Abstract
Retinoblastoma (RB) is a retinal cancer of childhood. RB survivors tend to develop additional tumors later in life, although the physical mechanisms of RB metastatic spread are largely unknown. One step in metastasis through the blood stream is tumor cell adherence to the blood vessel wall through specific receptor:ligand interactions. Yet, human RB cell lines RB143 and WERI-Rb27 do not express selectin ligands or beta-2 integrins and cannot directly interact with inflamed endothelium. In this study, we show that RB cells express ICAM-1, a beta-2 integrin ligand that correlates with metastasis and is preferentially co-expressed on RB cells that also express ABCG2, a stem cell marker associated with chemoresistance and metastasis. Based on the presence of ICAM-1+ RB cells, we tested the hypothesis that RB cells could be recruited to an E-selectin surface via attachment to activated polymorphonuclear cells (PMNs). We characterized the dynamic adhesion between RB cells and PMNs within E-selectin coated microtubes under a physiological range of wall shear stress values (0.2–5 dyn/cm2). We show that activated PMNs are necessary for the recruitment of RB cells through ICAM-1:LFA-1 binding. Results from this work may lead to new strategies that target the metastatic spread of tumor cells.
Keywords: Retinoblastoma, Cancer stem cell, Cell adhesion, Metastasis, ICAM-1, E-selectin, Neutrophils, ABCG2
INTRODUCTION
Retinoblastoma (RB) is a malignant tumor of the neural retina that affects approximately 250–350 children under the age of six every year in the United States.18 In some Central and South America countries, retinoblastoma is one of the most common solid tumor malignancies in children.26 Retinoblastoma usually manifests before the age of three and the tumors can grow locally within the eye as well as extend outside the globe by migrating down the optic nerve into the central nervous system and the cerebrospinal fluid. In addition, the tumors can spread through the vasculature to form metastases in distant organs. Patients with hereditary RB have a much higher risk for developing secondary malignancies later in life, mainly soft tissue sarcoma, osteosarcoma, and melanoma.4,10 Reports have indicated that the rate of secondary tumor formation in these patients is increased by radiation treatment and possibly in combination with chemotherapy.3 Chemoresistant, radiation-resistant stem-like cells exist in many forms of cancer20,22 and may relate to these secondary malignancies. One hallmark of many stem-like cell populations is the presence of ABCG2 (BCRP), a stem cell marker32 and a cell surface transmembrane pump that can confer resistance to a wide variety of chemotherapy agents, including anthracyclines, mitoxantrone, and camptothecins.17 The calcium-dependent transporter activity of ABCG2 has been used to identify and isolate stem-like cells from a variety of normal and malignant tissues based on the ability of ABCG2 positive cells to exclude Hoechst dye 3334.1,7,27 Seigel and colleagues have identified subpopulations of cancer stem-like cells in retinoblastoma that express stem cell markers such as ABCG2, as well as human embryonic stem cell markers, such as Nanog and Oct3/424,25 that provide an intriguing model for the study of tumor cell adhesion and metastasis.
The basic mechanisms of how neutrophils adhere to the vascular endothelium and migrate to inflammatory sites have been studied extensively over the past two decades. To start the neutrophil recruitment cascade, the initial capture of neutrophils from the blood-stream and rolling along the vessel wall is mediated by P-selectin and E-selectin presented by stimulated endothelial cells.11,12 Activated beta-2 integrins (CD11a/CD18 or LFA-1, and CD11b/CD18 or Mac-1) on neutrophils then facilitate the firm arrest of neutrophils by binding to ICAM-1 on endothelial cells.6 Circulating tumor cells such as colon carcinoma2 and acute myeloid leukemia19 express selectin ligands and integrins, which allow them to interact directly with the endothelium and metastasize through the blood stream. Other circulating metastatic cancer cells, however, have been found to adhesively associate with neutrophils in the circulation, to escape the circulation and engraft in the extravascular space.14,16 The similar properties shared between metastatic cancer cells and leukocytes such as the ability to interact with endothelium and cause basement membrane degradation suggests that leukocytes may play a role in promoting tumor growth and survival, as well as transendothelial migration.23,30 The interaction between cancer cells and leukocytes has been found in breast cancer, advanced gastric cancer, colon cancer, and malignant melanoma.15,21,31
It has been established that the accumulation of neutrophils at inflammatory sites is a collective phenomenon.8 Homotypic interactions between neutrophils were found to be responsible for enhancing the accumulation of neutrophils rolling on P-selectin surfaces in vitro.29 Walcheck et al. showed that transient tether formation between a rolling neutrophil and a cell suspended freely in the fluid can cause the rolling and capture of free-flowing cells near the surface.
It is not clear how retinoblastoma cells may adhere to the endothelium and subsequently extravasate through it under flow conditions in the microcirculation. In this study, we tested the hypothesis that retinoblastoma cancer stem cells can interact with a model endothelial surface via secondary recruitment by activated leukocytes, as a potential mechanism for metastatic spread.
MATERIALS AND METHODS
Reagents
FITC-conjugated mAb for anti-human CD 338 (ABCG2) clone 5D3, PE-conjugated mAb for anti-human CD 54 (ICAM-1) clone HCD54 and FITC-conjugated Mouse IgG2b, κ isotype control were purchased from BioLegend, (Sandiago, CA). PE-conjugated Mouse IgG1, κ isotype control was purchased from eBioscience (San Diego, CA). Recombinant E-selectin-IgG chimera was obtained from R&D Systems (Minneapolis, MN). Trypan blue stain (0.4%) was obtained from Lonza (Wilkersville, MD). Blotting grade blocker non-fat dry milk was obtained from Bio-Rad Laboratories (Hercules, CA). Protein-G was purchased from EMD Biosciences (San Diego, CA). Ca2+ and Mg2+ free HBSS (Invitrogen, Camarillo, CA, USA), Ca2+ and Mg2+ free DPBS (Invitrogen, Camarillo, CA, USA), calcium carbonate (Sigma Chemical Co., St. Louis, MO, USA), endotoxin free water (MO BIO Laboratories, Carlsbad, CA, USA), endotoxin free human serum albumin (Sigma Chemical Co., St. Louis, MO, USA), and low endotoxin (1 ng/mg), essentially globulin-free BSA (Sigma Chemical Co., St. Louis, MO, USA) were used to make buffer solutions for neutrophil isolation and flow experiments.
Retinoblastoma Cell Cultures
Retinoblastoma cell lines RB143 (a gift from Dr. Bruce Ksander) and WERI-Rb27 (a gift from Dr. John Ludlow) were maintained in Dulbecco’s Modified Eagle’s Medium (Sigma), with 20% calf serum for RB143 cells and 10% calf serum for WERI-Rb27 cells (Hyclone, Logan UT) at 37 °C in a 5% CO2 incubator.
Immunocytochemistry
The following primary antibodies were used for immunocytochemistry:
10 μg/mL rabbit anti-Nestin (Sigma), 4.16 μg/mL rabbit anti-ICAM-1 (Cell Signaling Technologies, Danvers, MA), 1 μg/mL goat anti-doublecortin (Santa Cruz Biotechnology, Santa Cruz, CA), 5 μg/mL mouse anti-CD147 (Neurothelin, EMMPRIN, Basigin; BD Pharmingen, Franklin Lakes, NJ), and 1:200 dilution of mouse anti-ABCG2 (Abcam, Cambridge, MA).
For diaminobenzidine (DAB) staining, RB143 cells were prepared as cytospins by centrifugation and resuspension in cytospin fixative (72% isopropyl alcohol, 19% acetone, 7.6% glycerol). After at least 10 min of fixation, the cell suspensions were spun onto slides at 1000g for 5 min in a Shandon Cytospin 2. Cytospins underwent immunocytochemistry using the following protocol: Cytospin slides were incubated for 5 min with PBS, 5 min with 3% H2O2 and rinsed twice with PBS. Cells were blocked for 15 min with 2% bovine serum albumin, followed by a PBS wash. Cells were incubated in primary antibody or isotype control antibody for 1 h, washed twice with PBS, and incubated with 1 μg/mL of appropriate biotinylated secondary antibody (anti-mouse, rabbit or goat from Vector Laboratories, Burlingame, CA) for 45 min. After two PBS washes, the cells were incubated for 20 min with horseradish peroxidase-labelled avidin (ABC Elite kit, Vector Laboratories, Burlingame, CA). The cells were washed twice with PBS and then treated with DAB (Zymed Laboratories, Carlsbad, CA) for 5 min to develop the brown reaction product. Cells were then viewed by brightfield microscopy with Nomarski optics (Nikon Eclipse, ES600).
For double immunofluorescent staining, cells were fixed and maintained in suspension. After PBS wash, cells were incubated at 4 °C with primary antibodies (mouse anti-ABCG2 and rabbit anti-ICAM-1 or iso-type control antibody) for 1 h, rinsed twice, then incubated for 1 h with a mixture of fluorescent secondary antibodies (2.5 μg/mL each of FITC anti-rabbit Ig and TRITC anti-mouse Ig, Sigma). Cells were rinsed, placed on a slide and coverslipped for viewing with fluorescence microscopy (Nikon Eclipse, ES600).
Flow Cytometry
Both RB143 and WERI-Rb27 cells were de-clumped prior to antibody incubation using an enzyme-free cell dissociation media that preserves the cell surface receptors rather than cleave them. After two washes with 1× DPBS, the cells were resuspended in 1× DPBS to a final concentration of 200,000–300,000 cells in each sample. Antibodies or appropriate isotype controls were added to cell suspensions and incubated over ice for 45 min. Following the incubations, the cells were washed three times with 1 mL of 1× DPBS to remove any unbound antibody. Flow cytometry samples were analyzed using an Accuri C6 flow cytometer (Accuri Cytometers Inc., Ann Arbor, Michigan, USA) and plots were created using the FlowJo v. 7.6.3 (Tree Star Inc., Ashland, Oregon) software package.
Preparation of Immobilized Protein Surfaces
Polyurethane microtubes with an inner diameter of 300 μm (Braintree Scientific Inc., Braintree, MA, USA) were cut to a length of 50 cm. Recombinant human E-selectin-IgG chimeric protein was dissolved in 1× PBS to a final concentration of 5 μg/mL. The microtube surface was first rinsed with 75% ethanol and then 1× PBS. The surface was subsequently incubated with 10 μg/mL of protein-G solution for 1.5 h, followed by a 2 h incubation with selectin chimera then blocked with 5% milk protein in PBS for 1 h. Control tubes were blocked with 5% milk protein in PBS for 1 h.
Neutrophil Isolation and Activation
Human peripheral blood was collected from consenting healthy adult donors (as per IRB-approved protocol). Neutrophils were isolated by centrifugation at 500g for 50 min at 23 °C using 1-Step Polymorphs (Accurate Chemical and Scientific Corporation, Westbury, NY, USA.), creating separate visible layers of plasma, mononuclear cells, neutrophils, and erythrocytes and platelets. The neutrophil layer was extracted and washed twice in Ca2+ and Mg2+ free HBSS to remove the polymorph residue, and any remaining red blood cells were lysed hypotonically with 1:6 and 10× PBS. Neutrophils were then resuspended at various concentrations of HBSS containing 0.5% HSA, 2 mM Ca2+, and 10 mM HEPES, buffered to 7.4. Prior to flow experiments, neutrophils were stimulated with 1nM IL-8 (R&D Systems Inc., Minneapolis, MN, USA) for 10 min at RT on a rocker (Rocker II Model 260350, Boekel Scientific).
Recruitment of Retinoblastoma Cells by Association with Neutrophils Under Flow
Microtubes with functionalized E-selectin surfaces were secured on the stage of a Olympus IX81 inverted microscope (Olympus America Inc., Melville, NY, USA). After mixing IL-8 stimulated neutrophils and CTG-labeled RB cells for 5 min on a rocker, the cell solution was introduced into the microtube using a syringe pump (KDS 230, IITC Life Science, Woodland Hills, CA) at a wall shear stress of 1.0 dyne/cm2. The dynamic adhesion between RB cells and human peripheral leukocytes was characterized over a physiological range of wall shear stress values (0.2–5 dyn/cm2).
Data Acquisition
Videos of flowing and adhering RB-neutrophil aggregates were recorded using a microscope-linked Hitachi CCD camera KP-M1AN (Hitachi, Japan) and a Sony DVD Recorder DVO-1000MD (Sony Electronics Inc., San Diego, California, USA). For each flow experiment, a 12 cm functionalized E-selectin surface was scanned and the aggregates were quantified and normalized. The average ratio of leukocytes to cancer cells per aggregate was measured as a function of shear stress, receptor density, and cell concentration.
Statistical Analysis
Adhesive interactions of RB143 and WERI-Rb27 cells with neutrophils at various ratios were quantified and plotted using Prism 5.0b for Microsoft (GraphPad Software, San Diego, CA, www.graphpad.com). Rolling cells were defined as those translating in the direction of flow with an average velocity less than 50% of the calculated hydrodynamic free stream velocity. Two-tailed unpaired t-test and ANOVA were employed with a significance level of α = 0.05 where applicable.
RESULTS
When RB143 and WERI-Rb27 cells were perfused through E-selectin coated microtube surfaces without neutrophils, neither demonstrated rolling adhesion as defined above. No expression of E-selectin ligands was found via flow cytometry in either the RB143 or WERI-Rb27 cell line (data not shown). There was, however, an ABCG2+ stem-like cell subpopulation within both cell lines that co-expressed high levels of ICAM-1 (Fig. 1). ICAM-1 is a beta-2 integrin ligand and a surface marker that correlates with tumor metastatic potential.28 As shown in Fig. 1, ~90% of ABCG2+ RB143 cells expressed ICAM-1, compared with less than 20% expression of ICAM-1 in ABCG2–cells. This co-staining for ABCG2 and ICAM-1 is also demonstrated in the micrograph of Fig. 1b.
FIGURE 1.
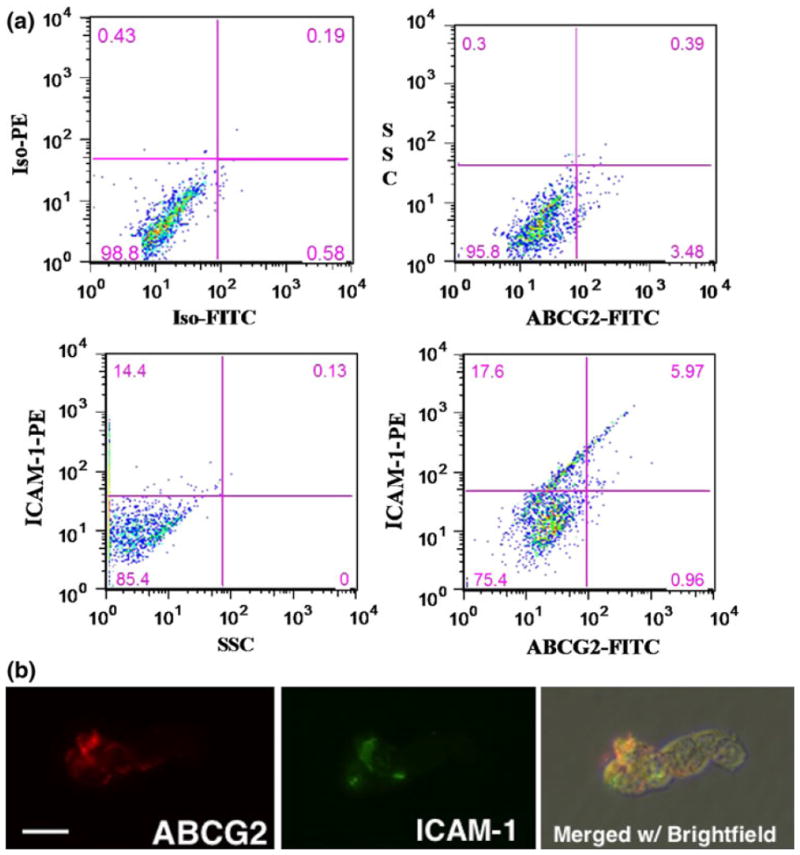
ABCG2+ RB cells preferentially express ICAM-1. (a) Detection of an ABCG2+/ICAM1+ subpopulation within RB143 cells labeled with FITC-conjugated anti-ABCG2 monoclonal antibody and PE-conjugated anti-CD54 (ICAM-1) monoclonal antibody. The upper left panel consists of isotype control antibodies (background), followed by panels for individual ABCG2 or ICAM-1 fluorescence. The lower right panel demonstrates double labeling and the degree of overlap between ABCG2 and ICAM-1. (b) Double immunofluorescent labeling of ABCG2 (red) and ICAM-1 (green). The same microscopic field is shown in all panels and merged with brightfield to display all cells in the field. Scale bar = 10 microns for all panels.
Results from the aggregation assay indicate that RB-leukocyte aggregate formation is ICAM-1 dependent (Fig. 2). In these experiments, RB143 and WERI-Rb27 cell concentrations were held constant at 1 million/mL while varying neutrophil concentrations from 2 million/mL to 5 million/mL. The total number of aggregates observed (including adhering and rolling/flowing) from all four different conditions (RB143 1:2, RB143 1:5, WERI 1:2, WERI 1:5) were not significantly different (p = 0.0814). Approximately 80% of the capture events in all four conditions were RB cells rolling or flowing with their anchoring neutrophils on the microtube surface. Significantly fewer events were observed in blocking and control experiments, as seen in Fig. 2. Overall, RB-neutrophil aggregate formation showed no correlation with initial cell ratios or cell lines (p = 0.814, ANOVA). We have also observed that some flowing neutrophils are captured by a retinoblastoma:neutrophil aggregate attached to the surface; such cells were recorded as “tethered” neutrophils since they were available for future collisions with flowing retinoblastoma cells.
FIGURE 2.
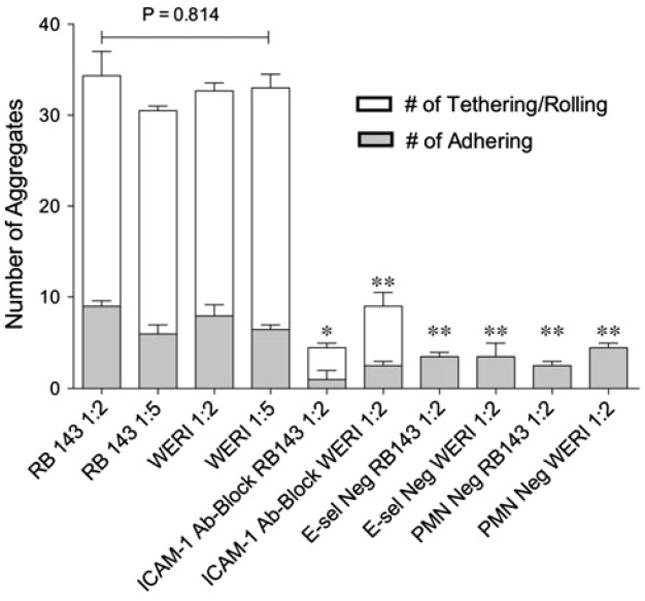
Aggregate formation is ICAM-1 specific and insensitive to initial cell ratios. RB143 and WERI-Rb27 retinoblastoma cell lines were used in the microtube aggregation assay. For both cell lines, RB cell concentration was held constant at 1 million/mL while neutrophil concentrations were varied from 2 million to 5 million/mL. Antibody blocking experiments were done using monoclonal anti-human ICAM-1 antibody. In the “E-selectin negative” experiments, the microtube surface was only incubated with protein G. In PMN-negative experiments, only RB cells were perfused in the functionalized E-selectin surface. ANOVA and student t-tests were used for statistical analyses. * p < 0.01; ** p < 0.005.
To further characterize the size of aggregates formed, the number of neutrophils that bound to RB cells in each aggregate (both adhering and rolling/flowing) was quantified for two experiments with RB143 cells, for two different initial RB143:neutrophil cell ratios of 1:2 and 1:5. The normalized results showed no significant difference in the number of aggregates captured. In the experiment in which the RB143:neutrophil initial ratio was 1:2, the aggregation profile exhibits an approximately binomial distribution, as shown in Fig. 3. Overall, the majority of aggregates captured were one-to-one (i.e., one RB with one neutrophil) binding events. 40 adhering one-to-one binding events were analyzed in which both RB and neutrophil were attached to the E-selectin surface (shown in Fig. 4). The orientation angle and center-to-center distance of the captured RB cell, relative to the anchoring neutrophil, was quantified for each observed heterotypic adhesion event. We found that in 90% of these one-to-one aggregates, RB cells and neutrophils formed angles between 0° and 180°, i.e., downstream to their anchoring neutrophils.
FIGURE 3.
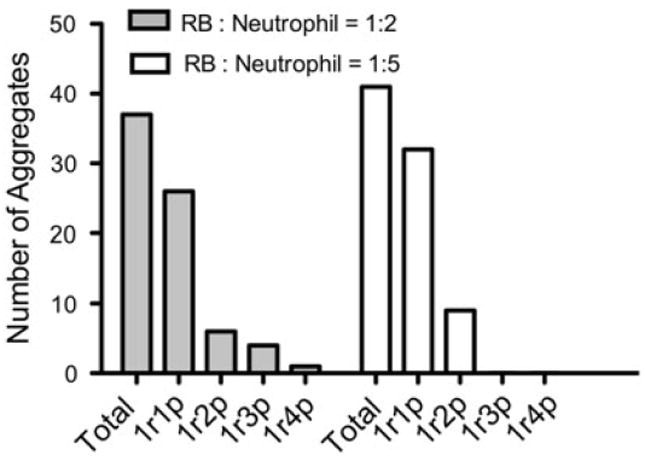
RB-leukocyte binding events are primarily one-to-one binding. RB143 concentration was held constant at 1 million/mL while neutrophil concentrations were varied from 2 million to 5 million/mL. 1r1p = one RB with one neutrophil observed in aggregates, etc. Student t test was used for statistical analysis. p = 0.885.
FIGURE 4.
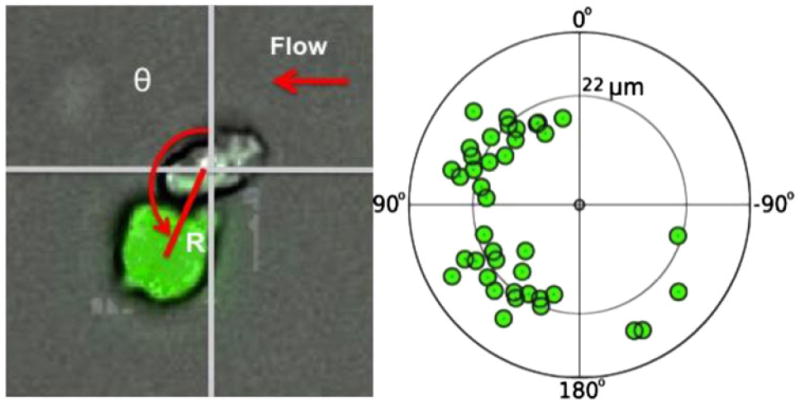
Bound RB is downstream to its anchoring leukocyte under physiological flow. 40 adhering one-to-one binding events were analyzed in which both the RB and neutrophil were attached to the E-selectin surface. The angle of association between RB cells and neutrophils was calculated by measuring the deviation of RB cells from a vertical line drawn straight upward from the center of the neutrophil. Average distance between the centers of RB cells and neutrophils was found to be 22 μm.
DISCUSSION
The goal of this study was to address the mechanism by which RB cells adhere to the endothelium that could allow subsequent extravasation and metastasis to distant sites. Although RB cells do not express selectin ligands or beta-2 integrins themselves, ABCG2+ RB stem-like cells do express ICAM-1 suggesting a role for leukocyte:RB adhesive interactions as a mechanism that can promotes RB arrest on the endothelium under flow.
ICAM-1 is expressed on a variety of primary tumors, metastases, and normal cells.5 In the present study, flow cytometry results suggest preferential expression of ICAM-1 on the surface of ABCG2+ RB143 cells. We found a similar trend in WERI-Rb27 cells (results not shown). This suggests the hypothesis that RB cells, especially ABCG2+ RB cancer stem-like cells, may be recruited to an E-selectin surface by first attaching to activated neutrophils via LFA-1: ICAM-1 binding (Fig. 5).
FIGURE 5.
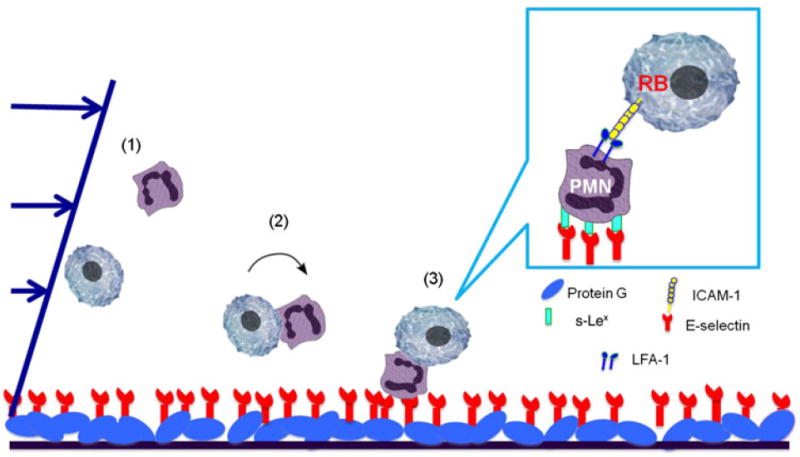
Hypothesis: RB cells are recruited by leukocytes in the bloodstream via LFA-1:ICAM-1 interactions. This illustration shows a three-step binding mechanism that facilitates the RB-neutrophil aggregate formation. (1) Neutrophils and RB cells are flowing in the microtube; (2) Neutrophils and RB cells interact and form aggregates; (3) The aggregates are firmly arrested to the surface through the neutrophil s-Lex:E-selectin binding.
The results from the microtube aggregation assay show that aggregate formation is insensitive to the initial ratio of neutrophils and RB cells. One might expect to observe more aggregates as the neutrophil concentration is increased from 2 to 5 million/mL for both RB cell lines. However, no significant increase in the number of aggregates was observed in experiments using RB143 nor WERI-Rb27 cells since all four conditions (RB143 1:2, 1:5; WERI-Rb27 1:2, 1:5) show similar trends in terms of the distribution of adhering and rolling/flowing aggregates. The significant decrease in the number of RB-neutrophil aggregates captured in the presence of ICAM-1 blocking antibodies suggests that the phenomenon of RB-neutrophil binding is highly ICAM-1 specific. In addition to the sensitivity and specificity, it was found that the majority of RB-neutrophil aggregates were one-to-one binding, i.e., once a neutrophil binds to a RB cell, it is less likely to recruit another RB cell, likewise the RB cells, once bound in one-to-one aggregates, are less likely to bind to a second neutrophil. This may be due to the relocation of receptors to the cell:cell contact area, as observed in neutrophil receptor relocation on the neutrophil surface.9,13 Furthermore, we observe that 90% of the bound RB cells are downstream to their anchoring neutrophils, which shares similarities to the secondary recruitment mechanism observed for neutrophil-neutrophil interactions.29
Interestingly, in the aggregation assay (Fig. 2), at least 20% of RB cell and neutrophil aggregates were observed to tether or roll rather than adhere to the E-selectin surface. This suggests a step-wise binding mechanism in which the RB cells and neutrophils first interact and form aggregates in flow, roll together on the surface through the neutrophil sialyl Lewis (s-Lex):E-selectin binding, and then firmly adhere to the surface (Fig. 5). Alternatively, the neutrophils could first adhere to the E-selectin surface and then recruit RB cells. However, the experiments in which we first flow activated neutrophils onto the E-selectin surface and then introduce RB cells showed no aggregate formation. This indicates that neutrophil recruitment of RB cells precedes neutrophil firm adhesion to E-selectin.
Since adhesion molecules, such as ICAM-1 expressed on ABCG2+ RB stem-like cells, are critical for invasion and metastasis, we propose anti-adhesion strategies as a potential adjunct to conventional cancer therapies. This study provides insight for future experimental designs investigating RB metastasis and developing therapeutic approaches to prevent secondary tumor formation in patients that survive a primary RB tumor.
CONCLUSIONS
In this study, it was discovered that ABCG2+ stem-like RB143 and WERI-Rb27 cells preferentially express ICAM-1, which acts as a bridge linking RB cells to leukocytes in the bloodstream via LFA-1:ICAM-1 interactions. It was found that neutrophil and RB cell aggregate formation is insensitive to initial cell ratio, highly ICAM-1 specific, and preferentially forms one-to-one aggregates (one neutrophil to one RB cell). Furthermore, it was hypothesized that a stepwise binding mechanism in which the neutrophils and RB cells first interact and form aggregates, roll on the E-selectin surface, and firmly adhere to the surface. Results from our work could lead to the design of anti-adhesion therapies that target metastatic cancer cells (especially cancer stem cells) and combat the invasive spread of cancer.
Acknowledgments
We gratefully acknowledge Sarah Hayes for technical support. We thank Dr. Bruce Ksander for providing the RB143 cell line and Dr. John Ludlow for the WERI-RB27 cell line. This work was supported by NIH Grant No. U54CA143876 (MRK, GMS). GMS is supported by R21CA127061 and a departmental challenge grant from Research to Prevent Blindness. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol. 2003;284:C977–C987. doi: 10.1152/ajpcell.00423.2002. [DOI] [PubMed] [Google Scholar]
- 3.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12:1237–1246. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96:357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SH, Seigel GM. Immunoreactivity of ICAM-1 in human tumors, metastases and normal tissues. Int J Clin Exp Pathol. 2009;2:553–560. [PMC free article] [PubMed] [Google Scholar]
- 6.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to Intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 7.Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65:6640–6650. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- 8.King MR, Hammer DA. Multiparticle adhesive dynamics: hydrodynamic recruitment of rolling leukocytes. Proc Natl Acad Sci. 2001;98:14919–14924. doi: 10.1073/pnas.261272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King MR, Sumagin R, Green CE, Simon SI. Rolling dynamics of a neutrophil with redistributed L-selectin. Math Biosci. 2005;194:71–79. doi: 10.1016/j.mbs.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Kleinerman RA, Tucker MA, Abramson DH, Seddon JM, Tarone RE, Fraumeni JF. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99:24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiological flow rates—dstinction from and prerequisite for adhesion through interins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence MB, Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 13.Lee D, King MR. Shear-induced capping of L-selectin on neutrophil surface during centrifugation. J Immunol Meth. 2007;328:97–105. doi: 10.1016/j.jim.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang S, Hoskins M, Khanna P, Kunz RF, Dong C. Effects of the tumor-leukocyte microenvironment on melanoma-neutrophil adhesion to the endothelium in a shear flow. Cell Mol Bioeng. 2008;1:189–200. doi: 10.1007/s12195-008-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang S, Slattery MJ, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregation, melanoma adhesion to the endothelium, and subsequent extravasation. Ann Biomed Eng. 2008;36:661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtenstein A. Stimulation of the respiratory burst of murine peritoneal inflammatory neutrophils by conjugation with tumor cells. Cancer Res. 1987;47:2211–2217. [PubMed] [Google Scholar]
- 17.Mao QC, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. Aaps J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols KE, Walther S, Chao E, Shields C, Ganguly A. Recent advances in retinoblastoma genetic research. Curr Opin Ophthalmol. 2009;20:351–355. doi: 10.1097/ICU.0b013e32832f7f25. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi M, Sato N, Sugimori H, Mori K, Oshimi K. A minor E-selectin ligand, CD65, is critical for extravascular infiltration of acute myeloid leukemia cells. Leuk Res. 2001;25:847–853. doi: 10.1016/s0145-2126(01)00036-4. [DOI] [PubMed] [Google Scholar]
- 20.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 21.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 23.Roland CL, Dineen SP, Toombs JE, Carbon JG, Smith CW, Brekken RA, Barnett CC. Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic xenografts. Exp Biol Med. 2010;235:263–269. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 24.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- 25.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–832. [PMC free article] [PubMed] [Google Scholar]
- 26.Shields CL, Meadows AT, Leahey AM, Shields JA. Continuing challenges in the management of retinoblastoma with chemotherapy. J Retin Vitr Dis. 2004;24:849–862. doi: 10.1097/00006982-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 28.Sun JJ, Zbou XD, Liu YK, Tang ZY, Feng JX, Zbou G, Xue Q, Chen J. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol. 1999;125:28–34. doi: 10.1007/s004320050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1—a mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol. 2001;280:C814–C822. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 31.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]


