Abstract
Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin in the brain, influencing neural development, plasticity, and repair (Chen et al., 2004; Thoenen, 1995). The BDNF gene contains a single-nucleotide polymorphism (SNP) called Val66Met. The Met allele interferes with intracellular BDNF-trafficking, decreases activity-dependent BDNF secretion, and consequently is often associated with a shift from plasticity to stability in neural circuits (Egan et al., 2003). We investigated the behavioral consequences of the presence of the Met allele by comparing how 40 heterozygous subjects with the Val/Met genotype and 35 homozygous subjects with the Val/Val genotype performed on visuomotor tasks (reaching and navigation) under two conditions: normal vision and completely left-right reversed vision. As expected, subjects did not differ in their short-term ability to learn the tasks with normal vision (p = 0.58). Intuitively, it would be expected that homozygous Val/Val subjects with a propensity for greater BDNF-induced activity-dependent plasticity would learn new tasks more quickly than heterozygous Val/Met subjects with decreased BDNF secretion (Gilbert, Li, & Piech, 2009). However, we found the opposite here. When short-term mechanisms of visuomotor adaptation were engaged to compensate for the misalignment of visual and somatomotor information created by the left-right reversal of vision, heterozygous Val/Met subjects learned significantly more quickly than their homozygous Val/Val counterparts (p = 0.027). Our results demonstrate the paradoxical finding that the presence of the Met allele, which is thought to promote cortical stability, here improves immediate visuomotor adaptation to left–right-reversed visual input.
Keywords: vision, visuomotor, prism adaptation, BDNF, plasticity
Introduction
Neural plasticity is the ability for neural circuitry to change in response to changes in input statistics through such mechanisms as the creation of new neural connections (structural plasticity) or the reweighting of extant connections (functional plasticity; Gilbert, Li, & Piech, 2009; Wandell & Smirnakis, 2009). Some neural systems require plasticity throughout life, such as the hippocampal memory network where dynamic neural circuits underlie the formation and maintenance of new memories. Other neural circuits, such as the visual system, require more stability once development is complete in order to effectively process incoming peripheral sensory information that must be maintained in a specific relationship between the environment and the cortical representation (e.g., the retinotopic map in V1; Brewer & Barton, 2014; Wandell & Smirnakis, 2009). At the interface between sensory and motor systems, plasticity is specifically required throughout life to compensate for changes in either system and thus maintain proper sensorimotor integration, as seen in visuomotor adaptation such as the vestibulo-ocular reflex arc (e.g., Gonshor & Jones, 1976a, 1976b; Lisberger, Miles, & Optican, 1983) or deviating prism adaptation (e.g., Barton, Lin, Asher, & Brewer, 2009; Keuroghlian & Knudsen, 2007; Knudsen & Brainard, 1991; Kohler, 1964; Lin, Barton, Asher, & Brewer, 2009; Linden, Kallenbach, Heinecke, Singer, & Goebel, 1999; Luaute et al., 2009; Miyauchi et al., 2004; Richter et al., 2002; Rode, Rossetti, Li, & Boisson, 1998; Rossetti et al., 1998; Sekiyama, Miyauchi, Imaruoka, Egusa, & Tashiro, 2000; Stratton, 1897; Sugita, 1996).
One of the pioneering studies of visuomotor adaptation to deviating prisms was done by Stratton (1897), who wore prism spectacles that flipped visual input up-down continuously for 8 days. On approximately days 5–6 of the experiment, Stratton described that he felt as if his motor skills had fully adapted to the inverted visual input. A number of studies have tried to replicate his findings over the past 100 years using both up-down inverting and left-right reversing prism goggles, but only recently have studies started to provide evidence about exactly how such adaptation to altered visual input may occur both behaviorally and in cortex using psychophysics (Kohler, 1964), electroencephalography (EEG) recording (Berndt, Franz, Bulthoff, Gotz, & Wascher, 2005; Sugita, 1996), functional neuroimaging (Barton et al., 2009; Lin et al., 2009; Linden et al., 1999; Miyauchi et al., 2004; Sekiyama et al., 2000), and single neuron recording on monkeys (Sugita, 1996). The behavioral measurements across these studies have consistently shown that subjects recover visuomotor skills to near-baseline levels after long-term, continuous adaptation (more than 7 days) to inverting or reversing prisms (Barton et al., 2009; Lin et al., 2009; Linden et al., 1999; Miyauchi et al., 2004; Richter et al., 2002; Stratton, 1897; Sugita, 1996). Interestingly, visuomotor adaptation to complete left-right reversing prisms (Barton et al., 2009; Lin et al., 2009; Miyauchi et al., 2004; Sugita, 1996) appears to be more difficult than to up-down inverting prisms (Linden et al., 1999; Richter et al., 2002; Stratton, 1897) or to prisms that produce only small deviations in visual input (e.g., 10° shift to the right to stay within a hemifield; Rode et al., 1998; Rossetti et al., 1998).
One outstanding aspect of many of these studies is the anecdotal discussion of differences in immediate adaptation to altered visual input across individuals. Such differences in behavioral adaptation likely arise from differences in cortical adaptation. As these differences seem to be most striking for deviating prisms that produce a complete left-right reversal of the visual input (Barton et al., 2009; Lin et al., 2009; Miyauchi et al., 2004; Sugita, 1996), investigating this dramatic condition of left-right reversal may provide insight into intersubject factors that could underlie subtle differences in response to therapy for cortical deficits, such as therapy with slightly (∼10°) deviating prisms in patients with left hemispatial neglect from stroke (Rode et al., 1998; Rossetti et al., 1998), or may unveil new ways to stimulate cortical plasticity for recovery from such cortical damage (Barton et al., 2009; Lin et al., 2009).
What might drive such differences in subjects' immediate and short-term abilities to adjust to dramatically altered visual input such as that created by left-right reversing prisms? One likely possibility may be a difference across individuals in factors affecting cortical plasticity such as the neurotrophin brain-derived neurotrophic factor (BDNF). Neurotrophins are a family of growth factors in the brain that play a key role in neural plasticity. As the most abundant neurotrophin in the brain, BDNF is widely expressed in cortex and influences a broad range of brain events related to developmental neuronal differentiation, synaptic plasticity, and neuronal survival in adulthood (Chen et al., 2004; Pearson-Fuhrhop, Kleim, & Cramer, 2009; Thoenen, 1995). The BDNF gene contains a single nucleotide polymorphism (SNP) in the prodomain called Val66Met (196 A/G; rs6265), in which methionine is substituted for valine at codon 66 (Chen et al., 2004; Egan et al., 2003). The Met allele is present in ∼30% of the population of the United States and ∼66% of the population of Japan (Shimizu, Hashimoto, & Iyo, 2004). The presence of the Met allele does not alter the structure of the final BDNF protein, but interferes with intracellular trafficking of the BDNF protein prior to its secretion from the cell. This alteration in trafficking decreases activity-dependent BDNF secretion (i.e., secretion in response to depolarization), but does not affect constitutive secretion (i.e., constant secretion not under environmental control; Chen et al., 2004; Egan et al., 2003). This decreased activity-dependent secretion is associated with a shift from plasticity to stability in cortical circuitry (Egan et al., 2003).
Here, we investigate the consequences of the presence of the Met allele for the Val66Met BDNF SNP on the short-term visuomotor adaptation to an extreme alteration of visual input, which leads to a misalignment of visual and somatomotor information. We compare how 40 heterozygous subjects with the Val/Met genotype and 35 homozygous subjects with the Val/Val genotype perform on visuomotor tasks under two conditions: normal vision and completely left-right reversed vision produced by custom prism goggles. Our results demonstrate the paradoxical finding that the presence of the Met allele, which is thought to promote cortical stability, here improves immediate visuomotor adaptation to left-right reversed visual input.
Materials and methods
Experimental design
The study consisted of two visuomotor tasks, reaching and navigation, which the subjects performed under two conditions, normal vision (Condition 1: control goggles) and completely left-right reversed vision (Condition 2: prism goggles). Subjects underwent three phases of testing. The first phase was used to review and sign consent forms, undergo a blood draw for genetic screening, and complete a series of short questionnaires to collect demographic information and for visuomotor skill assessment. Subjects then spent the second phase performing baseline visuomotor tasks (Condition 1) wearing a pair of custom-built control goggles with a moderately-restricted field of view, but with no other visual alterations (Figure 1A). Finally, subjects spent the third phase performing for a second time the same visuomotor tasks as in the second phase, but this time donning custom-built prism goggles (Condition 2; Figure 1B), which produced a complete left-right reversal of visual input within the same restricted field of view as the control goggles.
Figure 1.
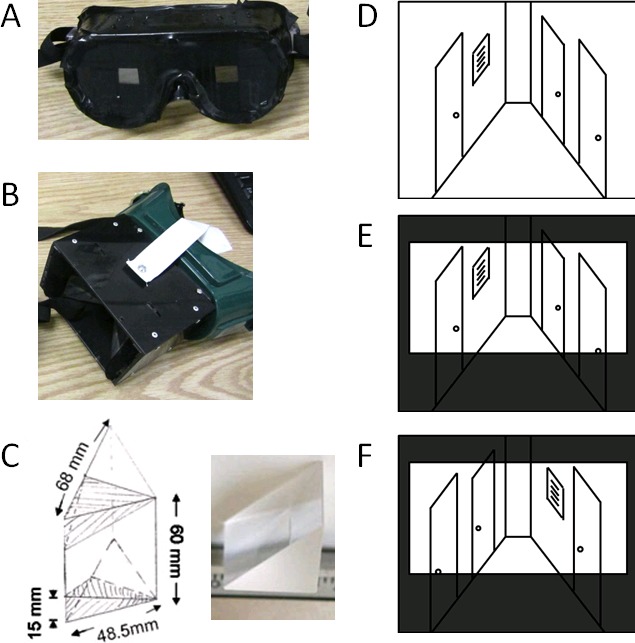
Custom goggles. (A) Custom control goggles were each constructed from clear safety goggles, which were covered with black electrical tape on the front and sides to restrict the field of view to match that of the prism goggles. (B) Prism goggles were each constructed using two right-angle prisms custom cut and polished from a clear acrylic cube (Tap Plastics, San Jose, CA). Frames were designed from a welder's mask base and heavy-duty black plastic. Goggles rested against each subject's forehead and nasion, held in place with adjustable elastic bands over the head (white straps). Black felt at the temples and within the base of the welder's mask prevented normal visual input from the periphery. Maximum field of view through the goggles was approximately 45°–55° vertically by 60°–77° horizontally. Prism position was manually adjusted for each subject to provide the maximum range of binocular vision. Subjects had normal depth perception from approximately 40 cm to infinity. (C) Prism dimensions (left) and close-up of one right-angle prism (right). (D–F) Cartoon Illustrations of Visual Alterations: (D) Normal, completely unaltered view of the world. (E) The view through the control goggles, which restrict the field of view to the same extent as the prism goggles, but have no other visual alterations. (C) The view through the prism goggles with left–right-reversed visual input and restricted field of view.
Goggle construction
The prism goggles were custom-built goggles that left-right mirror-reversed a subject's field of view (Figure 1B through D; Barton et al., 2009; Lin et al., 2009). They were each constructed using two right-angle prisms custom cut and polished from a clear acrylic cube (Figure 1C; Tap Plastics, San Jose, CA). Frames were designed from a welder's mask base and heavy-duty black plastic. Goggles rested against each subject's forehead and nasion, held in place with adjustable elastic bands over the head (white straps). Black felt at the temples and within the welder's base prevented normal visual input from the periphery. Maximum field of view through the goggles was approximately 45°–55° vertically by 60°–77° horizontally. Prism position was manually adjusted for each subject to provide the maximum range of binocular vision. Subjects had normal depth perception from approximately 40 cm to infinity. The control goggles were custom-built goggles with the same restricted field of view that were each constructed from clear safety goggles, which were covered with black electrical tape on the front and sides to restrict the field of view to match that of the prism goggles (Figure 1A, D through F). Control goggles were similarly held against each subject's head with adjustable elastic bands (black straps).
Subjects
Seventy-five subjects (39 female; 40 Val/Met heterozygotes and 35 Val/Val homozygotes) between the ages of 18 and 39 participated in this study for a modest honorarium of $10 per hour, for total of up to $30 for completion of the study. All subjects were of good health, had normal or corrected-to-normal vision, and reported being free of neurological and psychiatric disease, vertigo, balance problems, and psychoactive substances. All subjects also spoke English and were right-handed, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects did not significantly differ on a wide-ranging visuomotor skills inventory (see Questionnaires section). To minimize risk from potential falls while wearing the prism goggles, female participants reported not being pregnant, having early symptoms of menopause, having been diagnosed with premature ovarian failure, low bone density, or osteoporosis. The experimental protocol was approved by the Institutional Review Board and the Institute for Clinical and Translational Science (ICTS) Scientific Review Committee at the University of California, Irvine (UCI), in conjunction with ICTS biostatistical review, and informed consent was obtained from all subjects prior to beginning the experiments. At the end of the session or upon report of nausea arising from the left-right visual reversal, each subject was evaluated using the Graybiel Motion Sickness Diagnostic Criteria (Graybiel, Wood, Miller, & Cramer, 1968) and observed for ∼5–15 min to ensure a return of normal visuomotor function. If subjects reported significant nausea or vomiting induced by the prism goggles, they were removed from further testing.
Taking into account possible attrition and the expected distribution of the polymorphisms given the ethnic distribution at UCI (Shimizu et al., 2004; University of California, 2011), we determined a priori that our target sample size was 75 subjects. The sample size was designed to have a minimum of 25 subjects of each polymorphism to achieve approximately normally distributed data for the visuomotor measures. Our target sample size was drawn from recent published data from McHughen et al. (2010). These data evaluated performance on a driving-related computer game in relation to the BDNF genotype (Val66Met polymorphism present or not). The difference between genotype groups was significant (p = 0.044) across a total sample size of 25. There is no literature to date from which we could draw information about the specific expected standard deviation in visuomotor task performance during adaptation to a short-term complete reversal of visual input. However, recent studies of short- and long-term adaptation to deviating prism goggles (e.g., 10° deviation to the left) have typically used sample sizes of 13 (Sarri et al., 2008), 14 (Luaute et al., 2009; Richter et al., 2002), and 20–57 (Richter, Wennberg, & Raudsepp, 2007). We did not analyze the data until the completion of the data collection, so no specific criteria were used to stop subject enrollment other than our a priori estimate of target sample size.
Genetic screening
At the beginning of the experimental session, subjects underwent a 10 cc blood draw from the antecubital fossa of the left arm for genotyping of the Val66Met (rs6265) BDNF SNP at the facilities of the ICTS at UCI. Genomic DNA was extracted from leukocytes by standard DNA extraction procedures (Kleim et al., 2006; McHughen et al., 2010). The polymerase chain reaction (PCR) amplifications of the 274-bp fragment were carried out with the following forward and reverse primers: 5′-aaagaagcaaacatccgaggacaag-3′ 5′-attcctccagcagaaagagaagagg-3′, as described previously (Institute for Clinical and Translational Science, University of California, Irvine; Kleim et al., 2006; McHughen et al., 2010; Sen et al., 2003). BDNF SNP screening was performed with denaturing high-performance liquid chromatography (DHPLC) analysis on Transgenomics WAVE system (Transgenomic, Omaha, NE; for additional details, see McHughen et al., 2010; Oefner & Underhill, 1998). All homozygote mutant DNA samples detected by dHPLC were confirmed by sequencing (McHughen et al., 2010). Subjects were identified as having one of the following BDNF Val66Met SNP genotypes: Val/Val, Val/Met, or Met/Met. The two subjects with the homozygous Met/Met genotype were excluded from further analysis in this study. Genotype frequencies observed in our cohort (Val/Val: 0.45, Val/Met: 0.52, Met/Met: 0.03) were close to Hardy-Weinberg equilibrium, as expected from the mixed population of UCI, from which they were drawn (Shimizu et al., 2004; University of California, 2011).
Questionnaires
Subjects completed a set of extensive questionnaires detailing information about demographics and visuomotor skills (results are reported in Tables 1 and 2). In the Demographics Survey, subjects self-reported gender, age, education, and ethnicity. In the General Survey, subjects were asked to rate themselves on a series of questions related to visuomotor skills (e.g., sense of direction, motion sickness, footedness) using a five-point Likert scale. In the Skills Survey, subjects were asked to rate themselves on specific visuomotor skills with questions addressing at what they level performed each of the categories on four-point Likert scale, coded such that 1 = Rarely, 2 = Recreationally, 3 = Competitively, and 4 = Professionally. In addition, subjects were asked how many hours per week they spend doing each of the activities covered in the categories. Subjects were also asked to state specifically which sports, games, instruments, etc., they played (not reported here).
Table 1.
Demographic and Visuomotor Skills Survey Results. Notes: Demographics. Subjects self-reported age, education, and gender. A chi-square test was performed to test whether the ethnicity distribution of the subjects with the Val/Met genotype differed from the distribution expected based on that of the subjects with the Val/Val genotype. General Survey. Subjects were asked a series of questions on a 5-point Likert scale. Skills Survey. Subjects were asked “At what level do you” do each of the categories on 4-point Likert scale of self-ranked skill, coded such that 1 = “Rarely”, 2 = “Recreationally”, 3 = “Competitively”, and 4 = “Professionally”. Hours Spent Per Week. Subjects were asked how many hours per week they spend doing each of the activities covered in the categories. Subjects were also asked to state specifically which sports, games, instruments, etc. that they played (not reported here). A one-way analysis of variance (ANOVA) was performed to assess whether subjects with each genotype were matched on all factors except ethnicity (reported in Table 2).
| Subject Characterization |
Val/Val M (SD) |
Val/Met M (SD) |
dfb |
dfw |
F |
p |
| Demographics | ||||||
| Age in Years | 21.4 (1.8) | 22.2 (3.2) | 1 | 73 | 1.84 | 0.18 |
| Years of Education | 14.9 (1.3) | 15.5 (2.1) | 1 | 70 | 2.05 | 0.16 |
| Gender [Female/Total] | [18/35] | [21/40] | 1 | 73 | 0.01 | 0.93 |
| General Survey (Likert, 1-5) | ||||||
| Handedness (5 = right) | 4.7 (0.6) | 4.7 (0.5) | 1 | 73 | 0.04 | 0.84 |
| Footedness (5 = right) | 4.3 (0.9) | 4.3 (0.8) | 1 | 73 | 0.01 | 0.94 |
| Motion Sickness (5 = most) | 1.9 (0.8) | 2.1 (0.9) | 1 | 73 | 1.14 | 0.29 |
| Direction Sense (5 = best) | 4.0 (1.0) | 3.8 (0.9) | 1 | 73 | 0.82 | 0.37 |
| Hand-Eye Co-ord. (5 = best) | 4.0 (0.8) | 3.9 (0.9) | 1 | 73 | 0.83 | 0.36 |
| Driving Skill (5 = best) | 4.2 (0.9) | 4.1 (1.0) | 1 | 73 | 0.19 | 0.66 |
| Skills Survey (Likert, 1-4) | ||||||
| Sports (4 = best) | 1.9 (0.7) | 1.8 (0.7) | 1 | 73 | 0.14 | 0.48 |
| Video Games (4 = best) | 1.6 (0.6) | 1.7 (0.7) | 1 | 73 | 0.07 | 0.71 |
| Music (4 = best) | 1.3 (0.7) | 1.4 (0.8) | 1 | 73 | 0.12 | 0.73 |
| Other (4 = best) | 1.4 (0.5) | 1.4 (0.5) | 1 | 73 | 0.21 | 0.65 |
| Hours Spent per Week | ||||||
| Playing Sports | 4.4 (5.2) | 3.1 (3.5) | 1 | 70 | 1.58 | 0.21 |
| Playing Video Games | 4.1 (5.3) | 3.7 (7.1) | 1 | 71 | 0.07 | 0.80 |
| Playing Music | 1.9 (4.5) | 1.0 (2.7) | 1 | 70 | 1.07 | 0.30 |
| Doing Other Activities | 3.1 (4.8) | 1.5 (2.4) | 1 | 66 | 2.99 | 0.09 |
Table 2.
Ethnicity Results. Notes: Subjects self-reported ethnicity. A chi-square test was performed to test whether the ethnicity distribution of the subjects with the Val/Met genotype differed from the distribution expected based on that of the subjects with the Val/Val genotype.
| Subject Ethnicity |
Val/Val Total |
Val/Met Total |
df |
χ2 |
p |
| Caucasian | 19 | 11 | |||
| Asian | 6 | 20 | |||
| Other | 10 | 9 | |||
| 2 | 35.49 | < 0.001 |
Reaching task
The reaching task engaged visuomotor circuits by requiring subjects to repeatedly localize a small, randomly placed white circle flashed briefly in their field of view and subsequently use the right index finger to touch where the circle had been. Subjects were seated ∼45 cm in front of a touchscreen monitor (Planar 19-in. PT1910MX, resolution: 1280 × 1024) and were asked to fixate on a central fixation cross throughout each trial. For each condition (control goggles vs. prism goggles), subjects were asked to complete up to six blocks of 20 trials each. The number of blocks completed varied between subjects due to the onset of nausea in a subset of subjects, with 73 subjects completing at least four blocks of the reaching task and all 75 subjects completing at least three blocks.
Subjects initiated each trial by touching the ∼1° of visual angle black fixation cross in the center of the screen, after which a ∼1° white target disk appeared for 250 ms. Then the subject was required to touch, as quickly and accurately as possible within 1000 ms, the location on the screen where the white disk had appeared. The intertrial interval was determined by each subject, providing the potential for short breaks to prevent prism-induced vertigo as needed. Subjects were instructed to complete the trials as quickly as possible while remaining comfortable. The average break between blocks was ∼15 s. The target disks appeared in pseudorandom locations with the restriction that they appeared equally often in all four quadrants over the course of each block. After each trial, each subject was given feedback of the location of the white disk (∼1° of visual angle set of three concentric, black rings centered on the target location) and the actual touched location (∼1° of visual angle black disk). Stimuli were programmed in Matlab using Psychtoolbox (Brainard, 1997; Pelli, 1997).
Navigation task
The navigation task engaged visuomotor circuits by requiring subjects to walk to and use their right hands to touch seven targets placed ∼1.5 m above the floor on the walls to either side of a ∼2–m-wide, ∼ 18–m-long straight hallway as quickly as possible while maintaining a normal walking speed. The targets were ∼15 cm tall numbers printed in red ink on letter-size laminated paper and were numbered in order from 1–7, with even numbers placed on the left side of the hallway and odd numbers on the right (when viewed from the starting position). This arrangement forced the subjects to zig-zag back and forth to touch each number. The number 6 target was placed in an alcove on the left side of the hallway, sized ∼3 m × ∼1.8 m, with an obstacle of a concrete pillar aligned with the plane of the left hallway wall and centered with respect to the alcove. For each condition (Condition 1: control goggles vs. Condition 2: prism goggles), subjects performed two trials of an identical course (target locations did not vary between trials), measuring time to completion. By necessity, the navigation task trials were always last in the testing sessions (Conditions 1 and 2) due to the potential for the induction of nausea with walking under the short-term, left-right reversal of the visual field. Each trial of the navigation task in each condition was separated by at least 30 s and performed directly after one another with no other walking or tasks carried out by the subjects between the trials. Subjects were transported from the reaching task location and from the end point of the navigation task to the starting point using a rolling people-mover device pushed by the experimenters. Subjects were tended to by two experimenters at all times throughout each trial who served as spotters to prevent subjects from falling, hitting walls, or other navigation mishaps. Experimenters only warned subjects of immediate danger (e.g., “Wall!”) or skipped targets (e.g., “You missed target number three.”) and gave positive feedback of having touched the appropriate target (e.g., “Six!”). Experimenters otherwise did not interact with the subjects during the navigation task trials and remained outside their field of view.
Visuomotor task performance analysis
These two visuomotor tasks were chosen based on optimal measurements of visuomotor adaptation in the few previous studies of adaptation to left-right reversed visual input (e.g., Barton et al., 2009; Lin et al., 2009; Miyauchi et al., 2004). The effects of the left-right reversing prism goggles on balance and the vestibular system are so significant that they affect not only the navigation task, but also the reaching task, as it involves sitting upright and keeping head and arm orientation in proper position throughout the reaching task. Proprioception and balance are interestingly quite disturbed even at rest under the left-right visual reversal. In addition, unlike many other common tests of motor function (e.g., 9-hole Pegboard, Marble Board; McHughen et al., 2010), neither of the tasks measured in this study can be completed without using visual information (i.e., by relying solely on somatosensory input and thereby avoiding the left-right reversed visual input). For the particular paradigm of short-term adaptation to left-right reversing prisms that we investigate here, we are interested in differences between subject groups in the immediate effects of the reversed visual input on visuomotor adaptation. The analysis of the learning function to asymptotic levels for visuomotor tasks performed under left-right reversed visual input is not possible under short-term conditions, as studies of long-term adaptation to left-right reversing prisms have shown that the performance of subjects on the reaching and navigation tasks requires at least 7 to 10 days to return to a near-baseline asymptote of performance for both tasks (40–80 trials a day for 7–10 days for the reaching task; 1–3 trials per day for the navigation task, depending on nausea; Barton et al., 2009; Berndt et al., 2005; Lin et al., 2009). Of course, these subjects were also participating in visuomotor activity throughout each day outside of the experimental behavioral testing, so, if anything, these numbers of trials are a low estimate of the true asymptote. This time frame is also consistent with recent measurements of changes in cortical spatial representations during long-term adaptation to left-right reversed visual input (Barton et al., 2009; Lin et al., 2009; Luaute et al., 2009; Miyauchi et al., 2004; Sugita, 1996). Our primary interest in this study of short-term visuomotor adaptation is to examine differences between our subject groups in immediate the effects of an extremely taxing visual input (complete left-right reversal). Consequently, our primary analysis examines the changes in performance between the first and second exposures to each condition (control vs. prism goggles).
Although prism adaptation effects have been highly significant in previous literature, no study to date has measured these in combination with genetic polymorphisms. To compare subject genotype groups with adequate statistical power, we planned a priori to analyze these two tasks of visuomotor adaptation in a combined measurement, as we are not interested in how subjects adapt on each individual task, but rather are using these as a measure for more general visuomotor adaptation. To rule out differences between the groups other than those associated with the genetic differences between groups, we planned a priori to compare the groups on a wide range of factors. If any differences were found, based on the standard statistical criterion of p < 0.05, a follow-up posthoc analysis would be performed to rule out the influence that variable between the groups.
Results
Individual task measurements
Reaching task
The average distance between the target and where the subjects actually touched the touchscreen monitor is reported as distance error in Figure 2A and B in degrees of visual angle (dva). Dark gray bars or lines represent data from subjects with the Val/Met genotype, while light gray bars or lines represent data from subjects with the Val/Val genotype. In Figure 2B, as expected from studies of long-term adaptation to reversed visual input, distance error does not asymptote nor return to baseline over the experimental period, as shown across the first four blocks of trials for the reaching task. Note that the measurements in Figure 2B for both subject groups in Condition 1 are nearly overlaid on top of each other (bottom lines), as expected for the baseline visuomotor evaluation. The distance error measurements for just Blocks 1 and 2 are plotted in Figure 2A; these two blocks are used for the further analysis of error improvement in Figure 2D. We also measured the reaction time for the reaching task, which is shown in Figure 2C as the average amount of time a subject took to touch the touchscreen monitor in seconds, measuring from the time that the target disappeared to a maximum of 1 s. There is little difference between the performances of the two groups in Condition 1, when little-to-no visuomotor adaptation is required (Figure 2C). Note also the increase in reaction time for both groups in Condition 2 (∼20 msec), when subjects were required to adapt their movements to the altered visual environment. There was no evidence for a speed-accuracy trade-off. Improvement in the distance error for the Reaching Task (Block 2 – Block 1) is shown in Figure 2D, such that larger negative values are plotted upwards and indicate greater improvement.
Figure 2.
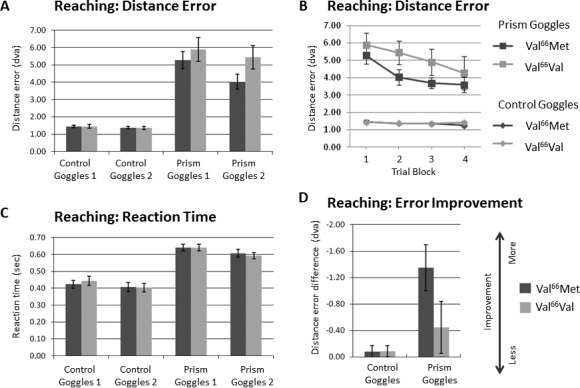
Reaching task performance. (A) Reaching: Distance Error Averages. The average distance between the target and where the subjects actually touched the touchscreen monitor is reported in degrees of visual angle (dva) for the first two blocks of trials for each condition. Dark gray bars represent data from subjects with the Val/Met genotype, while light gray bars represent data from subjects with the Val/Val genotype. (B) Reaching: Distance Error by Trial Block. The distance error is shown for the first four blocks of trials for the reaching task. Trial Blocks 1 and 2 are plotted in (A). Square markers represent trials done under condition-2: prism goggles (top two lines). Diamond markers represent trials done under condition-1: control goggles (bottom two lines). Dark gray lines in each set represent data from subjects with the Val/Met genotype, while light gray lines in each set represent data from subjects with the Val/Val genotype. Note that the measurements for both subject groups in condition-1: control goggles are nearly overlaid on top of each other, as expected for the baseline visuomotor evaluation. Time to return to baseline for the Reaching Task under the prism goggle condition has been measured in long-term, left–right-reversing prism adaptation studies in humans to take at least 7 to 10 days (Barton et al., 2009; Lin et al., 2009; Miyauchi et al., 2004). (C) Reaching: Reaction Time. The average amount of time the subject took to touch the touchscreen in seconds from the time that the target disappeared to a maximum of 1 s over the same set of trial blocks as shown in (A) for each condition. Note the lack of difference in condition-1: control goggles, when little-to-no visuomotor adaptation is required. Note also the slight improvement in condition-2: prism goggles (∼40 ms), when subjects were required to adapt their movements to the altered visual environment. There was no evidence for a speed-accuracy trade-off. (D) Reaching: Error Improvement. Block 2 – Block 1 difference in distance error, such that larger negative values (plotted upwards) indicate greater improvement. Larger negative values (plotted upwards) indicate greater combined improvement scores. Error bars indicate ±SEM.
Navigation Task
The average amount of time subjects took to touch all 10 targets in the Navigation Task is plotted for each trial (goggles 1 and 2) in each condition (control goggles and prism goggles) in Figure 3A. Improvement in Navigation Task time to completion (Trial 2 – Trial 1) is shown in Figure 3B. Note the similarity of the overall pattern of results for the two subject groups to the improvement in distance error for the Reaching Task in Figure 2D.
Figure 3.
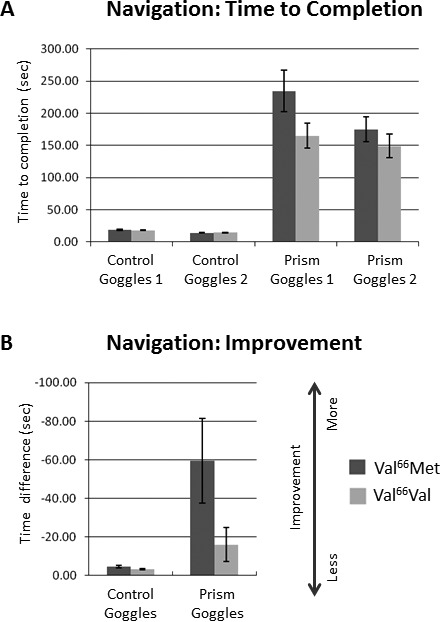
Navigation Task Performance. (A) Navigation: Time to Completion. The average amount of time subjects took to touch all 10 targets in the walking navigation task is plotted for each trial (goggles 1 and 2) in each condition (control goggles and prism goggles). (B) Navigation: Improvement. Block 2 – Block 1 difference in time to completion. Note the similarity of the overall pattern of results to the Reaching Task in Figure 2D. Other details are as in Figure 2.
Visuomotor task performance differed between subject groups under left-right reversed visual input
When wearing the control goggles, both tasks proved to require little effort to learn, as expected from the extensive experience by all subjects in everyday life with similar motor tasks (Figures 2–4). When wearing the prism goggles, which completely left-right reversed the visual field, both tasks were much more difficult and required considerable visuomotor adaptation (Figures 2-4).
Figure 4.
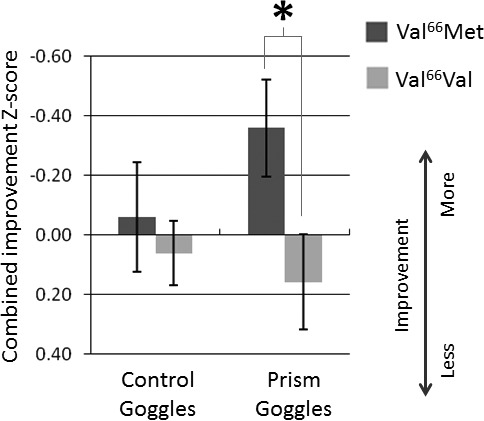
Combined improvement z-score. Improvement scores on visuomotor performance for the Reaching Task and the Navigation Task were transformed into z-scores and combined in order to assess statistical differences in visuomotor adaptation across both tasks between the subjects with the Val/Met and Val/Val genotypes. Larger negative values (plotted upwards) indicate greater combined improvement scores. Other details are as in Figure 2.
To assess statistical differences in immediate visuomotor adaptation between subjects with the Val/Val and Val/Met genotypes, average performance for the second block of trials (Reaching Task) or second trial (Navigation Task) was subtracted from the first block/trial, converted to z-scores, and combined for each condition. As expected, for Condition 1 when visuomotor adaptation is not strongly engaged, there is no difference in combined improvement z-score between subjects with the Val/Met genotype (M = −0.06, SD = 1.16) and subjects with the Val/Val genotype (M = 0.06, SD = 0.64; Condition 1-combined, two-tailed, independent-samples t test, t[73] = −0.56, p = 0.58; Figure 4). Counterintuitively, for Condition 2 when visuomotor adaptation is dramatically required, Val/Met subjects have significantly better combined improvement z-scores (M = −0.36, SD = 0.97) than Val/Val subjects (M = 0.16, SD = 0.93; Condition 2-combined, two-tailed, independent-samples t test, t[67] = −2.265, p = 0.027; Figure 4).
Subject groups did not differ significantly on other epigenetic factors
To rule out alternative explanations, we evaluated a number of other variables that might underlie the genotype-related differences observed for the visuomotor assessment of the two subject groups using extensive questionnaires detailing demographic information, self-rated visuomotor skills, and specific activities (Table 1; Graybiel et al., 1968; Oldfield, 1971). A one-way analysis of variance (ANOVA) was performed to assess whether subjects with each genotype were matched on all factors except ethnicity (Table 2), and no significant differences were found for the following factors (p > 0.05; Table 1): (a) Demographics: gender, age, years of education; (b) General assessment: handedness, footedness, motion sickness, direction sense, hand-eye coordination, driving skill; (c) Self-rated skill at sports, video games, and music; and (d) Hours spent per week playing sports, video games, music, or doing other activities.
Subject ethnicity
The distribution of ethnicities across the two subject groups was consistent with the expected demographics of the University of California, Irvine, student population (University of California, 2011). A chi-square test was performed to evaluate whether Val/Met subjects and Val/Val subjects differed in ethnic distribution. We used the ethnic distribution of the Val/Val genotype as the expected distribution for the Val/Met genotype and found that the Val/Met ethnic distribution was significantly different, χ2(2) = 35.49, p < 0.001 (Table 2). The two ethnicities which differed between the groups were Caucasian and Asian, whereas other ethnicities were almost identical (Table 2). This result is a replication of the previous findings that the Val/Met genotype is roughly twice as common in Asian populations relative to Caucasian populations (Shimizu et al., 2004; University of California, 2011). To rule out the possibility that the ethnic distribution and not the BDNF SNP genotype could explain the difference in visuomotor adaptation we observed in Condition 2 for the combined measurements, we performed a two-tailed, independent-samples t test on the Condition 2-combined z-scores between all Caucasian (N = 30) and Asian (N = 22) subjects, independent of BDNF SNP genotype. No significant difference was found for Condition 2-combined between Caucasian (M = −0.12, SD = 0.87) and Asian subjects (M = −0.09, SD = 1.10; t[50] = −0.103, p = 0.918).
Subject nausea
The time that subjects can tolerate the left-right visual reversal without prohibitive effects of nausea is very short (approximately 1 hour, depending on the subject). Twenty-six people dropped out by the end of the study due to nausea or vomiting. Typically, this happened during the Navigation Task, which was therefore scheduled at the end of the prism goggles testing session, so that we would not lose large portions of behavioral measurements for subjects who ended up developing nausea. Some subjects who were more susceptible to motion sickness developed nausea during blocks 4–6 of the Reaching Task, which also appears to affect the vestibular system under left-right reversed vision. Seventy-three subjects completed at least 4 blocks of the Reaching Task, and all subjects completed at least 3 blocks; thus, the analysis of improvement between blocks 1 and 2 of the Reaching Task was not affected by subject withdrawal. There was no other cause for withdrawal from the study.
Subjects were instructed to report nausea at the first sign of symptoms and were then evaluated with the Graybiel Motion Sickness Diagnostic Criteria (Graybiel et al., 1968) and removed from further data collection. The unique paradigm of the complete left-right visual reversal can produce significant, unavoidable nausea. It is therefore not possible to collect data without accounting for this expected complication and allowing for subject withdrawal due to nausea. Motion sickness and vestibular sensitivity to such conditions vary widely across people and have not been linked to differences in BDNF (e.g., Smith, 2000; Smith & Darlington, 1996). Here, a two-tailed, independent samples t-test revealed no significant difference in dropout rates between Val/Met heterozygotes (M = 0.43, SD = 0.5) and Val/Val homozygotes (M = 0.26, SD = 0.4; t(73) = 1.527, p = 0.13). Although p = 0.13 is not standardly considered to be statistically significant, we understand the concern some may have regarding any possible interaction between nausea and performance differences, given our unusual paradigm. To further rule this out, we performed an ANOVA with prism adaptation as the dependent variable, genotype as the independent variable, and nausea as a covariate. The effect of genotype was still significant, F(1, 66) = 4.199, p = 0.044.
One subject who did not follow the Navigation Task instructions was also removed from the analysis of Navigation Task performance. Data collected for these subjects for the questionnaires and up to the visuomotor task trial before withdrawal were included in the data analysis. No subjects were included in the analysis in condition-1: control goggles but not condition-2: prism goggles, or vice versa. That is, data for subjects who withdrew from the study were removed for that task or set of trials from both conditions.
Discussion
Here we have shown that the presence of the Met allele for the Val66Met BDNF SNP improves immediate visuomotor adaptation to left-right reversed visual input. These findings are in contrast to conventional thinking about the increased cortical stability associated with the presence of the Met allele. We would have predicted that the reduction of activity-dependent BDNF secretion arising from the Met allele would lead to a reduction of visuomotor adaptation speed due to decreased cortical plasticity. Along these lines, the presence of the Met allele has been suggested to correlate with such problems as memory impairments (Egan et al., 2003), abnormal cortical and cerebellar morphology (Agartz et al., 2006; Pezawas et al., 2004), increased susceptibility to such disorders as Alzheimer's disease (Ventriglia et al., 2002) and depression (Sen et al., 2003), and impaired motor learning and motor map plasticity (Kleim et al., 2006; McHughen et al., 2010). The two investigations of motor map plasticity used transcranial magnetic stimulation (TMS; Kleim et al., 2006) and fMRI (McHughen et al., 2010) to show that differences in cortical motor system function between Val/Val and Val/Met subjects, with increased error in motor learning and reduced motor map reorganization in Val/Met subjects.
Here we have measured a seemingly paradoxical positive effect of the Met allele, with Met carriers showing more rapid visuomotor adaptation to dramatically altered visual input. Although plasticity is necessary for visuomotor adaptation, is more plasticity always better? Intuitively, it would seem that a general decrease in cortical plasticity, as associated with the Met allele, should lead to slower or deficient visuomotor adaptation (Gilbert et al., 2009). In the short-term, however, plasticity must be disruptive — old connections must be replaced by or re-weighted in favor of the new connections arising in cortical adaptation and reorganization (Marrone & Petit, 2002). In a typical visual or somatomotor learning paradigm, the experimental task requires an improvement of discrimination or movement control which usually arises from a relatively small refinement of neural tuning and does not require a significant shift in cognitive strategy (e.g., Gilbert et al., 2009). In our experiment, however, we use an extreme alteration of visual input, leading to an interhemispheric disruption of visuomotor integration and possibly a greater dependence on a new cognitive strategy to complete the task. Rapid changes at the interface between the visual and motor systems at the neural level might lead to greater difficulty in developing a successful cognitive strategy.
Normally, reaching to a visual target is simple because of stable associations in the coordinate systems between the visual system and the somatomotor networks (Buneo, Jarvis, Batista, & Andersen, 2002). The reaching and navigation tasks in the control goggle condition are routine, likely employing a cognitive strategy of “see object in location A, reach or navigate to location A using well-learned motor commands.” This strategy has been learned over the subject's lifetime in the form of brain circuitry associating a point in the visual field with a set of motor commands for accurate trajectory planning through space. Tasks with the left-right reversing prism goggles are not routine, however, and subjects must shift from the normal cognitive strategy to a new one, which corresponds to “see object in location A, reach or navigate to location B, which is mirror-reversed from location A.” The prism goggle condition requires a subject to override normal, rapid motor commands and trajectory planning with a new cognitive strategy for a reversed visual world until a new mapping between visual space and motor space develops.
When the somatomotor networks are forced to associate with the visual system in a novel way, plasticity is engaged, disrupting the original, stable connections in favor of new connections (either structurally or functionally; e.g., Keuroghlian & Knudsen, 2007; Knudsen & Brainard, 1991; Marrone & Petit, 2002). While the new visuomotor association is being formed, a cognitive decision must override the normal motor response in favor of the new reversed-world strategy. In this situation, a more slowly changing cortical representation, as suggested by the presence of the Met allele, might actually predict faster visuomotor adaptation at the behavioral level in the short-term, because there might be less conflicting information output from the interface of the visuomotor networks for which a cognitive strategy must compensate. In other words, if the visuomotor interface changes more slowly, the subject may be able to more easily rely on a cognitive strategy to override the incorrect motor response in the prism goggle condition and respond with a left-right reversed movement. The evolutionary persistence across populations of the Met allele with the Val66Met BDNF SNP also supports such a possibility of beneficial effects for the Met allele (Shimizu et al., 2004). Some of the previous studies on the BDNF SNP have similarly shown interesting benefits associated with the presence of the Met allele, such as the preservation of grey matter in multiple sclerosis (Zivadinov et al., 2007), protection against neurocognitive dysfunction in systemic lupus erythematosus (Oroszi et al., 2006), and more efficient memory-based task switching in the elderly (Gajewski, Hengstler, Golka, Falkenstein, & Beste, 2011).
Conclusions
Our results suggest that visuomotor adaptation at the behavioral level under conditions of dramatically-altered visual input benefits in the short-term from a more stable cortical environment. The findings from this study provide a greater understanding of the role of BDNF in the interplay of cortical plasticity and stability that underlies differences in visuomotor adaptation across individuals. In addition, the understanding of the effects of such genetic differences on adaptation to short-term alterations in sensory input in the normal adult human could foster the development of therapies and tailor treatment for patients with damage to either the peripheral sensory visual pathways (e.g., retinal disease) or central cortical regions (e.g., stroke, traumatic brain injury).
Acknowledgments
This work was funded by grant R01 NS058755 (to S. C. C.), and by University of California, Irvine, startup funds (to A. A. B.). This work was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000153 to UCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Alexandra Dicaprio, Brian Minton, and Leah Breen for their help with subject recruitment and data collection and Mike Ward for his help with the construction of the prism goggles. A. A. B., S. C. C., and B. B. conceived of the project. A. T., M. H., G. A., and B. B. conducted the experiments. B. B. processed and analyzed the data with discussions with A. A. B. and S. C. C. The manuscript was written by B. B. and then revised by A. A. B. and S. C. C. and reviewed by all authors. The authors declare no competing financial interests.
Commercial relationships: none.
Corresponding author: Alyssa A. Brewer.
Email: aabrewer@uci.edu.
Address: Department Cognitive Sciences, University of California, Irvine, CA, USA.
Contributor Information
Brian Barton, Email: bbarton@uci.edu.
Andrew Treister, Email: atreiste@uci.edu.
Melanie Humphrey, Email: humphreymelanie@gmail.com.
Garen Abedi, Email: abedig@uci.edu.
Steven C. Cramer, Email: scramer@uci.edu.
Alyssa A. Brewer, Email: aabrewer@uci.edu.
References
- Agartz I., Sedvall G. C., Terenius L., Kulle B., Frigessi A., Hall H., et al. (2006). BDNF gene variants and brain morphology in schizophrenia. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 141B (5), 513–523 [DOI] [PubMed] [Google Scholar]
- Barton B., Lin L., Asher D. E., Brewer A. A. (2009). Alteration of Visuomotor Processing Following Left-Right Prism Adaptation. Vision Sciences Society; Journal of Vision. 9 (8): 763, http://www.journalofvision.org/content/9/8/763, doi:10.1167/9.8.763. [Abstract] [Google Scholar]
- Berndt I., Franz V. H., Bulthoff H. H., Gotz K. G., Wascher E. (2005). Effects of rearranged vision on event-related lateralizations of the EEG during pointing. Biological Psychology, 68 (1), 15–39 [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10 (4), 433–436 [PubMed] [Google Scholar]
- Brewer A. A., Barton B. (2014). Developmental Plasticity: FMRI Investigations into Human Visual Cortex. In Papageorgiou D. T., Smirnakis S. M., Christopoulos G. (Eds.), Functional Magnetic Resonance Imaging/Book 1. New York: In Tech; [Google Scholar]
- Buneo C. A., Jarvis M. R., Batista A. P., Andersen R. A. (2002). Direct visuomotor transformations for reaching. Nature, 416 (6881), 632–636 [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Patel P. D., Sant G., Meng C. X., Teng K. K., Hempstead B. L., et al. (2004). Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. The Journal of Neuroscience, 24 (18), 4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112 (2), 257–269 [DOI] [PubMed] [Google Scholar]
- Gajewski P. D., Hengstler J. G., Golka K., Falkenstein M., Beste C. (2011). The Met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiology of Aging, 32 (12), 2327 e2327–2319 [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Li W., Piech V. (2009). Perceptual learning and adult cortical plasticity. The Journal of Physiology, 587 (Pt 12), 2743–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A., Jones G. M. (1976. a). Extreme vestibulo-ocular adaptation induced by prolonged optical reversal of vision. The Journal of Physiology, 256 (2), 381–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonshor A., Jones G. M. (1976. b). Short-term adaptive changes in the human vestibulo-ocular reflex arc. The Journal of Physiology, 256 (2), 361–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A., Wood C. D., Miller E. F., Cramer D. B. (1968). Diagnostic criteria for grading the severity of acute motion sickness. Aviation, Space, and Environmental Medicine, 39 (5), 453–455 [PubMed] [Google Scholar]
- Keuroghlian A. S., Knudsen E. I. (2007). Adaptive auditory plasticity in developing and adult animals. Progress in Neurobiology, 82 (3), 109–121 [DOI] [PubMed] [Google Scholar]
- Kleim J. A., Chan S., Pringle E., Schallert K., Procaccio V., Jimenez R., et al. (2006). BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nature Neuroscience, 9 (6), 735–737 [DOI] [PubMed] [Google Scholar]
- Knudsen E. I., Brainard M. S. (1991). Visual instruction of the neural map of auditory space in the developing optic tectum. Science, 253 (5015), 85–87 [DOI] [PubMed] [Google Scholar]
- Kohler I. (1964). The Formation and Transformation of the Perceptual World. Psychological Issues, 12. [Google Scholar]
- Lin L., Barton B., Asher D.E., Brewer A.A. (2009). Visual Field Mapping of Visuomotor Adaptation to Prisms. Vision Sciences Society; Journal of Vision. 9 (8): 762, http://www.journalofvision.org/content/9/8/762, doi:10.1167/9.8.762. [Abstract] [Google Scholar]
- Linden D. E., Kallenbach U., Heinecke A., Singer W., Goebel R. (1999). The myth of upright vision. A psychophysical and functional imaging study of adaptation to inverting spectacles. Perception, 28 (4), 469–481 [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Miles F. A., Optican L. M. (1983). Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? The Journal of Neuroscience, 3 (6), 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luaute J., Schwartz S., Rossetti Y., Spiridon M., Rode G., Boisson D., et al. (2009). Dynamic changes in brain activity during prism adaptation. The Journal of Neuroscience, 29 (1), 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone D. F., Petit T. L. (2002). The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Research Reviews, 38 (3), 291–308 [DOI] [PubMed] [Google Scholar]
- McHughen S. A., Rodriguez P. F., Kleim J. A., Kleim E. D., Marchal Crespo L., Procaccio V., et al. (2010). BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex, 20 (5), 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S., Egusa H., Amagase M., Sekiyama K., Imaruoka T., Tashiro T. (2004). Adaptation to left-right reversed vision rapidly activates ipsilateral visual cortex in humans. Journal of Physiology - Paris, 98 (1-3), 207–219 [DOI] [PubMed] [Google Scholar]
- Oefner P. J., Underhill P. A. (1998). Current Protocols in Human Genetics. New York: John Wiley and Sons; [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9 (1), 97–113 [DOI] [PubMed] [Google Scholar]
- Oroszi G., Lapteva L., Davis E., Yarboro C. H., Weickert T., Roebuck-Spencer T., et al. (2006). The Met66 allele of the functional Val66Met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Annals of the Rheumatic Diseases, 65 (10), 1330–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson-Fuhrhop K. M., Kleim J. A., Cramer S. C. (2009). Brain plasticity and genetic factors. Topics in Stroke Rehabilitation, 16 (4), 282–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10 (4), 437–442 [PubMed] [Google Scholar]
- Pezawas L., Verchinski B. A., Mattay V. S., Callicott J. H., Kolachana B. S., Straub R. E., et al. (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. The Journal of Neuroscience, 24 (45), 10099–10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H., Magnusson S., Imamura K., Fredrikson M., Okura M., Watanabe Y., et al. (2002). Long-term adaptation to prism-induced inversion of the retinal images. Experimental Brain Research, 144 (4), 445–457 [DOI] [PubMed] [Google Scholar]
- Richter H., Wennberg P., Raudsepp Jaanus. (2007). The effects of inverting prisms on the horizontal-vertical illusion: a systematic effect of downward gaze. Experimental Brain Research , 183, 9–15 [DOI] [PubMed] [Google Scholar]
- Sarri M., Greenwood R., Kalra L., Papps B., Husain M., Driver J. (2008). Prism adaptation afteraffects in stroke patients with spatial neglect: pathological effects on subjective straight ahead but not visual open-loop pointing. Neuropsychologia, 7, 46 (4), 1069–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode G., Rossetti Y., Li L., Boisson D. (1998). Improvement of mental imagery after prism exposure in neglect: a case study. Behavioral Neurology, 11 (4), 251–258 [DOI] [PubMed] [Google Scholar]
- Rossetti Y., Rode G., Pisella L., Farne A., Li L., Boisson D., et al. (1998). Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature, 395 (6698), 166–169 [DOI] [PubMed] [Google Scholar]
- Sekiyama K., Miyauchi S., Imaruoka T., Egusa H., Tashiro T. (2000). Body image as a visuomotor transformation device revealed in adaptation to reversed vision. Nature, 407 (6802), 374–377 [DOI] [PubMed] [Google Scholar]
- Sen S., Nesse R. M., Stoltenberg S. F., Li S., Gleiberman L., Chakravarti A., et al. (2003). A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology, 28 (2), 397–401 [DOI] [PubMed] [Google Scholar]
- Shimizu E., Hashimoto K., Iyo M. (2004). Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 126B (1), 122–123 [DOI] [PubMed] [Google Scholar]
- Smith P. F. (2000). Pharmacology of the vestibular system. Current Opinion in Neurology, 13 (1), 31–37 [DOI] [PubMed] [Google Scholar]
- Smith P. F., Darlington C. L. (1996). Recent advances in the pharmacology of the vestibulo-ocular reflex system. Trends in Pharmacological Sciences, 17 (11), 421–427 [DOI] [PubMed] [Google Scholar]
- Stratton G. M. (1897). Vision without inversion of the retinal image. Psychological Review, 4 (4), 341–360 [Google Scholar]
- Sugita Y. (1996). Global plasticity in adult visual cortex following reversal of visual input. Nature, 380 (6574), 523–526 [DOI] [PubMed] [Google Scholar]
- Thoenen H. (1995). Neurotrophins and neuronal plasticity. Science, 270 (5236), 593–598 [DOI] [PubMed] [Google Scholar]
- University of California, I. (2011). UCI College Portrait - Demographics. from http://www.oir.uci.edu/portrait/uci-college-portrait.pdf [Google Scholar]
- Ventriglia M., Bocchio Chiavetto L., Benussi L., Binetti G., Zanetti O., Riva M. A., et al. (2002). Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer's disease. Molecular Psychiatry, 7 (2), 136–137 [DOI] [PubMed] [Google Scholar]
- Wandell B. A., Smirnakis S. M. (2009). Plasticity and stability of visual field maps in adult primary visual cortex. Nature Reviews Neuroscience, 10 (12), 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Weinstock-Guttman B., Benedict R., Tamano-Blanco M., Hussein S., Abdelrahman N., et al. (2007). Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Human Molecular Genetics, 16 (22), 2659–2668 [DOI] [PubMed] [Google Scholar]


