Abstract
Background
The purpose of this study was to assess the ability of intensity-modulated radiation therapy (IMRT) to achieve favorable disease-control rates while minimizing parotid gland doses in patients treated for small primary tumors of the oropharynx.
Methods
We retrospectively identified all patients who received IMRT as treatment for a small (< 4 cm) primary tumor of the oropharynx from October 2000 through June 2002. Tumor characteristics, IMRT parameters, and patient outcomes were assessed.
Results
Fifty-one patients met the criteria for our study. All patients had treatment to gross disease with margin (CTV1), and all but 1 had treatment to the bilateral necks. The most common treatment schedule (39 patients) was a once-daily fractionation of prescribed doses of 63 - 66 Gy to the CTV1 and 54 Gy to subclinical sites, delivered in 30 fractions. Twenty-one patients (40%) had gastrostomy tubes placed during therapy; in 4 patients, the tube remained in place for more than 6 months following completion of IMRT. The median follow-up was 45 months. The 2-year actuarial local-regional control, recurrence-free, and overall survival rates were 94%, 88%, and 94%, respectively.
Conclusions
These preliminary data suggest that treatment with IMRT results in favorable local-regional control of small primary oropharynx tumors. IMRT did not appear to have a more favorable acute toxicity profile in this group with respect to the use of a feeding tube; however, the mean dose of radiation delivered to the parotid gland by IMRT was decreased, as 95% of patients had a mean dose of < 30 Gy to at least one gland.
Introduction
Intensity-modulated radiation therapy (IMRT) offers several potential benefits to patients requiring irradiation for treatment of head and neck cancers. One such benefit is the ability of IMRT to conform closely to the tumor volume, avoiding or minimizing exposure to normal tissues where irradiation can cause chronic sequelae that can adversely affect a patient’s quality of life. For example, in the treatment of tumors near critical neural tissues, IMRT has been shown to improve target coverage without increasing radiation dose to the nervous system.1, 2 The use of IMRT in the treatment of head and neck cancer has also been shown to decrease the dose to the parotid glands, leading to better recovery of salivary flow.3 Several preliminary clinical experiences have been reported suggesting favorable outcomes for patients treated with IMRT.4-6 This report focuses on a subpopulation of patients with head and neck cancer, specifically those with early T-stage oropharynx cancer, to describe outcomes in a relatively homogenous subgroup.
As target volume definitions and techniques of IMRT have been evolving, we retrospectively reviewed our experience using IMRT as treatment for small primary tumors of the oropharynx to assess the parotid dose, disease-control rates relative to conventional radiation and the influence of the fractionation schemes used in our IMRT treatments.
Methods
Patient Selection
We searched the database of the Department of Radiation Oncology and identified all patients irradiated by IMRT from October 2000 to June 2002 as treatment for a small (< 4 cm) primary squamous or undifferentiated carcinoma of the oropharynx. This 20-month period was chosen to ensure a minimum 2-year duration of follow-up for our analysis.
We have previously described favorable outcomes for these patients with small tumors of the oropharynx, irrespective of AJCC staging.7 When we initiated our IMRT program, these patients were one of the first groups selected for treatment with IMRT. Patients with larger primary tumors were offered participation in ongoing trials investigating concurrent systemic therapy and radiation, and these trials (at that time) often did not allow the use of IMRT.
Study Design
Institutional Review Board approval was obtained prior to initiation of this retrospective chart review.
In October 2000, we had clinical release of an IMRT system, which uses a static gantry, step and shoot, multileaf collimation delivery system. Treatments for our study group were planned using the CORVUS treatment planning system (CORVUS ver. 4.0; Nomos Corporation, Pittsburgh, PA). Radiation was delivered via 6-MV photons generated by a Varian linear accelerator (Varian Medical Systems, Palo Alto, CA).
We reviewed the patients’ records to determine the prescribed and delivered doses of radiation to the clinical target volume (CTV) and the respective planning target volume (PTV); the doses delivered to each parotid gland; the use of a gastrostomy tube, which was a surrogate of acute toxicity; and disease-control and survival rates.
Statistical Analysis
Kaplan-Meier actuarial analyses were performed to determine disease-control and survival rates. Patients with local disease persistence following radiation or recurrence at the primary site were considered to have failed for the calculation of local control rates. Similar criteria were used for the determination of regional recurrence with the exception that patients with viable lymph node disease found at post-radiation neck dissections were not considered treatment failures for the calculation of regional (and loco-regional) control rates, or for recurrence-free survival rates. Loco-regional persistence or recurrence as defined above, or the development of hematogenous metastasis were considered failures for the calculation of recurrence-free survival rates.
Results
Patient and Tumor Characteristics
Table 1 lists the patient and tumor characteristics for this sample.
Table 1.
Patient and tumor characteristics
| Characteristic | Number |
|---|---|
| Sex | |
| Men | 44 |
| Women | 7 |
| Smokers | |
| Current | 11 |
| Former | 16 |
| Never | 24 |
| Alcohol Use | |
| > 10 drinks/week | 10 |
| <1 – 10 drinks/week | 22 |
| Rare/never | 19 |
| Primary-tumor site | |
| tonsil | 33 |
| base of tongue | 16 |
| pharyngeal wall | 2 |
| T stage | |
| 1 | 19 |
| 2 | 18 |
| x | 14 |
| N stage | |
| 0 | 8 |
| 1 | 7 |
| 2a | 10 |
| 2b | 14 |
| 2c | 1 |
| 3 | 2 |
| x | 9 |
Fifty-four patients met the eligibility criteria for this study. Three patients were excluded from our analysis: 1 had received IMRT to a boost field only, 1 received the boost with a conventional method and the third had been switched from a 70-Gy IMRT treatment plan to conventional radiation therapy at 14 Gy. The latter patient was unable to endure the treatment time required with IMRT. Therefore, the final patient sample was composed of 51 patients.
The median age of these 51 patients was 54 years (range 30 – 75 years). The cohorts in our previously reported series of irradiated patients with small primary oropharynx cancers had median ages of 57 and 61 years,7, 8 Tobacco and alcohol use are detailed in Table 1. Compared to a series of patients from our center with Stage I and II oropharynx cancer, the current study group had a higher percentage of non-smokers and non-drinkers.8
The predominant sites of primary tumor were tonsil (33 patients) and base of tongue (16 patients). The distribution of patients by tumor (T) stage was T1, 19 patients; T2, 18; and Tx, 14. The distribution by node (N) status was N0, 8 patients; N1, 7; N2, 25; N3, 2; and Nx, 9. Tx disease was diagnosed most commonly following tonsillectomy, and Nx disease was diagnosed most commonly following excisional node biopsy.
Chemotherapy and Surgery
Five patients, including the 2 patients with N3 disease, received chemotherapy. Four patients received chemotherapy concurrent with radiation therapy, 2 received neoadjuvant chemotherapy (one of whom also received concurrent chemotherapy), and one received adjuvant chemotherapy (following concurrent chemoradiation). Twelve patients had post-irradiation neck dissections. Ten of these patients had neck dissection specimens that were without pathologic evidence of disease.
IMRT: Volume definition, dose prescription, fractionation, and dose delivered
The high-dose target volume included gross disease with margin (CTV1). The minimum margin size was 5 mm; however, a larger margin was often used, particularly in cases in which the tumor borders were not well defined on computerized tomographic imaging. One patient was treated only to the CTV1 because there was concern that treatment to a larger volume might adversely affect the patient’s tolerance due to an immunocompromised state. In the remaining 50 patients, treatment was delivered to both sides of the neck. In 49 of these patients, the lower neck (levels 3 and 4) was treated with an anterior field matched to the inferior borders of the IMRT delivery. In the other patient, the entire neck was treated with IMRT.
Forty-seven patients were treated with once-daily fractionation. The most common fractionation scheme used in 39 patients was a prescription dose of 63 - 66 Gy to the CTV1 and 54 Gy to subclinical sites in both sides of the upper neck (CTV3), both delivered in 30 fractions. In 8 of these patients treated in 30 fractions, either with T1 or Tx (post-tonsillectomy) disease, the prescribed dose to the primary ranged from 63 – 65 Gy rather than 66 Gy. Conversely, 7 patients had CTV1 of gross adenopathy prescribed to 68 – 70 Gy in 30 fractions. Four patients CTV1 nodes were prescribed 66 Gy, but also received 2 – 4 Gy boost with electrons delivered in 1 to 2 fractions. Dose prescriptions to CTV1 of the primary tumors and gross adenopathy are detailed in Tables 2 and 3.
Table 2.
Prescription doses to CTV1 (Primary) in 51 patients treated with IMRT
| Patient number | Dose (Gy) | Fraction number |
|---|---|---|
| 31 | 66 | 30 |
| 8 | 63- 65 | 30 |
| 4 | 66 | 33 |
| 4 | 72 | 40 (concomitant boost) |
| 3 | 70 | 33 |
| 1 | 70 | 35 |
Table 3.
Prescription doses to CTV1 (Nodes) in 35 patients with gross adenopathy treated with IMRT
| Patient number | Dose (Gy) | Fraction number |
|---|---|---|
| 18 | 66 | 30* |
| 4 | 70 | 33 |
| 4 | 70 | 30 |
| 4 | 72 | 40 (concomitant boost) |
| 3 | 68 | 30 |
| 1 | 70 | 35 |
| 1 | 66 | 33* |
Four patients treated with electron boosts to gross adenopathy.
The other 8 patients treated with once-daily fractionation were treated to 2 to 2.19 Gy to the CTV1. The prescription doses ranged from 66 to 70 Gy in 32 to 35 fractions. Four of these 8 patients were prescribed 66 Gy to CTV1 primary and 4 were prescribed 70 Gy to CTV1 primary.
An intermediate volume, CTV2, which was additional margin around CTV1 of the primary tumor, was defined in 14 patients. The median prescribed dose to this volume was 60 Gy (range, 57 – 64 Gy).
Considering the neck as 2 distinct structures (left and right) for prescription purposes, 94 upper necks (47 patients) were treated with once-daily irradiation. Exclusive of the dose to the CTV1 (gross adenopathy and margin), the dose prescriptions were 50 Gy in 3 necks; 54 Gy in 69; 60 Gy in 18; and 66 Gy in 4.
The mean dose delivered to the CTV1 ranged from 66.8 to 73.9 Gy (median, 69.2 Gy). The mean percentage of the prescribed dose delivered to the CTV1 ranged from 97% to 111% (median, 104.5%) and was less than 100% in only 1 patient. The volume of the CTV1 receiving less than the prescribed dose ranged from 0% to 7.7 % (median, 1%). The volume of the PTV1 receiving less than the prescribed dose ranged from 0.3% to 27.3% (median, 7.6%). An account of the dosimetries by target volume is shown in Table 4.
Table 4.
Dose delivered to CTV1, PTV1 and parotid glands
| Minimum dose | Maximum dose | Mean dose range (median, SD) |
% volume below prescriptio n |
|
|---|---|---|---|---|
| CTV1 | 55.6 – 69.9 Gy | 60.7 – 80 Gy | 66.8 – 73.9 Gy (69.2, 1.8) |
0 – 7.7 |
| PTV1 | 28.8 – 65.5 Gy | 60.7 – 80 Gy | 0.3 – 27.3 | |
| Left parotid | 13.1 – 57.1 Gy (26.4, 8) |
|||
| Right parotid | 7.6 – 68.8 Gy (25.4, 10.1) |
|||
| Mean parotid* | 18.1 – 46 Gy (23.9, 6) |
|||
| Lower parotid** | 13.1 – 33.6 Gy (26.3, 4.1) |
|||
| Higher parotid** | 21.2 – 68.8 Gy (29.2, 10.1) |
SD – standard deviation.
Mean dose to both parotid glands for each patient.
Mean dose to each of the pair of parotid glands; Lower parotid is the lower dose of the pair; higher dose is the higher dose of the pair.
The concomitant boost fractionation schedule9 was used in the treatment of 4 patients. This boost fractionation involved 2 separate treatments. In the first plan 54 to 57 Gy was delivered to the CTV1 and the CTV3 in 30 fractions. In the second treatment, 15 to 18 Gy was delivered in 10 fractions to the CTV1 only. The second treatment is given as a second daily fraction for the last 10 days of the treatment.
Because CORVUS did not allow for separate identification of targets and avoidance structures that occupied the same pixels, we identified only the superficial parotid lobe in our outline and thus our dose analyses. The constraints set on the parotid gland were 26 Gy to each gland. These were relaxed in the event that target coverage was compromised by a strict constraint. The median dose to the parotid gland receiving the lower dose of the paired glands was 23.9 Gy (range, 13.1 – 33.6 Gy). The median dose to the parotid gland receiving the higher dose of the paired glands was 29.2 Gy (range, 21.2 – 68.8 Gy). In 76% of patients, at least 1 parotid gland was treated to a mean dose < 26 Gy, and in 95%, at least 1 gland was treated to < 30 Gy. Mean doses to the parotid glands are detailed in Table 4. The mandible dose limits were set so that the maximum dose to the mandible would not exceed the prescribed dose to the adjacent target. The larynx constraint was set at 30 –40 Gy, but in most cases the larynx was outside the IMRT portals and was blocked in the low-neck field.
Treatment Outcomes
The overall median duration of follow-up for our study population was 45 months (range, 15 to 63 months). The minimum duration of follow-up for patients still alive at the time this study was conducted was 27 months. All 5 of the patients who received chemotherapy are alive 37 – 50 months from diagnosis, and none have had disease recurrence.
Disease recurred in 8 patients of the 46 patients (17%) treated with radiation without chemotherapy. In 2 of these patients, including the one treated to the CTV1 only, the disease recurred locally. In 1 of these patients, the disease recurred at the local site and in the neck; in the other patient, the disease recurred in the primary site only. All these recurrences were in CTV1. One of these patients was treated to 66 Gy in 30 fractions, and the other was treated to 70 Gy in 35 fractions.
Overall, the 2-year actuarial local-control rate for this patient population was 96%. Both patients with primary recurrence had stage T2 primary disease. The 2-year local-control rate for patients with T1/Tx disease was 100% compared with 88% for patients with T2 disease.
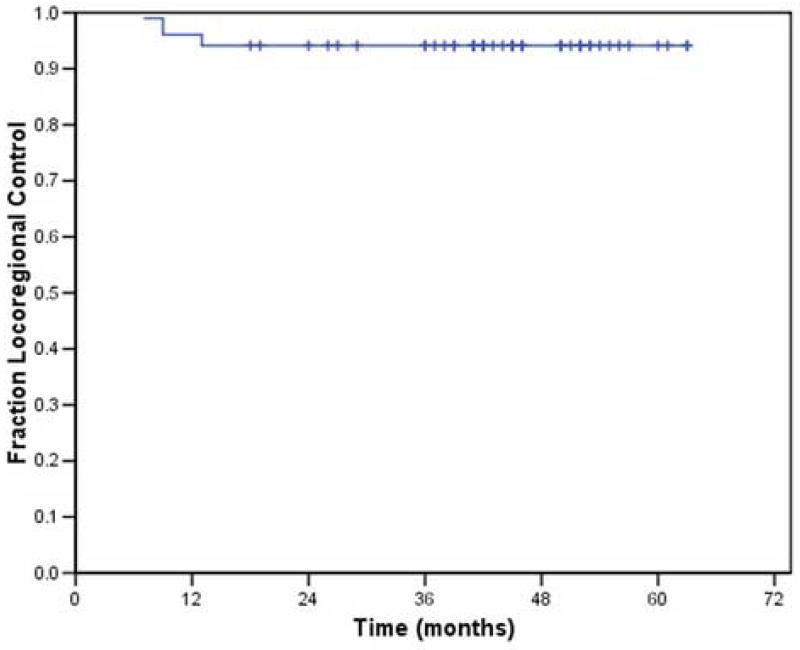
An additional patient had disease recurrence in the neck only. This patient presented with disease in levels 1 –4, and had retropharyngeal nodal disease as well. His disease recurred in multiple areas of the neck, including the carotid space. Thus, the disease recurred in the CTV1 and the remaining target volumes. The 2-year actuarial local-regional control rate was 93% (Figure 1).
Figure 1.
Local-regional control associated with the use of IMRT in the treatment of small (< 4 cm) oropharyngeal tumors.
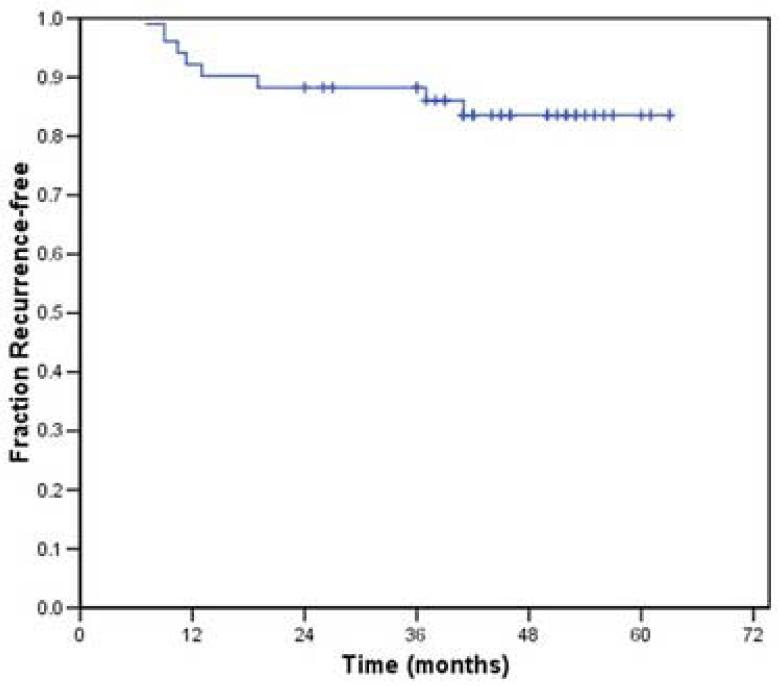
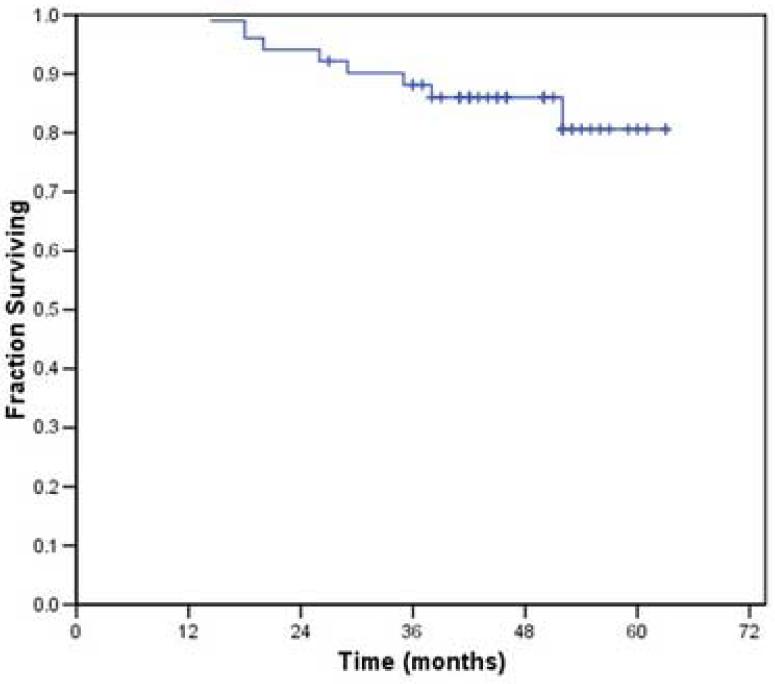
Distant metastasis occurred in 5 of the 43 patients who received radiation without chemotherapy and in whom local-regional control was achieved. The 2- and 3-year actuarial recurrence-free survival rates for this patient population were 87% and 84%, respectively; the 2- and 3-year overall survival rates were 93% and 87% respectively (Figures 2 and 3). At the time of analysis, 8 patients had died: 5 from disease recurrence, 2 from cardiopulmonary events and one from unknown causes. The latter three were disease-free at last follow-up. Two patients were alive with disease at last follow-up. One other patient underwent salvage surgery and reirradiation and was still alive with no evidence of disease 24 months following completion of the salvage therapy.
Figure 2.
Recurrence free survival associated with the use of IMRT in the treatment of small (< 4 cm) oropharyngeal tumors.
Figure 3.
Overall survival associated with the use of IMRT in the treatment of small (< 4 cm) oropharyngeal tumors.
Twenty-one patients (40%) required gastrostomy tubes for nutritional support. All were placed during therapy. In 4 of them, the tube was in place for more than 6 months. None required gastrostomy support one year post-therapy. Three of the 5 patients treated with chemotherapy plus IMRT required gastrostomies; however, none of them required the tube for more than 6 months.
Three patients have had chronic mild dysphagia with difficulty eating solids. Two patients have undergone post-radiation hyperbaric oxygen therapy; one for extraction prophylaxis and one for osteoradionecrosis. Two patients, both having radiation and neck dissections have chronic shoulder weakness and neck pain.
Discussion
The local-regional control rate of 93% achieved with IMRT in patients with small oropharynx cancers is comparable to the rates achieved with conventional radiation therapy techniques in similar series.7, 8 In those series, the 2-year local-regional control rates were 91%. Also similar to those studies, local-regional control with IMRT was T-stage dependent, as all local failures in this current series occurred in patients with T2 disease, and within CTV1. There were no geographic or marginal failures.
Other series have also reported favorable disease control rates of head and neck cancer treated with IMRT.4-6, 10 In contrast to those reports our study population was relatively homogeneous with regard to T and N stage and primary site of disease. In addition, patients in this report who are alive all have been followed for a minimum of 2 years.
IMRT did not appear to produce a more favorable acute toxicity profile in this patient group with respect to feeding tube use; however, the IMRT dose distributions markedly reduced the mean superficial lobe parotid dose as compared to former conventional techniques. In 95% of patients we were able to obtain a mean dose of <30 Gy to one gland. Using a conventional 3- field head and neck technique these patients typically would receive at least 40 Gy mean dose to the parotid gland11.
The majority of patients in this series were treated with relative hypofractionation at 2.2 Gy per fraction to the CTV1. Although intrigued by the fractionation schemes and results reported by Butler et al.,4 we were concerned that 2.4 Gy per fraction, which was used in their study, would be too dose intense. Our plan of 66 Gy delivered in 30 fractions (6 weeks) facilitated a dose and fractionation scheme that could be completed in the same time frame as plans with modest acceleration schedules, such as those reported by the Radiation Therapy Oncology Group (RTOG)12 and the Danish Head and Neck Cancer (DAHANCA) group,13 and thus should be biologically effective. IMRT allowed for larger doses per fraction to the tumor while exposing normal tissues to fractional radiation doses considered to be tolerable.
Although the fractionation scheme used in this study was considered appropriate for the majority of the patients and although this scheme was used in the recently completed RTOG H-0022 phase 2 trial evaluating IMRT for early-stage oropharynx cancer, prescribed doses were individualized for many patients. In our initial experience, several patients with bulky adenopathy had dose prescriptions of 68 to 70 Gy delivered to the gross adenopathy in 30 fractions. Although severe late toxicity has not been noted in these patients after nearly 4 years of follow-up, concern for the risk of late morbidity led to 2 changes in the treatment plan. In 3 patients, additional fractions were added via an electron boost rather than by exceeding the 2.2 Gy per fraction dose to nodes. In 4 other patients, we increased the overall number of fractions resulting in 70 Gy to the nodes, and 66 to 70 Gy to the primary site all in 33 fractions. Patients with T1 disease still received 66 Gy, and those with T2 disease received 70 Gy.
We were reluctant to administer 66 Gy in 30 fractions to patients who had received concurrent chemotherapy. Instead, this group of patients was typically treated with 70 Gy in 33 fractions, similar to the fractionation scheme described for nasopharyngeal carcinoma.14
Only 4 patients were treated with concomitant boost fractionation schedule9. The original treatment planning systems were unable to create a dose distribution combining the large field and boost field treatments, causing uncertainty in the plans that often had considerable heterogeneity. The additional treatment time required for delivery of IMRT, and thus the additional number of fractions used, and the use of a twice-daily scheme were also concerns. Although the latter factor remains a concern, the improvements in treatment planning systems now allow us to see the entire treatment as a single dosimetric entity.
While coined “concomitant boost”, this fractionation schedule does not truly deliver the boost treatment at the same time as the larger field treatment. Thus when planning a treatment using the classic concomitant boost fractionation schedule, two separate plans must be created though these 2 plans are ultimately combined to display a single composite dose distribution. This is in contrast to the capability of IMRT to truly deliver a simultaneous integrated boost (SIB) by delivering differing doses to different targets at risk during each fraction. Several reports have suggested that the SIB plans are superior to planning the boost separately.15, 16 In general, however, plans generated in these reports have relied on the initial optimization parameters established in dosimetric exercises. Our experience suggests that a concomitant boost schedule delivered via a sequential set of plans can meet the established set of constraints as well as SIB, if sufficient time is invested in individual plan optimization. Thus for patients with bulky primary tumors who receive radiation therapy alone, we still favor concomitant boost fractionation, which has a proven record of success in our institution in the treatment of intermediate-staged primary tumors of the oropharynx. 17, 18 Reports from Memorial Sloan-Kettering Cancer Center also describes positive experiences with IMRT and concomitant boost fractionation.1, 19
Despite providing a more conformal coverage of the tumor volume, the rate of significant acute toxicity associated with IMRT, as assessed by the rate of gastrostomy tube use, was 40%. However, for most patients, the gastrostomy tube was used for a relatively short period of time. Less than 10% of patients still required the tube 6 months after treatment, none for greater than one year, and only 3 patients had chronic difficulty swallowing solid foods. These observations are likely the result of a larger volume of mucosa receiving radiation, albeit a lower total dose, resulting in mucositis that often does not result in severe late toxicity. To minimize the dose to uninvolved mucosa, we now create avoidance structures within the oral cavity during the treatment-planning process.
We were able to maintain a mean dose of < 26 Gy to at least one parotid gland in over 75% of patients. We did not consistently correlate this outcome with subjective rates of xerostomia nor with objective measures of salivary flow; however, we are actively participating in RTOG H022 study in which a similar sample being treated with a similar dose fractionation scheme is being evaluated. This multi-institutional study will hopefully provide data to help us determine whether reducing the parotid dose can reduce the incidence of severe xerostomia.
This retrospective report adds to a growing body of literature suggesting that disease-control rates associated with IMRT are at least equivalent to those achieved with more conventional radiation delivery techniques.7, 8 IMRT did not appear to have a more favorable acute toxicity profile in this patient group with respect to feeding tube needs. We were however, often able to obtain dose distributions to the parotid gland that potentially can minimize the risk of severe xerostomia. We continue to treat the vast majority of our oropharynx patients with a split-beam technique20. Newer planning systems allow us to model the entire treatment in a single plan allowing us more flexibility in choice of fractionation, as well as combining the lower neck isodoses with the IMRT component of the treatment to minimize problems at the junctions. Our favorable results have led us to use IMRT to treat the vast majority of patients presenting to us for radiotherapy for small oropharynx tumors, as well as to use IMRT for most of our patients with more advanced oropharyngeal cancer.
Acknowledgments
This research was supported in part by Grant CA-06294 from the National Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 45th annual ASTRO meeting, October 19-23, 2003, in Salt Lake City, Utah.
References
- 1.Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharynx cancer. International Journal of Radiation Oncology, Biology and Physics. 2001;49:623–632. doi: 10.1016/s0360-3016(00)01389-4. [DOI] [PubMed] [Google Scholar]
- 2.Xia P, Fu KK, Wong GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. International Journal of Radiation Oncology, Biology and Physics. 2000;48:329–337. doi: 10.1016/s0360-3016(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World Journal of Surgery. 2003;27:832–837. doi: 10.1007/s00268-003-7105-6. [DOI] [PubMed] [Google Scholar]
- 4.Butler EB, Teh BS, Grant WHr, et al. Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. International Journal of Radiation Oncology, Biology and Physics. 1999;45:21–32. doi: 10.1016/s0360-3016(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 5.Chao KS, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. International Journal of Radiation Oncology, Biology and Physics. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and- neck cancer: the UCSF experience focusing on target volume delineation. International Journal of Radiation Oncology, Biology and Physics. 2003;57:49–60. doi: 10.1016/s0360-3016(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 7.Garden AS, Asper JA, Morrison WH, et al. Is concurrent chemoradiation the treatment of choice for all patients with stage III or IV head and neck cancer? Cancer. 2004;100:1171–1178. doi: 10.1002/cncr.20069. [DOI] [PubMed] [Google Scholar]
- 8.Selek U, Garden AS, Morrison WH, et al. Radiation therapy for early stage carcinoma of the oropharynx. International Journal of Radiation Oncology, Biology, Physics. 2004;59:743–751. doi: 10.1016/j.ijrobp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Peters LJ, Weber RS, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. International Journal of Radiation Oncology, Biology and Physics. 1990;19:1339–1345. doi: 10.1016/0360-3016(90)90341-g. [DOI] [PubMed] [Google Scholar]
- 10.Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. International Journal of Radiation Oncology, Biology and Physics. 2000;46:1117–1126. doi: 10.1016/s0360-3016(99)00550-7. [DOI] [PubMed] [Google Scholar]
- 11.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: Initial results. International Journal of Radiation Oncology, Biology and Physics. 2001;49:907–917. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 12.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group(RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. International Journal of Radiation Oncology, Biology and Physics. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. International Journal of Radiation Oncology Biology and Physics. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 15.Dogan N, King S, Emami B, et al. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. International Journal of Radiation Oncology, Biology and Physics. 2003;57:1480–1491. doi: 10.1016/s0360-3016(03)01569-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohan R, Wu Q, Manning M, et al. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. International Journal of Radiation Oncology, Biology and Physics. 2000;46:619–630. doi: 10.1016/s0360-3016(99)00438-1. [DOI] [PubMed] [Google Scholar]
- 17.Mak AC, Morrison WH, Garden AS, et al. Base-of-tongue carcinoma: Treatment results using concomitant boost radiotherapy. International Journal of Radiation Oncology, Biology and Physics. 1995;33:289–296. doi: 10.1016/0360-3016(95)00088-G. [DOI] [PubMed] [Google Scholar]
- 18.Gwozdz JT, Morrison WH, Garden AS, et al. Concomitant boost radiotherapy for squamous carcinoma of the tonsillar fossa. International Journal of Radiation Oncology Biology and Physics. 1997;39:125–135. doi: 10.1016/s0360-3016(97)00291-5. [DOI] [PubMed] [Google Scholar]
- 19.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: The Memorial Sloan-Kettering Cancer Center experience. International Journal of Radiation Oncology Biology and Physics. 2006;64:363–373. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Dabaja B, Salehpour MR, Rosen I, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: Comparison of split-field and whole-field techniques. International Journal of Radiation Oncology, Biology and Physics. 2005;63:1000–1005. doi: 10.1016/j.ijrobp.2005.03.069. [DOI] [PubMed] [Google Scholar]