
- 72.Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: A randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:201–206. doi: 10.4088/jcp.v68n0204. [DOI] [PubMed] [Google Scholar]
- 73.Johnson DR, Rosenheck R, Fontana A, et al. Outcome of intensive inpatient treatment for combat-related posttraumatic stress disorder. Am J Psychiatry. 1996;153:771–777. doi: 10.1176/ajp.153.6.771. [DOI] [PubMed] [Google Scholar]
- 74.Brodie JD, Figueroa E, Laska EM, et al. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–125. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- 75.Reich DB, Winternitz S, Hennen J, et al. A preliminary study of risperidone in the treatment of posttraumatic stress disorder related to childhood abuse in women. J Clin Psychiatry. 2004;65:1601–1606. doi: 10.4088/jcp.v65n1204. [DOI] [PubMed] [Google Scholar]
- 76.Bartzokis G, Lu PH, Turner J, et al. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry. 2005;57:474–479. doi: 10.1016/j.biopsych.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27:344–351. doi: 10.1097/JCP.0b013e3180ca86e5. Ovid Full Text Bibliographic Links. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deb S, Crownshaw T. The role of pharmacotherapy in the management of behaviour disorders in traumatic brain injury patients. Brain Inj. 2004;18:1–31. doi: 10.1080/0269905031000110463. [DOI] [PubMed] [Google Scholar]
- 79.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6:346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 80.Villegier AS, Lotfipour S, Belluzzi JD, et al. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology (Berl) 2007;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- 81.Greenwell TN, Walker BM, Cottone P, et al. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav. 2009;91:295–302. doi: 10.1016/j.pbb.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darracq L, Blanc G, Glowinski J, et al. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wee S, Mandyam CD, Lekic DM, et al. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simpson TL, Saxon AJ, Meredith CW, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 85.Petrakis IL, Poling J, Levinson C, et al. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biol Psychiatry. 2006;60:777–783. doi: 10.1016/j.biopsych.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 86.Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–226. doi: 10.1016/S0140-6736(98)05356-2. [DOI] [PubMed] [Google Scholar]
- 87.Johnson RE, Strain EC, Amass L. Buprenorphine: How to use it right. Drug Alcohol Depend. 2003;70(2 Suppl):S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 88.McFall M, Saxon AJ, Thompson CE, et al. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162:1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- 89.Young HE, Rosen CS, Finney JW. A survey of PTSD screening and referral practices in VA addiction treatment programs. J Subst Abuse Treat. 2005;28:313–319. doi: 10.1016/j.jsat.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Najavits LM, Sullivan TP, Schmitz M, et al. Treatment utilization by women with PTSD and substance dependence. Am J Addict. 2004;13:215–224. doi: 10.1080/10550490490459889. [DOI] [PubMed] [Google Scholar]
- 91.Brown PJ, Stout RL, Gannon-Rowley J. Substance use disorder-PTSD comorbidity. Patients’ perceptions of symptom interplay and treatment issues. J Subst Abuse Treat. 1998;15:445–448. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- 92.Back SE, Brady KT, Jaanimagi U, et al. Cocaine dependence and PTSD: A pilot study of symptom interplay and treatment preferences. Addict Behav. 2006;31:351–354. doi: 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 93.McNally RJ. Mechanisms of exposure therapy: How neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Najavits LM, Weiss RD, Shaw SR, et al. “Seeking safety”: Outcome of a new cognitive-behavioral psychotherapy for women with posttraumatic stress disorder and substance dependence. J Trauma Stress. 1998;11:437–456. doi: 10.1023/A:1024496427434. [DOI] [PubMed] [Google Scholar]
- 95.Najavits LM. Seeking Safety: A treatment Manual for PTSD and Substance Abuse. Guilford Press; New York: 2002. [Google Scholar]
- 96.Hien DA, Cohen LR, Miele GM, et al. Promising treatments for women with comorbid PTSD and substance use disorders. Am J Psychiatry. 2004;161:1426–1432. doi: 10.1176/appi.ajp.161.8.1426. [DOI] [PubMed] [Google Scholar]
- 97.Najavits LM, Schmitz M, Gotthardt S, et al. Seeking safety plus exposure therapy: An outcome study on dual diagnosis men. J Psychoactive Drugs. 2005;37:425–435. doi: 10.1080/02791072.2005.10399816. [DOI] [PubMed] [Google Scholar]
- 98.Weaver CM, Trafton JA, Walser RD, et al. Pilot test of seeking safety treatment with male veterans. Psychiatr Serv. 2007;58:1012–1013. doi: 10.1176/ps.2007.58.7.1012. [DOI] [PubMed] [Google Scholar]
- 99.Triffleman E, Carroll K, Kellogg S. Substance dependence posttraumatic stress disorder therapy. An integrated cognitive-behavioral approach. J Subst Abuse Treat. 1999;17:3–14. doi: 10.1016/s0740-5472(98)00067-1. [DOI] [PubMed] [Google Scholar]
- 100.Triffleman E. Gender differences in a controlled pilot study of psychosocial treatments in substance dependent patients with post-traumatic stress disorder: Design considerations and outcomes. Alcohol Treat Q. 2000;18:113–126. 360 Link. [Google Scholar]
- 101.Brady KT, Dansky BS, Back SE, et al. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. J Subst Abuse Treat. 2001;21:47–54. doi: 10.1016/s0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 102.Back SE, Dansky BS, Carroll KM, et al. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Description of procedures. J Subst Abuse Treat. 2001;21:35–45. doi: 10.1016/s0740-5472(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 103.Mills KL, Teesson M, Back SE, et al. Integrated Treatment for Substance Use and PTSD Using Exposure Therapy: Preliminary Findings. College on Problems of Drug Dependence; San Juan, Puerto Rico: 2008. [Google Scholar]
- 104.Department of Veterans Affairs VHA . Health Program Guidelines for the New Veterans Health Administration. Department of Veterans Affairs, Veterans Health Administration; Washington: 1999. [Google Scholar]
- 105.Donovan B, Padin-Rivera E, Kowaliw S. “Transcend:” Initial outcomes from a posttraumatic stress disorder/substance abuse treatment program. J Trauma Stress. 2001;14:757–772. doi: 10.1023/A:1013094206154. [DOI] [PubMed] [Google Scholar]
- 106.Soo C, Tate R. Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database Syst Rev. 2007:CD005239. doi: 10.1002/14651858.CD005239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]