Abstract
Aim
Long-chain acylcarnitines have been postulated to be sensitive biomarkers of acetaminophen (APAP)-induced hepatotoxicity in mouse models. In the following study, the relationship of acylcarnitines with other known indicators of APAP toxicity was examined in children receiving low-dose (therapeutic) and high-dose (‘overdose’ or toxic ingestion) exposure to APAP.
Materials & methods
The study included three subject groups: group A (therapeutic dose, n = 187); group B (healthy controls, n = 23); and group C (overdose, n = 62). Demographic, clinical and laboratory data were collected for each subject. Serum samples were used for measurement of APAP protein adducts, a biomarker of the oxidative metabolism of APAP and for targeted metabolomics analysis of serum acylcarnitines using ultra performance liquid chromatography–triple-quadrupole mass spectrometry.
Results
Significant increases in oleoyl- and palmitoyl-carnitines were observed with APAP exposure (low dose and overdose) compared with controls. Significant increases in serum ALT, APAP protein adducts and acylcarnitines were observed in overdose children that received delayed treatment (time to treatment from overdose >24 h) with the antidote N-acetylcysteine. Time to peak APAP protein adducts in serum was shorter than that of the acylcarnitines and serum ALT.
Conclusion
Perturbations in long-chain acylcarnitines in children with APAP toxicity suggest that mitochrondrial injury and associated impairment in the β-oxidation of fatty acids are clinically relevant as biomarkers of APAP toxicity.
Keywords: β-oxidation, acetaminophen, acylcarnitine, biomarker, clinical, hepatic, toxicity
Acetaminophen (APAP) is the most widely used drug for the treatment of pain and fever around the world. While relatively safe at therapeutic doses, APAP in high doses can cause hepatotoxicity and is recognized as a major cause of acute liver failure (ALF) in the USA and in many European countries [1]. APAP accounts for 50% of all cases of ALF in adults and 14% of cases in children in the USA [1,2]. While oxidative metabolism and depletion of hepatic glutathione are recognized to be critical early steps in the genesis of APAP-associated liver injury [3,4] other mechanisms of toxicity involving increased oxygen and/or nitrogen stress in mitochondria have been reported [5–7]. Recently, blood levels of metabolites involved in energy, urea and bile acid pathways were found to have strong correlations with hepatic necrosis scores and elevated ALT levels in APAP-induced liver injury in mice [8]. In addition, a modulatory role for hepatoprotective pathways such as the PPARα pathway has been identified [9–11]. PPARα regulates gene expression in mitochondrial fatty acid β-oxidation and upregulation of PPARα was hepatoprotective in the mouse model of APAP toxicity [11,12].
Acylcarnitines are intermediates in the mitochondrial α-oxidation of fatty acids and have been postulated as indicators of hepatotoxicity in APAP [9,13–15] and other drugs such as valproic acid [16] that are known to have effects on mitochondria. A liquid chromatography–mass spectrometry (LC/MS)-based metabolomics study of APAP metabolism in wild-type and Cyp2E1-null mice revealed that acylcarnitines can function as complementary biomarkers for APAP-induced hepatotoxicity [17]. The α-oxidation of fatty acids is facilitated by transport of activated fatty acids into the mitochondria via carnitine. With disruption of mitochondrial function, long-chain fatty acids accumulate and can be detected in blood [17,18].
We recently reported the elevation of long-chain acylcarnitines (palmitoyl-, oleoyl- and myristoyl-carnitines) in mice treated a high, nonfatal dose of APAP [18]. In order to examine the clinical relevance of data generated in animal models, the following study quantified acylcarnitines and other known indicators of APAP metabolism and toxicity in children with APAP poisoning.
Materials & methods
Patients
This was a multicenter study of APAP toxicity in children aged 2–18 years that was approved by the institutional review boards of all participating institutions. Following informed consent and assent when age appropriate, blood samples were collected from study subjects. The study included three subject subgroups as follows: group A (therapeutic dose group), defined as hospitalized children receiving APAP per standard of care; group B (control group), defined as healthy children with no use of APAP in the preceding 14 days; and group C (overdose group), defined as children requiring hospitalization for treatment of APAP overdose. Hospitalization of children with APAP overdose was determined according to published guidelines and included history of excessive dosing of APAP of >150 mg/kg and elevation of APAP in peripheral blood, plotted as a function of the time elapsed from the time of the overdose [19]. For subjects in group A, blood samples were collected prior to receipt of the first APAP dose ‘on study’ and thereafter at 8 and 24 h after the first dose of APAP, followed by convenience sampling throughout the period of hospitalization. APAP dosing, route and frequency were at the discretion of the treating physician for subjects in group A. A single blood sample was collected in group B subjects and admission and daily morning blood samples were collected in subjects in group C. Blood samples were centrifuged immediately after collection and the serum was stored at −80°C for batch analysis.
Clinical data
Demographic (age, gender, race, weight and height), clinical (APAP dose, route, dose interval, N-acetylcysteine [NAC] dose, route, cumulative dose and reason for hospitalization) and laboratory data (serum ALT and international normalized ratio [INR] for prothrombin time) were recorded in a specific data base designed for the study.
Laboratory analysis
APAP protein adducts in peripheral blood have been established as a biomarker of APAP toxicity in the animal model of APAP toxicity and in clinical samples [20–24]. Serum samples were analyzed for APAP protein adducts using previously reported high-performance LC with electrochemical detection methods [20–24]. Measurement of serum ALT was performed in clinical chemistry laboratories at the participating institutions using standardized methods.
Metabolomic analysis Exploratory analysis of serum acylcarnitines by rapid targeted metabolomics
In the initial approach, a targeted metabolomics approach using Waters’ ACQUITY UPLC® System with Xevo triple-quadrupole mass spectrometer (Waters, MA, USA) combined with the commercially available AbsoluteIDQ® p180 Kit (Biocrates Life Sciences AG, Austria) was used for rapid and quantitative analyses of 40 acylcarnitines according to the manufacturer’s protocol. The method combines the extraction of analytes with stable isotope dilution mass spectrometric analysis. The AbsoluteIDQ p180 Kit contains a 96-deep-well plate with a filter plate attached with sealing tape, as well as isotope-labeled internal standards and seven calibrators sufficient for quantitation of metabolites and three different quality control samples (low, medium and high spiked human plasma). The assay procedure has been previously described elsewhere [25–27].
Targeted analysis of serum acylcarnitines using ultra performance liquid chromatography–triple-quadrupole mass spectrometry
Targeted quantitative metabolite analysis was also performed using a previously published method [18]. Samples were removed from storage at −80°C and placed at −4°C for 2 h. A total of 50 µl of serum was spiked with isotope-labeled l-carnitine, acetyl-carnitine, myristoyl-carnitine, palmitoyl-carnitine and oleoyl-carnitine. The sample was then deproteinized with 300 µl of 3:1 mixture of acetone:MeOH (Optima grade, Thermo Fisher Scientific, MA, USA) and centrifuged for 15 min at 13,000 × g (4°C). The supernatant was transferred into a total recovery autosampler vial (Waters) and the solvent was evaporated under a stream of nitrogen. Samples were reconstituted in 50 µl of 50:50 acetonitrile:75% methanol. The chromatographic separation was carried out using an Acquity UPLC System equipped with a BEH C8 Column (2.1 × 100 mm, 1.7-µm particle size) and VanGuard C8 Pre-column (Waters). A total of 10 µl was injected and the flow rate was set to 0.4 ml/min with a column temperature of 40°C. Mobile phase A consisted of 90:10 water:acetonitrile with 0.5 g/l ammonium acetate adjusted to pH 7.4. Mobile phase B consisted of acetonitrile with 0.5 g/l ammonium acetate. The gradient started with 50% mobile phase B, held for 1 min, increasing to 86% at 5 min then to 100% at 7 min, after which initial conditions were restored at 8.5 min and the system was equilibrated for an additional 1.5 min. Blank injections were made between each sample injection. Mass spectrometric analysis was carried out on a Xevo triple-quadrupole instrument (Waters) operated in positive electrospray ionization mode. Multiple reaction monitoring was carried out using transitions previously optimized for each carnitine species by the direct infusion of standards. The capillary voltage was 4.5, and the source and desolvation temperatures were 150 and 400°C, respectively. The desolvation gas flow rate was 800 l/h.
Statistical analysis
The nonparametric Kruskal–Wallis test and post hoc comparisons by the method of Siegel and Castellan [28] was used to detect differences between the three subject groups. Mann–Whitney U test was used for pairwise comparisons between groups for toxicity, metabolism and metabolic markers. Pearson’s correlation coefficient was calculated to assess the relationship between the measures of toxicity in the overdose group. A p-value <0.05 was considered ‘significant’ for all analyses and a p-value between 0.05 and 0.1 was considered ‘tending towards statistical significance’ [29]. All statistical analyses were performed by SPSS Version 10.0 (SPSS Inc., IL, USA) and the open source statistical software package R. Multivariate data analysis was performed by the R package chemometrics [30] and SIMCA-P+ software (Umetrics, NJ, USA). The data matrix was transformed by autoscaling and a partial least squares discriminant analysis (PLS-DA) model was generated in order to identify acylcarnitines that closely associated with the toxicity status. Major latent variables in the data were represented in a scores scatterplot and the significant predictors were identified by their contribution to the PLS vectors in the loadings scatterplot as well as based on their variable importance on the projections (VIP) scores. Due to the convenience sampling design and variability of study duration between groups A (therapeutic dose) and C (overdose), time was analyzed as a continuous variable. Generalized least squares regression models were used to evaluate associations between acylcarnitines, ALT and APAP protein adducts, controlling for age and sex. A generalized autoregressive covariance matrix was imposed and continuous variables were parameterized in the regression model using restricted cubic splines.
Results
Table 1 summarizes the demographic and clinical parameters of the study subjects categorized as APAP therapeutic dose group (group A), healthy group with no recent APAP exposure (group B) and APAP overdose group (group C). Among the 62 patients in group C, 40 (65%) were judged to be ‘at risk’ for toxicity according to the Rumack nomogram. For the remaining 22 subjects, one had missing data and five had undetectable levels of APAP, but were judged to be at risk for toxicity based on elevations of ALT at the time of presentation to the hospital. An additional two subjects from the ‘no-risk group’ had co-ingestions with antihistamines, which are known to alter the pharmacokinetics of APAP. Thus, 14 of the study subjects in group C did not have readily identifiable reasons for treating the subjects with NAC by either the Rumack nomogram or by other clinical factors. All subjects in group C received treatment with NAC, but the time to NAC treatment was not available in three of the subjects. In the subjects with known time to NAC, 47 received NAC within 24 h of the overdose.
Table 1.
Demographic and clinical parameters of the group A, B and C subjects.
| Variables | Group A (n = 187) | Group B (n = 23) | Group C (n = 62) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 9.29 (5.59) | 8.30 (4.80) | 14.66 (3.47) |
| Median | 9.33 | 8.50 | 15.42 |
| Range | 1.0–18.67 | 1.17–16.17 | 1.50–18.25 |
| Gender, n (%) | |||
| Male | 103 (55) | 9 (39) | 13 (21) |
| Female | 84 (45) | 14 (61) | 49 (79) |
| Race, n (%) | |||
| White | 131 (70.05) | 15 (65.22) | 50 (80.65) |
| Asian | 4 (2.14) | ||
| African–American | 40 (21.39) | 4 (17.39) | 5 (8.06) |
| Other | 12 (6.41) | 4 (17.39) | 7 (11.29) |
| ALT (IU/l) | |||
| Mean (SD) | 69 (331) | 18.5 (6.7) | 554 (1287.7) |
| Median | 22 | 17 | 29* |
| Range | 6–3940 | 10–37 | 6–9909 |
| APAP protein adducts (nmol/ml) | |||
| Mean (SD) | 0.1 (0.2) | 0.005 (0.003) | 0.47 (0.87) |
| Median | 0.038 | 0.006 | 0.2* |
| Range | 0–2.1 | 0–0.01 | 0.02–7.9 |
Group A: therapeutic dose; group B: healthy controls; group C: overdose.
Statistically significant compared with group B at p < 0.05 (Mann–Whitney U test).
APAP: Acetaminophen; SD: Standard deviation.
Changes in APAP toxicity markers in children exposed to APAP therapeutic doses & overdoses
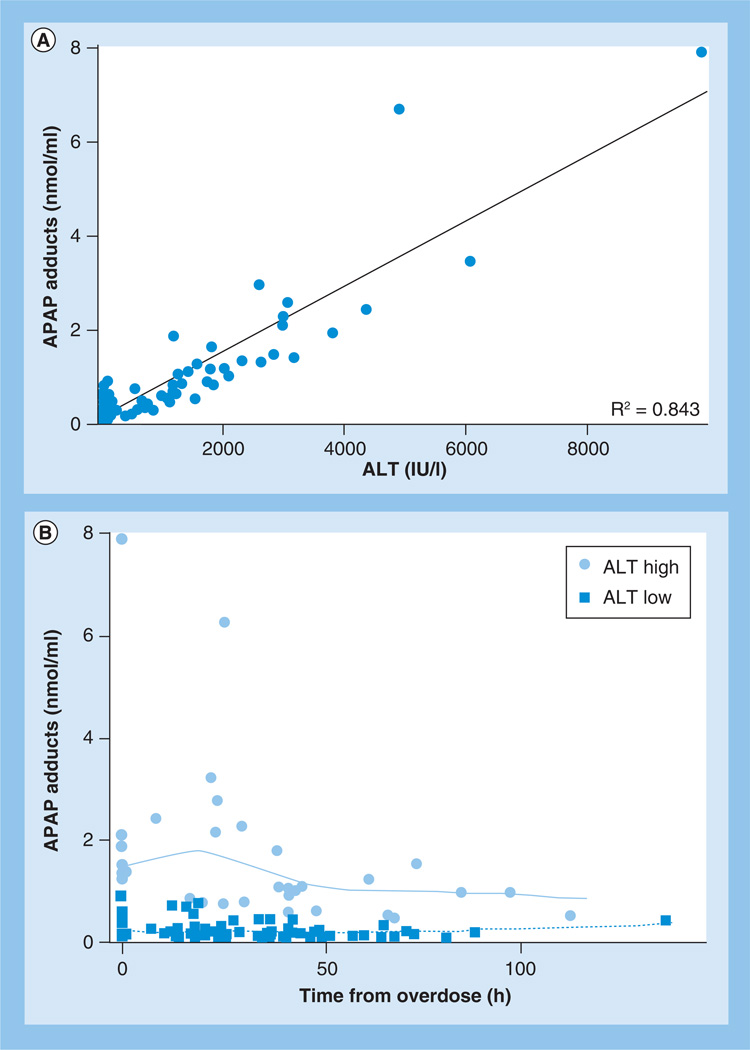
Summary data for ALT and APAP protein adducts are provided in Table 1. Due to the ‘as-needed’ dosing practice for APAP use in children, the analysis of the present study was limited to the initial 24 h of study participation for study group A. No differences were detected in median ALT values from baseline to 24 h (Table 1) and no significant differences were detected in ALT values between group A and group B (Table 1). As would be expected, median levels of ALT and APAP protein adducts were higher in group C than in groups A and B, and consistent with previous data, significant correlations with ALT and APAP protein adducts were observed in group C (R2 = 0.84, p < 0.05; Figure 1A). Stratification of group C children by peak ALT value (ALT >1000 IU/l) using a linear mixed-model analysis showed significant changes over time for APAP protein adducts (Figure 1B) in subjects with ALT >1000 and ≤1000 IU/l.
Figure 1. Relationship between acetaminophen protein adducts and ALT in children hospitalized for acetaminophen overdose.
(A) A significant correlation (R2 = 0.84; p < 0.05) was observed between ALT and APAP protein adducts. (B) Significant changes in adduct levels over time were observed in overdose subjects grouped based on their ALT levels (ALT high [>1000 IU/l] vs ALT low [≤1000 IU/l]).
APAP: Acetaminophen.
Exploratory analysis of acylcarnitines in the three groups of children
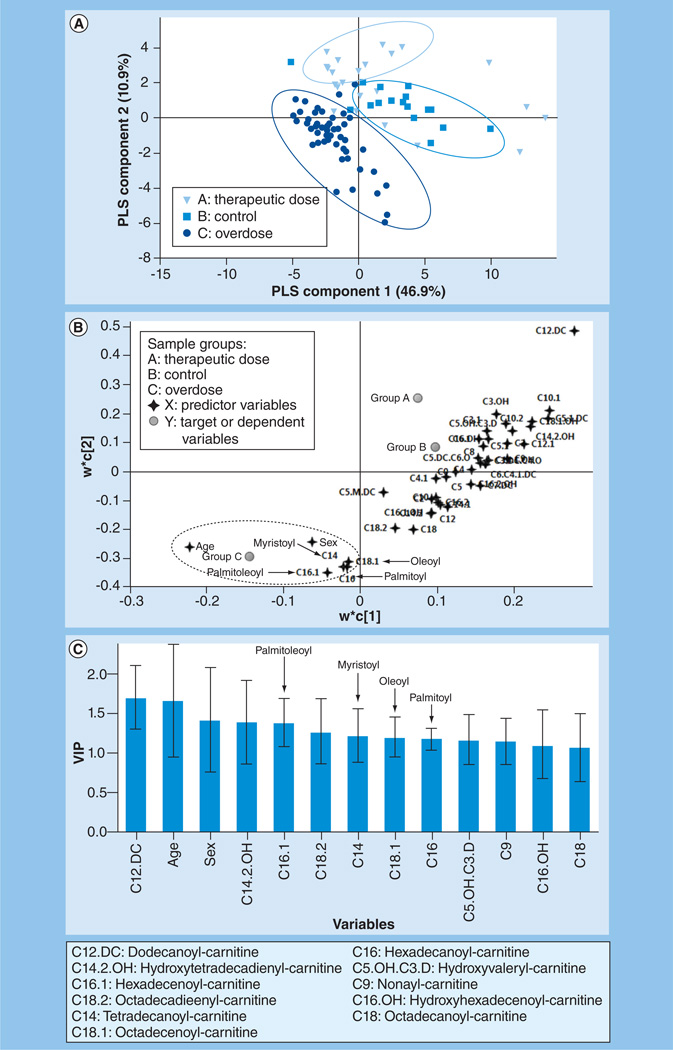
PLS-DA was performed to explore the maximum separation between the three subject groups based on the Biocrates Life Sciences AG data. The sample distribution pattern in the PLS-DA scores plot (Figure 2A) indicated clear separation among the three groups but the separation in group C was more pronounced than that of group A and B. This observation is consistent with the severity of toxicity status (ALT elevation) in group C versus groups A and B. The two-component PLS-DA model captured approximately 58% of the variation in X variables (predictor variables: acylcarnitines, age and sex) that correlated with approximately 40% of variation in the three groups. The goodness-of-predictability parameter (Q2[Y]) was approximately 35%, which was calculated by a seven-round internal cross-validation of the data. The model was further validated by repeated permutations (n = 500) of the sample identifiers. As shown in the corresponding loading plot (Figure 2B) and the VIP plot (Figure 2C), the major long-chain acylcarnitine species, palmitoyl-, oleoyl-, myristoyl-and palmitoleoyl-carnitines, were positively associated with the APAP overdose group and were among the significant contributors to the PLS-DA model (VIP >1.0) in addition to age and gender.
Figure 2. Partial least squares discriminant analysis model describing the relationship between the acylcarnitines (Biocrates Life Sciences AG), age and gender with the toxicity status of the three subject groups.
(A) Scores plot showing separation between the three subject groups along the two PLS components. The goodness of fit (R2) and goodness of predictability (Q2) of the model were approximately 58 and 35%, respectively. (B) The corresponding loadings plot of the PLS discriminant analysis scores plot shown in (A) demonstrating the relationship between the acylcarnitine predictors and age and gender along the two PLS components.
The plot shows that the long-chain acylcarnitines, oleoyl-, palmitoyl-, palmitoleoyl- and myristoyl-carnitines correlated with the overdose group (group C). (C) VIP plot of the significant predictors showing their pooled contribution over the PLS components. The acylcarnitines with VIP >1 are presented.
PLS: Partial least squares; VIP: Variable importance on the projections.
Quantitation of changes in long-chain acylcarnitines in the three groups of children
A separate analysis of free carnitine and the long-chain acylcarnitines was performed using ultra performance liquid chromatography–triple-quadrupole mass spectrometry (UPLC–TQ MS). Summary statistics of the concentrations (µM) of l-carnitine, acetyl-, myristoyl-, oleoyl- and palmitoyl-carnitines in the three subject groups are provided in Table 2. Among the long-chain acylcarnitines, concentrations of palmitoyl- and oleoyl-carnitine differed among the three groups (p < 0.001). Pairwise comparisons showed that oleoyl was increased in both the therapeutic and overdose groups compared with the control (p < 0.001). However, no differences in oleoyl-carnitine were observed between group A and group C. In addition, palmitoyl-carnitine levels were significantly higher (p < 0.001) in both of the APAP-exposed groups compared with the control. Conversely, acetyl-carnitine, a short-chain acylcarnitine, was significantly lower in the exposure groups (A and C) compared with control. Additionally, group C subjects with significant hepatotoxicity (ALT ≥1000 IU) had median peak levels of the three long-chain acylcarnitines that were between 1.2-and 2.5-fold higher than the control group (estimates of ratio of medians: oleoyl-carnitine, 2.49 [95% CI: 0.84–6.09]; palmitoyl-carnitine, 2.38 [95% CI: 1.06–3.92]; and myristoyl-carnitine: 1.22 [95% CI: 0.50–2.23].
Table 2.
Summary statistics of acylcarnitines by ultra performance liquid chromatography–triple-quadrupole mass spectrometry in the three subject groups.
| Metabolite | Group A (µM) | Group B (µM) | Group C (µM) | Kruskal–Wallis p-value |
|---|---|---|---|---|
| l-carnitine | ||||
| Mean (SD) | 16.28 (8.41) | 14.60 (5.43) | 15.08 (5.71) | Not significant |
| Median | 15.69 | 14.97 | 14.29 | |
| Range | 1.0–63.33 | 7.65–26.59 | 0.73–28.04 | |
| Acetyl-carnitine | ||||
| Mean (SD) | 4.09 (5.83) | 1.30 (0.81) | 2.83 (4.08) | 0.019 |
| Median | 2.24 | 1.24 | 1.26 | |
| Range | 0.03–53.59 | 0.20–3.59 | 0.05–24.09 | |
| Oleoyl-carnitine | ||||
| Mean (SD) | 0.87 (0.58) | 0.20 (0.28) | 0.64 (0.49) | <0.001 |
| Median | 0.81 | 0.12 | 0.62 | |
| Range | 0.02–3.88 | 0.02–1.17 | 0.01–1.76 | |
| Myristoyl-carnitine | ||||
| Mean (SD) | 0.02 (0.02) | 0.01 (0.01) | 0.02 (0.02) | Not significant |
| Median | 0.01 | 0.01 | 0.01 | |
| Range | 0–0.24 | 0–0.04 | 0–0.09 | |
| Palmitoyl-carnitine | ||||
| Mean (SD) | 0.15 (0.17) | 0.06 (0.04) | 0.13 (0.09) | <0.001 |
| Median | 0.11 | 0.06 | 0.12 | |
| Range | 0.01–1.49 | 0.01–0.17 | 0.02–0.38 | |
Group A: therapeutic dose, n = 187; group B: healthy controls, n = 23; group C: overdose, n = 62.
SD: Standard deviation.
Effect of age, sex & NAC treatment on long-chain acylcarnitines
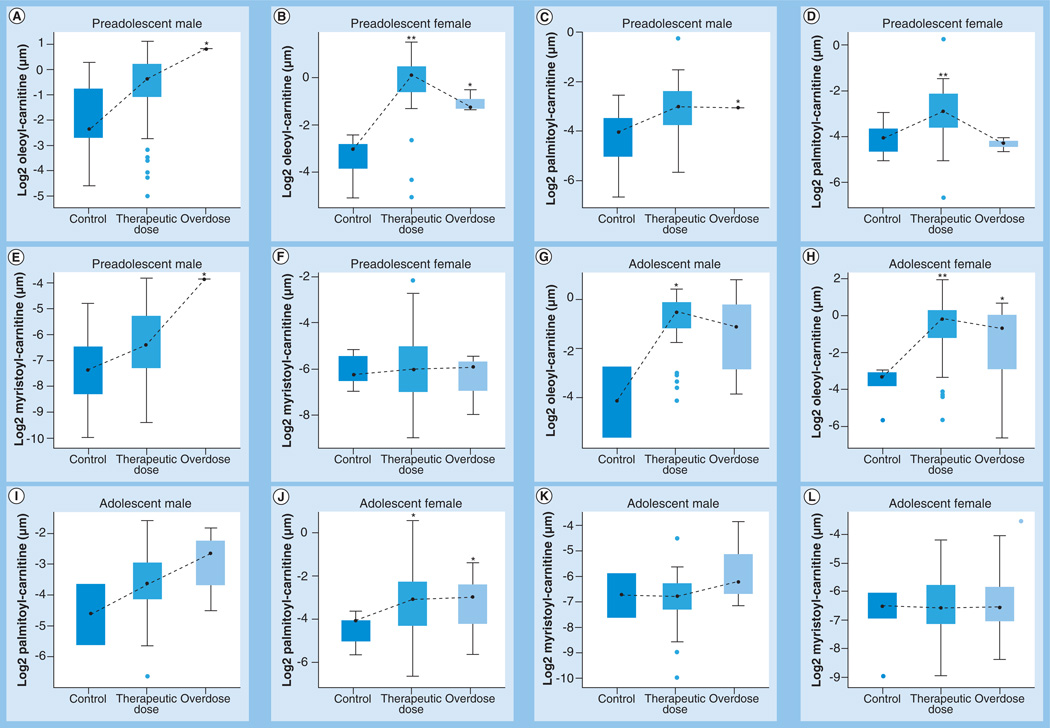
As age and sex were significant covariates in the PLS-DA model, the data were stratified by age and gender as preadolescent male (age <11 years; n = 73), preadolescent female (n = 51), adolescent male (age between 11 and 18 years; n = 52) and adolescent female (n = 96). Significant differences in acylcarnitines were observed between the APAP-exposed groups and controls (Figure 3). In both preadolescent males and females, oleoyl-carnitine was tending towards statistical significance(0.1 > p > 0.05) in the overdose group compared with control. In preadolescent females, both oleoyl- and palmitoyl-carnitines were significantly higher in the therapeutic dose group compared with control. In the adolescent group, oleoyl-carnitine was significantly different between the therapeutic dose group and control, while palmitoyl-carnitine was tending towards statistical significance in both APAP-exposed groups versus control in the females. Overall, after stratifying subjects for age and sex, the data showed significant increases in oleoyl- and palmitoyl-carnitines in the APAP-exposed groups compared with healthy controls.
Figure 3. Differences in the long-chain acylcarnitine levels in logarithmic scale between the three groups, stratified by age and gender (see facing page).
In the box plots, the upper ends represents the third quartile, lower ends the first quartile and the points in between the median. The upper whiskers represent the maximum within 1.5-times the interquartile range of the upper quartile while the lower whiskers represent the minimum within the 1.5-times the interquartile range of the lower quartile. Circles represent outliers. (A, C & E) Differences in oleoyl-, palmitoyl- and myristoyl-carnitines, respectively, in preadolescent (age <11 years) males. (B, D & F) Differences in oleoyl-, palmitoyl- and myristoyl-carnitines, respectively, in preadolescent females. (G, I & K) Differences in oleoyl-, palmitoyl- and myristoyl-carnitines, respectively, in adolescent (aged 11–18 years) males. (H, J & L) Differences in oleoyl-, palmitoyl- and myristoyl-carnitines, respectively, in adolescent females. Statistically significant increases in the two APAP dose groups compared with controls are indicated as follows: *0.05 < p < 0.1; **p < 0.05.
APAP: Acetaminophen.
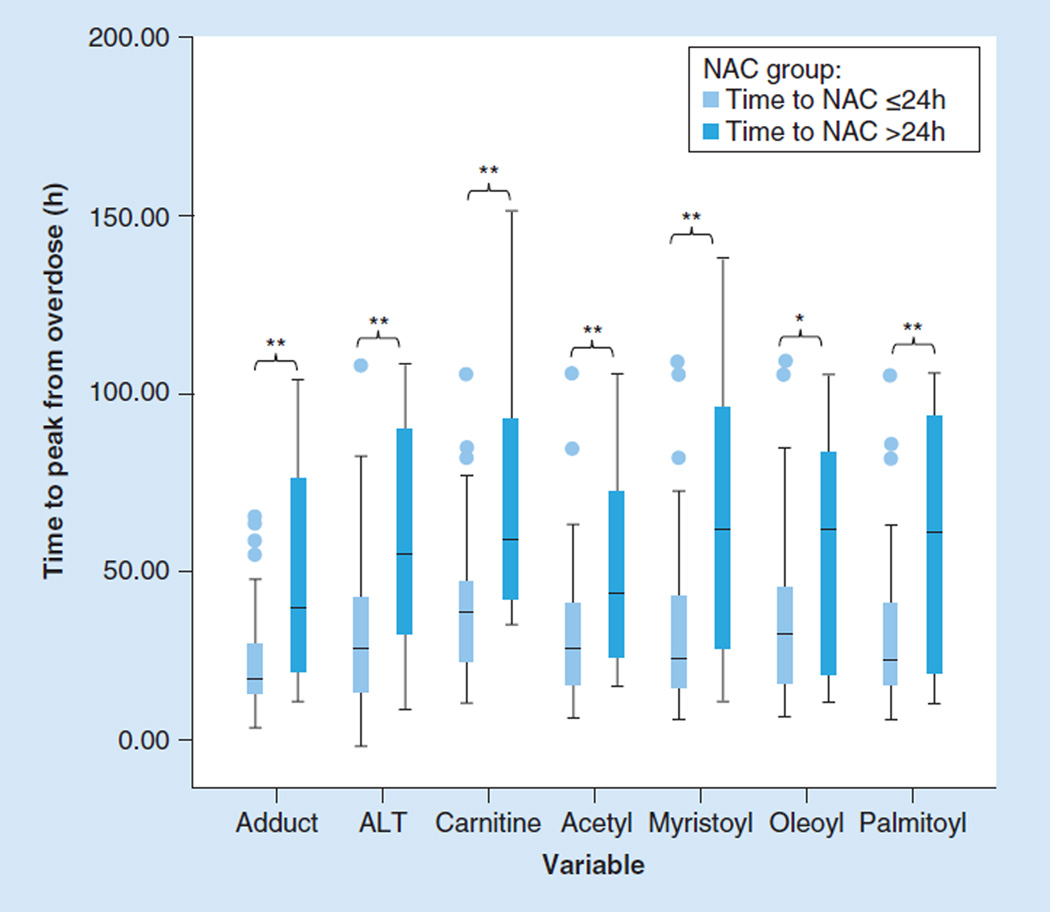
In the mouse model of APAP toxicity, oleoyl-, palmitoyl- and myristoyl-carnitines were observed to be significantly increased over the course of toxicity [16]. However, a major difference in the animal model and the clinical setting of APAP toxicity is the impact of treatment with NAC. It is well established that the efficacy of NAC treatment in preventing hepatotoxicity is highly dependent on the time the treatment is initiated related to the time of the overdose [31]. Thus, the effect of NAC treatment time (defined as time of first dose of NAC relative to the ingestion time), was examined in the group C cohort. Subjects were categorized by time to NAC treatment as early NAC (time to NAC ≤24 h) or late NAC (time to NAC >24 h). Among the 62 subjects in group C, 47 were early NAC, 12 were late NAC and three subjects had missing data on time to NAC, even though all subjects received NAC treatment. Significant differences were observed between the early and late NAC groups in time to achieve peak adduct and peak ALT levels as well as in the time to achieve peak levels of the long-chain acylcarnitines (Figure 4). It was further observed that among the toxicity and oxidative metabolism biomarkers, APAP protein adducts had the shortest time to peak, compared with carnitine, oleoyl- and palmitoyl-carnitine.
Figure 4. Statistically significant increases in ALT, acetaminophen protein adducts and acylcarnitine in subjects receiving late compared with early N-acetylcysteine treatment.
Late NAC treatment: time to NAC treatment from APAP ingestion >24 h; early NAC treatment: time to NAC treatment from APAP ingestion ≤24 h.
**p <0.05; *0.05 < p < 0.1
NAC: N-acetylcysteine.
Comparison of acylcarnitines to toxicity & metabolism biomarkers of APAP
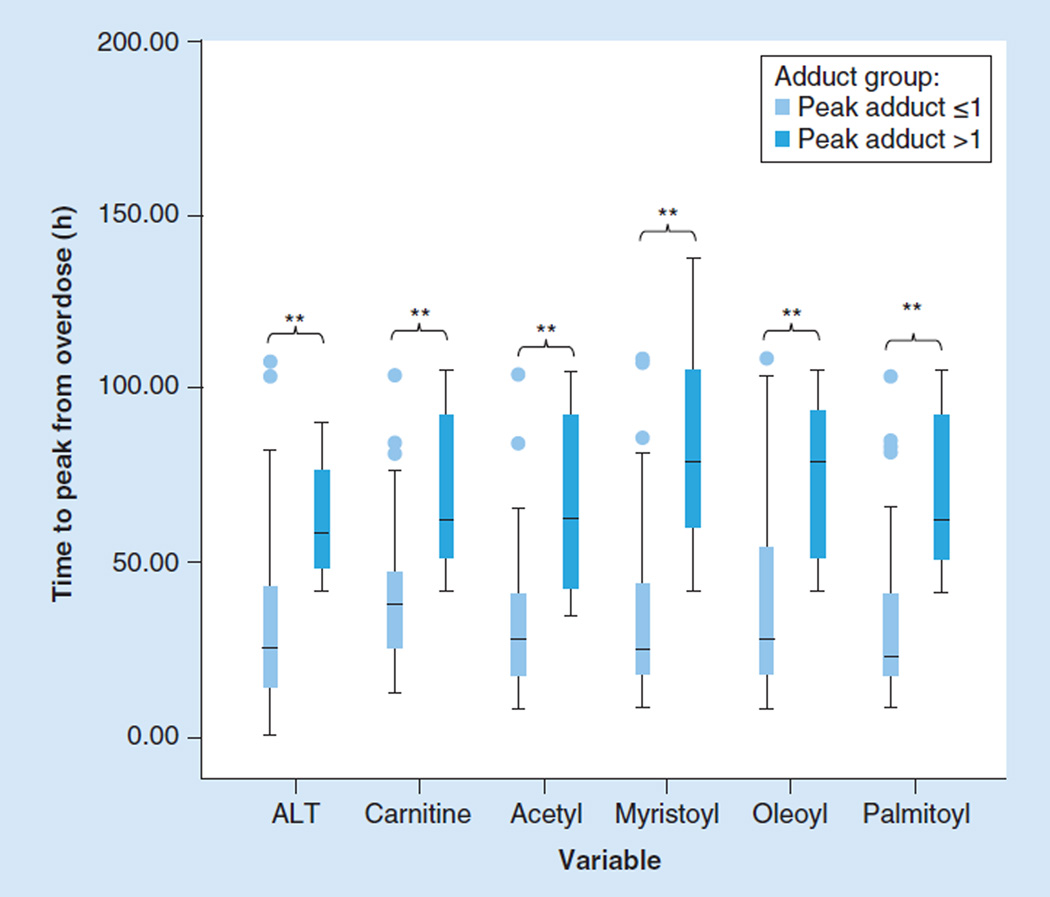
No significant associations were detected in generalized least squares regression models between the long-chain acylcarnitines, serum ALT and APAP protein adducts, while controlling for age and sex. While both APAP protein adducts and ALT levels significantly changed with time since the APAP ingestion (Figure 1B), the acylcarnitines, overall, did not have significant changes with time in the APAP overdose subjects, likely as a function of NAC treatment. In previous work, we showed that adduct levels were lower in subjects who received early treatment with NAC, compared with subjects who received later treatment with NAC [19]. In addition, we found that an APAP protein adduct level of >1.0 nmol/ml was very sensitive and specific for APAP toxicity in subjects with an ALT value >1000 IU/l [22]. Therefore, to further examine the relationship of acylcarnitines to toxicity (ALT values), the time to peak expression of the various parameters was examined. Times to achieve peak levels for each of the toxicity and metabolomic markers were significantly longer in children with APAP protein adduct levels >1.0 nmol/ml (Figure 5).
Figure 5. Evaluation of time to achieve peak level for ALT and acylcarnitines, stratified by an acetaminophen protein adduct level of 1.0 nmol/ml.
Boxplots showing significantly higher (**p < 0.05) ALT and acylcarnitine levels in subjects with high peak adduct levels, indicative of their higher toxicity status, in comparison with subjects with low peak adduct levels and low toxicity status.
Discussion
This study was initiated with the premise that serum biomarkers representing various mechanistic aspects identified in the preclinical model of APAP-induced hepatotoxicity may have potential application in the clinical setting of APAP overdose. Recent data have demonstrated the elevation of long-chain acylcarnitines (palmitoyl-,oleoyl- and myristoyl-carnitine) in mouse models of APAP-induced hepatotoxicity, indicating disruption of the fatty-acid β-oxidation pathway and mitochondrial dysfunction [17,18]. This study is the first to demonstrate significant increases in the long-chain acylcarnitines in children exposed to APAP in therapeutic doses or overdose. Exploratory metabolomic profiling of acylcarnitine levels using the AbsoluteIDQ p180 Kit showed significant changes in 13 measured acylcarnitines including the long-chain acylcarnitines palmitoyl-, oleoyl-, myristoyl- and palmitoleoyl-carnitine. The PLS-DA scores plot showed clear separation between the three sample groups based on the acylcarnitines; however, the separation was more pronounced in the overdose group, consistent with the severity of toxicity in this group, as indicated by ALT and adduct levels. We validated our findings from the PLS-DA model using a quantitative, targeted metabolomic approach using UPLC–TQ MS. Among the long-chain acylcarnitines, only palmitoyl- and oleoylcarnitine showed significant overall changes with APAP exposure compared with the control group. Furthermore, in subjects with significant hepatotoxicity (ALT ≥1000 IU), median peak levels of the three long-chain acylcarnitines were 1.2–2.5-fold above those of the control group.
Given the sporadic nature of sample collection in the subjects with therapeutic or APAP overdose (as dictated by the convenience sampling design of the study), the sample set was heterogeneous and the three groups differed significantly in age and sex, factors identified to be significant covariates in the exploratory PLS-DA model. Hence, the sample groups were stratified based on age and sex to further examine perturbations in acylcarnitines. After stratification, significant increases in oleoyl- and palmitoyl-carnitine levels persisted in the APAP-exposed groups compared with the healthy controls.
The impact of NAC treatment in the overdose subjects was of interest since NAC is the commonly used antidote for APAP toxicity [31]. NAC functions to replace the sulfhydryl pool that is depleted early in APAP toxicity. The treatment effect of NAC in the clinical setting is highly dependent of the time elapsed between the APAP overdose and the initiation of NAC treatment[31]. Ideally, NAC should be administered within 10 h of the overdose. In the APAP overdose subjects, the mean time from APAP ingestion to first serum sample available for metabolomic analysis was approximately 23 h and the mean time from ingestion to receipt of NAC was approximately 20 h. Therefore, most of the samples from the overdose subjects represented post-NAC samples. Hence, the data were examined as a function of time to NAC treatment. In three subjects for whom pre- and post-NAC acylcarnitine measurements were available, a ‘post-NAC’ decline in long-chain acylcarnitines was observed (data not shown).
A recent small study performed in two clinical centers examined acylcarnitines in 16 adults with APAP toxicity (mean ALT of ~6000 IU/l)[32]. No differences were found in the levels of three acylcarnitines (palmitoyl-, linoleoyl- and oleoylcarnitine) in the adult subjects despite significant ALT elevation. The authors of this report attributed the study findings to the effect of NAC treatment. However, specific information on the time of NAC treatment relative to the time of the overdose was not included in the report, serial sampling in APAP toxicity patients was limited to six patients and the study did not compare acylcarnitine profiles to the metabolism biomarker (APAP protein adducts). In addition, the study population in the adult study only examined acylcarnitines in patients with ALT elevation, while the present study examined acylcarnitines in patients regardless of ALT status.
Several limitations of the current study should be addressed. The study was designed to dovetail with clinical practice to the extent possible in order to minimize additional blood draws in hospitalized children. Thus, group A consisted of children who were ill and additional analysis of changes in acylcarnitines as a function of disease versus APAP exposure were not possible in this limited data set. Nevertheless, group A is representative of a clinically relevant population of patients, that is, ill children for whom the use of APAP is clinically indicated and frequently prescribed. In addition, no significant statistical differences in peak ALT values were detected between groups A and B. To further understand the relationship of acylcarnitines to low-dose APAP exposure in children, the administration of low doses of APAP to healthy children could be compared with the APAP overdose population in future studies.
Conclusion
In conclusion, this study examined the association of APAP-induced hepatotoxicity and longchain acylcarnitines in children with APAP toxicity. High-resolution LC/MS-based targeted metabolomics identified the elevation of oleoyl- and palmitoyl-carnitines in children with APAP exposure and children following APAP overdose. In children with APAP overdose, acylcarnitines were higher in children with APAP protein adducts >1.0 nmol/ml, the previously determined toxicity threshold for APAP protein adducts[22]. In addition, time to peak expression of APAP protein adducts was shorter than that of the acylcarnitines. While the specificity and rapidity of appearance for APAP protein adducts may make them preferential as a diagnostic biomarker for APAP hepatotoxicity, the acylcarnitines provide a functional and circulating biomarker associated with mitochondrial dysfunction that can be assessed in a clinical setting using the LC/MS-based metabolomic approach described here. Further study of acylcarnitines in nonhospitalized children receiving APAP is needed to understand the clinical significance of acylcarnitine elevations following low-dose APAP exposure and potentially, susceptibility to APAP-associated liver injury.
Future perspective
The association of acylcarnitine elevations with determinations of oxidative drug metabolism in APAP toxicity has application for future studies designed to understand susceptibility to APAP toxicity. For example, pediatric diseases or conditions known to be associated with impaired mitochondrial function could be examined with this approach in the future. In addition, similar approaches could be applied to study other drugs (e.g., valproic acid) that are widely used in children and are known to have effects on mitochondria and to cause liver injury [16]. Therefore, future studies should examine metabolomicbased metabolites in relation to indicators of drug metabolism and liver injury.
Executive summary.
Changes in acetaminophen toxicity markers in children exposed to acetaminophen therapeutic doses & overdoses
-
▪
While ALT remained unaltered in the therapeutic dose group of acetaminophen (APAP) exposure compared with controls, both ALT and APAP protein adducts were significantly higher in the overdose group compared with the control.
Exploratory analysis of acylcarnitines in the three groups of children
-
▪
Metabolomic analysis using the Biocrates Life Sciences AG kit, followed by targeted quantitative analysis using ultra performance liquid chromatography–triple-quadrupole mass spectrometry, showed significant increases in oleoyl- and palmitoyl-carnitines with APAP exposure (therapeutic and overdose).
Quantitation of changes in long-chain acylcarnitines in the three groups of children
-
▪
In the overdose group, subjects with significant hepatotoxicity, as indicated by ALT levels >1000 IU, showed 1.2–2.5-fold increase in peak acylcarnitine levels compared with the control group.
Effect of age, sex & N-acetylcysteine treatment on long-chain acylcarnitines
-
▪
When stratified by age and sex, oleoyl- and plamitoyl-carnitines were significantly higher with APAP exposure compared with controls.
-
▪
Significant differences in acylcarnitines were observed in the overdose children as a function of time to initiation of N-acetylcysteine (NAC) treatment. Acylcarnitines were higher in children with delayed NAC treatment time (NAC >24 h).
Comparison of acylcarnitines to toxicity & metabolism biomarkers of APAP
-
▪
Peak levels of APAP protein adducts occurred before peak elevations of acylcarnitines and ALT.
Acknowledgements
The authors are indebted to their medical and nursing colleagues as well as the children and their parents who agreed to take part in this study. The following site coordinators participated in this study: Lee Howard, Arkansas Children’s Hospital Research Institute, Section of Clinical Pharmacology and Toxicology, Arkansas Children’s Hospital; Missi Thomas, Kosair Charities Pediatric Clinical Research Unit/University of Louisville/Kosair Children’s Hospital; Michael Venneman, Children’s Mercy Hospital and Clinics; Elaine Williams, Children’s National Medical Center; Amanda Hodge and Tonia Polanski, Akron Children’s Hospital; Juli Kidd, Cook Children’s Health Care System; Evan R Hempel, KAI Research, Inc, an Altarum Company.
This work was funded in part by a grant (R01 DK75936 to LP James) from the National Institutes of Diabetes, Digestive and Kidney Diseases and the Arkansas Biosciences Institute, which is funded by Arkansas Tobacco Settlement Funds. LP James is part owner of Acetaminophen Toxicity Diagnostics, LLC and has a patent pending for the development of a commercial assay for measurement of acetaminophen protein adducts.
Footnotes
Disclaimer
The views presented in this article are those of the authors and do not necessarily reflect those of the NIH, Arkansas Biosciences Institute or the US FDA. No official endorsement is intended nor should be inferred.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Contributor Information
Sudeepa Bhattacharyya, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA; Arkansas Children’s Hospital Research Institute, Little Rock, AR 72202, USA.
Ke Yan, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Lisa Pence, Division of Systems Biology, National Center for Toxicological Research, US Food and Drug Administration, Jefferson, AR 72079, USA.
Pippa M Simpson, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Pritmohinder Gill, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA; Arkansas Children’s Hospital Research Institute, Little Rock, AR 72202, USA.
Lynda G Letzig, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA; Arkansas Children’s Hospital Research Institute, Little Rock, AR 72202, USA.
Richard D Beger, Division of Systems Biology, National Center for Toxicological Research, US Food and Drug Administration, Jefferson, AR 72079, USA.
Janice E Sullivan, Departments of Pediatrics & Pharmacology & Toxicology, Kosair Charities Pediatric Clinical Research Unit, University of Louisville, Louisville, KY 40202, USA; Kosair Children’s Hospital, Louisville, KY 40202, USA.
Gregory L Kearns, Division of Pediatric Pharmacology, Medical Toxicology & Therapeutic Innovation, Children’s Mercy Hospital, Kansas City, MO 64108, USA.
Michael D Reed, Division of Clinical Pharmacology & Toxicology, Department of Pediatrics, Northeast Ohio Medical University, Akron, OH 44038, USA; The Rebecca D Considine Research Institute, Akron Children’s Hospital, Akron, OH 44308, USA.
James D Marshall, Children’s Health Care System, Fort Worth, TX 76104, USA.
John N Van Den Anker, Division of Pediatric Clinical Pharmacology, Children’s National Medical Center, Washington, DC 20010, USA.
Laura P James, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA; Arkansas Children’s Hospital Research Institute, Little Rock, AR 72202, USA; Departments of Pharmacology & Toxicology, University of Arkansas for Medical Sciences, Little Rock, AR 72205, USA.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J. Pediatr. 2006;148(5):652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973;187(1):185–194. [PubMed] [Google Scholar]
- 4.Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187(1):211–217. [PubMed] [Google Scholar]
- 5.Reid AB, Kurten RC, McCullough SS, et al. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005;312(2):509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- 6. Jaeschke H, Gores GJ, Cederbaum AI, et al. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. Addresses specific mechanisms of hepatotoxicity.
- 7.Agarwal R, MacMillan-Crow LA, Rafferty TM, et al. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J. Pharmacol. Exp. Ther. 2011;337(1):110–116. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Ando Y, Ahlbory-Dieker D, et al. Systems biology investigation to discover metabolic biomarkers of acetaminopheninduced hepatic injury using integrated granscriptomics and metabolomics. J. Mol. Biomark. Diagn. 2013;(S1) 002. [Google Scholar]
- 9.Chen C, Hennig GE, McCann DJ, et al. Effects of clofibrate and indocyanine green on the hepatobiliary disposition of acetaminophen and its metabolites in male CD-1 mice. Xenobiotica. 2000;30(11):1019–1032. doi: 10.1080/00498250010002252. [DOI] [PubMed] [Google Scholar]
- 10.Shankar K, Vaidya VS, Apte UM, et al. Type 1 diabetic mice are protected from acetaminophen hepatotoxicity. Toxicol. Sci. 2003;73(2):220–234. doi: 10.1093/toxsci/kfg059. [DOI] [PubMed] [Google Scholar]
- 11. Manautou JE, Hoivik DJ, Tveit A, et al. Clofibrate pretreatment diminishes acetaminophen’s selective covalent binding and hepatotoxicity. Toxicol. Appl. Pharmacol. 1994;129(2):252–263. doi: 10.1006/taap.1994.1250. Investigates the mechanistic basis for peroxisome proliferators-mediated protection against acetaminophen (APAP) hepatotoxicity.
- 12.Manautou JE, Emeigh Hart SG, et al. rotection against acetaminophen hepatotoxicity by a single dose of clofibrate: effects on selective protein arylation and glutathione depletion. Fundam. Appl. Toxicol. 1996;29(2):229–237. doi: 10.1006/faat.1996.0026. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Krausz KW, Idle JR, et al. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J. Biol. Chem. 2008;283(8):4543–4559. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi H, Li F, Krausz KW, Qu A, Johnson CH, Gonzalez FJ. Targeted metabolomics of serum acylcarnitines evaluates hepatoprotective effect of Wuzhi tablet (Schisandra sphenanthera extract) against acute acetaminophen toxicity. Evid. Based Complement. Alternat. Med. 2013;2013:985257. doi: 10.1155/2013/985257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Sun J, Petrova K, et al. Metabolomics evaluation of the effects of green tea extract on acetaminophen-induced hepatotoxicity in mice. Food Chem. Tox. 2013;62:707–721. doi: 10.1016/j.fct.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Werner T, Treiss I, Kohlmueller D, et al. Effects of valproate on acylcarnitines in children with epilepsy using ESI–MS/MS. Epilepsia. 2007;48(1):72–76. doi: 10.1111/j.1528-1167.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Krausz KW, Shah YM, et al. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminopheninduced hepatotoxicity. Chem. Res. Toxicol. 2009;22(4):699–707. doi: 10.1021/tx800464q. Reveals the mechanistic aspects of APAP toxicity by serum metabolomics and shows irreversible inhibition of fatty acid oxidation by APAP in the mouse model.
- 18.Bhattacharyya S, Pence L, Beger R, et al. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Metabolites. 2013;3(3):606–622. doi: 10.3390/metabo3030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James LP, Capparelli EV, Simpson PM, et al. Network of Pediatric Pharmacology Research Units, National Institutes of Child Health and Human Development. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin. Pharmacol. Ther. 2008;84(6):684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davern TJ, 2nd, James LP, Hinson JA, et al. Measurement of serum acetaminophenprotein adducts in patients with acute liver failure. Gastroenterology. 2006;130(3):687–694. doi: 10.1053/j.gastro.2006.01.033. Demonstrates APAP protein adducts as a highly sensitive and specific biomarker of APAP toxicity.
- 21.Khandelwal N, James LP, Sanders C, et al. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53(2):567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab. Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James LP, Chiew A, Abdel-Rahman SM, et al. Acetaminophen protein adduct formation following low-dose acetaminophen exposure: comparison of immediate-release vs extended-release formulations. Eur. J. Clin. Pharmacol. 2013;69(4):851–857. doi: 10.1007/s00228-012-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muldrew KL, James LP, Coop L, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30(4):446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BH, Broadhurst DI, Mandal R, et al. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS ONE. 2012;7(12):e50520. doi: 10.1371/journal.pone.0050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS ONE. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illig T, Gieger C, Zhai G, et al. A genomewide perspective of genetic variation in human metabolism. Nat. Genet. 2010;42(2):137–141. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences (2nd Edition) McGraw-Hill, NY, USA: 1988. [Google Scholar]
- 29.Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. Brooks/Cole, CA, USA: 2009. [Google Scholar]
- 30.Varmuza K, Filzmoser P. Introduction to Multivariate Statistical Analysis in Chemometrics. Fl, USA: Taylor & Francis-CRC Press; 2009. [Google Scholar]
- 31. Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N. Engl. J. Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. Evaluates efficacy of APAP antidote. N-cetylcysteine in APAP overdose.
- 32.McGill RM, Li F, Sharpe MR, et al. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2013 doi: 10.1007/s00204-013-1118-1. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]