Abstract
Background
Patients on in-center nocturnal hemodialysis therapy typically experience higher interdialytic weight gain (IDWG) than patients on conventional hemodialysis therapy. We determined the safety and effects of decreasing dialysate sodium concentration on IDWG and blood pressure in patients on thrice-weekly in-center nocturnal hemodialysis therapy.
Study Design
Quality improvement, pre-post intervention.
Settings & Participants
15 participants in a single facility.
Quality Improvement Plan
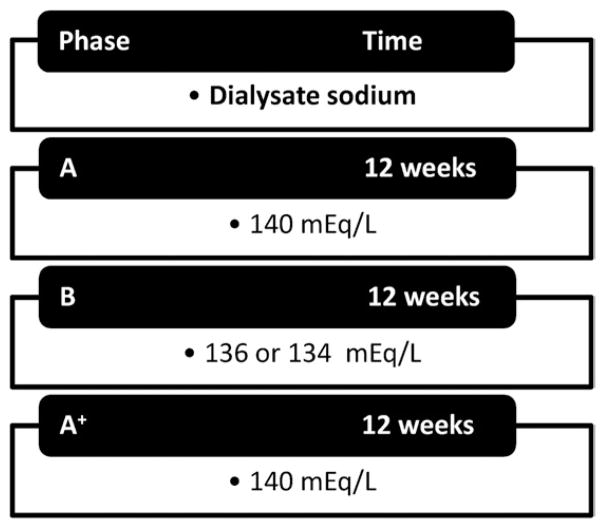
Participants underwent three 12-week treatment phases, each with different dialysate sodium concentrations, as follows: phase A, 140 mEq/L; phase B, 136 or 134 mEq/L; and phase A+, 140 mEq/L. Participants were blinded to the exact timing of the intervention.
Outcomes
IDWG, IDWG/dry weight (IDWG%), and blood pressure.
Measurements
Outcome data were obtained during the last 2 weeks of each phase and compared with mixed models. The fraction of sessions with adverse events (eg, cramping and hypotension) also was reported.
Results
IDWG, IDWG%, and predialysis systolic blood pressure decreased significantly by 0.6 ± 0.6 kg, 0.6% ± 0.8%, and 8.3 ± 14.9 mm Hg, respectively, in phase B compared with phase A (P < 0.05 for all comparisons). No differences in predialysis diastolic and mean arterial or postdialysis blood pressures were found (P > 0.05 for all comparisons). The proportion of treatments with intradialytic hypotension was low and similar in each phase (P = 0.9). In phase B compared with phase A, predialysis plasma sodium concentration was unchanged (P > 0.05), whereas postdialysis plasma sodium concentration decreased by 3.7 ± 1.9 mEq/L (P < 0.05).
Limitations
Modest sample size.
Conclusion
Decreasing dialysate sodium concentrations in patients undergoing thrice-weekly in-center nocturnal hemodialysis resulted in a clinical and statistically significant decrease in IDWG, IDWG%, postdialysis plasma sodium concentration, and predialysis systolic blood pressure without increasing adverse events. Prolonged exposure to higher than required dialysate sodium concentrations may drive IDWG and counteract some of the purported benefits of “go-slow” (longer session length) hemodialysis.
INDEX WORDS: Nocturnal hemodialysis, interdialytic weight gain, sodium gradient, dialysate sodium, sodium set point, hypertension
Excessive fluid retention measured as higher absolute interdialytic weight gain (IDWG) or IDWG indexed by the estimated dry weight (IDWG%) is associated with increased morbidity1 and mortality.2–5 Fluid retention in patients on conventional hemodialysis therapy often is attributed to the inability to achieve target (dry) weight owing to the rapid ultrafiltration rates typically required during a 3- to 4-hour session length, complicated by intradialytic hypotension. Among the purported benefits of “go-slow” (extended session length) hemodialysis is the ability to provide lower ultrafiltration rates, in theory facilitating volume removal without associated intradialytic hypotension. Interestingly, patients undergoing nocturnal hemodialysis (typically prescribed for 6–8 hours) have been observed to upregulate IDWG over time6,7 to levels higher than those seen with conventional hemodialysis.8 The drifting upward of IDWG in patients on nocturnal hemodialysis therapy generally has been attributed to more liberal fluid intake in response to the greater capacity for ultrafiltration. In the cycle of IDWG and nocturnal hemodialysis, the “chicken and egg” question of which comes first has not been disentangled.
Volume overload in patients on hemodialysis therapy is caused primarily by excessive sodium intake. However, the importance of intradialytic sources of salt, such as higher than required dialysate sodium concentrations, has been underappreciated.9 Sodium is removed during hemodialysis by both diffusion and convection (ultrafiltration). The contribution of diffusion and the direction of sodium transfer depend on the difference between dialysate and plasma sodium concentrations, the so-called sodium gradient.10,11 If the sodium gradient is positive, plasma sodium concentration after hemodialysis generally will increase12 due to a diffusive flux of sodium to the patient.
The predialysis plasma sodium concentration trends toward hyponatremia13 and varies from patient to patient, but appears to be stable over time within patients.13–17 In the early years of maintenance hemodialysis therapy, dialysate solutions contained <130 mEq/L of sodium.17,18 With advances in dialytic technology, session lengths were shortened and dialysate sodium concentrations often were increased to ≥140 mEq/L,17,18 resulting in positive sodium gradients in most patients. Unintentionally, the increase in prescribed dialysate sodium concentration may have been triggering a vicious cycle, with larger IDWG due to thirst stimulation by higher postdialysis plasma sodium concentrations.12,16,17
Patients in our in-center nocturnal hemodialysis program typically were treated with dialysate sodium concentrations of 140 mEq/L, as were patients on conventional hemodialysis therapy. As others have reported, we noticed that IDWGs were drifting upward over time and consistently were higher, patient by patient, compared with each patient’s experience on conventional hemodialysis therapy. Because of concerns about the negative effects of higher dialysate sodium concentrations, we decided to institute a unit-wide quality improvement project in the 16 prevalent patients on in-center nocturnal hemodialysis therapy. We hypothesized that decreasing the dialysate sodium concentration to achieve a sodium gradient ≤2 mEq/L would result in lower IDWG, IDWG%, and blood pressure without increasing the frequency of adverse events in patients undergoing thrice-weekly in-center nocturnal hemodialysis.
METHODS
Quality Improvement Plan
All prevalent patients with dialysate sodium prescriptions ≥140 mEq/L were included. Participants were aware that changes in dialysate prescription would be made, but were blinded to the timing to avoid bias in reporting symptoms or in IDWG. Intradialytic blood pressure was monitored more frequently to protect patients’ well-being. The project was approved by the dialysis provider’s clinical leadership, medical director of the facility, and all treating nephrologists. It is important to emphasize that this quality improvement project was implemented to address an important clinical problem, increased IDWG, observed in the in-center nocturnal hemodialysis patients. Because the intervention, decreased dialysate sodium concentration, was not a new approach but rather a reintroduction of a well-established dialysate sodium prescription routinely used in the past, this project was not considered human subject research.
Each patient underwent 3 consecutive 12-week treatment phases in the following order: A, B, and A+. In phase A, all except one participant dialyzed with a standard dialysate sodium concentration of 140 mEq/L (one participant dialyzed with 145 mEq/L). In phase B, the dialysate sodium concentration was decreased to 136 mEq/L. In 3 participants with plasma sodium levels <135 mEq/L, dialysate sodium concentration was decreased further by 1 mEq/L biweekly until reaching 134 mEq/L. In phase A+, dialysate sodium concentration was changed back in all participants to the standard prescription of 140 mEq/L to evaluate whether changes induced by altering dialysate sodium concentrations would reverse or persist (Fig 1).
Figure 1.
Study design.
We dialyzed all participants with Fresenius F-180 or F-200 dialyzers, using Fresenius 2008K dialysis machines (Fresenius Medical Care North America, www.fmcna.com). We used automated internal blood pressure modules, model TM2910FR (Fresenius Medical Care North America), and Tronix floor scale model 6102 (Scale-Tronix, www.scale-tronix.com). The standard nocturnal hemodialysis prescription included session length of 8 hours 3 times per week, with a blood flow rate of 300 mL/min, dialysate flow rate of 500 mL/min, and dialysate solution containing the following concentrations: sodium, 140 mEq/L; bicarbonate, 35–40 mEq/L; potassium, 1–3 mEq/L; calcium, 2.5 mEq/L; and magnesium, 1.0 mEq/L. A dialysate glucose concentration of 200 mg/dL was used during the entire phase A and the first 4 weeks of phase B. It was then decreased to 100 mg/dL due to a company-wide introduction of 100-mg/dL glucose dialysate. All participants received dietary counseling to limit salt intake to ≤5 g/d according to the facility’s standard of care. Dietary intake was not measured or estimated. Participants were evaluated by the treating physician and dietitian at least monthly, more frequently if IDWG was >4% of dry weight. Dry weight was prescribed and determined by the participant’s treating nephrologist, and changes were allowed as clinically indicated.
We used the electronic medical record to retrospectively collect data for demographics, vintage (time since initiation of dialysis therapy), nocturnal vintage (time since starting the nocturnal hemodialysis program), urine volume, dialysis access, dialysate sodium concentration, antihypertensive medications, blood pressure (predialysis, intradialytic, and postdialysis), and pre- and postdialysis weight. We also collected data for the administration of normal (0.9%) or hypertonic (3%) saline solution and the occurrence of symptoms, including headache, cramps, nausea, and vomiting.
Blood samples were collected before the first weekly treatment (Monday or Tuesday) according to the standard protocol for monthly laboratory monitoring and processed at Satellite Laboratory Services (www.ascendclinical.com). Laboratory tests included pre- and postdialysis plasma sodium (measured by indirect ion-selective electrode), glucose, albumin, and serum urea nitrogen. Pre- and post serum urea nitrogen values were used for formal urea kinetic modeling, which included estimated total-body water and normalized protein equivalent of nitrogen appearance.
Definitions
We estimated IDWG as the difference between predialysis weight and postdialysis weight of the previous hemodialysis session, calculated as the average of the last 6 interdialytic periods in each phase. IDWG% was calculated as IDWG divided by the target weight and multiplied by 100. We calculated dry weight for each phase as the average postdialysis weight from the last 6 sessions, whereas dry weight at baseline corresponds to the first day of the study. Blood pressure was calculated as the average sitting blood pressures from the last 6 hemodialysis sessions in each phase. Adverse events included intradialytic hypotension, defined as a decrease in systolic blood pressure >20 mm Hg requiring the administration of normal or hypertonic saline solution with or without the presence of symptoms, and cramps, defined as severe enough to promote the provision of normal or hypertonic saline solution. We calculated mean pre- and postdialysis plasma sodium concentrations by averaging monthly predialysis plasma sodium concentrations available in each phase. We calculated sodium gradient as the lowest dialysate sodium concentration minus the mean predialysis plasma sodium concentration from each phase.
Statistical Analysis
Continuous variables are summarized as mean ± standard deviation or median with interquartile range. Categorical variables were expressed as proportions. We used Pearson correlation coefficients to evaluate relationships among sodium gradient, IDWG, IDWG%, and blood pressure. To minimize the possibility of a carryover effect, we analyzed data from the last 2 weeks of each study phase, with one exception (in one participant who moved, only the last 1 week of phase B was included). We used linear mixed models to compare differences in dependent variables (IDWG, IDWG%, and blood pressure) by study phase (fixed factor) accounting for repeated participant observations (random effect). Different covariance structures were explored to obtain the best fitted model based on the smallest akaike information criterion value. When P was <0.05, further analysis was performed with Dunnett post hoc test for multiple comparisons, setting phase A as the reference period. We used generalized estimating equations to compare the proportion of treatments with intradialytic hypotension and cramps during the last 2 weeks of each phase, adjusting for within-patient correlation. We also reported data from the first 2 weeks and the entire phase B to evaluate the temporal effect of decreasing dialysate sodium concentration. We considered P < 0.05 to be statistically significant. We conducted statistical analyses with SAS EG, version 4.2 (www.sas.com).
RESULTS
We enrolled 16 participants in the thrice-weekly in-center nocturnal hemodialysis program. One participant died of sudden cardiac death before completing phase B. This patient was found dead at home after being discharged from a 3-day hospitalization for uncontrolled diabetes. She was last dialyzed in the in-center nocturnal program 6 days before her death. We report results from 15 participants in phases A and B and 14 in phase A+ because one participant relocated.
Demographics and clinical characteristics of subjects are listed in Table 1. Generally, participants were relatively young, more likely to be men, and of longer vintage than the broader population of patients cared for at Satellite. All except one participant had no residual renal function and 4 participants had nocturnal dialysis vintages of 3 months or less. Per study design, the sodium gradient was decreased by 4.8 mEq/L in phase B. Dry weight was changed in the 3 study phases (Table 2). In phase B, dry weight was increased by ≥3 kg in 3 participants. There was a statistically significant difference in postdialysis plasma sodium concentration among the 3 phases (P < 0.001; Table 2). Postdialysis sodium concentration decreased significantly in phase B compared with phase A and was not significantly different in phases A and A+, but remained higher than the predialysis sodium concentration in all 3 phases. Predialysis plasma sodium concentration was not significantly different in phases A and B, but was higher in phase A+ (Table 2). Of note, 2 participants in phase A+ who dialyzed through a catheter (locked with gentamycin/sodium citrate) had unusually high single predialysis sodium concentrations of 145 and 146 mEq/L.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Value |
|---|---|
| Age (y) | 51.1 ± 14.0 |
| Men | 11 (73.3) |
| Black | 5 (33.3) |
| Diabetes | 7 (46.7) |
| Dialysis vintage (mo) | 62 (31; 79) |
| Nocturnal dialysis vintage (mo) | 9 (3; 10) |
| Access | |
| Arteriovenous fistula | 10 (66.7) |
| Arteriovenous graft | 2 (13.3) |
| Catheter | 3 (20.0) |
| Dry weight (kg) | 87.1 ± 22.3 |
| Body water volumea (L) | 36.1 ± 7.7 |
| No residual renal function | 14 (93.3) |
| Glucose (mg/dL) | 127.5 ± 71.0 |
| Albumin (g/dL) | 3.9 ± 0.2 |
| spKt/V | 3.3 ± 0.9 |
| nPNA | 1.0 ± 0.2 |
| No. with no antihypertensive drugs | 2 |
| No. of antihypertensive drugs/participant | 2.1 ± 1.3 |
Note: N = 15. Values are expressed as mean ± standard deviation, number (percentage), or median (25th; 75th percentile). Conversion factors for units: serum glucose in mg/dL to mmol/L, ×0.05551; albumin in g/dL to g/L, ×10.
Abbreviations: nPNA, normalized protein equivalent of nitrogen appearance; spKt/V, single-pool Kt/V per session.
Measured by urea kinetic model.
Table 2.
Plasma Sodium Concentration and Outcomes in Each Study Phase
| Phase A (n = 15) | Phase B (n = 15) | Phase A+ (n = 14) | Pa | |
|---|---|---|---|---|
| Dialysate [Na] (mEq/L) | 140b | 134c or 136 | 140 | |
| [Na] gradient (mEq/L) | 4.2 ± 3.2 | −0.6 ± 1.5 | 2.9 ± 2.6 | |
| Dry weight (kg) | 86.8 ± 21.3 | 87.9 ± 21.6 | 87.2 ± 22.2 | |
| Pre-HD [Na] (mEq/L) | 136.2 ± 3.1 | 136.2 ± 2.1 | 137.1 ± 2.6d | 0.01 |
| Post-HD [Na] (mEq/L) | 142.1 ± 1.5 | 138.4 ± 1.5e | 141.1 ± 1.5 | −0.001 |
| IDWG (kg) | 4.2 ± 0.8 | 3.6 ± 0.6e | 4.1 ± 0.9 | 0.02 |
| IDWG% | 5.0 ± 1.0 | 4.4 ± 1.2e | 5.0 ± 1.8 | 0.03 |
| Pre-HD blood pressure | ||||
| Systolic (mm Hg) | 159.0 ± 22.0 | 150.7 ± 24.7e | 150.3 ± 30.2d | 0.03 |
| Diastolic (mm Hg) | 89.7 ± 11.6 | 84.0 ± 10.6 | 85.1 ± 15.4 | 0.1 |
| MAP (mm Hg) | 112.8 ± 13.3 | 106.2 ± 14.0 | 106.8 ± 19.8 | 0.07 |
| Post-HD blood pressure | ||||
| Systolic (mm Hg) | 128.6 ± 15.7 | 126.1 ± 14.6 | 132.5 ± 18.5 | 0.5 |
| Diastolic (mm Hg) | 77.6 ± 11.0 | 74.3 ± 11.0 | 80.9 ± 9.1 | 0.2 |
| MAP (mm Hg) | 94.6 ± 11.6 | 91.6 ± 11.2 | 98.0 ± 11.2 | 0.3 |
Note: Measurements are expressed as mean ± standard deviation.
Abbreviations and definitions: HD, hemodialysis; IDWG, interdialytic weight gain; IDWG%, IDWG divided by estimated dry weight, then multiplied by 100; MAP, mean arterial pressure; [Na], sodium concentration; [Na] gradient, dialysate sodium concentration minus the pre-HD plasma sodium concentration.
P values represent comparison among the 3 study phases.
One participant dialyzed in phase A with dialysate sodium concentration of 145 mEq/L.
Three participants dialyzed in phase B with dialysate sodium concentrations of 134 mEq/L.
P < 0.05 comparing phase A+ versus phase A.
P < 0.05 comparing phase B versus phase A.
We found a statistically significant difference in IDWG (P = 0.02) and IDWG% (P = 0.03) among all 3 phases. Compared with phase A, in phase B, both IDWG and IDWG% decreased significantly by 0.6 ± 0.6 kg and 0.6% ± 0.8%, respectively (P < 0.05 for both comparisons; Table 2). After dialysate sodium concentration was increased to 140 mEq/L in phase A+, IDWG and IDWG% also increased.
We found a statistically significant difference in predialysis systolic blood pressure among the 3 phases (P = 0.03; Table 2). It decreased significantly by 8.3 ± 14.9 mm Hg in phase B compared with phase A (P < 0.05; Table 2) and remained lower in phase A+ compared with phase A. No statistically significant difference in predialysis diastolic or mean arterial blood pressure was noted among the 3 phases. However, they both decreased, by 6.6 ± 11.9 and 5.7 ± 10.8 mm Hg, respectively, in phase B compared with phase A (P > 0.05 for both comparisons; Table 2) and remained lower in phase A+. No significant differences in postdialysis blood pressures were found (P > 0.05 for all comparisons; Table 2).
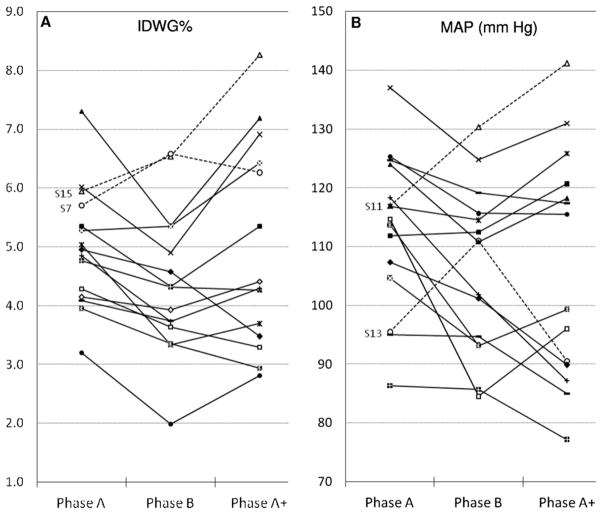
Interestingly, in the absence of changes in antihypertensive drug regimens, 2 participants (patients 7 and 15) improved their predialysis blood pressures despite increased IDWG or IDWG%. Two additional participants, both using 3 antihypertensive drugs, experienced higher predialysis blood pressures (patients 11 and 13) without increasing IDWG (Fig 2).
Figure 2.
(A, B) Individual changes in intradialytic weight gain (IDWG) indexed by the estimated dry weight (IDWG%) and predialysis mean arterial pressure (MAP) throughout the different study phases. (A) Dashed lines represent participants (patients 15 and 7) with increased IDWG%, but decreased predialysis blood pressures in phase B. (B) Dashed lines represent participants (patients 11 and 13) who experienced higher predialysis blood pressures without increasing IDWG% in phase A.
The number and percentage of treatments with either symptomatic or asymptomatic intradialytic hypotension were low and similar during the last 2 weeks of each study phase (P = 0.9; Table 3). The number of treatments with cramps was zero. The first 2 weeks after dialysate sodium concentration was decreased, the number and percentage of treatments with hypotension or cramps increased to 7 (8%) for both events. Five participants had a single treatment with adverse events and only one had a repeated episode of both hypotension and cramps. All adverse events resolved with normal saline solution administration and none required hypertonic saline solution (Table 3).
Table 3.
Treatments With Intradialytic Hypotension and Cramps in Each Study Phase
| Phase A (last 2 wk) | Phase B
|
Phase A+ (last 2 wk) | Pa | |||
|---|---|---|---|---|---|---|
| Entire Phase | First 2 wk | Last 2 wk | ||||
| Intradialytic hypotension | 4 (4.5) | 24 (4.5) | 7 (8.0) | 3 (3.5) | 3 (3.6) | 0.9 |
| Asymptomatic | 3 (3.4) | 9 (1.7) | 1 (1.1) | 1 (1.2) | 3 (3.6) | 0.8 |
| Symptomatic | 1 (1.1) | 15 (2.8) | 6 (6.8) | 2 (2.3) | 0 (0) | — |
| Cramps | 0 (0) | 11 (2.1) | 7 (8.0) | 0 (0) | 0 (0) | — |
| Total no. of treatments | 89 | 529 | 88 | 86 | 83 | — |
Note: Values are expressed as number (percentage).
P values represent comparison between the last 2 weeks of the 3 study phases; some P values are not available because of zero adverse events in 1 phase.
Additionally, we found direct correlations at baseline between sodium gradient and IDWG (r = 0.45; P = 0.09) and IDGW% (r = 0.76; P = 0.001). We also found a significant direct correlation between IDWG and predialysis systolic blood pressure (r = 0.52; P = 0.05), but the correlation between IDWG% and predialysis systolic blood pressure was not significant (r = 0.19; P = 0.5).
DISCUSSION
We achieved significant decreases in IDWG (and IDWG%) and predialysis systolic blood pressure in participants on thrice-weekly in-center nocturnal dialysis therapy with the simple strategy of decreasing dialysate sodium concentration. These findings are consistent with previously reported studies of patients on conventional hemodialysis therapy15,19 and likely are attributed to lower postdialysis plasma sodium concentrations achieved by decreasing sodium gradients.14,16
Improved outcomes in participants on longer hemodialysis regimens compared with those undergoing conventional hemodialysis have been reported by observational studies in Europe20 and recently in a US cohort.8 Higher IDWG or IDWG% has been associated with morbidity1 and mortality2,3 in patients on conventional hemodialysis therapy. The higher IDWG reported in patients on nocturnal hemodialysis therapy6–8 may attenuate potential benefits of extended session length. Consequently, strategies to restrict intradialytic salt influx by decreasing dialysate sodium concentration would be a reasonable adjunct (and easier in some respects) to moderation of dietary sodium intake.
Under present hemodialysis practices, >80% of sodium is removed by convection and only 15%–20% is removed by diffusion.16,21 To prevent diffusive sodium transport across the dialysis membrane, a “eunatremic” dialysate sodium concentration of 1.5–5 mEq/L less than the plasma sodium concentration measured by indirect ion-selective electrode would be required.22 This could explain why postdialysis plasma sodium concentrations in our patients during phase B were still higher than predialysis sodium concentrations because the mean sodium gradient was only −0.6 ± 1.5 mEq/L.
Unequal sodium concentrations between plasma and dialysate measured by indirect ion-selective electrode occur because plasma water makes up 93% of the whole plasma volume, whereas constituting all of the total dialysate volume. In vivo, the Gibss-Donnan effect compensates for this concentration difference17 because plasma-diffusible sodium is decreased by 5% due to binding to negatively charged nondiffusible plasma proteins.16,17 Because these 2 factors tend to cancel each other,23 the exact value measured in plasma was used to calculate sodium gradient, as has been proposed previously.16 This allows for a practical and reproducible approach in routine clinical practice. Alternatively, novel technologies, such as online conductivity monitoring,21,24,25 have been proposed to individualize dialysate sodium composition.
Predialysis blood pressures decreased with lower dialysate sodium concentrations, but were almost unchanged after increasing dialysate sodium concentrations to 140 mEq/L in phase A+ in the absence of changes in antihypertensive medications. A potential explanation, particularly given the relatively limited experience with nocturnal hemodialysis in some participants, is the “lag phenomenon,” which refers to changes in blood pressure independent of decreases in extracellular volume that have been reported to occur up to 8 months after reaching an arbitrary ideal dry weight.20,26,27 The lag phenomenon has been linked to low peripheral resistance,28,29 mediated by the release of nonosmotically active sodium stored in the connective tissue from blood vessels,30 a process that potentially could take several months.
A few participants in our study showed a decrease in blood pressure with an increase in IDWG or an increase in blood pressure despite a decrease in IDWG. Previous studies of patients on conventional hemodialysis therapy31,32 have reported a decrease in either blood pressure or IDWG with lowering of dialysate sodium concentration, but not in both. Although we would expect an improvement in both blood pressure and IDWG, any therapeutic effect could be masked by the severity of the hypertension and incomplete adherence to antihypertensive medications and sodium restriction. These data also raise the question of volume-independent effects of sodium exposure on blood pressure. In a recent analysis of a large cohort of prevalent hemodialysis patients, a 1% increase in IDWG was associated with just a 1-mm Hg increase in systolic blood pressure,33 suggesting that weight gain may only modestly affect blood pressure, at least in the short term.
Clinicians and investigators have raised the concern of provoking intradialytic hypotension with lower dialysate sodium concentrations. In this quality improvement report, the proportion of treatments with intradialytic hypotension and the fraction of participants affected during the last 2 weeks of the low dialysate sodium phase were low and similar compared with the higher dialysate sodium concentration phases. However, we noted a higher proportion of treatments with intradialytic hypotension, all responsive to saline solution administration, the first 2 weeks after decreasing dialysate sodium concentration. These results could indicate an adaptation period that perhaps could be attenuated with an even more gradual decrease in dialysate sodium concentration over time or by using biofeedback systems that modulate blood volume contraction by adjusting the ultrafiltration and dialysate conductivity.25,34 Other investigators have expressed concern that lower dialysate sodium concentrations could result in frank hyponatremia. In our patients, after decreasing the sodium dialysate concentration to reach a sodium gradient ≤2 mEq/L, mean postdialysis plasma sodium concentration remained within the reference range. However, these data need to be confirmed in larger studies.
One participant enrolled in this study was found dead at home 1 day after being discharged from the hospital, where she was admitted for severe hyperglycemia found before a planned dialysis access surgery. This patient had multiple comorbid conditions, including severe uncontrolled diabetes, hypertension, ortho-static hypotension, diastolic dysfunction, and reentrant tachyarrhythmia. She underwent basilic vein transposition with no complications after controlling her hyperglycemia. Her plasma sodium concentration increased from 131 mEq/L on admission in the presence of a blood glucose level of 488 mg/dL to 134 mEq/L on the day of discharge. While in the hospital, she underwent 2 conventional hemodialysis treatments, both with dialysate sodium concentrations of 140 mEq/L. Of note, she was last dialyzed uneventfully in the in-center nocturnal program with a dialysate sodium concentration of 136 mEq/L 6 days before she died. The cause of death was considered to be unrelated to the nocturnal hemodialysis prescription.
Some researchers have postulated the existence of a stable unique predialysis plasma sodium concentration for each patient on hemodialysis therapy; the so-called “sodium set-point.”13,14,17 Consistent with this hypothesis, we found no change in predialysis plasma sodium concentrations after the dialysate sodium concentration was decreased in phase B. In phase A+, predialysis plasma sodium concentration increased modestly, although this increase may have been spurious due to inadequate washout of participants’ catheter lock solution (hypertonic sodium citrate).35
Similarly to previous reported studies of patients on conventional hemodialysis therapy,14,15 we also found a highly significant direct correlation between sodium gradient and IDWG%. These data support the strategy of decreasing dialysate sodium concentrations to decrease sodium gradients, with the clinical goal to prevent thirst stimulation and therefore decrease fluid intake as previously postulated.16,17
The intervention and results of our study must be taken in the context of a quality improvement project in a selected and small population that limits our ability to generalize our results. We could not determine actual sodium balance because we did not measure or estimate dietary sodium intake and did not determine the actual sodium concentration in dialysate or ultrafiltrate. We could not control for the potential effect of variable dry weight and dialysate glucose concentration in the different study phases. Moreover, we had limited precision in the measurement of blood pressure. A potential bias owing to the unblinded dialysis staff and the possibility that participants could have noticed the change in dialysate sodium concentration on the machines’ screens cannot be excluded. Future studies should more formally measure or estimate sodium balance and obtain more precise and time-averaged assessments of blood pressure. Ideally, studies of patients on alternative hemodialysis modalities (eg, home-based hemodialysis, both thrice weekly and more frequent, as well as conventional in-center hemodialysis) should be performed. Whereas decreasing dialysate sodium concentrations in patients treated with conventional hemodialysis using shorter session lengths (ie, 3–4 hours) also could result in lower IDWG and blood pressure, the potential risk of increased adverse events (eg, cramping and hypotension) will need to be evaluated.
In summary, patients on thrice-weekly in-center nocturnal hemodialysis therapy who were exposed to progressively lower dialysate sodium concentrations experienced lower IDWG (and IDWG%) and predialysis systolic blood pressure compared with treatment using baseline dialysate sodium concentrations of 140 mEq/L. These results are consistent with several other studies suggesting that higher sodium gradients (differences between predialysis plasma and dialysate sodium concentrations) contribute to higher IDWG and potentially to associated complications, such as left ventricular remodeling and heart failure. Although the optimal target IDWG and blood pressure in patients on hemodialysis therapy are unknown, modification of dialysate sodium concentration should be considered as a potential therapeutic maneuver to limit “dialysis-associated weight gain” through individualized hemodialysis therapy.
Acknowledgments
We thank the dedicated staff of the in-center nocturnal hemodialysis program, who provide excellent quality care, and our patients, who have entrusted themselves to our care and taught us more about end-stage renal disease than textbooks possibly could.
Support: Dr Schiller, Ms Sun, and Ms Doss are employees of Satellite Healthcare. During his fellowship at Stanford University, Dr Munoz Mendoza was the recipient of the Satellite Hans Wolf Research fellowship award.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Holmberg B, Stegmayr BG. Cardiovascular conditions in hemodialysis patients may be worsened by extensive interdialytic weight gain. Hemodial Int. 2009;13(1):27–31. doi: 10.1111/j.1542-4758.2009.00335.x. [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119(5):671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saran R, Bragg-Gresham JL, Rayner HC, et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64(1):254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 4.Leggat JE, Jr, Orzol SM, Hulbert-Shearon TE, et al. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis. 1998;32(1):139–145. doi: 10.1053/ajkd.1998.v32.pm9669435. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62(5):1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 6.Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42(suppl 1):13–17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 7.Troidle L, Hotchkiss M, Finkelstein F. A thrice weekly in-center nocturnal hemodialysis program. Adv Chronic Kidney Dis. 2007;14(3):244–248. doi: 10.1053/j.ackd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Lacson E, Jr, Wang W, Lester K, Ofsthun N, Lazarus JM, Hakim RM. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220–226. doi: 10.2215/CJN.06070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penne EL, Levin NW, Kotanko P. Improving volume status by comprehensive dietary and dialytic sodium management in chronic hemodialysis patients. Blood Purif. 2010;30(1):71–78. doi: 10.1159/000317124. [DOI] [PubMed] [Google Scholar]
- 10.Pozzoni P, DI Filippo S, Pontoriero G, Locatelli F. Effectiveness of sodium and conductivity kinetic models in predicting end-dialysis plasma water sodium concentration: preliminary results of a single-center experience. Hemodial Int. 2007;11(2):169–177. doi: 10.1111/j.1542-4758.2007.00165.x. [DOI] [PubMed] [Google Scholar]
- 11.Kimura G, Van Stone JC, Bauer JH, Keshaviah PR. A simulation study on transcellular fluid shifts induced by hemodialysis. Kidney Int. 1983;24(4):542–548. doi: 10.1038/ki.1983.191. [DOI] [PubMed] [Google Scholar]
- 12.Flanigan MJ, Khairullah QT, Lim VS. Dialysate sodium delivery can alter chronic blood pressure management. Am J Kidney Dis. 1997;29(3):383–391. doi: 10.1016/s0272-6386(97)90199-2. [DOI] [PubMed] [Google Scholar]
- 13.Peixoto AJ, Gowda N, Parikh CR, Santos SF. Long-term stability of serum sodium in hemodialysis patients. Blood Purif. 2010;29(3):264–267. doi: 10.1159/000274460. [DOI] [PubMed] [Google Scholar]
- 14.Keen ML, Gotch FA. The association of the sodium “set-point” to interdialytic weight gain and blood pressure in hemodialysis patients. Int J Artif Organs. 2007;30(11):971–979. doi: 10.1177/039139880703001105. [DOI] [PubMed] [Google Scholar]
- 15.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66(3):1232–1238. doi: 10.1111/j.1523-1755.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 16.Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(2):522–530. doi: 10.2215/CJN.03360807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanigan MJ. Role of sodium in hemodialysis. Kidney Int Suppl. 2000;76:S72–S78. doi: 10.1046/j.1523-1755.2000.07609.x. [DOI] [PubMed] [Google Scholar]
- 18.Flanigan M. Dialysate composition and hemodialysis hypertension. Semin Dial. 2004;17(4):279–283. doi: 10.1111/j.0894-0959.2004.17327.x. [DOI] [PubMed] [Google Scholar]
- 19.Sayarlioglu H, Erkoc R, Tuncer M, et al. Effects of low sodium dialysate in chronic hemodialysis patients: an echocardiographic study. Ren Fail. 2007;29(2):143–146. doi: 10.1080/08860220601095785. [DOI] [PubMed] [Google Scholar]
- 20.Charra B. From adequate to optimal dialysis long 3 × 8 hr dialysis: a reasonable compromise. Nefrologia. 2005;25(suppl 2):19–24. [PubMed] [Google Scholar]
- 21.Lambie SH, Taal MW, Fluck RJ, McIntyre CW. Online conductivity monitoring: validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO J. 2005;51(1):70–76. doi: 10.1097/01.mat.0000150525.96413.aw. [DOI] [PubMed] [Google Scholar]
- 22.Flanigan MJ. How should dialysis fluid be individualized for the chronic hemodialysis patient? Sodium. Semin Dial. 2008;21(3):226–229. doi: 10.1111/j.1525-139X.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- 23.Depner TA, Ing TS. Toxic fluid flux? Am J Kidney Dis. 2010;56(1):1–4. doi: 10.1053/j.ajkd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Moret KE, Beerenhout CH, Kooman JP. Variations in pre-dialytic plasma conductivity in dialysis patients: effect on ionic mass balance and blood pressure. ASAIO J. 2011;57(1):53–61. doi: 10.1097/MAT.0b013e3182078b66. [DOI] [PubMed] [Google Scholar]
- 25.Manlucu J, Gallo K, Heidenheim PA, Lindsay RM. Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: a pilot study using a biofeedback software system. Am J Kidney Dis. 2010;56(1):69–76. doi: 10.1053/j.ajkd.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Shaldon S, Vienken J. The long forgotten salt factor and the benefits of using a 5-g-salt-restricted diet in all ESRD patients. Nephrol Dial Transplant. 2008;23(7):2118–2120. doi: 10.1093/ndt/gfn175. [DOI] [PubMed] [Google Scholar]
- 27.Charra B, Bergstrom J, Scribner BH. Blood pressure control in dialysis patients: importance of the lag phenomenon. Am J Kidney Dis. 1998;32(5):720–724. doi: 10.1016/s0272-6386(98)70147-7. [DOI] [PubMed] [Google Scholar]
- 28.Shaldon S. An explanation for the “lag phenomenon” in drug-free control of hypertension by dietary salt restriction in patients with chronic kidney disease on hemodialysis. Clin Nephrol. 2006;66(1):1–2. doi: 10.5414/cnp66001. [DOI] [PubMed] [Google Scholar]
- 29.Luik AJ, Charra B, Katzarski K, et al. Blood pressure control and hemodynamic changes in patients on long time dialysis treatment. Blood Purif. 1998;16(4):197–209. doi: 10.1159/000014335. [DOI] [PubMed] [Google Scholar]
- 30.Titze J. Water-free sodium accumulation. Semin Dial. 2009;22(3):253–255. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 31.Daugirdas JT, Al-Kudsi RR, Ing TS, Norusis MJ. A double-blind evaluation of sodium gradient hemodialysis. Am J Nephrol. 1985;5(3):163–168. doi: 10.1159/000166927. [DOI] [PubMed] [Google Scholar]
- 32.Thein H, Haloob I, Marshall MR. Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrol Dial Transplant. 2007;22(9):2630–2639. doi: 10.1093/ndt/gfm220. [DOI] [PubMed] [Google Scholar]
- 33.Inrig JK, Patel UD, Gillespie BS, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis. 2007;50(1):108–118. 118 e101–104. doi: 10.1053/j.ajkd.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basile C, Giordano R, Vernaglione L, et al. Efficacy and safety of haemodialysis treatment with the Hemocontrol biofeedback system: a prospective medium-term study. Nephrol Dial Transplant. 2001;16(2):328–334. doi: 10.1093/ndt/16.2.328. [DOI] [PubMed] [Google Scholar]
- 35.Lamb EJ, Abbas NA. Spurious hypernatraemia and Citra-Lock. Ann Clin Biochem. 2007;44(pt 6):579. doi: 10.1258/000456307782268219. [DOI] [PubMed] [Google Scholar]