Abstract
There has been a dramatic increase in the number of genetic tests available but few tests have practice guidelines. In addition, many tests have become available outside of genetics clinics through direct-to-consumer (DTC) companies and several offer tests not considered standard of care. To address several practical challenges associated with the rapid introduction of clinical and DTC genetic tests, we propose that genetic counselors and geneticists organize expert panels in their institutions to discuss the integration of new tests into patient care. We propose the establishment of Genetic Testing Integration Panels (GTIPs) to bring together local experts in medical genetics, genetic counseling, bioethics and law, health communication and clinical laboratory genetics. We describe key features of this approach and consider some of the potential advantages and limitations of using a GTIP to address the many clinical challenges raised by rapidly emerging clinical and DTC genetic tests.
Keywords: genetic testing, direct-to-consumer genetic testing (DTC), genetic tests, Genetic Testing Integration Panels (GTIPs), ethical issues, whole genome sequencing, multiplex tests
Managing a deluge of new genetic tests
Recent advances in human genetics have produced exponential increases in the number of genetic tests available to clinicians. In 1999, the year the White House announced the completion of the initial draft of the human genome, there were approximately 700 clinical genetic tests available (GeneTests). By June 2011, over 2300 genetic tests were listed in the GeneTests database, an increase of more than 200% (GeneTests). To our knowledge, no other medical specialty has been faced with the introduction of several hundred new tests in such a short time period. This dramatic expansion in genetic tests available to clinicians has not been accompanied by proper vetting of all tests nor a corresponding increase in the number of practice guidelines. Without such guidance, individual genetic counselors, clinical geneticists, and other healthcare professionals face challenging decisions about the utilization of novel genetic tests, particularly when a test’s sensitivity and clinical implications have not been firmly established by peer-reviewed studies.
Very few genetic tests have been formally evaluated by either of the two major professional societies that support clinical genetics professionals in the United States, the American College of Medical Genetics (ACMG) and the National Society of Genetic Counselors (NSGC). As of June 2011, the ACMG had released 15 disease/phenotype-specific standards and guidelines, 27 practice guidelines, and 23 policy statements (American College of Medical Genetics 2011a, b, c). The NSGC, as of June 2011, had issued 11 practice guidelines and 13 position statements, several of which deal with broader issues (e.g. nondiscrimination or recurrent miscarriages) and not testing for specific genetic conditions (National Society of Genetic Counselors 2011a, b). Many of these ACMG and NSGC guidelines and policy statements were published several years ago, and may no longer be up-to-date given advances in testing for specific genetic conditions, declining sequencing costs and the emergence of new methodologies to test for hundreds of conditions simultaneously, and changing societal attitudes about genetic testing. Indeed, in a rapidly changing field like genetics, it is no longer reasonable to expect that professional societies can establish test-specific practice guidelines that keep pace with the introduction of new tests. The development of practice standards and guidelines is a time-consuming process, often taking years and requiring multiple expert and committee reviews prior to their adoption by an appropriate medical board or professional organization.
The lack of professional guidance on the use of genetic tests and oversight of genetic testing has also been recognized by the federal government. Efforts to address these issues included the appointment of the Secretary’s Advisory Committee on Genetic Testing (SACGT) in 1999 (Secretary’s Advisory Committee on Genetic Testing) and subsequently the Secretary’s Advisory Committee on Genetics, Health and Society (SACGHS) in 2002 (Secretary’s Committee on Genetics, Health and Society). In addition, EGAPP (Evaluation of Genomic Applications in Practice and Prevention) was initiated by the Centers for Disease Control and Prevention (CDC) in 2004 to establish an evidence-based process for evaluating genetic tests and their clinical application (Teutsch et al. 2009). The SACGT and SACGHS committees have since been disbanded. As of September 2011, EGAPP had issued eight evidence reports and six recommendations regarding use of genetic tests (Evaluation of Genomic Applications in Practice and Prevention Initiative 2011a, b). The genetic testing reports and recommendations of these groups took years to complete for just a few conditions and while helpful, have still left major gaps in providing guidance about the use of genetic tests.
The introduction of multiplex tests and eventually whole genome sequencing, which yield a large volume of results, means that the traditional approach to pre- and post-test counseling and informed consent will need to be reassessed and new approaches developed (Sharp 2011). As daunting as these challenges may seem, they are perhaps more difficult still when one considers that many genetic tests are now available outside of traditional clinical settings. A number of direct-to-consumer (DTC) genetic testing companies have been established over the past several years. According to the Genetics and Public Policy Center, as of May 28, 2010, 29 DTC companies were offering a range of genetic testing products and services. (Genetics & Public Policy Center 2010). While some of the tests offered DTC are also offered in clinical settings, many are not, including nutrigenomic tests, tests for specific traits (e.g. athletic ability) and tests evaluating risks of developing complex diseases (e.g. diabetes) based on association studies for which there currently is not sufficient evidence to support clinical use.
There are many factors to consider in deciding whether genetic testing is clinically indicated, which specific test is most appropriate, and which laboratory to utilize (Faucett and Ward 2009; Uhlmann 2009). For DTC tests, patients can decide to have genetic testing done independently without any involvement from a healthcare provider and without consideration of their personal or medical history to determine whether testing is in fact clinically indicated. Patients may have DTC genetic testing and then contact their healthcare providers to request assistance in interpreting test results (Giovanni et al. 2010; Powell et al. 2011). The healthcare provider is placed in the difficult situation of not being consulted before the patient initiated genetic testing and then being asked afterwards to assess the potential implications of results from tests that are not considered standard of care. Of concern is also the problem of misattributed equivalence where patients and physicians can potentially think the results of a DTC genomic test are as robust and accurate as a clinically ordered genetic test (Eng and Sharp, 2010). Patients may also be falsely reassured by DTC results which put them at low risk and as a result, decline clinically recommended genetic testing.
The volume of data in genetic test reports can amount to a number of pages and be overwhelming to both patients and healthcare providers. Most healthcare providers, including specialists in genetics, have limited familiarity with DTC tests and the population-based genome-wide association studies (GWAS) that are used to establish the provided risk estimates. In addition, knowledge of statistics may be limited and understanding differences between risks presented as relative risks, absolute risks and odds ratios may be confusing. Compounding interpretation is the fact that the same sample sent to different companies can yield different results (Ng et al. 2009; United States Government Accountability Office 2010). Although existing genetics professional organizations’ statements on DTC testing all urge caution in using these tests, none provide specific guidance about how to counsel the patient seated in front of you requesting interpretation of their test results (American College of Medical Genetics 2008; American Society of Human Genetics 2007; National Society of Genetic Counselors 2007). As a result, individual practitioners are left to struggle with the interpretation of DTC results on their own.
To address some of the practical challenges associated with the significant increase in clinical and DTC genetic tests and rapid pace of their introduction, we propose that genetic professionals consider organizing expert panels in their institution or local genetics community to discuss testing issues and begin developing guiding principles and points to consider for the use in patient care of new genetic tests and even existing genetic tests with no practice guidelines. These expert panels can complement the work of genetics professional organizations and inform the development of future practice guidelines.
We propose the creation of local Genetic Testing Integration Panels (GTIPs) that draw together available experts in medical genetics, genetic counseling, bioethics and law, health communication and clinical laboratory genetics. We see GTIPs as providing a local mechanism to obtain input on global issues regarding the use of genetic tests and helping to address the absence of guidance resulting from the rapid pace of test introduction and lack of practice guidelines. We describe key features of this approach and consider some of the potential advantages and limitations of using a GTIP to address global testing issues and assist in managing the many clinical challenges raised by rapidly emerging clinical and DTC genetic tests.
Origins of the approach: From translational bioethics research to genomic integration
The approach we describe has its origins in a research study examining genetic professionals’ views regarding the return of diagnostic results from new forms of genetic testing titled “Presenting Diagnostic Results from Large-Scale Clinical Mutation Testing” (R01 HG004500; PI: Sharp RR). This study, funded by the Ethical, Legal and Social Implications Program of the National Human Genome Research Institute, sought to characterize how genetics professionals view the types of diagnostic possibilities and results that should be discussed with patients in the context of very large-scale forms of mutation analysis. Participants in this R01 study were invited to participate in a series of four two-hour Working Group meetings over an 18-month period in which they were asked to: 1) consider the potential utility of various clinical applications of highly multiplexed forms of genetic testing, 2) discuss practical challenges associated with the interpretation and communication of results from highly multiplexed forms of genetic testing, 3) identify ethical and legal considerations raised by the use of genomic tests in patient care settings, and 4) describe their overall levels of enthusiasm and concern regarding emerging forms of genomic testing. The more global aim of the study was to develop effective pre- and post-test counseling strategies for discussing results from highly multiplexed forms of genetic testing.
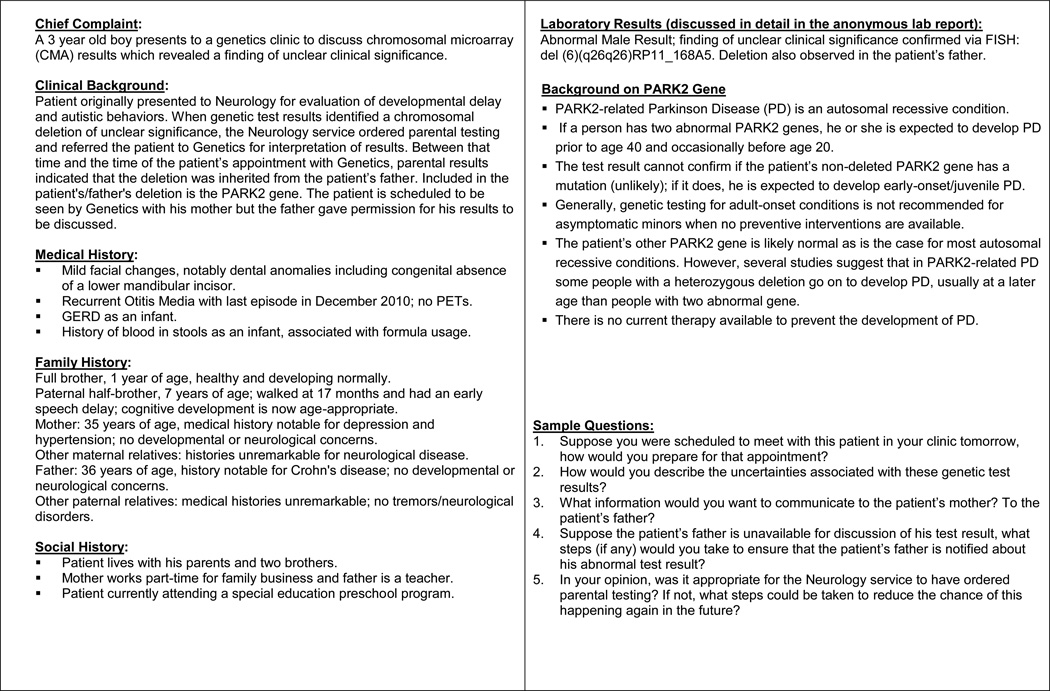
To increase the practical relevance of Working Group discussions, detailed case studies were used to examine how genetic professionals would approach challenging clinical situations, such as how to disclose and explain results to patients when genetic testing is done for a very large number of genetic conditions, how to address ambiguous results or how to return incidental findings that are not directly relevant to the testing indication. These case studies included a summary of a hypothetical patient’s case history, as well as an anonymous laboratory report. Participants were asked to consider how they would prepare for a clinical appointment in which they would present these test results to a patient and provide counseling about their interpretation. To allow for an extended and clinically nuanced discussion of relevant practical considerations, each two-hour session focused on just 2-3 case studies. Individual case studies examined currently available genetic tests and were developed to examine both clinically administered and DTC products (Figure 1).
Figure 1.
A sample clinical case scenario to facilitate GTIP discussions of chromosomal microarray results.
Working Groups were established in six US cities where there are well established programs in medical genetics and translational genomic research: Ann Arbor, Michigan; Baltimore, Maryland; Cleveland, Ohio; Denver, Colorado; Philadelphia, Pennsylvania; and Seattle, Washington. To promote diversity across study sites, we selected these cities based on their geographical location, current training programs in medical genetics and genetic counseling, genomic research programs, and clinical practice settings (e.g. pediatric vs. adult clinics, public vs. private institutions, etc.). At each site, we recruited nine to twelve specialists in genetics to serve as members of these Working Groups. In addition to clinical geneticists and genetic counselors, Working Groups typically included specialists in bioethics, health communication, law, primary-care medicine, and directors of clinical genetics laboratories. Working Group discussions were undertaken as a research activity, which was approved by the appropriate Institutional Review Boards at each study site. Participants provided written consent to participate in the study as a Working Group member and received a modest honorarium for their participation.
Sessions were facilitated by the principal investigator (RRS). Working Group discussions were audiotaped and transcribed to enable qualitative analysis. Although data analysis is ongoing, we recognized that these Working Groups could be useful outside of research settings, as a unique forum for exploring how novel genetic tests might be integrated into clinical practice. This opinion was voiced by several Working Group participants as well, who commented that they would like to continue meeting after the study ended: “We want to try to continue. … We don’t all get together [very often] and just to have these discussions with the different perspectives has been very valuable.”
We found that these Working Groups provided a neutral environment where the challenges raised by new genetic testing options could be openly discussed. Most of the Working Groups, whether from the same or different institutions, included genetic professionals who tended not to work closely together and practiced in different clinics including, adult, pediatrics, cancer and reproductive genetics. The Working Groups also included experts from outside the genetics community, who brought different perspectives than genetics clinicians and through their input and clarifying questions helped participants think more globally about current and future approaches to genetic testing and patient care. For several Working Group members, these sessions also provided them with their first opportunity to review multiplexed genetic test results and reports from DTC testing laboratories.
We believe that having an outside facilitator frame questions for discussion promoted an openness among participants and helped to “level the playing field” with respect to differences of power that can exist between physicians and non-physicians and between those in medical and non-medical professions. The result was a rich discussion of relevant issues, in which all participants were encouraged and felt empowered to share their respective opinions without fear of dismissive reactions by others and in a supportive setting that encouraged creative thinking. Working Group discussions also fostered a more academic engagement of relevant challenges, with much greater emphasis on intellectual debate and discussion than is typically available within the confines of a specific genetics clinic. Of note, there was considerable consistency of approach across the six Working Groups, particularly with respect to their identification of pertinent challenges associated with emerging clinical and DTC genetic tests. In particular, Working Group participants consistently drew attention to challenges of pre-test counseling for tests that generate a diverse range of results, such as test products that evaluate both rare and common disease associations. Similarly, participants consistently expressed concerns about their ability to stay abreast of new DTC testing options and interpret results from these tests.
Creating a Genetic Testing Integration Panel (GTIP)
Based on our personal experiences working with clinical genetic professionals in the context of the study described above, and the feedback we received from our Working Group members, we recommend that genetic counselors and geneticists consider establishing a GTIP at their institution. The purpose of the GTIP would be to establish an environment in which genetic professionals can come together with experts outside their respective clinics and personal areas of expertise to consider how best to manage the many clinical issues and implementation challenges associated with emerging clinical and DTC genetic tests.
A well-organized GTIP could provide advice on both system integration challenges and genetic counseling dilemmas associated with novel genetic tests (Table 1). In addition to considering these global issues, a GTIP could provide the opportunity for clinicians to share their experiences and approaches using novel genetic tests and factors considered and addressed pre- and post-testing. The GTIP could provide a forum for multiple clinicians and experts to weigh in on cases that pose particularly difficult interpretive, diagnostic or ethical challenges. Some of the practical issues a GTIP might consider include: How should the many potential results from multiplex forms of genetic testing be discussed in pre-test counseling sessions? How many genetic findings or genetic risk factors is it reasonable to review in a single counseling session? What communication methods tend to be most effective in promoting patient retention of large amounts of genetic information over time? In addition, GTIPs may be useful in establishing standard approaches to system integration challenges, such as whether DTC results should be routinely included in a patient’s medical record or how to determine appropriate utilization of costly genetic tests and evolving tests, such as whole-genome or exome sequencing.
Table I.
Examples of clinical challenges raised by multiplexed and DTC genetic tests.
| System Integration Challenges |
|
| Genetic Counseling Challenges |
|
These panels of local experts in clinical genetics could incorporate many of the key elements of the Working Groups described above. For example, GTIPs could include genetics professionals working in different clinical settings, directors of clinical genetics laboratories, and specialists in related fields such as bioethics and health communication; GTIP discussions could also be organized around case studies that highlight dilemmas or specific challenges associated with emerging clinical and DTC genetic tests; and, finally, GTIPs could be moderated by someone who is not directly involved in the day-to-day operations of a genetics clinic to encourage more open communication among members. This way of structuring a GTIP also has parallels to tumor boards commonly used to engage local experts in discussions of difficult cases in oncology.
Since some genetic tests are ordered routinely by non-geneticists, it is important that GTIPs also include one or more physicians in primary care, such as pediatrics or obstetrics. Where applicable, GTIPs should also include genetic professionals across multiple local institutions, to encourage open exchange of ideas. If relevant experts are not available locally, outside experts might be able to participate by telephone or by computer using Skype or a similar program. Since a primary aim of a GTIP is to foster creative thinking about difficult questions, it is important to have a range of disciplines and institutional perspectives included. Lacking that diversity, GTIPs may simply perpetuate current thinking and be less likely to generate new approaches to managing novel genetic tests.
We anticipate that many GTIPs will choose to focus on global genetic testing issues, such as whether and how genetic testing should be integrated into patient care. In addition, similar to a tumor board, challenging patient-care issues might be brought forward by GTIP members or non-members for discussion. Nonetheless, discussions of actual patient test reports by GTIPs would not take the place of a clinically indicated genetics referral or further investigation by the ordering physician or laboratory into the significance of the test results. For example, if a neurologist orders a chromosome microarray analysis and obtains an abnormal result, the patient may benefit from a referral to a genetics clinic for discussion of the clinical significance of the genetic finding and to determine whether additional medical evaluations or genetic testing of the patient and/or other family members is indicated.
Based on experiences with our Working Groups, we also recommend that GTIPs be limited to no more than ten to twelve members. This number should accommodate most of the relevant areas of expertise, while enabling good discussion with all participants having an opportunity to participate. This target size is also reasonable since schedule conflicts may arise and not all members will be able to attend every GTIP meeting, resulting in a typical meeting of eight to ten individuals, based on our research project experience. In addition, designating a facilitator for each meeting, who is responsible for preparing either a list of specific issues to address or questions to discuss based on relevant cases, can help to ensure success over time. Initially, a GTIP might meet quarterly or as determined by the level of interest that exists among participants.
We believe genetic counselors are well positioned to take the lead in establishing GTIPs at their institutions. Genetic counselors are often responsible for researching and coordinating genetic testing for patients and are generally knowledgeable about who are some of the experts at their institution, given their clinical roles and skills at identifying resources. As experts in clinical genetics, clinical geneticists and genetic counselors should lead efforts to develop “best practices” and guidelines for the use of DTC and other emerging clinical genetic tests. In addition, establishing a GTIP can serve other important functions, such as the promotion of communication across genetics clinics and more global discussions of appropriate genetic testing integration institutionally in specialties and primary care.
Fostering intra-institutional dialogue and laying the groundwork for professional standards
At an institutional level, genetics clinics may be in several different departments. In each clinic, the types and numbers of genetic tests ordered can vary significantly, with some tests ordered regularly and others ordered periodically or only on rare occasions. The test issues to address can also vary depending on the clinic setting (e.g. prenatal versus pediatric), the testing indication (e.g. symptomatic versus asymptomatic), the patient, insurance coverage and other factors. Even though clinics see different patient populations, there are genetic tests that are utilized across clinics (e.g. testing for connective tissue disorders, such as Marfan syndrome, may be done in prenatal, pediatric and adult genetics clinics). Time and institutional politics may limit general discussions about genetic testing practices. While individual clinics may meet on a regular basis with their clinical teams to go over cases and specific clinic issues, there are few opportunities for all genetics clinics at an institution to convene. There may be clinical genetics case conferences and journal clubs but these forums generally do not present opportunities to discuss the integration of genetic advances into clinical practice and generally do not involve non-geneticists. Time is already limited to address specific clinical issues let alone think globally about how genetic services and genetic testing should be integrated more widely in healthcare. There can also be “turf” issues as well, with some providers feeling that some genetic tests are more squarely in their areas of expertise. GTIPs can provide a safe harbor in which to examine what might otherwise be viewed as a potentially divisive range of issues around genetic testing.
Establishing a GTIP has the key benefit of bringing together a diverse group of local experts to consider testing issues. GTIPs provide an opportunity to highlight issues of concern across genetic testing environments and provide a recurrent forum in which to discuss these issues. GTIPs can provide an effective venue for more general reflection about the emergence of new forms of genomic testing and incorporation into patient care in addition to facilitating the development of strategies for addressing specific genetic testing dilemmas.
Ongoing debates about the regulation of DTC tests have highlighted how these tests raise issues that extend well beyond patient management to include a broader range of professional, ethical, and societal issues — and thus require input from a more diverse set of experts than are typically present in the day-to-day operations of a genetics clinic. GTIPs can provide an institutional forum in which clinical geneticists and genetic counselors can engage these difficult questions in consultation with clinical genetics laboratory directors and specialists in bioethics, health communication and law. To the extent that the creation of formal practice guidelines can take years, GTIPs may be viewed as a “stop gap” approach that allows institutions to develop interim guidance on the use of genetic tests in a more timely and efficient manner.
Genetic Testing Integration Panels initially could be established at individual institutions. Over time, if this model gains wider acceptance, organizations such as the ACMG and NSGC could provide resources to support GTIP efforts, including a secure website to share discussion questions, discuss outcomes, share GTIP recommendations, and disseminate key documents and useful case studies. Open sharing of this information would help save time for GTIPs and avoid duplication of efforts. GTIP members could also meet with other GTIP members and ACMG leadership at annual conferences. Genetic counselors who participate on GTIPs might also consider forming a Special Interest Group (SIG) within NSGC and could meet with NSGC leadership at annual conferences. By sharing the work of GTIPs through a secure website and through discussions with ACMG and NSGC leadership and potentially through publications and submitted abstracts to conferences, the work of GTIPs could be more widely disseminated and provide information useful for the development of practice guidelines. The GTIP model could also be adapted more broadly in medicine through the formation of other Testing Integration Panels (TIPs), potentially creating an effective forum for considering the integration of other new diagnostic modalities, particularly in areas of medicine that are changing quickly.
Potential implementation challenges
As an emerging model of professional deliberation, there are several unresolved questions about how best to structure a GTIP. One question is who should provide leadership on these multidisciplinary panels. While a genetic counselor or clinical geneticist could effectively facilitate a GTIP, based on our experiences in the study described above, we recommend that organizers consider the potential value added by having these sessions led by an “outsider” to the relevant genetic clinics. An outside advisor is unlikely to have clinical interests in the resolution of practical questions, which may allow that individual to lead meetings in an unbiased way and provide a more impartial perspective if tensions arise.
Given competing time demands, it is essential to consider how to encourage participants’ attendance at GTIP meetings and establish the “buy in” necessary to sustain these efforts over time. At the outset, it will be critical that support from genetics clinics be assessed, but as with any clinical endeavor, the success of a GTIP will depend on the extent to which those involved find it to be a useful activity that improves patient care and thereby supports their personal involvement.
There are several other important issues to consider in establishing GTIPs including: selection process and terms of GTIP membership, procedures for determining GTIP meeting agendas, development of clinical cases for discussion and inclusion of cases by non-GTIP members, documentation of GTIP meetings, and communication of GTIP deliberations with relevant medical communities. To the extent that many existing genetics clinics are located in major academic medical centers, we envision GTIPs beginning at those centers of academic excellence. As experience is gained and the GTIP model is better developed over time, this approach could be applied more broadly in community-based hospitals and other clinical settings. Of note, the GTIP model we propose as a starting point is based on our earlier research project, which was done in several academic medical centers. Successful translation of our GTIP model to other clinical settings will require more experience and potential modifications of this approach that leverage the strengths of the local environment.
Conclusion
The fact is DTC genetic tests are here to stay and whole-genome sequencing will become a clinical reality in a few years. At this point in time, few of us in the genetics community have had patients bring in DTC genetic test results for interpretation or have had patients inquire about this type of testing (Giovanni et al. 2010; Hock et al. 2011). As we heard in our Working Groups, even genetics professionals may be overwhelmed by the amount of data in these reports and find it difficult to interpret the data and effectively communicate results to their patients. Specialists in genetics need to be prepared to handle the vast amount of genetic test results generated by these multiplex and DTC tests and ensure that thoughtful consideration is given to the potential integration of these tests into healthcare. Genetic specialists also need to determine the types of genetic tests that require genetics expertise to order and interpret versus tests that can be ordered by other specialists and primary care providers.
The importance of establishing an institutional setting in which these multidisciplinary discussions can occur is clear when one considers the pace at which new diagnostic technologies and genetic tests are being introduced. This remarkable expansion of genetic testing options makes it very unlikely that an individual clinician will be able to keep pace with the changes taking place in medical genetics. The pace at which new genetic tests are emerging also challenges traditional approaches to guiding clinical practices by expert review, such as the issuing of practice recommendations by professional societies. Now is the time for the genetics community to initiate more robust, practically oriented conversations about the integration of new clinical and DTC tests into patient care. Establishing a GTIP can help clinicians address the gap in available practice guidelines and provide an effective forum for discussion of these challenging and complex issues.
Acknowledgments
This work was funded by grant number R01HG004500 from the National Human Genome Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Human Genome Research Institute or the National Institutes of Health. We thank the participants who generously gave their time to share their experiences and opinions with us.
References
- American College of Medical Genetics. [Accessed July 1, 2011];Disease/phenotype-specific standards and guidelines. 2011a Available from: http://www.acmg.net/AM/Template.cfm?Section=Laboratory_Standards_and_Guidelines&Template=/CM/HTML.Display.cfm&ContentID=6439.
- American College of Medical Genetics. [Accessed July 1, 2011];Practice guidelines. 2011b Available from: http://www.acmg.net/AM/Template.cfm?Section=Practice_Guidelines&Template=/CM/HTMLDisplay.cfm&ContentID=6518.
- American College of Medical Genetics. [Accessed July 1, 2011];Policy statements. 2011c Available from: http://www.acmg.net/AM/Template.cfm?Section=Policy_Statements&Template=/CM/HTMLDisplay.cfm&ContentID=6042.
- American College of Medical Genetics. [Accessed July 1, 2010];ACMG statement on direct-to-consumer genetic testing. 2008 doi: 10.109701.GIM.0000106164.59722.CE. Available from: http://www.acmg.net/AM/Template.cfm?Section=Policy_Statements&Template=/CM/ContentDisplay.cfm&ContentID=2975. [DOI] [PubMed]
- American Society of Human Genetics. ASHG statement on direct-to-consumer genetic testing in the United States. [Accessed July 1, 2011];American Journal of Human Genetics. 2007 81:635–637. Available from: http://www.ashg.org/pdf/dtc_statement.pdf. [Google Scholar]
- Eng C, Sharp RR. Bioethical and clinical dilemmas of direct-to-consumer personal genomic testing: The problem of misattributed equivalence. Science Translational Medicine. 2010;2(17):1–5. doi: 10.1126/scitranslmed.3000214. [DOI] [PubMed] [Google Scholar]
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative. [Accessed October 2, 2011];Evidence Reports. 2011a Available from: http://www.egappreviews.org/workingrp/reports.htm.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative. [Accessed October 2, 2011];Recommendations. 2011b Available from: http://www.egappreviews.org/recommendations/index.htm.
- Faucett WA, Ward PA. Understanding genetic testing. In: Uhlmann WR, Jane L Schuette, Beverly M Yashar, editors. A guide to genetic counseling. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2009. pp. 283–311. [Google Scholar]
- Genetics and Public Policy Center. [Accessed June 30, 2011];DTC Genetic Testing Companies. 2010 Available from: http://www.dnapolicy.org/resources/AlphabetizedDTCGeneticTestingCompanies.pdf.
- Gene Tests: Medical Genetics Information Resource (database online) [Accessed June 30, 2011];Copyright, University of Washington, Seattle. 1993 – 2010. Available from: http://www.genetests.org.; Growth of laboratory directory. [Accessed June 30, 2011]; Available from: http://www.ncbi.nlm.nih.gov/projects/GeneTests/static/whatsnew/labdirgrowth.shtml.
- Giovanni MA, Fickie MR, Lehmann LS, Green RC, Meckley LM, Veenstra D, Murray MF. Health-care referrals from direct-to-consumer genetic testing. Genetic Testing and Molecular Biomarkers. 2010;14(6):817–819. doi: 10.1089/gtmb.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock KT, Christensen KD, Yashar BM, Roberts JS, Gollust SE, Uhlmann WR. Direct-to-consumer genetic testing: An assessment of genetic counselors’ knowledge and beliefs. Genetics in Medicine. 2011;13(4):325–332. doi: 10.1097/GIM.0b013e3182011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Society of Genetic Counselors. [Accessed July 1, 2011];Practice guidelines. 2011a Available from: http://www.nsgc.org/Publications/PracticeGuidelines/tabid/313/Default.aspx.
- National Society of Genetic Counselors. [Accessed July 1, 2011];Position statements. 2011b Available from: http://www.nsgc.org/Media/PositionStatements/tabid/330/Default.aspx.
- National Society of Genetic Counselors. [Accessed July 1, 2011];Position statements. Direct-to-consumer genetic testing. 2007 Available from: http://www.nsgc.org/Media/PositionStatements/tabid/330/Default.aspx.
- Ng PC, Murray SS, Levy S, Venter JC. An agenda for personalized medicine. Nature. 2009;461(7265):724–726. doi: 10.1038/461724a. [DOI] [PubMed] [Google Scholar]
- Powell KP, Cogswell WA, Christianson CA, Dave G, Verma A, Eubanks S, Henrich VC. Primary care physicians’ awareness, experience and opinions of direct-to-consumer genetic testing. Journal of Genetic Counseling. 2011 doi: 10.1007/s10897-011-9390-9. Epub ahead of print July 16. [DOI] [PubMed] [Google Scholar]
- Secretary’s Advisory Committee on Genetic Testing (SACGT) Documents. [Accessed October 2, 2011]; Available from: http://oba.od.nih.gov/SACGHS/sacgt_documents.html#GT_DOC007.
- Secretary’s Advisory Committee on Genetics, Health and Society (SACGHS) Archives. [Accessed October 2, 2011]; Available from: http://oba.od.nih.gov/SACGHS/sacghs_home.html. [Google Scholar]
- Sharp RR. Downsizing genomic medicine: Approaching the ethical complexity of whole-genome sequencing by starting small. Genetics in Medicine. 2011;13(3):191–194. doi: 10.1097/GIM.0b013e31820f603f. [DOI] [PubMed] [Google Scholar]
- Teutsch SM, Bradley LA, Palomaki GE, Haddow JE, Piper M, Calonge N, Dotson WD, Douglas MP, Berg AO EGAPP Working Group. The evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: methods of the EGAPP working group. Genetics in Medicine. 2009;11(1):3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann WR. Thinking it all through: Case preparation and management. In: Uhlmann WR, Jane L Schuette, Beverly M Yashar, editors. A guide to genetic counseling. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2009. pp. 93–131. [Google Scholar]
- United States Government Accountability Office. Direct-to-consumer genetic tests: Misleading test results are further complicated by deceptive marketing and other questionable practices. [Accessed July 1, 2011];Testimony before the Subcommittee on Oversight and Investigations, Committee on Energy and Commerce, House of Representatives. Statement of Gregory Kunz, Managing Director Forensic Audits and Special Investigations. GAO-10-847T-1-29. 2010 Available from: http://www.gao.gov/new.items/d10847t.pdf.