Abstract
Many industrially important reactions use immobilized enzymes in non-aqueous, organic systems, particularly for the production of chiral compounds such as pharmaceutical precursors. The addition of a spacer molecule (“tether”) between a supporting surface and enzyme often substantially improves the activity and stability of enzymes in aqueous solution. Most “long” linkers (e.g. polyethylene oxide derivatives) are relatively hydrophilic, improving the solubility of the linker-enzyme conjugate in polar environments, but this provides little benefit in non-polar environments such as organic solvents. We present a novel method for the covalent immobilization of enzymes on solid surfaces using a long, hydrophobic polytryptophan tether. Candida antarctica lipase B (CALB) was covalently immobilized on non-porous, functionalized 1-μm silica microspheres, with and without an intervening hydrophobic poly-DL-tryptophan tether (n ≈ 78). The polytryptophan-tethered enzyme exhibited 35 times greater esterification of n-propanol with lauric acid in the organic phase and five times the hydrolytic activity against pnitrophenol palmitate, compared to the activity of the same enzyme immobilized without tethers. In addition, the hydrophobic tethers caused the silica microspheres to disperse more readily in the organic phase, while the surface-immobilized control treatment was less lipophilic and quickly settled out of the organic phase when the suspensions were not vigorously mixed.
Keywords: Immobilization, Candida antarctica lipase B, hydrophobic tethers, poly-DL- tryptophan, enzyme-catalyzed organic synthesis, silica microspheres
INTRODUCTION
Interest in the use of enzymes as catalysts for organic synthesis reactions has increased over the past few decades. Many enzymes remain active and functional in non-aqueous or organic phases (Zaks and Klibanov, 1985; Halling and Kvittingen, 1999), and typically exhibit higher stereospecificity and stability than in an aqueous environment. A number of explanations for this behavior in organic solvents have been suggested, including increased stability of enzyme structure, mitigation of pH and ionic interactions, and improved solubility of lipophilic substrates and products (Klibanov, 2001). Although the specific activity of enzymes in organic systems is typically much lower than in aqueous systems, advances in the efficient preparation of enzymes for use in organic solvents have allowed enzyme activities within an order of magnitude of aqueous systems to be achieved (Ru et al., 2001, Lee and Dordick, 2002).
In organic media, normally hydrolytic enzymes can be “reversed” to catalyze synthesis reactions, often with very high stereospecificity. By reducing the amount of water in the system, hydrolytic side reactions that can interfere with syntheses are restricted. In addition, reactants and products of synthetic reactions are often much more soluble in organic solvents than in water. A wide variety of pharmaceutical precursors and other commercially important compounds are produced by enzyme-catalyzed processes in organic and semi-aqueous systems (Bommarius and Riebel, 2004; Hudson et al., 2005; Mahmoudian, 2007; Dreyer et al., 2007).
Direct linkage of enzymes to surfaces is often reported to cause significant loss of activity when compared to the free enzyme, although hyperactivation (e.g. Palomo et al., 2002) following immobilization is not uncommon. Many physical and chemical effects contribute to the activity changes, including changes in molecular structure during coupling, steric hindrance of access to the catalytic sites, and physical denaturation caused by adsorption or proximity to the solid-liquid interface (Norde, 1986). Steric and interfacial effects in aqueous systems are often reduced by incorporating a “spacer arm” linker between the support and enzyme (Cao, 2005), which should be “long enough to promote effective separation of the enzyme from the support”, and sparsely distributed on the support to avoid the creation of a “spacer wall” and a new steric hindrance problem (Guisán, et al., 1997).
Stark and Holmberg (1989) studied the activity of Rhizopus lipase immobilized on tresylated silica with or without a hydrophilic polyethylene oxide (PEO, 34 units) spacer. Although the hydrolytic activity in aqueous solution was doubled by the presence of the PEO spacer arm, there was no significant effect on transesterification activity in non-aqueous solutions. The authors concluded that the hydrophilic spacer arm was incompatible with the organic reaction medium, and preferentially adsorbed to the support rather than extending away from the surface.
Despite the obvious conclusion that a lipophilic spacer might improve activity in organic media, few previous studies have explored the use of long (i.e. tens to hundreds of repeat units) hydrophobic polymers to tether enzymes to surfaces for use in non-aqueous systems. The aim of this study was to demonstrate that long, hydrophobic linkers can increase the activity of enzymes in organic media, offering another tool to supplement the existing repertoire of immobilization methods, and potentially increasing the efficiency of non-aqueous enzymatic synthesis of commercially important compounds.
Choice of Enzyme and Hydrophobic Linker
Lipases are lipophilic, hydrolytic enzymes that are used in organic media to catalyze numerous industrial and pharmaceutical processes (Bommarius and Riebel, 2004; Mahmoudian, 2007). A representative enzyme, Candida antarctica lipase B (CALB), was chosen for use in this study because an immobilized form of this enzyme is widely used to prepare a variety of compounds in organic media. In addition, CALB does not possess a hydrophobic “lid” covering the catalytic site, and, unlike most lipases, does not exhibit “interfacial activation” caused by large structural changes that expose the catalytic site in the presence of a hydrophobic interface. For example, CALB was found to have the same synthetic activity when adsorbed on a fully hydrophobic polystyrene carrier, or on n-alkyl-modified hydrophilic polymethacrylate matrices (Petkar, et al., 2006).
Heterobifunctional molecules are most desirable for a linker, as they allow a controlled, stepwise conjugation reaction that prevents polymerization and self-conjugation of the target molecules. Homopolymers of amino acids (HPAAs) are available in a variety of molecular weights and side-chain compositions. These synthetic polypeptides have terminal –NH2 and – COOH groups, so the conjugation methods are similar to that of enzymes. A random copolymer of D- and L-tryptophan was selected for this study because the indole side-chain is relatively unreactive, and tryptophan is among the most hydrophobic of the amino acids (Karplus, 1997).
If side-chain interactions are minimal, most isotactic hydrophobic polypeptides will spontaneously adopt a mostly α-helical or β-sheet conformation in non-aqueous environments. This is primarily due to the “self-solvation” of the peptide backbone caused by H-bonding between the peptide C=O and N–H groups (Chipot and Pohorille, 1998; Efremov, et al., 1999; Nguyen et al., 2004). While alternating copolymers of D-/L-amino acids with bulky side-chains do adopt regular helical structures (Hesselink and Scheraga, 1972), the random copolymer of D-and L-tryptophan used in this study is not expected to adopt any regular structure, due to the steric exclusion of the bulky side groups extending randomly from both sides of the peptide backbone (Krause, et al., 2000). Therefore, we assumed a loose random coil conformation for poly-DL-tryptophan; its relatively high solubility in organic solvents also implies a lack of ordered structure.
Materials And Methods
Non-porous silica microspheres (1 μm nominal diameter) were obtained from Fiber Optic Center (New Bedford, MA). Lipase B from Candida antarctica (CALB) was a gift from Novozymes North America (Franklinton, NC). The liquid enzyme preparation (Novozymes NS81020 CALB-L, 4 mL) was dialyzed (10 kDa MWCO) against distilled water (3 changes, 2 liters each) at 4°C, then lyophilized and stored at -20°C until reconstituted with buffer. Novozym™ 435 immobilized lipase and poly-DL-tryptophan (14.5 kDa by LALLS, n ≈ 78) were obtained from Sigma-Aldrich (St. Louis, MO), and used without further refinement. The γ- aminopropyltriethoxysilane (APTES) was kept under argon in a desiccator, and was used as received. All solvents and reagents used for the immobilization procedures were kept over molecular sieves (4 Å) or desiccants to remove excess water. Unless otherwise noted, all other reagents and solvents were obtained from Pierce (Rockford, IL), VWR (Westchester, PA), or Sigma-Aldrich, and were of the highest practical purity.
Preparation of Carboxylated Silica Microspheres (SiO2-COOH)
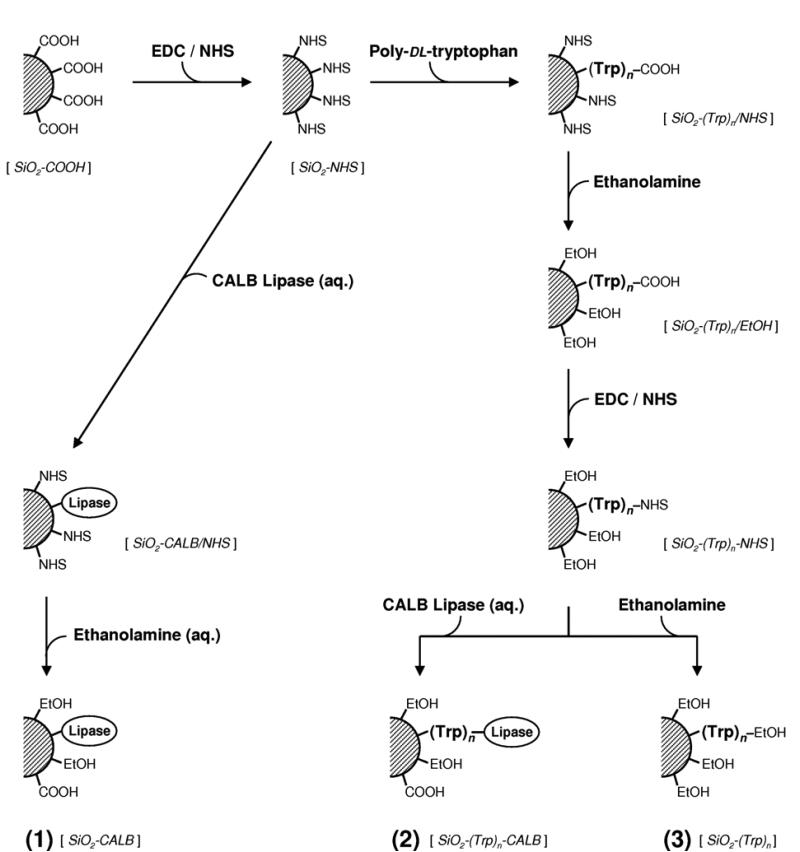
Non-porous 1-μm silica microspheres (SiO2) were functionalized with APTES in dry toluene with triethylamine (Et3N) as catalyst, by the “Am-2” method described by Kovalchuk, et al. (2006). The resulting SiO2-NH2 was suspended in three volumes of 0.1 M succinic anhydride and 0.2 M Et3N in dry DMF, and stirred vigorously for 2 hours at 37°C to convert the surface amino groups to carboxyl (–COOH) groups. After extensive washes with DMF, 0.1 N HCl, and HPLC-grade water, then dried under vacuum at 50-60°C and stored in a desiccator. The resulting carboxylated silica, SiO2-COOH, was the starting point for all further syntheses (Figure 1).
Figure 1.
Synthesis of surface-immobilized lipase [SiO2-CALB, 1]; poly-DL-tryptophan-tethered lipase [SiO2-(Trp)n-CALB, 2]; and poly-DL-tryptophan control [SiO2-(Trp)n, 3]. All steps were carried out in dry DMSO, except those noted as aqueous (0.1M PBS, pH 6.5).
Non-Aqueous Activation of SiO2-COOH with EDC/NHS
Carboxylated silica was washed twice with 0.1 N HCl to ensure complete protonation of the carboxyl groups, twice with water, and once with ethanol. The silica was then dried under vacuum at 100-110°C for 1 hour. Aliquots (1 mL) of a solution of N-hydroxysuccinimde (NHS, 0.15 M) and 1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide (EDC, 0.3 M) in dry DMSO were added to 0.45 g samples of the dry, protonated SiO2–COOH. The slurry was mechanically rotated overnight at 37°C, then washed 3x with DMSO to remove excess reactants. The activated SiO2-NHS-ester carrier was then immediately conjugated with the primary –NH2 groups of the polytryptophan tether.
Immobilization of Poly-DL-Tryptophan Tethers on SiO2-NHS
In order to achieve submonolayer coverage of hydrophobic tethers, polytryptophan was added in limiting amounts. The target coverage was five tether molecules for each enzyme molecule in a theoretical monolayer (2.8x10-7 moles of enzyme per gram of 1 μm microspheres). A 30 mg/mL solution of poly-DL-tryptophan was made in dry DMSO. Triethylamine (100 mM) was added to the linker solution to ensure that the polypeptide terminal –NH2 groups were deprotonated. The activated SiO2-NHS samples were resuspended in 500 µL of dry DMSO, and 350 μL of the (Trp)n solution was added. The slurry was mechanically rotated overnight at 37°C.
Samples of the SiO2-(Trp)n conjugate were taken for quantification of immobilized mass by TGA. These were washed at least five times with DMSO to remove excess (Trp)n, then incubated for 4 hours at 37°C with a solution of ammonium hydroxide (NH4OH) in water (pH 10.5) to hydrolyze the remaining NHS-esters from the surface carboxyl groups. The samples were then washed extensively with water and ethanol prior to thermogravimetric analysis.
Capping of SiO2-(Trp)n Surface NHS-Esters with Ethanolamine
Conjugation of the limited (Trp)n tethers with SiO2-NHS leaves a very large number of unreacted NHS-esters on the silica surface. To quench these excess active groups, 0.3 mmole of ethanolamine in 50 μL of dry DMSO was added to the SiO2-(Trp)n conjugates, and allowed to react at 37°C overnight. The resulting hydroxyl-capped SiO2-(Trp)n was then washed 3x with DMSO, 3x with water, and 3x with ethanol, then dried at 90°C under vacuum for 12 hours. The resulting (Trp)n–modified carrier was stored under argon in a desiccator until used.
Immobilization of Lipase on NHS-Activated SiO2-COOH and SiO2-(Trp)n Carriers
The C-terminal tether carboxyl groups on SiO2-(Trp)n carriers were activated with EDC (0.15 M) and NHS (0.3 M) in dry DMSO (1 mL) as described above. An identical activation procedure was also performed on SiO2-COOH carriers to provide a “surface-immobilized” lipase control treatment. Following the EDC/NHS activation reaction, the SiO2-NHS and SiO2-(Trp)n-NHS carriers were washed with dry DMSO (5x) to remove excess reagents.
A 10 mg/mL solution of CALB was prepared with cold (4°C) 0.1 M phosphate buffered saline (PBS, 150 mM NaCl) at pH 6.5. Each of the NHS-activated silica carriers was then resuspended in 200 μL of DMSO, and 1 mL of cold CALB enzyme solution (10 mg CALB) was added to each tube. The tubes were quickly vortexed to disperse the silica, and then incubated for 24 hours at 4°C with rotation. These conditions decrease the rate of hydrolysis of the NHS esters (Hermanson, 1996), and favor the covalent attachment of the N-terminal amino group of the protein instead of the ε-amino group of lysine residues (Sélo, et al., 1996). After 24 hours, 1.2 mmole of ethanolamine was added to the SiO2-CALB (1) and SiO2-(Trp)n-CALB (2) conjugates to cap any remaining NHS esters, and further incubated at 37°C for four hours. The silica-enzyme conjugates were washed six times with 0.1M PBS (pH 7.4) to remove excess ethanolamine and loosely bound enzyme, then stored in the same buffer at 4°C until used for hydrolytic or synthetic activity measurement. A linker-only control was produced by repeating the above procedure for synthesis of SiO2-(Trp)n-NHS. Instead of enzyme solution, an excess of ethanolamine in dry DMSO was added to cap the terminal NHS esters on the linkers. The resulting enzyme-free SiO2-(Trp)n conjugate (3) was also tested for hydrolytic and synthetic activity.
Thermogravimetric Analysis (TGA) of Silica Conjugates
The total mass of immobilized molecules on the silica surface was determined by thermogravimetric analysis. Samples were washed extensively with appropriate solvent (e.g. buffer or DMSO) to remove weakly-bound molecules, then with distilled water to remove salts. Excess water was removed by washing and storage in dry ethanol. Aliquots containing 10-15 mg of solids in ethanol were loaded directly into the ceramic pan of the TGA instrument (TA Instruments model 2950-HR, New Castle, DE). Excess ethanol was removed by heating at 50°C for 10 min, and adsorbed water was removed at 110°C (60 min, to constant mass corresponding to 100% dry weight). The temperature was then increased by 5°C/min to 700°C, and then held for 90 minutes to reach constant mass. Compressed air (breathing grade) was used for the purge gas to provide complete oxidation and combustion of the surface coating.
Lipase Hydrolytic Activity Assay
The hydrolytic (aqueous) activity of free and immobilized lipase was determined by a modification of the method of Jain, et al. (2005). A substrate mixture of 1.0 mM p-NPP (added as 0.050 M p-NPP in dry CH3CN) was prepared in PBS with 1% (v/v) Triton™ X-100. This substrate mixture was briefly heated to 60°C to dissolve the p-NPP, and then cooled to 37°C before use.
For the free enzyme, 500 μL of substrate solution was added to 500 μL of CALB (0.1 mg/mL in 0.1 M PBS, pH 7.0) at 37°C. A blank was prepared with enzyme-free buffer; a 100% standard was made by substituting p-nitrophenol for the p-NPP substrate. After rotating the samples for 7.5 minutes at 37°C, the absorbance of the liberated yellow p-nitrophenolate ion at 405 nm was measured.
For immobilized enzymes, silica-protein conjugates (30 mg) were suspended in 0.1 M PBS (pH 7.0) to a total liquid volume of 500 μL. The tubes were warmed to 37°C, and the reaction initiated by addition of 500 μL p-NPP substrate. After sufficient time for color development (5-10 min), the tubes were centrifuged for 30 seconds at 14,000xg, and the absorbance of the supernatants at 405 nm was measured. The immobilized enzymes were then washed with PBS to remove excess p-NPP and surfactant and stored in PBS at 4°C. The enzyme specific activity in both cases was computed as μmoles of p-NP– liberated per mg of enzyme protein per minute.
Lipase Synthesis Activity Assay
The non-aqueous synthetic activity of the immobilized enzymes was evaluated by the esterification of n-propanol and lauric acid to propyl laurate, by a modification of a standard analytical method (Anonymous, 2001). Samples (~200mg) of silica-protein conjugates in cold 0.1 M PBS (pH 7.0) were centrifuged in 4 mL glass vials fitted with PTFE/silicone/PTFE septa, and the excess buffer was removed. Additional buffer was added to make the total liquid volume to 150 μL (5% v/v); this relatively high water concentration was necessary to produce a stirrable slurry of the microspheres. A magnetic stir bar and 10 mmole of lauric acid flakes were added to each vial, and the contents shaken to disperse the silica on the solid acid. The reaction was started by adding 10 mmole of n-propanol to each vial, after which the vials were securely capped and immersed in a circulating water bath at 60°C for 1 hour, with vigorous magnetic stirring to keep the silica carriers suspended.
Quantitative 1H NMR was used to determine the extent of the organic synthesis reaction (Griffiths and Irving, 1998; Weber, et al., 2002; Maiwald, et al., 2004). At the end of the assay period, silicone-free hypodermic syringes (Foresti and Ferreira, 2005) were used to dilute 100 μL samples from the reaction vials with 0.9 mL CDCl3 containing 0.5% TMS. The diluted samples were injected into 1.5 mL microcentrifuge tubes, centrifuged at 14,000xg for 60 seconds, and the supernatant transferred to NMR tubes. The 1H-NMR spectra of the samples were recorded on a 300 MHz Bruker AC-300 spectrometer. The conversion was calculated from the ratio of the integrals of the propyl ester methylene peak (δ ≈ 4.03 ppm) and of the alcohol methylene peak at δ ≈ 3.62 ppm. The specific synthetic activity was calculated as μmoles of propyl laurate formed per milligram of enzyme protein per minute.
Following completion of the reaction, the carriers were washed several times with n-propanol to remove excess lauric acid and propyl laurate, then with PBS (pH 7.0) to remove the organic solvent and allow rehydration of the enzyme. The washed silica-enzyme conjugates were kept in buffer at 4°C between assays, and washed with fresh PBS before each successive trial. To avoid confounding effects of changes in tether hydrophobicity caused by surfactants, the X-100-contacted silica from the hydrolysis assays was never used for synthesis reactions.
Total Protein (BCA) Assay of Storage Buffers
Unused silica-enzyme conjugates were stored for three months in initially protein-free PBS at 4°C. These samples were centrifuged at 14,000g for two minutes, and the total protein concentration of the supernatant was analyzed by the BCA method (Smith, e al., 1985) using a microplate assay kit (Pierce, Rockford, IL). As CALB exhibits a lesser response in this assay than bovine serum albumin (BSA; data not shown), standards curves were made using dialyzed CALB diluted in PBS instead of BSA.
Surfactant Desorption of Lipase
To further investigate the role of adsorbed lipase on the hydrophobic (Trp)n tethers, an additional SiO2-(Trp)n treatment was prepared as previously described. The terminal –COOH groups of the tethers on a portion of this (Trp)n-modified silica were activated as before with EDC/NHS in DMSO, while an identical sample was treated only with DMSO (i.e. no activation). After incubation with the lipase solution, a portion of the silica that had been activated with EDC/NHS was further subjected to two washes with 1% sodium dodecyl sulfate (SDS) in PBS (pH 7.4). All three samples were washed extensively with PBS to remove weakly-bound protein or surfactants. The hydrolytic activity against p-NPP in the presence of 0.5% X-100 was then measured over several cycles. The catalysts were washed five times with PBS after each run to remove the reactants and any desorbed protein.
RESULTS AND DISCUSSION
Effects of Hydrophobic (Trp) n Tethers on Silica Suspensions
Conjugation of the hydrophobic (Trp)n linkers to the silica microspheres changes the behavior of suspensions of the particles. Although the number of (Trp)n molecules was limited to prevent the creation of a true close-packed monolayer, hydrophobic interactions in the form of flocculation and aggregation of the (Trp)n-coated silica was observed in aqueous buffers. Thin layers of the hydrophobic silica particles also tended to form at air-water and solid-water interfaces. The SiO2-(Trp)n carriers were more readily dispersed and formed more stable suspensions in organic solvents than the untreated silica. The SiO2-(Trp)n conjugates were also more easily dispersed in aqueous buffer after exposure to X-100 in the hydrolytic assay, suggesting that the hydrophobic tethers were coated with the surfactant molecules.
Clumping of enzymes in organic phase reactions is known to limit the rate and extent of reaction by hindering mass transport to the immobilized enzymes (Pencreac’h and Baratti, 1996; Foresti, et al., 2005). Although the presence of polytryptophan tethers on silica allow it to be dispersed rapidly in the organic medium of the synthesis assay, the SiO2-CALB conjugate was also sufficiently lipophilic to be mostly dispersed after a short time by vigorous magnetic stirring. While the increased wetting afforded by the (Trp)n tethers certainly improves the dispersal of catalyst in organic systems, this macroscopic phenomenon may not completely explain the marked increase in activity exhibited by SiO2-(Trp)n-CALB.
Immobilization of (Trp)n Tethers and Lipase on Functionalized Silica
Non-porous silica microspheres were chosen to maximize surface area while avoiding pore diffusion and size exclusion effects. The EDC/NHS conjugation chemistry was found to be successful in a non-aqueous system; the use of anhydrous solvents allows the conjugation of otherwise insoluble hydrophobic molecules such as (Trp)n, and eliminates the competing hydrolytic side reactions that occur in aqueous systems.
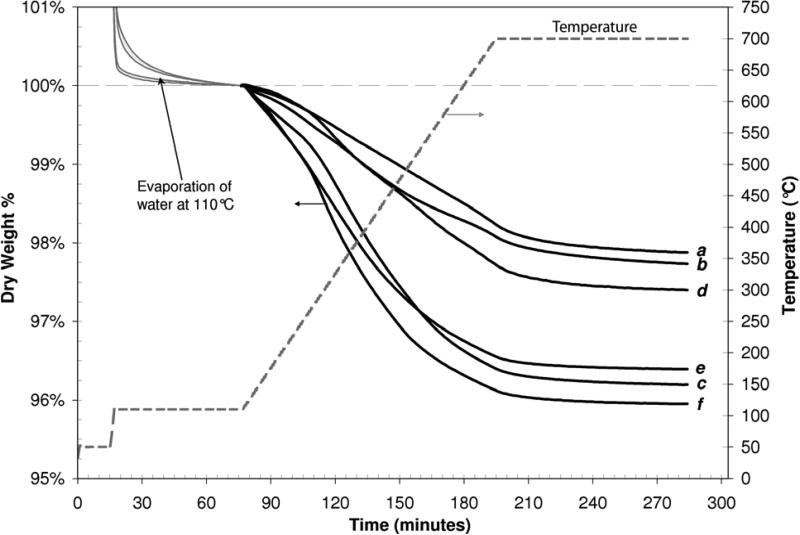
Thermogravimetric analysis has been used to quantify various immobilized groups on silica (Cestari and Airoldi, 1995; Maitra, et al., 2003). The TGA of SiO2-CALB and SiO2-(Trp)n-CALB samples reproducibly quantified the increase in immobilized mass as successive groups were linked to the silica carriers (Figure 2). The total immobilized protein (Table I) on the silica carriers was computed using the molecular weight of the (Trp)n provided by Sigma-Aldrich, and the purified CALB enzyme as determined by MALDI (ABI 4700 Proteomics Analyzer). The amount of lipase on SiO2-CALB was determined from the difference between Figure 2e (dry wt% lost by SiO2-CALB at 700°C) and Figure 2b (the measured baseline wt% lost by the SiO2-COOH carriers alone); the total immobilized protein per gram of carriers was then calculated from the known dry weight of each sample. A similar calculation was used to determine the mass of (Trp)n tethers (difference between Figures 2d and b), and the mass of lipase immobilized on the tethers (Figures 2f and b) on SiO2-(Trp)n-CALB.
Figure 2.
Thermogravimetric analysis of immobilized mass on non-porous silica microspheres. Curves shown are representative of a) SiO2-NH2, b) SiO2-COOH, c) SiO2-(Trp)n/COOH, d) SiO2-(Trp)n/EtOH (3), e) SiO2-CALB (1), and f) SiO2-(Trp)n-CALB (2).
Table I.
Immobilized mass, synthetic and hydrolytic activities of free, surface-immobilized and (Trp)n-tethered Candida antarctica lipase B. Enzyme activities are normalized to the mass of enzyme protein.
| Enzyme Treatment | Immobilized Protein (mg/g silica) | Hydrolytic Activitya (μ mole/min·mg CALB) | Synthetic Activityb |
|---|---|---|---|
| Free CALB | $ | 335 ± 4 | $ |
| SiO2-(Trp)n | 3.2 mg (Trp)n/g | $ | $ |
| SiO2-CALB | 12.7 mg CALB/g | 21 ± 0.4 | 1 |
| SiO2-(Trp)n-CALB | 14.3 mg CALB/g | 102 ± 10.9 | 35 |
0.5 mM p-nitrophenol palmitate in PBS with 0.5% (v/v) Triton™ X-100, pH 7.0, 37°C (n = 2).
10 mmole each of n-propanol and lauric acid with 5% (v/v) PBS (pH 7.0), 60°C, 1 hr.
An unexpected but repeatable decrease in the total immobilized mass was observed after the ethanolamine capping of remaining surface NHS-esters on the (Trp)n–modified silica (Figures 2d and c). We attribute this mass loss to desorption of weakly bound (Trp)n during the non-aqueous capping and subsequent washing steps, and used the TGA mass loss of the ethanolamine-capped silica (SiO2-EtOH/(Trp)n), Figure 2d) to determine the amount of (Trp)n immobilized on the surface (3.2 mg tethers/g silica). These data indicate that four enzymes were immobilized for each tether molecule, which is clearly not possible if all of the lipase was covalently linked at the C-terminus of the tethers.
The number of molecules that can be covalently immobilized at or near the silica surface is theoretically limited to less than a close-packed monolayer. Unlike self-assembly processes that can create highly ordered dense films by surface diffusion, covalently linked proteins are unable to redistribute themselves once attached to the surface. Attachment of a protein to the surface sterically hinders access to neighboring attachment points, making efficient packing unlikely. However, when long, mobile end-activated linkers are present, the volume exclusion effect is lessened, and multiple points of attachment could be formed. This “multipoint attachment” is believed to increase the stability of enzymes in aqueous systems (Guisán, et al., 1997); it is reasonable to assume that a similar effect would also occur in non-polar solvents.
The amount of (Trp)n presented for immobilization was limited in order to produce a sparse brush. Proteins typically do not penetrate dense brushes of polymers such as PEO, due to the unfavorable thermodynamic effect of compressing the brush (Jeon, et al., 1991). However, Lipases are known to adsorb strongly at hydrophobic interfaces (Palomo et al., 2002; Foresti, et al., 2005), and the calculated surface density of the tethers (ca. 20 nm2/tether) is similar to the footprint of the lipase molecule (ca. 18 nm2/lipase). The strongly hydrophobic sparse tethers should facilitate the adsorption of lipase into the brush layer from the aqueous solution.
Total protein assays of the silica-enzyme storage buffers indicated that almost no protein was desorbed from the SiO2-CALB or SiO2-(Trp)n treatments. In contrast, small amounts of protein were found in the SiO2-(Trp)n-CALB storage buffer. When tested with p-NPP, this buffer exhibited a very slight hydrolytic activity (data not shown). These results support the adsorption of lipase onto the (Trp)n-modified silica.
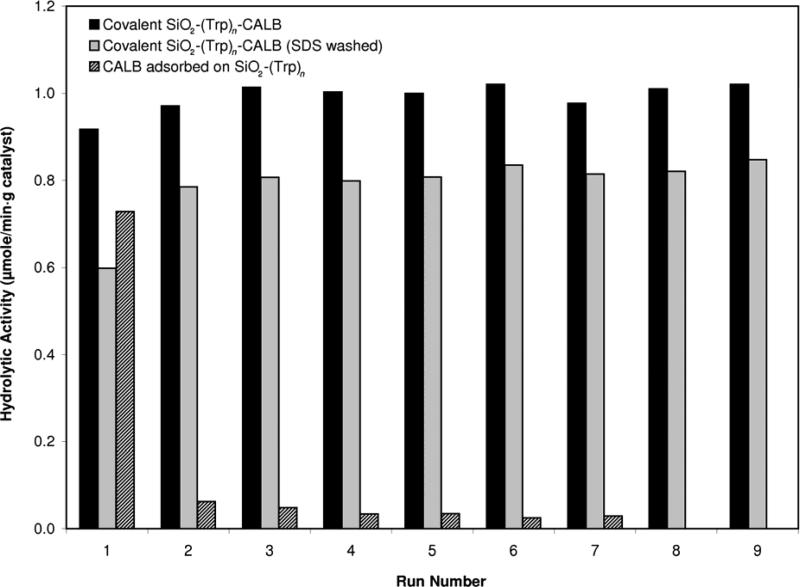
Initially, the lipase adsorbed on the non-activated tethers had similar activity to the covalently-linked samples, but rapidly dropped to nearly negligible levels (Figure 3). This indicated that the lipase did adsorb on the (Trp)n-coated silica, but was easily displaced by a weak surfactant. In contrast, both covalently-linked treatments maintained a constant level of activity over nine cycles under the same conditions. This suggests that the majority of the active lipase in these samples was covalently linked, and not merely adsorbed. As expected, exposure to SDS had a negative effect on the activity of the lipase, probably due to partial denaturation or desorption of enzyme by the surfactant (Creigthon, 1993).
Figure 3.
Aqueous p-NPP hydrolysis activity of adsorbed (striped bars) and covalently-bound lipase on (trp)n-modified silica, during repeated reactions in the presence of 0.5% (v/v) Triton™ X-100. Prior to the first use, the covalent treatments were washed with 1% SDS in PBS (gray bars), or with PBS only (black bars). All samples were washed with PBS between runs.
These results show that lipophilic enzymes are adsorbed onto the relatively flexible (Trp)n tethers during the aqueous immobilization step. Once entrapped in the sparse brush, multiple hydrophobic associations between the end-activated tethers and the enzyme surface would stabilize the enzyme in a highly active form, until covalent linkages could be formed. This is consistent with the protein elutability observed for adsorbed lipase and the similar activity of all treatments in Figure 3, but does not explain the high enzyme:tether ratio. In 2004, Chiou and Wu described the previously undocumented activation of hydroxyl groups by EDC, leading to formation of an activated species that is chemically similar to the o-acylisourea formed with carboxyl groups. These authors found that large amounts of Candida rugosa lipase could be stably immobilized on chitosan hydroxyls by this mechanism. The surface of the (Trp)n-modified silica in the present study was capped with hydroxyl groups, then exposed to high concentrations of EDC and NHS. The adsorbed lipase could then be covalently linked to the surface as well as the activated ends of the tethers, producing the observed excess of lipase immobilized on the SiO2-(Trp)n carriers.
Effects of Hydrophobic (Trp)n Tethers on Activity of Immobilized Lipase
The surface-immobilized enzyme retained only 6% of the specific hydrolytic activity of the free enzyme. The presence of hydrophobic (Trp)n tethers increased both the hydrolytic and synthetic activity of CALB, when compared to the surface-immobilized enzyme (Table I). In aqueous solution, the specific p-NPP hydrolysis rate of the (Trp)n-tethered CALB enzyme was five times greater than the surface-immobilized lipase. The observed hydrolytic activity indicates that the immobilized lipase maintains some activity under both immobilization treatments.
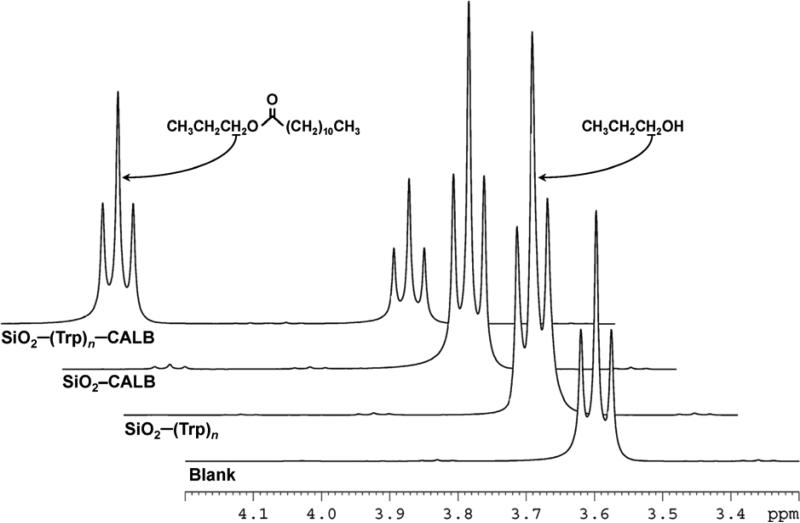
Figure 4 shows the 1H NMR spectra for the first of a series of propyl laurate esterification reactions in which the catalyst was washed and reused (Figure 5, discussed below). As npropanol is esterified, the peak associated with the alcoholic methylene (CH3CH2CH2OR, R=H or laurate) shifts downfield. The specific activity of the immobilized enzyme was calculated from the ratio of the integrated areas of these well-resolved triplet peaks. The (Trp)n-tethered lipase exhibited greater esterification activity in each of the organic synthesis assays, with an initial activity 35 times higher than SiO2-CALB (Table I). This increase in activity of the (Trp)ntethered lipase cannot be specifically attributed to the presence of the polytryptophan, as no substantial hydrolytic or synthetic activity was observed for silica modified only with (Trp)n.
Figure 4.
1H NMR spectra of the first run in a series of propyl laurate synthesis assays. Ester synthesis activity is computed from the ratio of the integrated areas of the propyl laurate (δ ≈ 4.03 ppm) and n-propanol (δ ≈ 3.61 ppm) methylene peaks.
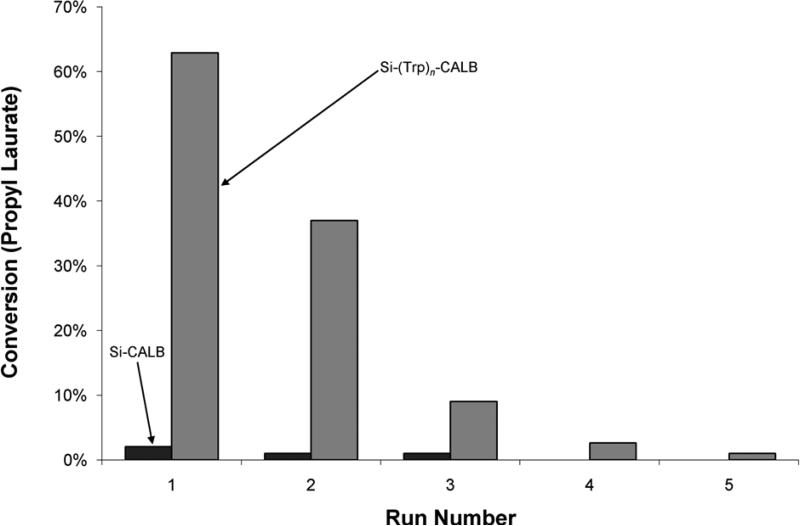
Figure 5.
Propyl laurate synthesis by Candida antarctica lipase B immobilized at the silica surface (dark gray) and with poly-DL-tryptophan tethers (light gray) during repeated reactions with the same catalyst (washed with n-propanol and PBS between runs).
For comparison, the activity of Novozym™ 435 (CALB adsorbed on a high surface-area macroporous PMMA) was found to be 5,000 units/g of catalyst under the same reaction conditions, while the SiO2-(Trp)n-CALB preparation exhibited an activity of 440 units/g of silica. While Mei, et al. (2003) have shown that the enzyme loading of Novozym 435 is limited to the outer 80-100 μm of the particle surface, this still affords an immobilization surface area that is much larger than that of the non-porous microspheres. The actual mass loading of commercial Novozym 435 is not specified, although Chen, et al. (2007) observed loadings of ca. 8 wt% for CALB adsorption on similar PMMA resins. In comparison, the non-porous SiO2-(Trp)n-CALB treatment has approximately 1.4 wt% of CALB.
Because the surface beneath the tethers is terminated with hydroxyl groups, it is reasonable to postulate the existence of a thin layer of water at the silica surface. This layer of water would be maintained throughout the post-immobilization washing and in the synthetic assays, and could partially stabilize the proteins by forming an organic-aqueous interface at the base of the brush layer. A water film surrounding the surface-immobilized enzymes could also produce an artificially low substrate concentration in the microenvironment of the immobilized lipase because of partitioning effects of hydrophobic lauric acid and propyl laurate molecules (Mora-Pale, et al., 2007). This effect could be mitigated by the presence of the (Trp)n tethers, perhaps by interrupting the water film, or adsorption of the substrate/product molecules on the hydrophobic polypeptides.
Similar amounts of enzyme were present on both silica-enzyme conjugates, and both the surface-immobilized and tethered lipase exhibited hydrolytic activity. The increased synthetic activity is presumably due to increased solvation in the organic phase rather than an inactivation of the surface-immobilized lipase. The hydrophobic indole side-chains of the polytryptophan linker are expected to be solvated in an organic medium, and the lipase would be extended toward the bulk phase, thus avoiding the surface effects discussed earlier. In the aqueous phase assay, the (Trp)n linkers appear to be coated with the X-100 surfactant, allowing the linkers to remain somewhat solvated and extended despite their high hydrophobicity. The bulky randomly-oriented side-chains of the tryptophan residues would also be expected to help to prevent the collapse of the random coil into a compact structure on the surface, even in unfavorable solvents.
Stability of Immobilized Lipase Upon Repeated Reuse
As discussed above, the covalently-immobilized enzymes were quite stable during hydrolytic reactions (Figure 3). However, repeated experiments using the same catalyst in organic synthesis reactions showed a decrease in activity with each re-use of the silica-enzyme preparations (Figure 5). This is not unprecedented for organic syntheses; for example, Mahmoudian (2007) considered a loss of 50% activity within nine cycles by commercial adsorbed CALB (Novozym™ 435) to be “excellent stability”. While the activity of the (Trp)n-tethered lipase was initially very high, both treatments were essentially inactivated (i.e. achieved < 1% conversion) by the fifth experiment. To further investigate the reasons for this deactivation, the same experiment was repeated with fresh catalyst, and the first run was extended to seven hours at 60°C. After this incubation period, the conversion by the SiO2-(Trp)n-CALB had increased to 85%, while the SiO2-CALB achieved 3% conversion. However, when these 7-hour catalysts were reused, conversion by the SiO2-(Trp)n-CALB decreased to less than 3%, and the SiO2-CALB exhibited less than 1% conversion. Essentially no activity was observed in subsequent assays with either catalyst. Repeated reactions using Novozym™ 435 also showed a large loss of activity with each successive reuse of the catalyst under the same experimental conditions (data not shown).
Although desorption of lipase entrapped in the brush layer could be responsible for the observed deactivation of the SiO2-(Trp)n-CALB catalyst, the immobilized mass on the inactivated catalyst did not decrease appreciably from that of the unused catalyst after five reactions (data not shown). The tether-immobilized lipase appears to be stably linked and loss of enzyme by desorption does not explain the inactivation of the catalyst in the synthesis reaction. The catalyst deactivation was ascribed to thermal denaturation caused by the high temperature (60°C) and water content (5% v/v) of this assay. The water activity, aw, of the system is well-known to strongly influence the non-aqueous reaction rates of lipases and other enzymes by changing the reaction equilibrium and hydration state of the enzyme and support (Wehjte, et al., 1997; Ma, et al., 2002; Mora-Pale, et al., 2007). The effects of even small changes in water content, while critically important to enzyme activity at relatively low aw, are of less consequence in a water-saturated system as in this study.
As the esterification reaction proceeds, it releases water and further increases the water concentration. At moderate conversions, the mixture became a biphasic emulsion (water was present above saturated levels) in which (Trp)n-lipase preparations were well dispersed. At high conversions, the excess of water may shift the reaction equilibrium and decrease the forward reaction rate, as water is a reactant in the reverse hydrolytic reaction. Increased water could also cause partitioning effects with hydrophobic substrates and products. Even with these effects, the extent of reactions catalyzed by the CALB immobilized with (Trp)n tethers was much greater than that of the surface immobilized lipase.
Although a number of researchers have used CALB in anhydrous solvents at temperatures up to 70°C (Mahapatro, et al., 2003; Persson, et al., 1999), others reported rapid inactivation of immobilized CALB at 50°C in the presence of bulk water (e.g. Palomo, et al., 2002). The synthesis assay used in this work is based on a method that is used to assess the specific activity of commercial immobilized lipase products, but it was found unsuitable for repeated experiments with long reaction times.
CONCLUSIONS
The present study describes the novel use of the polypeptide poly-DL-tryptophan, (Trp)n, as a long-chain hydrophobic tether for Candida antarctica lipase B immobilized on non-porous silica microspheres. The specific activity of CALB-catalyzed hydrolysis and organic synthesis reactions was substantially increased by the hydrophobic (Trp)n tethers, although the tethers alone do not measurably catalyze either reaction. In addition, the presence of (Trp)n on the carrier surface improved the wetting and dispersion of the silica carriers in organic solvents. The increased activity is probably due to reduced clumping as well as improved solvent accessibility and mobility of the tethered lipase molecules. Inactivation of the lipase during the synthesis assay was attributed to the high temperature and water content of the reaction. Even with this inactivation, the (Trp)n-tethered enzyme retained considerably higher synthetic activity than the surface-immobilized control over several cycles of reuse.
Currently, we are working to address the role of hydrophobic tether conformation (e.g. random coil vs. α-helix), composition and length on enzyme activation in organic solvents. Future work should investigate the effect of hydrophobic tethers on the activity and stability of other enzymes (particularly those with interfacial activation) and other organic reactions should be investigated. The absolute surface hydrophobicity, effect of water activity, and adsorption isotherms of water and organic solvents on the (Trp)n-modified carriers should also be examined. Comparison of these results with those of commercial enzyme preparations may help to clarify the mechanism of the observed increase in enzyme activity on (Trp)n-silica. The current study indicates that the use of hydrophobic polypeptide tethers to immobilize enzymes for non-aqueous synthesis could provide appreciable improvements in the manufacture of pharmaceutical intermediates, functional food ingredients, biofuels, and fine chemicals.
ACKNOWLEDGMENTS
The authors wish to thank Novozymes North America for graciously providing the CALB enzyme used in this study. We also thank Dr. Joe McGuire and Kelsey Yee for many invaluable suggestions. This publication was made possible, in part, by the Mass Spectrometry Facilities and Services Core of the Environmental Health Sciences Center, Oregon State University, grant number P30 ES00210, National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
BRIEFS. Immobilization of Candida antarctica lipase B on silica microspheres with an intervening hydrophobic poly-DL-tryptophan linker increased the hydrolytic (aqueous) and synthetic (non-aqueous) activity of the enzyme, when compared to the same lipase immobilized without tethers.
References
- Anonymous Lipase activity based on ester synthesis – PLU, propyl-laurate synthesis. [Oct. 2001];Novozymes Analytical Method F-9600369. 2001 [Google Scholar]
- Bommarius AS, Riebel BR. Biocatalysis: Fundamentals and Applications. Wiley-VCH Verlag; Weinheim, GmbH: 2004. pp. 339–372. [Google Scholar]
- Cao L. Carrier-bound immobilized enzymes. Wiley-VCH Verlag; Weinheim, GmbH: 2005. pp. 169–316. [Google Scholar]
- Cestari AR, Airoldi C. A new elemental analysis method based on thermogravimetric data and applied to alkoxysilane immobilized on silicas. J Therm Anal Calorim. 1995;44(1):77–81. [Google Scholar]
- Chen B, Miller ME, Gross RA. Effects of porous polystyrene resin parameters on Candida antarctica lipase B adsorption, distribution, and polyester synthesis activity. Langmuir. 2007;23(11):6467–6474. doi: 10.1021/la063515y. [DOI] [PubMed] [Google Scholar]
- Chiou S-H, Wu W-T. Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials. 2004;25(2):197–204. doi: 10.1016/s0142-9612(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Chipot C, Pohorille A. Folding and translocation of the undecamer of poly-L-leucine across the water-hexane interface: a molecular dynamics study. J Am Chem Soc. 1998;120(46):11912–11924. doi: 10.1021/ja980010o. [DOI] [PubMed] [Google Scholar]
- Creigthon TE. Proteins: Structures and molecular properties. 2nd ed. W. H. Freeman; New York: 1993. p. 26. [Google Scholar]
- Dreyer S, Lembrecht J, Schumacher J, Kragl U. Enzyme catalysis in nonaqueous media: past, present and future. In: Patel RN, editor. Biocatalysis in the pharmaceutical and biotechnology industries. CRC Press; Boca Raton: 2007. pp. 791–827. [Google Scholar]
- Efremov RG, Nolde DE, Vergoten G, Arseniev AS. A solvent model for simulations of peptides in bilayers. I. Membrane-promoting α-helix formation. Biophys J. 1999;76(5):2448–2459. doi: 10.1016/S0006-3495(99)77400-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti ML, Alimenti GA, Ferreira ML. Interfacial activation and bioimprinting of Candida rugosa lipase immobilized on polypropylene: effect on the enzymatic activity in solvent-free ethyl oleate synthesis. Enzyme Microb Technol. 2005;36(2-3):338–349. [Google Scholar]
- Foresti ML, Errazu A, Ferreira ML. Effect of several reaction parameters in the solvent-free ethyl oleate synthesis using Candida rugosa lipase immobilised on polypropylene. Biochem Eng J. 2005;25(1):69–77. [Google Scholar]
- Foresti ML, Ferreira ML. Frequent analytical/experimental problems in lipase-mediated synthesis in solvent-free systems and how to avoid them. Anal Bioanal Chem. 2005;381(7):1408–1425. doi: 10.1007/s00216-005-3087-6. [DOI] [PubMed] [Google Scholar]
- Griffiths L, Irving AM. Assay by nuclear magnetic resonance spectroscopy: quantification limits. Analyst. 1998;123(5):1061–1068. [Google Scholar]
- Guisán JM, Bastida A, Blanco RM, Fernández-Lafuente R, García-Junceda E. Immobilization of enzymes on glyoxyl agarose: Strategies for enzyme stabilization by multipoint attachment. In: Bickerstaff GF, editor. Methods in biotechnology, Vol. 1: Immobilization of enzymes and cells. Humana Press; Totowa: 1997. pp. 277–288. [Google Scholar]
- Halling P, Kvittingen L. Why did biocatalysis in organic media not take off in the 1930s? Trends Biotechnol. 1999;17(9):343–344. doi: 10.1016/s0167-7799(99)01331-1. [DOI] [PubMed] [Google Scholar]
- Hermanson GT. Bioconjugate techniques. Academic Press; San Diego: 1996. pp. 139–140.pp. 175–176. [Google Scholar]
- Hesselink FT, Scheraga HA. On the possible existence of ±-helical structures of regular-sequence D,L copolymers of amino acids: conformational energy calculations. Macromolecules. 1972;5(4):455–463. [Google Scholar]
- Hudson EP, Eppler RK, Clark DS. Biocatalysis in semi-aqueous and nearly anhydrous conditions. Curr Opin Biotechnol. 2005;16(6):637–643. doi: 10.1016/j.copbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Jain P, Jain S, Gupta MN. A microwave-assisted microassay for lipases. Anal Bioanal Chem. 2005;381(7):1480–1482. doi: 10.1007/s00216-005-3105-8. [DOI] [PubMed] [Google Scholar]
- Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide: I. Simplified theory. J Colloid Interf Sci. 1991;142(1):149–166. [Google Scholar]
- Karplus PA. Hydrophobicity regained. Prot Sci. 1997;6(6):1302–1307. doi: 10.1002/pro.5560060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov AM. Improving enzymes by using them in organic solvents. Nature. 2001;409(6817):241–246. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- Kovalchuk T, Sfihi H, Kostenko L, Zaitsev V, Fraissard J. Preparation, structure and thermal stability of onium- and amino-functionalized silicas for the use as catalysts supports. J Colloid Interf Sci. 2006;302(1):214–229. doi: 10.1016/j.jcis.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Krause E, Bienert M, Schmieder P, Wenschuh H. The helix-destabilizing propensity scale of D-amino acids: the influence of side chain steric effects. J Am Chem Soc. 2000;122(20):4865–4870. [Google Scholar]
- Lee M-Y, Dordick JS. Enzyme activation for nonaqueuous media. Curr Opin Biotechnol. 2002;13(4):376–384. doi: 10.1016/s0958-1669(02)00337-3. [DOI] [PubMed] [Google Scholar]
- Ma L, Persson M, Adlercreutz P. Water activity dependence of lipase catalysis in organic media explains successful transesterification reactions. Enzym Microb Technol. 2002;31(7):1024–1029. [Google Scholar]
- Mahapatro A, Kalra B, Kumar A, Gross RA. Lipase-catalyzed polycondensations: effect of substrates and solvent on chain formation, dispersity and end-group structure. Biomacromolecules. 2003;4(3):544–551. doi: 10.1021/bm0257208. [DOI] [PubMed] [Google Scholar]
- Mahmoudian M. A decade of biocatalysis at Glaxo Wellcome. In: Patel RN, editor. Biocatalysis in the pharmaceutical and biotechnology industries. CRC Press; Boca Raton: 2007. pp. 53–102. [Google Scholar]
- Maitra P, Ding J, Huang H, Wunder SL. Poly(ethylene oxide) silananted nanosize fumed silica: DSC and TGA characterization of the surface. Langmuir. 2003;19(21):8994–9004. [Google Scholar]
- Maiwald M, Fischer HH, Kim Y-K, Albert K, Hasse H. Quantitative high-resolution on-line NMR spectroscopy in reaction and process monitoring. J Magn Reson. 2004;166(2):135–146. doi: 10.1016/j.jmr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Mei Y, Miller L, Gao W, Gross RA. Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules. 2003;4(1):70–74. doi: 10.1021/bm025611t. [DOI] [PubMed] [Google Scholar]
- Mora-Pale JM, Pérez-Munguía S, González-Mejía JC, Dordick JS, Bárzana E. The lipase-catalyzed hydrolysis of lutein diesters in non-aqueous media is favored at extremely low water activities. Biotechnol Bioeng. 2007;98(3):535–542. doi: 10.1002/bit.21417. [DOI] [PubMed] [Google Scholar]
- Nguyen HD, Marchut AJ, Hall CK. Solvent effects on the conformational transition of a model polyalanine peptide. Prot Sci. 2004;13(11):2909–2924. doi: 10.1110/ps.04701304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norde W. Adsorption of proteins from solution at the solid-liquid interface. Adv Colloid Interf Sci. 1986;25:267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- Palomo JM, Muñoz G, Fernández-Lorente G, Mateo C, Fernández-Lafuente R, Guisán JM. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): immobilization, hyperactivation and stabilization of the open form of lipases. J Mol Catal B Enzym. 2002;19-20(1):279–286. [Google Scholar]
- Pencreac'h G, Baratti JC. Hydrolysis of p-nitrophenyl palmitate in n-heptane by the Pseudomanas cepacia lipase: a simple test for the determination of lipase activity in organic media. Enzyme Microb Technol. 1996;18(6):417–422. [Google Scholar]
- Persson BA, Huerta FF, Bäckvall J-E. Dynamic kinetic resolution of secondary diols via coupled ruthenium and enzyme catalysis. J Org Chem. 1999;64(14):5237–5240. doi: 10.1021/jo990447u. [DOI] [PubMed] [Google Scholar]
- Petkar M, Lali A, Caimi P, Daminati M. Immobilization of lipases for non-aqueous synthesis. J Mol Catal B Enzym. 2006;39(1):83–90. [Google Scholar]
- Ru MT, Wu KC, Lindsay JP, Dordick JS, Reimer JA, Clark DS. Towards more active biocatalysts in organic media: increasing the activity of salt-activated enzymes. Biotechnol Bioeng. 2001;75(2):187–196. doi: 10.1002/bit.1178. [DOI] [PubMed] [Google Scholar]
- Sélo I, Négroni L, Créminon C, Grassi J, Wal JM. Preferential labeling of α-amino N-terminal groups in peptides by biotin: application to the detection of specific anti-peptide antibodies by enzyme immunoassays. J Immunol Methods. 1996;199(2):127–138. doi: 10.1016/s0022-1759(96)00173-1. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stark M-J, Holmberg K. Covalent immobilization of lipase in organic solvents. Biotechnol Bioeng. 1989;34(7):942–950. doi: 10.1002/bit.260340709. [DOI] [PubMed] [Google Scholar]
- Weber H, Brecker L, de Souza D, Griengl H, Ribbons DW, Weber HK. Online NMR for monitoring biocatalysed reactions – the use of lipases in organic solvents. J Mol Catal B: Enzym. 2002;19-20(1):149–157. [Google Scholar]
- Wehtje E, Costes D, Adlercreutz P. Enantioselectivity of lipases: Effects of water activity. J Mol Catal B Enzym. 1997;3(5):221–230. [Google Scholar]
- Zaks A, Klibanov AM. Enzyme-catalyzed processes in organic solvents. Proc Natl Acad Sci USA. 1985;82(10):3192–3196. doi: 10.1073/pnas.82.10.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]