Abstract
The energy demand imposed by physical exercise on the components of the oxygen transport and utilization system requires a close link between cellular and external respiration in order to maintain ATP homeostasis. Invasive and non-invasive experimental approaches have been used to elucidate mechanisms regulating the balance between oxygen supply and consumption during exercise. Such approaches suggest that the mechanism controlling the various subsystems coupling internal to external respiration are part of a highly redundant and hierarchical multi-scale system. In this work, we present a “systems biology” framework that integrates experimental and theoretical approaches able to provide simultaneously reliable information on the oxygen transport and utilization processes occurring at the various steps in the pathway of oxygen from air to mitochondria, particularly at the onset of exercise. This multi-disciplinary framework provides insights into the relationship between cellular oxygen consumption derived from measurements of muscle oxygenation during exercise and pulmonary oxygen uptake by indirect calorimetry. With a validated model, muscle oxygen dynamic responses is simulated and quantitatively related to cellular metabolism under a variety of conditions.
Keywords: Cellular metabolism, Modeling, Multi-scale approach, Oxygen transport, Oxidative phosphorylation, Systems biology
INTRODUCTION
The energy demand imposed by physical exercise on the components of the oxygen transport and utilization system requires a close link between cellular and external respiration in order to maintain adenosine triphosphate (ATP) homeostasis. At the onset of exercise, an immediate increase in the rate of production of (ATP) in active skeletal muscle fibers is required to meet the increased rate of ATP utilization (metabolic demand). Mechanical power output of muscle – measured on a cycle ergometer – responds quickly (τPower < 2 s to a step change in work rate, indicating an even faster response of the ATP production rate by contracting muscles. In contrast, the corresponding dynamic responses of pulmonary oxygen uptake (VO2p) and muscle oxygen uptake (VO2m)29,38,47,50,55 are much slower (τVO2 ~ 30 s). This slower response reflects the ATP production rate from oxidation of reducing equivalents in active muscle. While the steady states for ATP demand and ATP supply through oxidative metabolism match perfectly after ~2–3 min at submaximal work rates, their transient responses differ by at least an order of magnitude.
Noninvasive measurements of VO2p dynamics can characterize differences in onset kinetics of healthy and chronically ill individuals.7,8,49 These measurements, however, not only reflect cellular metabolism, but also the effect of components of the delivery system that transports oxygen from the external environment to the mitochondria (e.g., pulmonary ventilation, diffusion from alveoli to blood, transport in blood – dissolved and bound to hemoglobin – to tissues, diffusion from blood to cells). Because measurements of VO2p dynamics do not accurately indicate the cellular O2 availability and utilization rate in contracting muscle, they are insufficient for deducing mechanisms of metabolic control at the cellular level.
To investigate factors controlling the rate of pulmonary oxygen uptake at exercise onset, oxygen uptake has been measured directly across the femoral bed during cycling in humans29 and across isolated whole skeletal muscle preparations in dogs.24–27,29 These whole-muscle studies have provided valuable insights about the effect of altering convective oxygen delivery and peripheral oxygen diffusion on the dynamics of muscle oxygen uptake during moderate and heavy intensity exercise. Nevertheless, they also have some inherent limitations. When a whole muscle is stimulated in lieu of voluntary muscle contraction, the fiber-type recruitment pattern is different. Furthermore, controlling bulk oxygen delivery does not necessarily control the matching of perfusion to metabolism. Measurements of the oxygen content in the arterial and venous blood do not reflect the actual amount of oxygen available at the mitochondrial level. Local oxygen availability (i.e., cellular PO2) in intact muscle tissue is difficult to assess during exercise in these whole-tissue preparations.
Cellular oxygen availability can be assessed in muscle of contrasting fiber types with phosphorescence quenching9 or in intact working muscle with either near-infrared spectroscopy28 or 1H magnetic resonance spectroscopy.36,60 However, these methods lack specificity when measuring the dynamics of cellular oxygen consumption in a large muscle group during exercise. Nevertheless, intracellular oxygen consumption can be estimated using 31P magnetic resonance spectroscopy by using PCr dynamics as a proxy (within 10%) to muscle oxygen consumption dynamics.3,53,54,65 A more suitable approach to investigate mechanisms controlling respiration is to conduct simultaneous measurements of putative intramuscular control mediators (PCr, Cr, Pi, ATP, ADP) dynamics and VO2p dynamics while performing exercise involving a large muscle group (e.g., quadriceps). This approach provides a large change in VO2p at exercise onset and reliable VO2p dynamic responses which can be compared to those of PCr.53,65
Even if measurements at each scale (i.e., organelle, cellular, tissue/organ, and whole organism level) were experimentally feasible with the appropriate time resolution, these must be integrated into a coordinated system for data interpretation and for elucidation of mechanisms of metabolic control and regulation. For quantitative understanding, integration of transport and metabolic processes at the cellular, tissue/organ, and whole-body levels requires a formal theoretical framework. As a complement to experimental studies of oxygen uptake/consumption at various exercise intensities and biological levels, we used a mathematical model to integrate data and to simulate responses at the cellular and tissue level. The models integrated in this study have been validated previously.42,62
The “systems biology” approach employed here in linking external to internal respiration uses both experimental data from non-invasive methods and computational models to understand complex phenomena and biological systems underlying physiological function and ATP homeostasis. For initial model development to analyze exercise responses, we used data at the whole body and tissue level. In addition, we measured pulmonary oxygen uptake (indirect calorimetry) and muscle oxygenation (near-infrared spectroscopy) dynamics during exercise in healthy volunteers.42 Based on these data, we developed a computational model of oxygen transport and metabolism in skeletal muscle that can simulate and predict muscle oxygen consumption dynamics during exercise. 42 In this study, we integrated our model with a model of energy balance in myocytes41,62 to provide a more mechanistic expression for oxidative phosphorylation, 35 which is linked to the ATPase and creatine kinase (CK) reactions.62 With this model, muscle oxygen consumption dynamics at the cellular level can be estimated during exercise. Finally, model simulations are used to provide insight with respect to concentration and flux rate dynamics of key metabolites (PCr, ATP, ADP, O2) participating in oxidative phosphorylation and ATP homeostasis.
METHODS
Model Development
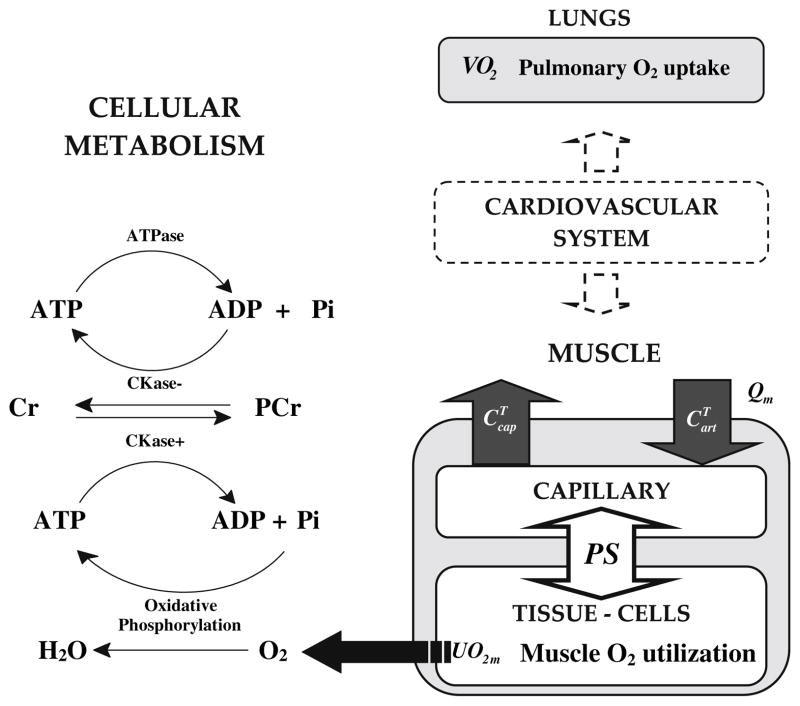
Oxygen utilization rate in skeletal muscle (UO2m) is linked to oxygen uptake rate in lungs (VO2p) by transport processes within the tissues and via the cardiovascular system (Fig. 1). To analyze the dynamic relationship between UO2m and VO2p, a mathematical model is needed. In an earlier approach to developing this relationship, simulations were based on a model42 that approximated oxygen utilization in working skeletal muscle as a mono-exponential function of work rate.10 This model was used to analyze dynamic responses to exercise at several levels of intensity.42 With this simple model, however, distinct metabolic processes involved during exercise stimulus cannot be evaluated. Therefore, a more mechanistic model is needed that describes oxidative phosphorylation by incorporating ATPase and CK reactions. Such a metabolic model has been developed previously.62 Here, this model of oxidative phosphorylation in myocytes is combined with a model of oxygen transport and utilization42 for analysis of responses to exercise.
FIGURE 1.
Oxygen utilization and transport between lungs and skeletal muscle and cellular metabolism during exercise.
Mass Transport Balances
Oxygen concentration dynamics in skeletal muscle are represented by compartmental mass balances. These balance equations involve total oxygen ( ) to free oxygen ( ) in the capillaries and tissues:
| (1) |
| (2) |
Based on relationships between total, bound, and free oxygen concentrations, these equations have an equivalent representation with derivatives of free oxygen (Appendix I). The muscle blood flow, Qm(t), the capillary-tissue transport coefficient represented as the permeability – surface area product, PS(t), and the oxygen utilization, UO2m(t), depend on exercise intensity. The rate of muscle oxygen uptake is defined as (All variables and symbols are defined in the “List of Symbols”).
Metabolic Reaction Balances
The metabolic reaction processes of oxidative phosphorylation during an exercise stimulus have been reported in Fig. 1 and can be expressed as the concentration dynamics of ATP and PCr:
| (3) |
| (4) |
where β is the P:O2 ratio in vivo and the reaction fluxes ϕj are functions of the ATP and PCr concentrations, which must satisfy the mass balances for conservation of adenosine and creatine:
| (5) |
| (6) |
The metabolic flux of oxygen is nonlinear related to the ADP and oxygen concentrations:
| (7) |
where the rate coefficient, , depends on exercise intensity. The metabolic flux for the ATPase reaction is proportional to the ATP concentration:
| (8) |
where the reaction rate coefficient, , depends on exercise intensity. The forward and reverse reaction fluxes of CK are nonlinearly related to the coupled concentrations of Cr, PCr, ADP, and ATP:62
| (9) |
| (10) |
The contributions of glycogenolysis and glycolysis to ATP synthesis are not included in the mathematical model Eq. (3) at this stage of model development. Thus, results obtained from simulations with this incomplete model need to be interpreted accordingly.
The reaction processes, Eqs. (3) and (4), are related to the transport processes, Eqs. (1) and (2), through the rates of oxygen metabolism, ϕOxPhos, and oxygen utilization:
| (11) |
which involves a tissue volume, Vtis, and depends on the exercise intensity j. The total muscle volume is defined as Vm = Vcap + Vtis where Vm is computed as a fraction of the whole body mass (49%) and Vcap, Vtis are each computed as a fraction of the Vm.42
Exercise-dependent Functions
In response to a step increase in work rate from a steady-state warm up condition, the dynamic response of blood flow at exercise intensity j is assumed to be exponential:42
| (12) |
where is the steady-state value during warm-up, is the increase in blood flow, and is the time constants of muscle blood flow, and tW is the initial time. In response to exercise, blood flow increases, which increases the rate of capillary-tissue transport:11
| (13) |
where ΔPS = PSE − PSR, PSR and QC are constants. For a description of their meaning refer to the List of Symbol.
Model Simulation
For comparison of simulated and experimental responses of oxygen obtained from human exercise experiments at several intensities, muscle oxygen saturation is computed:42
| (14) |
The parameters Tm (total amount of Hb and Mb), Vcap and Vtis are constants independent of exercise intensity. The initial conditions for the simulations of the dynamic responses are specified at a warm-up steady state (Appendix II):
| (15) |
To simulate responses to exercise, the model equations were solved numerically using a robust algorithm for stiff ordinary differential equations (DLSODE, http://www.netlib.org/odepack/).31
Parameter Estimation
Values of most parameters in the model equations above have been determined previously and reported in Tables 1A, B and 2.42,62 Others are evaluated directly from steady-state relationships ( , PSR) (Table 3) or by optimal estimation ( ) (Table 4).
TABLE 1.
(A) Values of muscle oxygen utilization, , and pulmonary oxygen uptake, , (mmol min−1) at various exercise intensities (j = R,W,M,H,V)42 and (B) Values of blood flow at various exercise intensities (j = R,W), mean response times (j = M,H,V) and compartment volumes V(L) for model simulations.42
| Panel A | ||||||||||||
| Muscle oxygen utilization | ||||||||||||
| Parameter |
|
|
|
|
|
|||||||
| Mean ± SD | 2 ± 0.2 | 15 ± 4 | 58 ± 18 | 83 ± 18 | 109 ± 13 | |||||||
| Pulmonary oxygen uptake | ||||||||||||
| Parameter |
|
|
|
|
|
|||||||
| Mean ± SD | 9.8 ± 0.9 | 23.6 ± 3.5 | 70.7 ± 17 | 96 ± 15 | 122 ± 11.6 | |||||||
| Panel B | ||||||||||||
| Parameter |
|
|
|
|
|
Vcap | Vtis | |||||
| Mean ± SD | 0.8 ± 0.1 | 3.4 ± 0.4 | 21.3 ± 2 | 24 ± 1 | 24 ± 1 | 2.3 ± 0.3 | 31 ± 5 | |||||
TABLE 3.
Means (n = 7) of reaction rate coefficients for ATPase at different exercise intensities (j = R,W,M,H,V) and of permeability-surface area at rest (L min−1).
| Parameter |
|
|
|
|
|
PSR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 0.048 ± 0.008 | 0.36 ± 0.04 | 1.4 ± 0.3 | 2 ± 0.3 | 2.6 ± 0.4 | 113 ± 26 |
TABLE 4.
Mean (n = 7) parameter values for exercise intensity j = M,H,V: change in muscle blood flow from warmup; maximal rate of oxidative phosphorylation .
| Parameter |
|
|
|
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 5.6 ± 2.2 | 7.6 ± 2 | 9.7 ± 2 | 45 ± 15 | 45 ± 15 | 44 ± 16 |
From experiments, the values of Vtis and are determined so that at each exercise intensity we can evaluate ϕOxPhos from Eq. (11). Under steady-state conditions and assuming β = 6 (32), Eqs. (3) and (4) simplify to
| (16) |
Combining this with Eq. (8) and given a constant value of CATP, due to the ATP homeostasis, we can evaluate (see Table 3). To calculate PSR, we consider Eqs. (1) and (2) at steady state under resting conditions:
| (18) |
From experiments, and are determined. By simultaneous solution of Eqs. (17) and (18), incorporating the relationship , we estimate PSR and .
The parameters and must be estimated for each subject at each exercise intensity. For this purpose, we find the parameter values that yield the best fit of the model output dynamics (from numerical solution of the model equations) to the experimental data. Specifically, for each subject at each exercise intensity, we minimize a least-squares objective function:
| (19) |
where N is the number of data points. The objective function is minimized by numerical optimization using adaptive, non-linear algorithm (DN2FB, http://www.netlib.org).19
RESULTS
In response to different exercise intensities, simulations represent dynamic responses of muscle ATP and PCr concentrations, oxygen saturation, StO2m, ATPase (ϕATPase), oxidative phosphorylation (ϕOxPhos), and net CK ( ) flux rates. By fitting of simulated StO2m to experimental data, optimal estimates were obtained for the maximal flux rate of oxidative phosphorylation and change in muscle blood flow of seven subjects at exercise intensities j = M,H,V. From model simulations, mean response times (τVO2m, τUO2m, τPCr) were computed for muscle O2 uptake, muscle O2 utilization, and PCr, respectively.
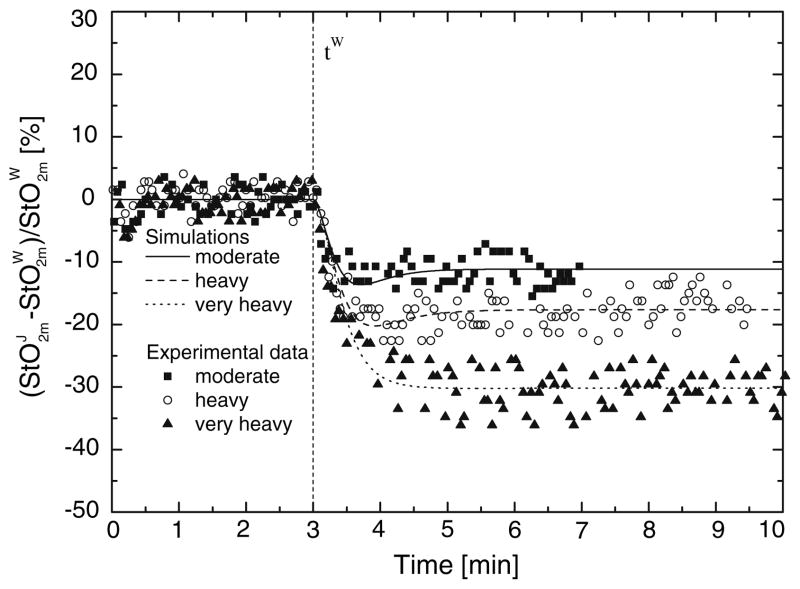
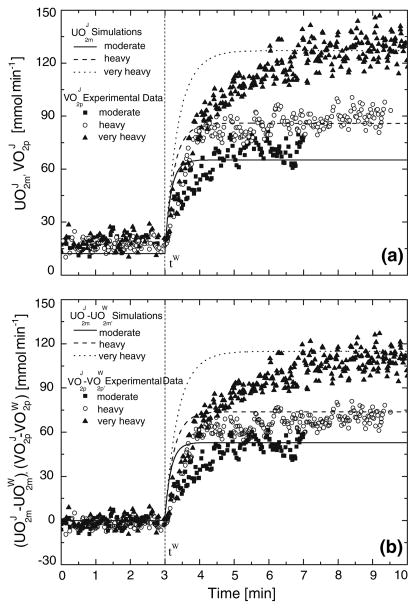
Typical StO2m step responses to a change in exercise intensity relative to a warm-up steady state, , for one subject are presented in Fig. 2. The decrease in StO2m is greater for a higher exercise intensity. Referring to the same subject, model simulations of responses to increased exercise intensities (j = M,H,V) are compared to measured (Fig. 3). Dynamic responses of are faster than the dynamic responses of for all subjects. The corresponding mean response times are compared in Table 5.
FIGURE 2.
Relative oxygen saturation in muscle, : for representative subject responses to step changes from a warm-up steady-state condition (W) to a steady state during moderate, heavy, and very heavy exercise (j = M,H,V). Model output compared with experimental data.42
FIGURE 3.
Comparison between dynamic responses of experimental pulmonary oxygen uptake and simulated muscle oxygen consumption for a representative subject.42 Forcing functions are step changes from a warm-up steady-state condition (W) to a work rate of moderate, heavy, and very heavy intensity exercise (j = M,H,V). Muscle oxygen consumption and pulmonary oxygen uptake are represented (a) in absolute terms as and , respectively and (b) as absolute changes from warm-up steady-state values, i.e., and , respectively.
TABLE 5.
Mean (N = 7) response times for exercise intensity j = M,H,V of muscle oxygen uptake , muscle oxygen utilization and PCr dynamics .
| Mean ± SD | Mean ± SD | Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
18 ± 3 |
|
18 ± 2 |
|
20 ± 3 | |||
|
|
14 ± 3 |
|
15 ± 4 |
|
18 ± 4 | |||
|
|
14 ± 3 |
|
15 ± 4 |
|
18 ± 4 | |||
From optimal estimation of from seven subjects, their mean values are independent of exercise intensity (Table 4). In contrast, the mean change in muscle blood flow increased by about 80% with higher exercise intensity.
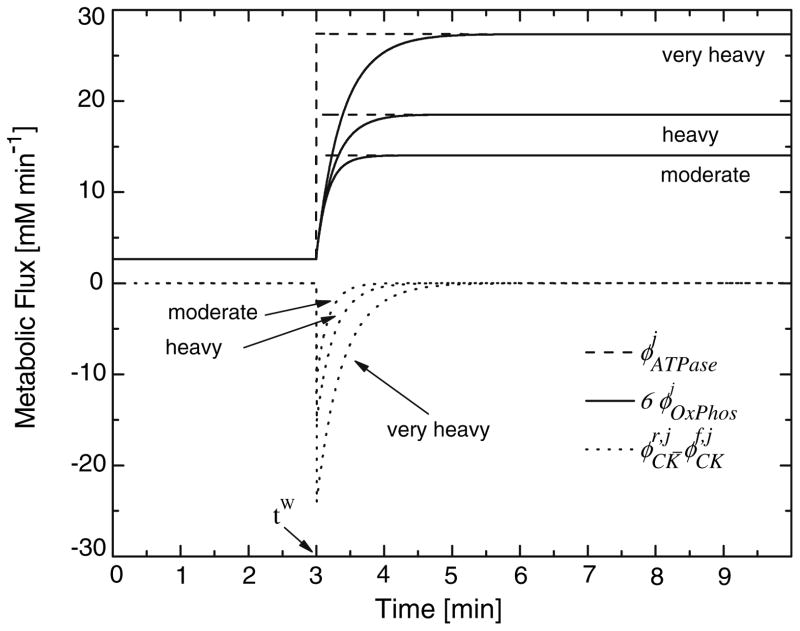
Model simulated metabolic flux rates ϕOxPhos and in response to a step change in ϕATPase (Table 3), corresponding to a given exercise intensity, are presented in Fig. 4. As expected (Eq. 3), at all exercise intensities, 6ϕOxPhos reaches the same steady as ϕATPase. Accordingly to the direct proportionality of ϕOxPhos and (Eq. 11), the mean response time of ϕOxPhos given by τUO2m (Table 5) increases by about 35% from moderate to very high intensity exercise. While ϕOxPhos increases monotonically to the steady state, shows an initial step decrease and then increases in exponential manner towards zero. The dynamic responses of the and ϕOxPhos fluxes are closely balanced to guarantee ATP homeostasis.
FIGURE 4.
Simulation results for representative subject for the fluxes of ATPase, , oxygen phosphorylation, and creatine kinase, . The simulations represent the dynamic between the warm-up steady state and the three levels of exercise intensity: moderate, heavy, and very heavy (j = M,H,V).
The CADP at steady-state, during exercise at all intensities, reached in our simulations (i.e., Figs. 2–5), consistently increases from moderate to very heavy intensity of exercise (M, 2.32 · 10−3 mM; H, 5.26 · 10−3 mM; V, 1.45 · 10−2 mM).
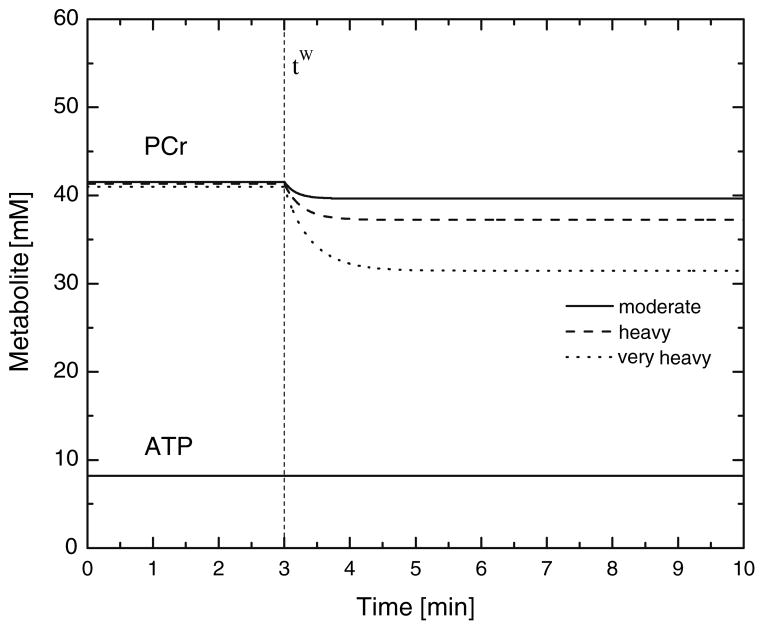
FIGURE 5.
Simulation results for representative subject. Comparison of absolute variation between the PCr break-down and the ATP consumption. The simulations are carried out starting from the warm-up steady state to the three levels of exercise intensity: moderate (continuous), heavy (dashed) and very heavy (dotted).
The ATP concentration change is small compare to the PCr concentration change which decreases in response to a change in exercise intensity (Fig. 5). The mean response time of PCr, τPCr, shows a change with exercise that is similar to the change of τUO2m. Model simulations were also performed assuming that the Michaelis-Menten parameter Km is sufficiently small in Eq. (7) such that ϕOxPhos is independent of oxygen concentration. In response to exercise, the mean response times for and CPCr(τUO2m, τPCr) are nearly the same for moderate, heavy and very heavy exercise (13, 13, 15 s). These response times are about 2–3 s less than those for which ϕOxPhos depends on oxygen concentration (Table 5). Also, for ϕOxPhos with no oxygen dependence, the mean values of (44 (M), 43 (H), 37 (V) mM min−1) compared to those given in Table 4 differ only for the very heavy intensity (j = V). Although decreased from 44 to 37 mM min−1 without oxygen dependence, the mean response time of muscle oxygen uptake τVO2m did not change.
A reduction of the muscle volume, Vm, from 40 to 9 l leads to greater amplitude changes of ϕATPase, net fluxes, CPCr and about a 50% increase in τPCr and τUO2m. Also, the estimated in this range lies between 45 and 25 mM min−1 and was independent of exercise intensity.
DISCUSSION
A mathematical model based on mass balances and metabolic and transport fluxes was successfully developed and applied to study the interacting regulation of cellular respiration and bioenergetics required to maintain ATP homeostasis during step transitions from rest to work rate intensities representing moderate, heavy, and very heavy exercise on a cycle ergometer in humans. This model couples oxygen transport and utilization in skeletal muscle42 to a model of cellular metabolism and energetics.62 The latter model, which describes oxidative phosphorylation, ATPase and CK reactions in finger and wrist flexors during an imposed stimulus, was adapted to investigate cellular energetics in large muscle groups.
In this study, transport and metabolic processes at the onset of exercise were quantified in the steady state and during the exercise transition by comparing model simulations with measurements of pulmonary oxygen uptake and muscle oxygen saturation at different exercise intensities on a cycle ergometer. Then, the dynamics of the metabolic flux rates of ϕOxPhos, ϕATPase, and were simulated for each of the seven subjects at three levels of exercise intensity.
Internal and External Respiration
The regulation of cellular oxygen consumption in skeletal muscle during exercise in vivo depends on electron flow, proton pumping, metabolic fluxes of NADH, ADP-dependent feedback control and oxygen delivery.16 At the cellular level, the dynamics of oxidative phosphorylation to a step increase in energy demand is expected to be fast (<450 ms) based on studies with isolated mitochondria in vitro.12,58 This response time is at least two orders of magnitude faster than in vivo measurements64,65 of oxygen uptake dynamics at the mouth (~45 s). Since direct in vivo measurements of muscle oxygen consumption dynamics (UO2m) are difficult to obtain in humans during exercise, an indirect estimate is obtained from pulmonary oxygen uptake (VO2p) based on measurable variables at the airway opening.66 Under non-steady state conditions, however, VO2p cannot provide a reliable estimate of muscle oxygen consumption because of differences in dynamics at exercise onset.37 Experimentally, Grassi et al.29 found no significant differences in response dynamics between muscle and pulmonary uptake, VO2m and VO2p, during the transition from light to moderate intensity exercise. The methodology used for measuring VO2m dynamics across the femoral bed during exercise may not be sufficiently accurate because the VO2m response depends on lumped measurements of oxygen content in the femoral vein and bulk measurements of femoral blood flow,61 which may result in having slow muscle oxygen uptake dynamics close to that of pulmonary oxygen uptake. Several studies have considered whether the observed dynamics of oxygen uptake at the onset of exercise is the manifestation of an “inertia” in the rate of O2 delivery to the muscle fibers18 or of an intrinsic slowness of the intracellular oxidative metabolism.23,28
Linking Simulated and Measured Fluxes
The model developed here takes into account the dynamic interplay of oxygen delivery (via convection and diffusion),42 and mechanisms of cellular energy metabolism occurring in skeletal muscle during exercise. 62 Specifically, muscle oxygen saturation (Fig. 2) and pulmonary oxygen uptake (Fig. 3) measurements were linked using a mathematical model to quantify the metabolic fluxes involved in cellular respiration in skeletal muscle during rhythmic voluntary contractions on a cycle ergometer.
According to the model of cellular energy metabolism proposed by Vicini and Kushmerick,62 ϕOxPhos is coupled to the metabolic fluxes of and (Fig. 4). This model describes how the dynamics of net PCr breakdown ( ) results in a drop of CPCr to supply the amount of ATP (Fig. 5) that ϕOxPhos cannot synthesize rapidly enough (Fig. 4) to maintain ATP homeostasis at exercise onset (Fig. 5). Similarly, to Vicini and Kushmerick,62 we described cellular energy balance in skeletal muscle without including the glycolytic pathway as a source of ATP synthesis. Under certain conditions, such as maximal or supramaximal exercise, as well as at the onset of high intensity exercise, the glycolytic contribution to ATP synthesis may be significant. The results of our simulation at the onset of heavy and very heavy intensity exercise would most likely be different, were the glycolytic contribution be taken into account. Specifically, we speculate that the rate of phosphocreatine breakdown would be reduced in proportion to the net rate of lactate formation from glycolysis throughout the exercise period.
The simulated dynamic response of muscle oxygen utilization (indicated by ϕOxPhos) was always significantly faster than the response of pulmonary oxygen uptake at all intensities (Fig. 3).
The dynamic response τUO2m of simulated UO2m or ϕOxPhos was always faster than of measured VO2p at all exercise intensities ( , M: 65 ± 7 s; 100 ± 24 s; 82 ± 31 s).42 Furthermore, τUO2m based on model simulation in which ϕOxPhos depends on oxygen concentration Eq. (7) was 2–3 s larger than τUO2m obtained using an empirically derived exponential function for UO2m.42 This difference vanished when ϕOxPhos did not include oxygen dependence. The dependence of ϕOxPhos on oxygen concentration may become evident at low oxygen concentration values, especially at high-intensity exercise.
It should be noted that without the oxygen dependence, the mean estimated is reduced from 44 to 37 mmol l−1 min−1. Therefore, the original expression for ϕOxPhos proposed by Vicini and Kushmerick62 was modified in the present model by including an oxygen dependency, which is important at higher exercise intensities where oxygen could be very low and affect metabolism.67–69 During exercise, oxygen dependency affects the dynamics of muscle oxygen utilization, but does not affect muscle oxygen uptake because the venous oxygen concentration dynamics is not greatly affected. It is worth noticing that, in our model, we assume the rate of ATP production associated with oxidative phosphorylation to be six times the rate of oxygen consumption (i.e., β = 6). Although the P/O2 ratio may be 5 or 6, as proposed in some authors,45,56 a recent review suggests values between 3 and 6.32 Nevertheless, when different values of β were used in the simulations, the estimated dynamics of oxidative phosphorylation remain the same at all exercise intensities, even though it affected the value of and the estimation of .
The kinetic parameters of cellular metabolism Eq. (9–10) were previously estimated for nerve stimulation of finger and wrist flexors.62 The same parameter values were used in this study even though muscles engaged in bicycle exercise were voluntarily activated. Nevertheless, model simulations of the dynamics of oxidative phosphorylation and PCr, characterized by the mean response times τUO2m and τPCr, are in agreement with previously published studies performed in human subjects under similar conditions.53
The model equations account for cellular ATPase, oxidative phosphorylation and CK fluxes variations in skeletal muscle during exercise, where the cellular respiration is regulated by feedback control with dependence from ADP and oxygen concentrations.13–15,67–69 It is worth to note that, in the literature, several feedback and feed forward control models have been proposed to describe the regulation of cellular respiration in vivo, such as (1) feedback control using a Michaelis-Menten relationship between oxidative phosphorylation and [ADP], (2) higher-order feedback control using an expression in which the Hill coefficient is greater than 1, or (3) a more fundamental expression of relating oxidative phosphorylation to the free energy of ATP hydrolysis.34,41,63 Furthermore, some other scientists have proposed feed forward mechanisms to control oxidative phosphorylation.2,40 However, the experimental data available in the present study are not sufficient to address this issue; therefore, we adopted the approach successfully applied previously to in vivo data obtained by NMR spectroscopy.62
The computation of CADP at steady-state during constant work rate exercise (M: 2.74 · 10−3 mM; H: 5.94 · 10−3 mM; V: 1.54 · 10−2 mM), based on the equilibrium assumption using the CK equilibrium constant Keq = 177 (pH = 7, T = 38 °C)57 and CATP, CPCr, CCr obtained from simulations using full kinetic expressions for CK (Eqs. 9–10), is consistent with the CADP values simulated (M: 2.32 · 10−3 mM; H: 5.26 · 10−3 mM; V: 1.45 · 10−2 mM). Moreover, our approach allows for dynamic information on the metabolic fluxes, which is useful to investigate the mechanisms regulating the dynamics of oxidative phosphorylation during exercise.
Model Transport Parameter
In this model, mass transport of oxygen between capillary blood and tissue depends on permeability-surface area coefficient PS, which varies with muscle blood flow (Eq. 13) that in turn depends on exercise intensity.11 The dependency of PS on allows the model to simulate resting and exercise conditions. Although the expression used to describe the variation of PS with work rate is phenomenological, it is consistent with tissue oxygen concentration values at rest and in agreement with the hypothesis of a capillary recruitment during exercise.33 Regardless whether there is capillary recruitment during exercise or just an increase in blood flow rate through the already recruited capillaries, in our simulations, PS coefficient must be set to a sufficiently high value to ensure enough oxygen supply to contracting muscle fibers to match the energy demand at all intensities.42 The parameter QC that affects the relationship (Eq. 13), must be sufficiently small so that simulations of arterio-venous oxygen differences are consistent with the blood flow increase with exercise intensity. Also, from Eq. (12), the blood flow increases between steady states, were in agreement with those found previously at all intensities.42
Limitations of Experimental Data
Model simulations indicate that a reduction of the amount of active muscle, Vm, from 40 to 9 l and thus a reduction of capillary and tissue volume (Vcap, Vtis) engaged during exercise, affects the temporal profile of the metabolites, the maximum rate of oxidative phosphorylation and the mean response time of cellular oxygen utilization . In particular, the smaller the Vm values are the faster the dynamics of oxygen concentration in capillary blood and in tissue appears, for a fixed value of . The value estimated by Vicini and Kushmerick62 (30 mM min−1) falls in the range of values determined in our simulations (45–25 mM min−1) when varying Vm between 40 and 9 l. The estimation of the parameter is limited by the experimental uncertainty in determining active muscle volume and the microvascular volume distribution during exercise. In the present study, the volume of muscle recruited by each subject during exercise was assumed to be a percentage of the whole body volume; thus, a more accurate determination of the functional relationship between active muscle volume engaged and work rate is needed. Experimental measurements of key metabolites, such as PCr in large muscle groups, could be coupled to the model developed to estimate the active muscle volume unknown. Future model development will take into consideration motor unit recruitment pattern accounting for an increase of muscle volume during exercise.
Another limitation related to the unknown muscle volume engaged during exercise is the lack of accuracy and specificity of NIRS measurements in contracting skeletal muscle. Specifically, the limitation of using NIRS technique to evaluate StO2m in skeletal muscle is its lack of discriminatory power to distinguish among the relative contributions of skin, adipose tissue,43 capillaries, and small arterioles and venules to the NIRS signal obtained from an uncertain volume of the region investigated. Therefore, the absolute values may be misleading with respect to the rate of adjustment of oxidative metabolism. Changes in capillary blood volume can affect the concentrations of oxygenated and deoxygenated hemoglobin measured by NIRS and cause variability in the evaluation of oxygen consumption. 10,18 Another potential source of interference is the relative contribution of Hb and Mb44,60 to the NIRS signal. Most studies focus on Hb changes, since it has been reported that intracellular Mb accounts for less than 10% of the total NIRS signal.28 Currently, available NIRS instrumentation cannot accurately determine the relative contribution of myoglobin (Mb) to the total NIRS signal.
Future Directions
The complexity of the model required in future developments depends on the experimental information available and hypotheses to be tested. The validity of model simulations depends on the accuracy and precision associated with estimating unknown parameters. In modeling the linkage between whole body, tissue, and cellular processes with mass balances, the analysis of dynamic responses depends on the estimated volumes of the tissues.
In practice, if the observation scale is reduced to the microvascular level, the volume of perfused tissue under consideration is uncertain. Furthermore, the spatial distribution and temporal variation of blood flow and oxygen concentration can have a significant effect on the interpretation of the measurements.48 With appropriate experimental data, more general detailed models,5,6,17,22 can take into account (a) spatial distribution of transport and reaction processes in the capillaries and extra-vascular tissue, (b) intracellular compartmentation,46 and (c) structural and metabolic characteristics of the tissue (e.g., muscle fiber types). Specifically, for human studies of skeletal muscle metabolism in response to exercise, improvements in the analysis of pulmonary and muscle oxygen dynamics could be made by experiments that simultaneously combine invasive and non-invasive techniques. 18,28,29,39,50,59 The variables to be measured are venous oxygen content, muscle blood flow, and muscle oxygenation during exercise. Measurements of muscle oxygen utilization should be as close as possible to the site of cellular respiration in order to assess the dynamics of oxidative phosphorylation in vivo during exercise. Measurements of 31P and 17O by magnetic resonance spectroscopy could provide key species concentrations in oxidative phosphorylation.16
Nowadays measurements of pulmonary oxygen uptake, VO2p are used to make inferences on the bioenergetic processes involved during exercise. The VO2p average measured in our population at the end of constant work rate exercise may be expressed as percent of the maximal pulmonary oxygen uptake.42: 49 ± 11%, 68 ± 9% and 87 ± 6% at moderate, heavy and very heavy exercise intensity, respectively.
Presence of the slow component was discernable from our data during heavy and very heavy intensity exercise. To date, there is no unequivocal explanation for such behavior because several factors can lead to an excess VO2p during high intensity constant work rate exercise, as already reported by several authors.4,20,21,52 The predominant portion of the slow component (86%) may be associated with the contracting muscles,51 whereas fiber recruitment at higher level may affect the pattern of VO2p measured at the mouth. Fiber recruitment may only contribute in part to the slow component. The model developed here does not incorporate components that may lead to a slow component for VO2; however, it can be enhanced to enable testing of putative mechanisms resulting in excess oxygen consumption during high intensity exercise.
Additionally, if dynamic measurements of O2, CO2, and pH were obtained in the venous blood perfusing the contracting muscle, then the effect of acid-base regulation1,17 could be modeled to investigate the control of respiration during exercise. Although direct in vivo measurements of UO2m are not feasible during exercise involving a large muscle mass, indirect evaluation of UO2m can be achieved with a multi-scale systems biology approach. This would combine computational modeling and simulation with experimental data with many types of measurements at the whole body, tissue, and cellular levels.
TABLE 2.
| Transport model
|
Metabolic model
|
||||||
|---|---|---|---|---|---|---|---|
| Notation | Unit | Value | Notation | Unit | Value | ||
| KHb | (mM)−2.7 | 7800.7 | CCr,tot | (mM) | 42 | ||
| KMb | (mM)−1 | 308.6 |
|
(mM) | 8.2 | ||
|
|
(mM) | 0.135 |
|
(mM min−1) | 3008.6 | ||
|
|
(mM) | 3.375 × 10−2 30 |
|
(mM min−1) | 6000 | ||
| Crbc,Hb | (mM) | 5.18 | Kb | (mM) | 1.11 | ||
| Cmc,Mb | (mM) | 0.5 | Kp | (mM) | 3.8 | ||
| Hct | (−) | 0.45 | Kia | (mM) | 0.135 | ||
| QC | (L min−1) | 8a | Kib | (mM) | 3.9 | ||
| Vcap | (L) | 7% Vm | Kiq | (mM) | 3.5 | ||
| Vtis | (L) | 93% Vm | KADP | (mM) | 0.058 | ||
| Wmc | (− | 0.75 | Km | (mM) | 7 × 10−4 17 | ||
| PSE | (L min−1) | 20,000 | |||||
This work.
Acknowledgments
We thank Dr. Asit K. Saha for providing thoughtful and constructive criticisms. This research was supported by the grant GM-66309-01 from the National Institute for General Medical Science (NIGMS) of the National Institute of Health (NIH) for establishing the Center for Modeling Integrated Metabolic Systems (MIMS) at Case Western Reserve University.
LIST OF SYMBOL
- CA,tot
Total concentration of ADP and ATP (mM)
- CADP
Concentration of ADP in tissue (mM)
- CATP
Concentration of ATP in tissue (mM)
- CCr,tot
Total Concentration of PCr and Cr (mM)
- CCr
Concentration of Cr in tissue (mM)
- CPCr
Concentration of PCr in tissue (mM)
- Crbc,Hb
Concentration of Hb in the red blood cell (mM)
- Cmc,Mb
Concentration of Mb in myocyte (mM)
Bound oxygen concentration in artery, capillary and tissue (mM)
Free oxygen concentration in artery, capillary and tissue (mM)
Total oxygen concentration in artery, capillary and tissue (mM)
- Hct
Hematocrit (fraction of red blood cells in blood) (−)
- kATPase
ATPase rate constant (min−1)
- KHb
Hill constant at which Hb is 50% saturated by O2 (mM−n)
- KMb
Hill constant at which Mb is 50% saturated by O2 (mM−1)
- KADP
CK constant (mM)
- Kb
CK constant (mM)
- Kia
CK constant (mM)
- Kib
CK constant (mM)
- Kiq
CK constant (mM)
- Km
Michaelis Menten constant (mM)
- Kp
CK constant (mM)
- n
Hill coefficient (−)
- PS
Permeability-surface area product (L min−1)
- PSE
Maximal value of permeability-surface area product (L min−1)
- PSR
Permeability-surface area product at rest (L min−1)
- QC
Muscle blood flow constant in Eq. (13) (L min−1)
- Qm
Muscle blood flow (L min−1)
- Scap,Hb
Oxygen hemoglobin saturation in blood capillary (−)
- StisMb
Oxygen myoglobin saturation in muscle tissue (−)
- StO2m
Muscle oxygen saturation (−)
- Tm
Total amount of Hb and Mb (mmol)
- t
Time (min)
- tW
Time at the onset of the exercise (min)
- UO2m
Muscle oxygen utilization (mmol min−1)
Maximal forward flux of CK reaction (mM min−1)
Maximal reverse flux of CK reaction (mM min−1)
- Vmax
Maximal flux of oxidative Phosphorylation (mM min−1)
- Vcap, Vtis
Anatomical volume of capillary and tissue (L)
- VO2m, VO2p
Muscle and pulmonary oxygen uptake (mmol min−1)
- WR
Work rate (watt)
- Wmc
Myocyte volume fraction (−)
- ΔPS
Amplitude of permeability-surface area (L min−1)
- ΔQm
Amplitude of response of muscle blood flow rate (L min−1)
Greek letters
- ϕATPase
ATPase metabolic flux (mM min−1)
CKase forward metabolic flux (mM min−1)
CKase reverse metabolic flux (mM min−1)
- ϕOxPhos
Oxydative phosphorylation metabolic flux (mM min−1)
- τPCr
Time constant of the PCr kinetics (s)
- τPower
Time constant of the mechanical power (s)
- τQm
Time constant of the muscle blood flow (s)
- τUO2m
Time constant of the muscle oxygen utilization (s)
- τVO2m
Mean response time of the muscle oxygen uptake (s)
- τVO2p
Mean response time of the pulmonary oxygen uptake (s)
Superscript
- B
Bound oxygen concentration
- F
Free oxygen concentration
- H
Heavy condition
- j
Exercise intensity
- M
Moderate condition
- R
Resting condition
- T
Total oxygen concentration
- V
Very heavy condition
- W
Warm-up condition
APPENDIX I
The oxygen mass balances of Eqs. (1) and (2) require a relationship between total oxygen ( ) to free oxygen ( ) in the capillaries and tissues.42 To relate the total oxygen concentration to free oxygen concentration , we consider oxygen in free and (hemoglobin) bound forms in arterial and capillary blood ( ) and in free and (myoglobin) bound forms in muscle tissue ( ). The total oxygen concentrations in arterial and capillary blood and in muscle tissue are the sums of the corresponding free and bound oxygen concentration as:
| (A.1) |
which are related by local chemical equilibrium. In blood the relation is
| (A.2) |
In tissue the relation is
| (A.3) |
These relations depend on Hb and Mb concentrations in red blood cell and myocyte (Crbc,Hb, Cmc,Mb) and their respective volume fractions (Hct, Wmc) and oxygen saturations (Scap,Hb, Stis,Mb). From (A.1), we get the differential relationships:
| (A.4) |
where
| (A.5) |
Using the relationships above, Eq. (1) can be expressed as:
| (A.6) |
Similarly, we can express Eq. (2) as
| (A.7) |
It should be noted that this spatially lumped, two-compartment model of oxygen transport and metabolism simulates the dynamic changes of oxygen concentration in capillary blood and tissue cells within the skeletal muscle during exercise assuming perfect mixing. Thus, under this assumption, is equivalent to the free oxygen concentration at the end of the capillary, as well as to the venous oxygen concentration.
APPENDIX II
The values of the concentrations under steady-state conditions at any exercise condition j must satisfy the following equations:
| (A.8) |
| (A.9) |
| (A.10) |
| (A.11) |
| (A.12) |
| (A.13) |
These 6 equations involve eight concentrations. Therefore, for any j we can specify two concentrations and solve for the other six. At rest, j = R, we can specify and CCr,tot, then solve for the other concentrations including CA,tot. Under the warm-up conditions, j = W, we can specify CA,tot and CCr,tot then solve for the other concentrations including and .
References
- 1.Andreassen S, Rees SE. Mathematical models of oxygen and carbon dioxide storage and transport: interstitial fluid and tissue stores and whole-body transport. Crit Rev Biomed Eng. 2005;33:265–298. doi: 10.1615/critrevbiomedeng.v33.i3.20. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS. Myocardial energy metabolism in health and disease cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 3.Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- 4.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- 5.Beard DA, Bassingthwaighte JB. Modeling advection and diffusion of oxygen in complex vascular networks. Ann Biomed Eng. 2001;29:298–310. doi: 10.1114/1.1359450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard DA, Schenkman KA, Feigl EO. Myocardial oxygenation in isolated hearts predicted by an anatomically realistic microvascular transport model. Am J Physiol Heart Circ Physiol. 2003;285:H1826–H1836. doi: 10.1152/ajpheart.00380.2003. [DOI] [PubMed] [Google Scholar]
- 7.Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H926–H932. doi: 10.1152/ajpheart.00059.2002. [DOI] [PubMed] [Google Scholar]
- 8.Behnke BJ, McDonough P, Musch TI, Poole DC, Arena R. Comparison of oxygen uptake on-kinetics calculations in heart failure. Med Sci Sports Exerc. 2003;35:708–709. doi: 10.1249/01.MSS.0000058444.43322.05. [DOI] [PubMed] [Google Scholar]
- 9.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binzoni T, Colier W, Hiltbrand E, Hoofd L, Cerretelli P. Muscle O2 consumption by NIRS: a theoretical model. J Appl Physiol. 1999;87:683–688. doi: 10.1152/jappl.1999.87.2.683. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell JH, Martin GV, Raymond GM, Bassingthwaighte JB. Regional myocardial flow and capillary permeability-surface area products are nearly proportional. Am J Physiol. 1994;267:H654–H666. doi: 10.1152/ajpheart.1994.267.2.H654. [DOI] [PubMed] [Google Scholar]
- 12.Chance B, Jamieson D, Coles H. Energy-linked pyridine nucleotide reduction: inhibitory effects of hyperbaric oxygen in vitro and in vivo. Nature. 1965;206:257–263. doi: 10.1038/206257a0. [DOI] [PubMed] [Google Scholar]
- 13.Chance B, Leigh JS, Jr, Kent J, Mccully K, Nioka S, Clarkii BJ, Maris JM, Graham T. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA. 1986;83:9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I kinetics of oxygen utilization. J Biol Chem. 1955;217:383–394. [PubMed] [Google Scholar]
- 15.Chance B, Williams GR, Holmes WF, Higgins J. Respiratory enzymes in oxidative phosphorylation. V A mechanism for oxidative phosphorylation. J Biol Chem. 1955;217:439–452. [PubMed] [Google Scholar]
- 16.Chung Y, Mole PA, Sailasuta N, Tran TK, Hurd R, Jue T. Control of respiration and bioenergetics during muscle contraction. Am J Physiol Cell Physiol. 2005;288:C730–C738. doi: 10.1152/ajpcell.00138.2004. [DOI] [PubMed] [Google Scholar]
- 17.Dash RK, Bassingthwaighte JB. Simultaneous blood-tissue exchange of oxygen, carbon dioxide, bicarbonate, and hydrogen ion. Ann Biomed Eng. 2006;34:1129–1148. doi: 10.1007/s10439-005-9066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- 19.Dennis JE, Gay DM, Welsch RE. An adaptive non linear least squares algorithm. ACM, Trans Math Software. 1981;7:348–383. [Google Scholar]
- 20.Engelen M, Porszasz J, Riley M, Wasserman K, Maehara K, Barstow TJ. Effects of hypoxic hypoxia on O2 uptake and heart rate kinetics during heavy exercise. J Appl Physiol. 1996;81:2500–2508. doi: 10.1152/jappl.1996.81.6.2500. [DOI] [PubMed] [Google Scholar]
- 21.Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev. 1996;24:35–71. [PubMed] [Google Scholar]
- 22.Goldman D, Popel AS. Computational modeling of oxygen transport from complex capillary networks. Relation to the microcirculation physiome. Adv Exp Med Biol. 1999;471:555–563. doi: 10.1007/978-1-4615-4717-4_65. [DOI] [PubMed] [Google Scholar]
- 23.Grassi B. Regulation of oxygen consumption at exercise onset: is it really controversial? Exerc Sport Sci Rev. 2001;29:134–138. doi: 10.1097/00003677-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- 25.Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated in-situ canine muscle. J Appl Physiol. 1998;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- 26.Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin D, Gladden BL. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol. 2002;538:195–207. doi: 10.1113/jphysiol.2001.012984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden BL. Role of convective O2 delivery in determining VO2 on-kinetics in canine muscle contracting at peak VO2. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- 28.Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- 29.Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- 30.Guyton AC, Hall JE. The Textbook of Medical Physiology. 10. Philadelphia: W.B., Saunders Company; 2000. Transport of oxygen and carbon dioxide in the blood and body fluids; pp. 463–473. [Google Scholar]
- 31.Hindmarsh AC. ODEPACK a systematized collection of ode solvers. In: Stepleman RS, editor. Scientific Computing. Amsterdam: North Holland; 1983. pp. 55–64. [Google Scholar]
- 32.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2005;1706:1–11. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Honig CR, Odoroff CL, Frierson JL. Capillary recruitment in exercise: rate, extent, uniformity, and relation to blood flow. Am J Physiol. 1980;238:H31–H42. doi: 10.1152/ajpheart.1980.238.1.H31. [DOI] [PubMed] [Google Scholar]
- 34.Jeneson JAL, Westerhoff HV, Brown TR, Van Echteld CJA, Berger R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physiol. 1995;268:C1474–C1484. doi: 10.1152/ajpcell.1995.268.6.C1474. [DOI] [PubMed] [Google Scholar]
- 35.Jeneson JAL, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem. 1996;271:27995–27998. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 36.Jue T, Tran TK, Mole P, Chung Y, Sailasuta N, Hurd R, Kreutzer U, Kuno S. Myoglobin and O2 consumption in exercising human gastrocnemius muscle. Adv Exp Med Biol. 1999;471:289–294. doi: 10.1007/978-1-4615-4717-4_35. [DOI] [PubMed] [Google Scholar]
- 37.Kemp G. Kinetics of muscle oxygen use, oxygen content, and blood flow during exercise. J Appl Physiol. 2005;99:2463–2468. doi: 10.1152/japplphysiol.00709.2005. [DOI] [PubMed] [Google Scholar]
- 38.Knight DR, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effect of inspired O2 concentration on leg lactate release during incremental exercise. J Appl Physiol. 1996;81:246–251. doi: 10.1152/jappl.1996.81.1.246. [DOI] [PubMed] [Google Scholar]
- 39.Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD. Relationship between body and leg VO2 during maximal cycle ergometry. J Appl Physiol. 1992;73:1114–1121. doi: 10.1152/jappl.1992.73.3.1114. [DOI] [PubMed] [Google Scholar]
- 40.Korzeniewski B. Regulation of ATP supply in mammalian skeletal muscle during resting state intensive work transition. Biophys Chem. 2000;83:19–34. doi: 10.1016/s0301-4622(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 41.Kushmerick MJ. Energy balance in muscle activity: simulations of ATPase coupled to oxidative phosphorylation and to creatine kinase. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:109–123. doi: 10.1016/s0305-0491(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 42.Lai N, Dash RK, Nasca MM, Saidel GM, Cabrera ME. Relating pulmonary oxygen uptake to muscle oxygen consumption at exercise onset: in vivo and in silico studies. Eur J Appl Physiol. 2006;97:380–394. doi: 10.1007/s00421-006-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Niwayama M, Shiga T, Kudo N, Takahashi M, Yamamoto K. Influence of a fat on muscle oxygenation measurement using near-IR spectroscopy: quantitative analysis based on two-layered phantom experiments and Monte Carlo simulation. Front Med Biol Eng. 2000;10:43–58. doi: 10.1163/15685570052061531. [DOI] [PubMed] [Google Scholar]
- 44.Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 45.Nelson D, Cox M. Lehninger Principles of Biochemistry. 3. New York: Worth Publishers; 2000. [Google Scholar]
- 46.Ovadi J, Saks V. On the origin of intracellular compartmentation and organized metabolic systems. Mol Cell Biochem. 2004;256–257:5–12. doi: 10.1023/b:mcbi.0000009855.14648.2c. [DOI] [PubMed] [Google Scholar]
- 47.Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol. 2001;533:891–902. doi: 10.1111/j.1469-7793.2001.t01-1-00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pittman RN. Oxygen supply to contracting skeletal muscle at the microcirculatory level: diffusion vs. convection. Acta Physiol Scand. 2000;168:593–602. doi: 10.1046/j.1365-201x.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 49.Poole DC. VO2 kinetics in COPD patients under hyperoxic conditions. J Appl Physiol. 1996;80:1070–1072. doi: 10.1152/jappl.1996.80.3.1070. [DOI] [PubMed] [Google Scholar]
- 50.Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol. 1992;72:805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- 51.Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- 52.Pringle JSM, Doust JH, Carter H, Tolfrey K, Campbell IT, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity ‘submaximal’ exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol. 2003;89:289–300. doi: 10.1007/s00421-003-0799-1. [DOI] [PubMed] [Google Scholar]
- 53.Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ. Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol. 2002;93:2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- 55.Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salway JG. Metabolism at a Glance. 3. Blackwell Publishing Professional; 2004. [Google Scholar]
- 57.Teague WE, Jr, Dobson GP. Effect of temperature on the creatine kinase equilibrium. J Biol Chem. 1992;267:14084–14093. [PubMed] [Google Scholar]
- 58.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 59.Tordi N, Mourot L, Matusheski B, Hughson RL. Measurements of cardiac output during constant exercises: comparison of two non-invasive techniques. Int J Sports Med. 2004;25:145–149. doi: 10.1055/s-2004-819949. [DOI] [PubMed] [Google Scholar]
- 60.Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, Jue T. Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol. 1999;276:R1682–R1690. doi: 10.1152/ajpregu.1999.276.6.R1682. [DOI] [PubMed] [Google Scholar]
- 61.Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- 62.Vicini P, Kushmerick MJ. Cellular energetics analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. Am J Physiol Cell Physiol. 2000;279:C213–C224. doi: 10.1152/ajpcell.2000.279.1.C213. [DOI] [PubMed] [Google Scholar]
- 63.Westerhoff HV, Van Echteld CJ, Jeneson JA. On the expected relationship between Gibbs energy of ATP hydrolysis and muscle performance. Biophys Chem. 1995;54:137–142. doi: 10.1016/0301-4622(94)00129-8. [DOI] [PubMed] [Google Scholar]
- 64.Whipp BJ, Casaburi R. Characterizing O2 uptake response kinetics during exercise. Int J Sports Med. 1982;3:97–99. doi: 10.1055/s-2008-1026070. [DOI] [PubMed] [Google Scholar]
- 65.Whipp BJ, Rossiter HB, Ward SA, Avery D, Doyle VL, Howe FA, Griffiths JR. Simultaneous determination of muscle 31P and O2 uptake kinetics during whole body NMR spectroscopy. J Appl Physiol. 1999;86:742–747. doi: 10.1152/jappl.1999.86.2.742. [DOI] [PubMed] [Google Scholar]
- 66.Whipp BJ, Ward SA, Rossiter HB. Pulmonary O2 uptake during exercise: conflating muscular and cardiovascular responses. Med Sci Sports Exerc. 2005;37:1574–1585. doi: 10.1249/01.mss.0000177476.63356.22. [DOI] [PubMed] [Google Scholar]
- 67.Wilson DF, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977;233:C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]
- 68.Wilson DF, Erecinska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys. 1979;195:485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]
- 69.Wilson DF, Owen CS, Holian A. Control of mitochondrial respiration: a quantitative evaluation of the roles of cytochrome c and oxygen. Arch Biochem Biophys. 1977;182:749–762. doi: 10.1016/0003-9861(77)90557-4. [DOI] [PubMed] [Google Scholar]