Abstract
Visual localization is based on the complex interplay of bottom-up and top-down processing. Based on previous work, the posterior parietal cortex (PPC) is assumed to play an essential role in this interplay. In this study, we investigated the causal role of the PPC in visual localization. Specifically, our goal was to determine whether modulation of the PPC via transcranial direct current stimulation (tDCS) could induce visual mislocalization similar to that induced by an exogenous attentional cue (Wright, Morris, & Krekelberg, 2011). We placed one stimulation electrode over the right PPC and the other over the left PPC (dual tDCS) and varied the polarity of the stimulation. We found that this manipulation altered visual localization; this supports the causal involvement of the PPC in visual localization. Notably, mislocalization was more rightward when the cathode was placed over the right PPC than when the anode was placed over the right PPC. This mislocalization was found within a few minutes of stimulation onset, it dissipated during stimulation, but then resurfaced after stimulation offset and lasted for another 10–15 min. On the assumption that excitability is reduced beneath the cathode and increased beneath the anode, these findings support the view that each hemisphere biases processing to the contralateral hemifield and that the balance of activation between the hemispheres contributes to position perception (Kinsbourne, 1977; Szczepanski, Konen, & Kastner, 2010).
Keywords: visual localization, spatial attention, transcranial electrical stimulation, interhemispheric competition, position perception
Introduction
To determine the spatial position of an object, observers must integrate position information from the object's components, such as edges, borders, and other structural elements. This process is typically accurate and the perceived position is close to the physical position of the target (He & Kowler, 1991; Kowler & Blaser, 1995). However, there are many factors that contribute to a divergence between the perceived and physical position, including its retinal eccentricity (Müsseler, van der Heijden, Mahmud, Deubel, & Ertsey, 1999), its motion trajectory (Krekelberg, 2001; Krekelberg & Lappe, 2001), changes in frame of reference (Bridgeman, Peery, & Anand, 1997), and adaptation (Whitaker, McGraw, & Levi, 1997). Visual localization has also been shown to be modulated by experimental manipulations of attention, which can yield improved accuracy (Bocianski, Müsseler, & Erlhagen, 2010; Fortenbaugh & Robertson, 2011) and reliability (Prinzmetal, Amiri, Allen, & Edwards, 1998), but also induce illusory shifts in position perception (Kosovicheva, Fortenbaugh, & Robertson, 2010; Suzuki & Cavanagh, 1997; Tsal & Bareket, 1999; Wright, Morris, & Krekelberg, 2011).
While the details of the neural circuitry underlying visual localization are largely unknown, many studies identify the posterior parietal cortex (PPC) as a key area. For instance, the PPC has been linked to both attention (Corbetta & Shulman, 2002) and spatial processing (Fink et al., 2000; Morris, Chambers, & Mattingley, 2007; Morris, Kubischik, Hoffmann, Krekelberg, & Bremmer, 2012; Szczepanski & Kastner, 2013). In this study we investigated the causal involvement of the PPC in visual localization. We used transcranial direct current stimulation (tDCS) over the PPC of healthy human volunteers and investigated how the stimulation affected the centroid estimation of a one-dimensional (1-D) horizontal random dot pattern (RDP). We reasoned that an imbalance in the activity of the PPC in the two hemispheres could—potentially through the mechanisms of attention—induce spatial mislocalization as suggested by theories of interhemispheric competition (J. D. Cohen, Romero, Servan-Schreiber, & Farah, 1994; Kinsbourne, 1977; Szczepanski et al., 2010). Current understanding of neural excitability modulation by tDCS (Nitsche & Paulus, 2000) suggests that excitability increases beneath the anode, while excitability decreases beneath the cathode. We placed one electrode over the left PPC and the return electrode over the right PPC (dual tDCS) to maximize the imbalance between left and right PPC excitability (Giglia et al., 2011), and thereby maximize a potential behavioral effect. Specifically, we reasoned that an anode placed over the right PPC combined with a cathode over the left PPC (we refer to this montage as rPPCa) should increase excitability of the right PPC and decrease excitability of the left PPC. If the allocation of attention were driven by a linear combination of the activation levels across both PPCs, the rPPCa montage would increase the allocation of attention to the left visual field and, based on our previous behavioral findings (Wright et al., 2011), induce leftward localization compared to stimulation with the reverse polarity (rPPCc). Our experiments confirmed this hypothesis and in the Discussion section we interpret these findings in terms of interhemispheric competition as well as other aspects of spatial processing known to reside in the PPC.
The same, admittedly somewhat simplistic, logic predicts that the rPPCa montage should induce leftward localization when compared to a more traditional sham stimulation control. Our experiments did not confirm this prediction, and we present possible explanations for this finding in the Discussion.
A final motivation for the experiments in this study was our recent finding that alternating current stimulation reduces visual adaptation and is particularly effective when applied during but not before the presentation of a visual stimulus (Kar & Krekelberg, 2014). This inspired us to not only use a typical tDCS design that measured the aftereffects of stimulation by applying stimulation before the start of the behavioral trials, but also a design in which stimulation was applied during the behavioral experiment. We found that the behavioral effect was similar in amplitude regardless of whether the stimulation was applied before or during task performance. However, this experiment revealed a novel and interesting time course: The behavioral effects had a rapid onset and then dissipated over ∼10 min, even with continuing stimulation. After tDCS offset the behavioral effects resurfaced and then dissipated again in ∼10–15 min.
Methods
This study consisted of two main experiments. In both experiments, subjects located the centroid of a 1-D RDP with different applications of tDCS over the PPC. In the first experiment, we applied tDCS prior to all experimental trials (tDCS-Before) and in the second experiment we applied tDCS concurrently with experimental trials (tDCS-During). All experimental procedures were approved by the Institutional Review Board of Rutgers University and followed international guidelines for the ethical treatment of human subjects as expressed in the Declaration of Helsinki. All subjects provided written informed consent and reported normal or corrected-to-normal vision.
Participants
Twelve subjects (all right-handed; six male; age range 18–34 years) participated in both experiments. Subject 1 was an author (JMW); all other subjects were naive to the purpose of the experiments.
In the tDCS-Before experiment, the performance of one subject deviated largely from the remainder of the subjects. This subject had an effect of tDCS that was opposite in sign to 9 of the remaining 11 subjects and more than 3 SDs from the population mean. Therefore, we excluded this subject from all further data analysis for the tDCS-Before experiment.
Transcranial direct current stimulation
We applied tDCS using an STG4002 stimulus generator (Multi Channel Systems, Reutlingen, Germany) with a pair of saline-soaked sponges attached to conductive rubber electrodes (7.6-cm diameter). The current was 1 mA for 15 min prior (tDCS-Before) or during (tDCS-During) the presentation of visual stimuli. This resulted in a maximum current density of 0.02 mA/cm2, which is within the current safety guidelines (Iyer et al., 2005; Nitsche, Liebetanz et al., 2003). We increased and decreased the current linearly over a period of 10 s at the start and end of tDCS, respectively, which has been shown to reduce subject discomfort (Nitsche, Liebetanz, et al., 2003). We placed the two electrodes (anode and cathode) over the locations of P3 and P4 (in accord with the international 10–20 method for electroencephalographic (EEG) electrode placement). There was no separate reference electrode in this dual montage. Given the large size of the electrodes, the spread of current from these electrodes (Datta et al., 2009; Kar & Krekelberg, 2012), and the nominal location of the PPC (Dambeck et al., 2006; Herwig, Satrapi, & Schonfeldt-Lecuona, 2003; Hilgetag, Theoret, & Pascual-Leone, 2001; Pourtois, Vandermeeren, Olivier, & de Gelder, 2001; Sack et al., 2002), these montages are expected to generate significant electric fields in each subject's PPC.
There were three stimulation conditions and for simplicity, we refer to the first two conditions based on the type of stimulation over P4; (a) cathode placed over P3 and anode placed over P4 (right PPC anode, or rPPCa, for short); (b) anode placed over P3 and cathode placed over P4 (rPPCc), and (c) sham stimulation. Sham stimulation consisted of a total of 20 s of stimulation; in the first 10 s the current intensity increased to 1 mA and then decreased back to 0 mA in the remaining 10 s. We surveyed four of the naive subjects after each session and asked them to classify what type of stimulation they received; they were unable to distinguish between sham and stimulation sessions.
Experimental procedure
Centroid localization task
In each of the experiments, the task was to estimate the centroid of a 1-D RDP (Figure 1B). We manipulated exogenous attention by cueing subjects to one side of the visual display, either to the left (Left-Cue condition) or right (Right-Cue condition) of fixation. In a baseline condition, we cued subjects to both sides of the display (Bilateral-Cue condition). This kept the visual display and the temporal structure of the task as similar as possible between the cue conditions, thus avoiding confounding the influence of spatial attention with temporal uncertainty (Morris et al., 2010).
Figure 1.
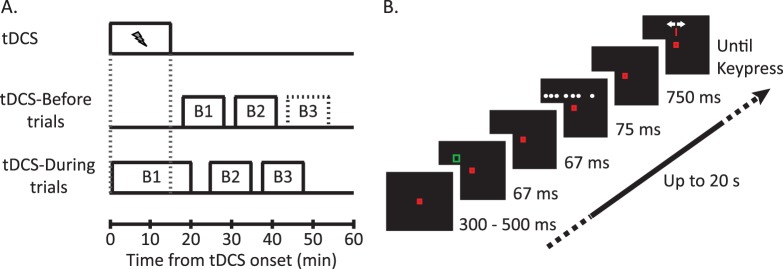
Experimental paradigm. (A) In both experiments tDCS was applied for 15 min at the start of a session (top trace). In the tDCS-Before experiment (middle trace) subjects completed at least two blocks of trials following tDCS offset. In the tDCS-During experiment, trials began 20 s after tDCS onset and subjects completed an additional two blocks of trials after tDCS offset. (B) Example trial (Left-Cue condition). Subjects fixated centrally until the RDP disappeared. A noninformative cue appeared at an eccentricity of 8.08° just prior to the onset of the RDP. After 750 ms from the offset of the RDP, a cursor appeared at the center of the grid and subjects moved the cursor to the perceived centroid location.
Visual stimuli were shown on a Sony FD Trinitron (GDM-C520; Sony, New York City, NY) CRT monitor that measured 40° × 30° with a resolution of 1024 × 768 pixels and a refresh rate of 120 Hz. We presented the stimuli using custom software, Neurostim (http://neurostim.sourceforge.net), and subjects viewed the display from a distance of 57 cm. A head-mounted Eyelink II eye tracker system (SR Research, Mississauga, Canada) recorded eye movements and tracked the pupils of both eyes at a sample rate of 500 Hz. To reduce head movements, subjects used individually molded bite bars.
The 1-D RDP consisted of seven small white (76 cd/m2) squares (0.20° × 0.20°) on a black (0.4 cd/m2) background. On each trial, seven unique dot positions, selected from a grid of 32 possible dot positions, appeared on the display. The grid extended from −15.5° to 15.5° relative to the vertical midline at a constant height of 3° above the horizontal midline. Each dot location in the grid was 1° away from its nearest horizontal neighbor(s). The actual centroids of the RDPs across all trials approximated a normal distribution with a mean of 0° and a standard deviation of 1.5°.
A green square outline (1° × 1°; line width: 0.12°) served as an exogenous cue for attention. This cue appeared at an eccentricity of 8.08° in either (or both) the left or right visual field and was centered over two grid locations; (−7.5°, 3°) and (7.5°, 3°), respectively.
The central fixation stimulus was a small red square (0.16° × 0.16°), which remained visible for the duration of each trial at the center of the display. Subjects maintained fixation within a 2.5° × 2.5° square at the center of the display until the RDP disappeared. Trials in which subjects failed to fixate appropriately were terminated immediately and repeated at a random time later within the block. One block contained a minimum of 120 trials with the three cue conditions interleaved.
Each trial began when the subject fixated the central fixation point. After a variable delay (300–500 ms), the attentional cue(s) appeared for 67 ms (eight frames) just prior (134 ms) to the appearance of the RDP. This cue-target interstimulus interval maximizes effects of exogenous attention on behavioral performance (Cheal & Lyon, 1991; Muller & Rabbitt, 1989) and induces shifts in perceived centroid location (Wright et al., 2011). The RDP remained visible for 75 ms (nine frames). A cursor (red vertical line 0.04° × 0.5°) appeared at the center of the grid of dots (0°, 3°) 750 ms after target offset. Subjects then located the centroid, i.e., average position, of all dots presented on a trial by moving the cursor to the centroid using a computer mouse in their right hand and then clicking the left button (Figure 1B).
Experiment 1: tDCS-Before
Each session began with subjects seated in a darkened room for 15 min while receiving rPPCa, rPPCc, or sham tDCS. During tDCS, subjects viewed a black visual display (0.4 cd/m2) and were allowed to listen to music. After the stimulation period, subjects performed the centroid localization task in a minimum of two blocks of trials for a total of at least 200 trials (Figure 1A).
Experiment 2: tDCS-During
In these sessions, subjects received 15 min of tDCS (rPPCa, rPPCc, or sham) while they performed the centroid localization task after a short delay (20 s) at the beginning of the session to avoid interference from the initialization of stimulation. Subjects continued completing experimental trials for 5 min following tDCS offset (approximately 400–500 trials in total). After this 20-min period for each stimulation type, subjects completed two blocks of experimental trials without stimulation for a total of at least 200 additional trials (Figure 1A). The experimental task was the same as in Experiment 1.
Session ordering
All subjects completed a minimum of 12 sessions: six tDCS-Before and six tDCS-During sessions. We used a repeated measures design, therefore, for each experiment; subjects completed two sessions per stimulation condition, i.e., rPPCa, rPPCc, and sham. Six subjects completed all tDCS-Before sessions prior to tDCS-During sessions. We used the same randomized order of stimulation conditions per subject in each of these experiments so that differences could not be attributed to session ordering. However, we recognized that there could be a training effect by completing all tDCS-Before sessions prior to the tDCS-During sessions. Therefore, in the remaining subjects we interleaved the tDCS-Before and tDCS-During sessions and assigned the stimulation conditions randomly across subjects and experiments. We did not find any qualitative differences due to session ordering between subject groups; therefore, we analyzed all subjects together.
Initially, subjects received between 1 to 2 hr (three to six blocks) of training on the localization task. Data collected during these training blocks are not reported here; however, we ensured that accuracy and the correlation of subject responses to the actual centroid at the end of training were comparable to subsequent measures during the experiment. After these practice runs, subjects participated in only one session per day.
Data analysis
Population response error functions over time
We first determined the mean response error relative to the actual centroid as a function of time per stimulation session and subject. To do this, we grouped behavioral responses in a single session into nonoverlapping time bins of 250 s. We then determined the mean response error for each time bin that contained at least 30 trials. To account for time gaps introduced by breaks between sessions, we used the interp1 function in Matlab 7.14 (MathWorks, Natick, MA) to interpolate between the bins using a shape-preserving piecewise cubic spline. We did not extrapolate beyond the first or last time point in a session. We then used the interpolated functions and averaged the mean responses per time point across sessions to generate one time course per subject and montage.
To determine the difference in behavioral responses between rPPCa and rPPCc stimulation, we subtracted the subject-specific rPPCa interpolated time course from the rPPCc interpolated time course. Therefore in the resultant time course, positive values indicate that the perceived centroid in the rPPCc stimulation condition was more to the right relative to the rPPCa stimulation condition. We performed a similar analysis between the rPPCa (rPPCc) and sham conditions where positive values indicate that the perceived centroid during rPPCa or rPPCc stimulation was more to the right relative to the sham condition. To view the effect of stimulation across the population, we determined the median response across subjects at each time point. We only included time points with data from nine or more subjects. The error bars represent the median absolute difference between the subject response and the population median scaled by the square root of the number of subjects, and significance of individual data points was tested with Wilcoxon signed-rank tests.
Significance tests
We first verified if our sample met conditions of normality using the Jarque-Bera method (jbtest function in Matlab 7.14). If the sample violated assumptions of normality we used nonparametric significance tests and report the median and range of the data in lieu of parametric measures. The main population level analysis of significance was based on a repeated measures ANOVA (rmANOVA) with the following within-subject factors: stimulation type (rPPCa, rPPCc), cue location (Left-, Right-, Bilateral-Cue), and centroid position (more than 2.5° left, less than 2.5° left, less than 2.5° right, more than 2.5° right of fixation).
Partial correlation difference
The location of the centroid and the bisection point of the outermost dots are correlated in our stimulus. Hence even if subjects actually performed a bisection task, they could still perform reasonably well on the centroid task. To disentangle the influence of the centroid from the bisection point on the behavioral responses of each subject, we calculated the pairwise partial correlations between the behavioral response, the centroid, and the bisection point. We reasoned that the partial correlation with the highest value identified the response strategy that subjects most likely utilized across trials. To assess the statistical significance of the difference between these partial correlation values, we compared the actual difference in partial correlations with a null distribution created by 1,000 random shuffles of the behavioral responses per subject. A pcd was considered statistically significant if it exceeded the 95th percentile of this null distribution.
Results
Subjects reported the centroid of a briefly presented 1-D RDP. We applied tDCS through electrodes placed over the left and right PPC. There were three stimulation conditions: anode over right-PPC combined with cathode over left-PPC (rPPCa), anode over left-PPC combined with cathode over right-PPC (rPPCc), and sham stimulation.
Before showing the influence of tDCS, we first present an analysis of the subjects' performance on the behavioral task that confirms they can reliably assess the centroid of our 1-D dot stimulus, and that their localization behavior is consistent with previous reports.
Task performance: Sham
As a general measure of task performance, we determined subject response bias and variability relative to the actual centroid for the sham condition regardless of cue condition. The response bias, defined as the mean of the absolute difference between subject responses and the actual centroid across trials, was 0.38° (SE = 0.10°) across subjects. The variable error, defined as the standard deviation of the subject response error across subjects, was 1.81° (SE = 0.06°). Across subjects, the Pearson correlation between the behavioral responses and the actual centroid ranged from 0.74 to 0.91 (p < 0.001). This confirms that—similar to two-dimensional (2-D) dot displays (Wright et al., 2011)—subjects reliably estimated the centroid of the 1-D stimulus. We also analyzed the subject response error while maintaining the sign of the error and found that most subjects showed a rightward bias, which was individually significant in six subjects, t(≥700) > 2.85, p < 0.01, d > 0.11. Three subjects showed a significant leftward bias, t(≥700) < −5.09, p < 0.01, d < −0.19. This is similar to the variability seen in previous line bisection studies (Jewell & McCourt, 2000).
Consistent with our previous findings using 2-D RDPs (Wright et al., 2011), we found that the attentional cue significantly shifted perceived location as revealed by a main effect of cue location (rmANOVA; F[2, 20] = 4.22, p = 0.03, ηp2 = 29.68, see Methods). Subjects' responses were more leftward in the Left-Cue condition (M = 0.06, SE = 0.09) relative to either the Bilateral- (M = 0.25, SE = 0.10) or Right-Cue conditions (M = 0.29, SE = 0.10). One of our goals was to investigate whether this pattern of mislocalization induced by exogenous cues could also be generated by transcranial stimulation of the PPC. Finally, subjects had a foveal bias as revealed by a main effect of centroid position, F(3, 30) = 8.35, p < 0.001, ηp2 = 45.50. The magnitude of this foveal bias increased for more peripheral centroids (M = 0.97, SE = 0.05) compared to more foveal centroids (M = 0.56, SE = 0.04). Such a foveal bias has been reported previously (Mateeff & Gourevich, 1983; O'Regan, 1984; Stork, Musseler, & van der Heijden, 2010; van der Heijden, van der Geest, de Leeuw, Krikke, & Musseler, 1999).
Experiment 1: tDCS-Before
Next, we investigated how tDCS over the PPC affected localization. As discussed above, current views of tDCS suggest that excitability is increased underneath the anode and excitability is decreased underneath the cathode. Furthermore, the current evidence supports the view that each PPC mainly allocates attention and responds predominantly to visual stimuli in the contralateral visual hemifield (see Discussion). Given these assumptions, the most sensitive analysis to detect whether tDCS of the left and right PPC affects localization is to compare the sessions where the anode was placed over the right PPC and the cathode over the left PPC (the rPPCa condition) with the sessions in which the anode and cathode were reversed (rPPCc condition). Below we will present those results first, and then drill down to further comparisons between stimulation and sham.
In this experiment stimulation was applied before subjects completed experimental trials. We subtracted the average response error in the rPPCa condition from the average response error in the rPPCc condition for each subject. Positive differences indicate a shift in the perceived centroid to the right under rPPCc stimulation relative to rPPCa stimulation. A population level repeated measures ANOVA (see Methods) revealed a significant main effect of stimulation, F(1, 10) = 10.86, p = 0.008, ηp2 = 52.06. At the single subject level, 9 out of 11 subjects had a positive difference (M = 0.09, SE = 0.03) and the effect was individually significant in three subjects, t(≥720) ≤ −1.99, p < 0.05, d ≤ −0.1 (Figure 2).
Figure 2.
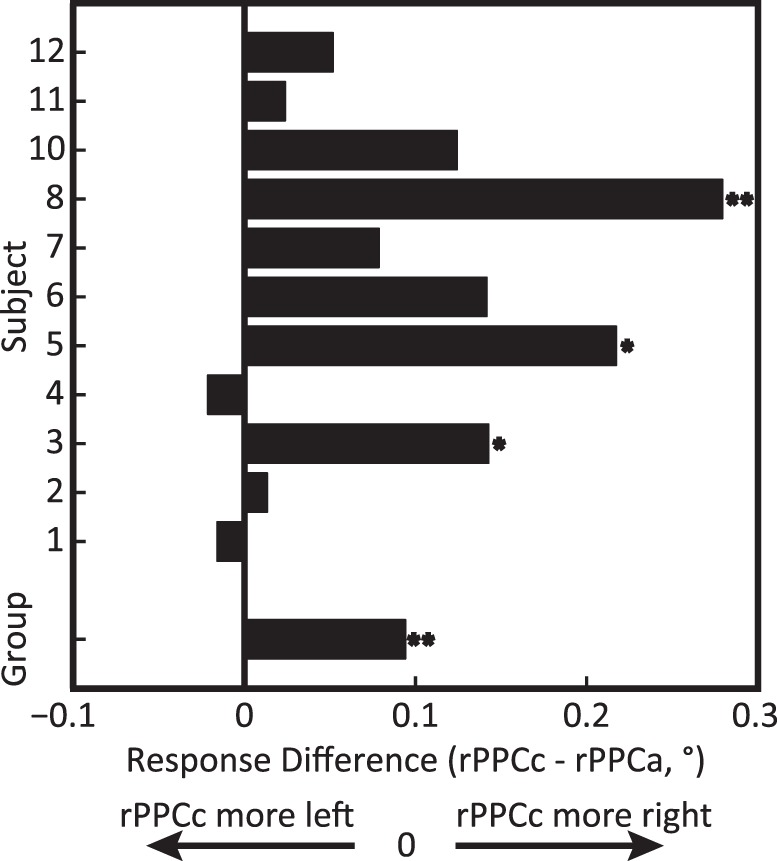
Experiment 1: tDCS-induced mislocalization after tDCS, comparing rPPCc and rPPCa stimulation. Bars show the difference in average response errors between the rPPCc and rPPCa conditions for each subject and the group average (bottom bar). Positive values indicate rPPCc responses that were shifted more to the right relative to the rPPCa responses. One asterisk denotes individual significance with p < 0.05 and two asterisks denotes p < 0.01 (see Methods, Significance tests). The rPPCc montage shifted perceived centroid location rightward compared to the rPPCa montage, supporting the involvement of the PPC in localization.
We did not find a significant interaction between montage and cue-location, F(2, 20) = 0.92, p = 0.42, ηp2 = 8.39, hence we found no evidence that stimulation was more or less effective depending on the locus of attention. This was further supported by a control analysis in which we investigated only the Bilateral-Cue condition and found that the influence of stimulation was qualitatively the same as in the full data set. Similarly, there was no significant interaction between montage and centroid position, F(2, 20) = 1.58, p = 0.22, ηp2 = 13.61. Given this lack of significant interactions we pooled the data across cue-location and centroid location for all further analyses.
Figure 3 shows the time course of the behavioral effect of tDCS (see Methods, Population response error functions over time). As before, positive values indicate that the perceived centroid shifted more rightward under rPPCc stimulation compared to rPPCa stimulation. The aftereffects of stimulation dissipated within approximately 15 min. In principle, this dissipation could be confounded by fatigue or other stimulation-independent factors that affected overall performance on the task. To exclude this possibility, we compared performance in the first and second block of trials in the sham condition and found no significant differences in the mean response error (Wilcoxon signed-rank test; Z = −0.78, p = 0.43) or the variable error (Z = −1.33, p = 0.18). We conclude that the temporal dissipation shown in Figure 3 can be ascribed to the waning influence of the tDCS stimulation.
Figure 3.
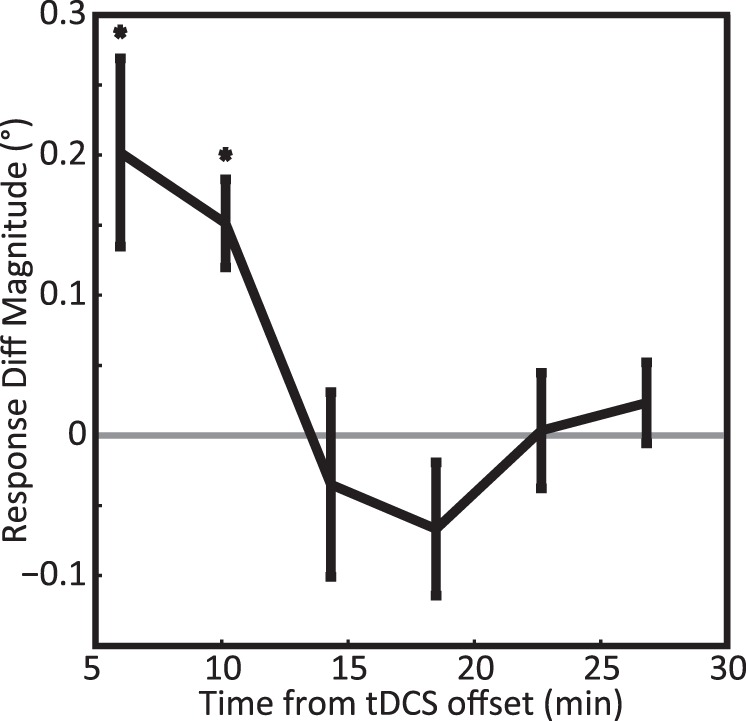
Experiment 1: Time course of response errors after tDCS. The black curve shows the response differences, rPPCc – rPPCa, as a function of time, averaged across all subjects. Positive values indicate that rPPCc stimulation shifted the perceived centroid rightward relative to rPPCa stimulation. One asterisk denotes significance at p < 0.05. The graph shows that the aftereffect of tDCS dissipated over a period of ∼15 min.
The above-described mislocalizations may also result if tDCS affected the subject's eye position. We monitored fixation and aborted trials in which eye movement strayed beyond 1.25° from the fixation point, but this leaves a window of error that allows for small deviations in eye position. For example, if rPPCc caused the eye position to deviate slightly to the left, dot positions could appear more rightward yielding a rightward mislocalization relative to rPPCa especially if rPPCa induced opposite effects in eye position. We therefore examined the horizontal displacement in eye position during the presentation of the RDP. A population level repeated measures ANOVA (see Methods) revealed no main effect of stimulation, F(1, 10) = 0.37, p = 0.56, ηp2 = 3.24; attention cue, F(2, 20) = 1.93, p = 0.17, ηp2 = 14.95; or centroid position, F(3, 30) = 0.85, p = 0.48, ηp2 = 7.19, on eye position. Limiting the analysis only to trials within 15 min of tDCS offset also did not reveal any significant effects. Therefore, we conclude that our results are not a result of changes in eye position.
Figure 4 compares performance in the rPPCa and rPPCc conditions to sham stimulation. Somewhat surprisingly, we found that the sign of the directional bias was the same in the rPPCa and rPPCc conditions for most subjects. Across the population this effect was highly significant (sign test; p < 0.01). Given that the subjects also had idiosyncratic biases in the sham condition (see Task Performance-Sham), we investigated whether those biases could predict the effect of tDCS. The correlation between the sign of the bias in the sham condition (left/right) and the sign of the effect of tDCS, however, was not significant, r(9) = 0.24, p = 0.48.
Figure 4.
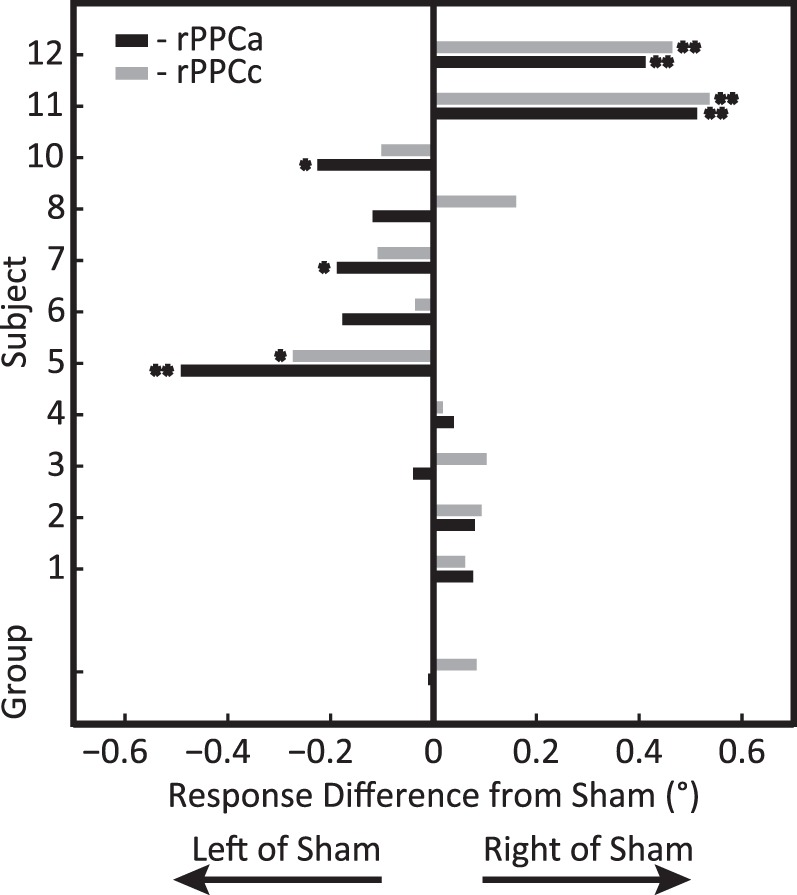
Experiment 1: tDCS-induced mislocalization after tDCS comparing rPPCc (gray) and rPPCa (black) to sham stimulation for each subject and the group average (bottom bars). Positive values indicate rightward shifts relative to sham. Asterisks indicate significance (*p < 0.05; **p < 0.01) for a specific subject and stimulation condition compared to sham. This graph shows that the sign of the behavioral effect differed across subjects, but that rPPCc effects were typically more rightward than rPPCa effects (see also Figure 2).
Experiment 2: tDCS-During
In the first set of experiments, tDCS was applied before the subjects performed the task. In other words, the behavioral effects we reported were aftereffects of tDCS. This mimics the typical use in many clinical studies, but there is increasing evidence that tDCS specifically targets populations of neurons that are active (Kar & Krekelberg, 2013). Based on this we performed a second set of experiments in which tDCS was applied concurrently with the task.
Following the analysis of Figure 3 we again determined the time course of the stimulation effect, subtracting the effect of rPPCa from rPPCc stimulation (Figure 5). The behavioral effect was largest at the start of tDCS and dissipated over approximately 8 min of ongoing stimulation. The behavioral effect increased again once stimulation had ended, and lasted approximately 10 min following stimulation. Even though the latter phase of the tDCS-During experiment is not an exact replication of the tDCS-Before experiment, its time course (including the magnitude) is similar to that shown in Figure 3.
Figure 5.
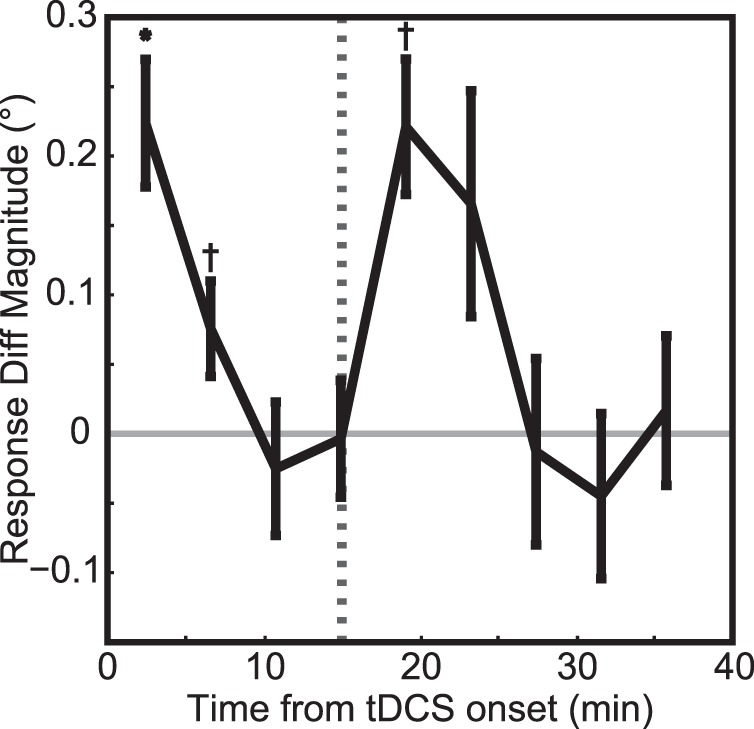
Experiment 2: Time course of response errors during and after tDCS. The black curve shows the response differences, rPPCc – rPPCa, as a function of time, averaged across all subjects (see Methods, Population response error functions over time). Positive values indicate that rPPCc stimulation shifted the perceived centroid rightward relative to the rPPCa stimulation condition. The dashed line indicates tDCS offset. One asterisk denotes significance with p < 0.05 and a cross denotes a trend at p < 0.10. This figure shows that tDCS induced both short-term effects that dissipated even while current was applied and an aftereffect that lasted ∼10 min (as in Figure 3).
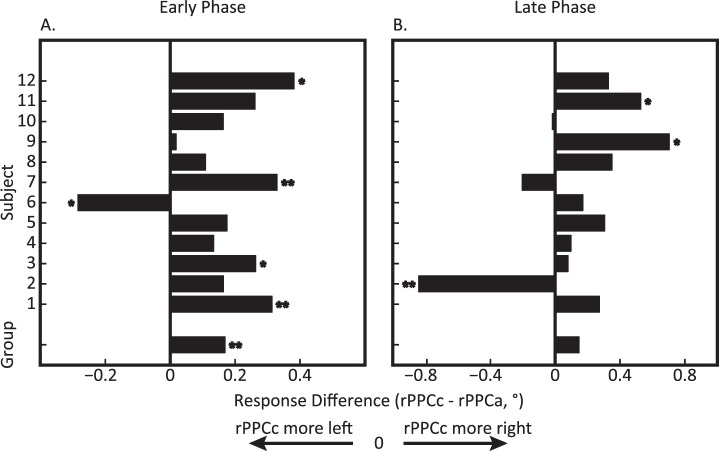
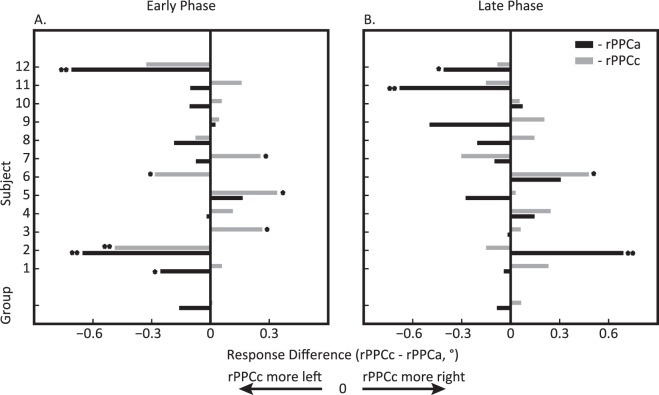
Figure 6 shows the response error differences for individual subjects in the early (A) and late (B) phases. This graph shows that the rightward shift when comparing rPPCc to rPPCa is found consistently across subjects both during tDCS and immediately after tDCS, F(1, 10) = 10.69, p = 0.008, ηp2 = 49.29. However, separate population repeated measures ANOVAs on each phase revealed a significant effect of stimulation in the early, F(1, 10) = 15.67, p = 0.002, ηp2 = 58.75, but not in the late phase, F(1, 10) = 0.83, p = 0.38, ηp2 = 7.03, due to Subject 2 who displayed a large deviation, more than 2 SDs, from the rest of the group. Given the consistency in the overall direction of the effect, the large intersubject variability in the comparison with sham stimulation (Figure 7) is remarkable.
Figure 6.
Experiment 2: tDCS-induced mislocalization during and after tDCS comparing rPPCc and rPPCa stimulation for each subject and the group average (bottom bar). (A) Early phase during tDCS: 0–8 min after tDCS onset and (B) late phase after tDCS offset: 2–10 min after tDCS offset). Positive values indicate rPPCc responses that were shifted more to the right relative to the rPPCa responses. These graphs show that rPPCc tDCS typically induced rightward shifts compared to rPPCa tDCS across both the early and late phase. One asterisk denotes individual significance with p < 0.05 and two asterisks denotes p < 0.01.
Figure 7.
Experiment 2: tDCS-induced mislocalization during and after tDCS comparing rPPCc (gray) and rPPCa (black) to sham stimulation for each subject and the group average (bottom bars). (A) Early phase during tDCS: 0–8 min after tDCS onset and (B) late phase after tDCS offset: 2–10 min after tDCS offset. Positive values indicate rightward shifts relative to sham. These graphs show a large degree of intersubject variability when comparing stimulation to sham, but—as shown in Figure 6—a consistently rightward shift when comparing rPPCc and rPPCa. One asterisk denotes individual significance with p < 0.05 and two asterisks denotes p < 0.01.
Similar to the tDCS-Before experiment there were no systematic deviations in eye position that would explain these behavioral effects. We performed two population level repeated measures ANOVAs (see Methods) to demonstrate this. The first used trials in the early phase (0–8 min following tDCS onset) and the second in the late phase (2–10 min following tDCS offset). Both analyses showed no main effect of stimulation, F(1, 10) < 0.47, p > 0.51, ηp2 < 4.04; attention cue, F(2, 20) < 2.04, p > 0.15, ηp2 < 15.62; or centroid position, F(3, 30) < 2.07, p > 0.12, ηp2 < 15.81, on eye position.
Task strategy: Control analysis
Although we instructed subjects to determine the centroid of each RDP, it is possible that subjects instead utilized only the positions of the two outermost dots and localized the bisection point. This would make our task similar to traditional line bisection tasks. Because the bisection point is correlated with the centroid location, accurate performance on the centroid task (shown above) does not exclude a bisection strategy. The strategy followed by the subject is relevant for our definition of localization error. For instance, if a subject actually performed bisection, but we defined errors with respect to the true centroid, our measure could be insensitive, or even biased. We performed a number of analyses to rule out such possible confounds.
To determine which of the two strategies subjects employed we used a partial correlation analysis (see Methods, Partial correlation difference). We first used the sham trials regardless of cue condition. In four subjects the partial correlation between the behavioral responses and the actual centroid was higher than the partial correlation between the responses and the bisection point (0.40 < pcd < 1.05, p < 0.001). We infer that these subjects most likely adopted a true centroid localization strategy. Three subjects showed the reverse pattern (−0.44 < pcd < −0.36, p < 0.001). It is possible that these subjects adopted a bisection strategy. The remaining four subjects showed no significant difference (|pcd| < 0.09, p > 0.10). Analyzing the partial correlation values across the rPPCa and rPPCc conditions showed that the response strategies typically remained consistent across montages (8 out of 11 subjects).
Finally, we investigated whether a subject-specific definition of localization error (i.e., relative to the bisection point for subjects that appear to follow a bisection strategy, and to the centroid for subjects that appear to follow a centroid strategy) affected any of our results. It did not, neither for the tDCS-Before nor for the tDCS-During experiment. For simplicity, we therefore defined error for all subjects as the mismatch between the actual and the reported centroid for all analyses.
Discussion
Our experiments investigated the causal involvement of the PPC in visual localization. We showed that tDCS with electrodes placed over the left and right PPC altered visual localization. Specifically, placing the anode over the left PPC and the cathode over the right PPC induced a rightward shift in perceived centroid location relative to the reverse montage. This finding was consistent across subjects and occurred whether the stimulation was applied well before or during the performance of the localization task. Surprisingly, behavioral effects dissipated during the application of tDCS, but resurged after stimulation offset to dissipate again over a period of ∼10–15 min.
Below we first discuss the novel insight our experiments provide about tDCS, and how uncertainties inherent in tDCS affect our interpretation of the data. Finally, we discuss a number of potential mechanisms that could underlie the behavioral effects induced by tDCS.
The tDCS method
The behavioral effects of tDCS vary based on multiple factors: electrode size, placement, current amplitude, current duration, etc. (Nitsche et al., 2008). For example, a recent study has shown that 2 mA of tDCS for 20 min over the right intraparietal sulcus altered selective attention, whereas 1 mA of current did not (Moos, Vossel, Weidner, Sparing, & Fink, 2012). Sparing and colleagues (2009), on the other hand, found differences in visual detection and line bisection with only 1 mA of tDCS for 10 min over the PPC. A direct comparison is difficult since effects may be task-specific, other stimulation parameters, such as electrode size, differed between the experiments, and because current flow within the brain depends on idiosyncratic brain folding (Datta, Baker, Bikson, & Fridriksson, 2011; Wagner et al., 2007). We interpret these findings as showing that a relatively large degree of variability is expected both within and across tDCS studies.
In addition, the little that is known about the modes of action of tDCS at the neural level leads one to expect a high degree of complexity. For instance, cell morphology and cell orientation with respect to the applied field affects the outcome in terms of membrane depolarization measured in-vitro (Radman, Ramos, Brumberg, & Bikson, 2009). Our previous behavioral findings (Kar & Krekelberg, 2014) as well as unpublished observations in the macaque monkey (Kar & Krekelberg, 2013), furthermore suggest that electrical stimulation affects cells in a state dependent (inactive/active/adapted) manner. As a consequence, the net effect of stimulation in-vivo is not easy to predict and may well include neural changes that are not well described by changes in excitability.
One specific potential explanation for the large intersubject variability when comparing stimulation to sham is that the electrical fields induced by tDCS are idiosyncratic due to individual differences in brain folding (Datta et al., 2009; Datta, Zhou, Su, Parra, & Bikson, 2013; Wagner et al., 2007). If the orientation of the induced field in a critical subregion of the PPC is opposite to that induced in another subject, one would predict quite different (potentially opposite) changes in excitability and therefore potentially opposite behavioral effects. The finding that the difference between our two stimulation conditions is nevertheless consistent across subjects can be attributed to the fact that the fields generated in the rPPCa condition are oriented approximately opposite to those generated in the rPPCc condition (limited only by the accuracy of electrode placement). Hence, for each subject, if excitability in a subregion of the PPC increased during rPPCa, one would expect it to decrease during rPPCc. This neural consistency should be reflected in behavioral consistency, which is indeed what we found (Figures 2 and 6). Taken together this analysis suggests that due to the idiosyncratic nature of induced electric fields, a comparison of tDCS and sham conditions across subjects should be interpreted with caution, but that some intersubject variability can be removed by comparing montages in which the anode and cathode are reversed.
Effects of tDCS over time
Previous studies have shown that the aftereffects of tDCS can last for a few minutes up to 2 hr (Mielke et al., 2013; Nitsche, Nitsche, et al., 2003; Nitsche & Paulus, 2001; but see Floel et al., 2012). The duration appears to depend on the behavioral paradigm, the electrode montage, as well as other stimulation parameters. In our experiments the aftereffects were relatively short-lived (<15 min) and, even more interestingly, we observed that the behavioral effects dissipated during the application of tDCS. This time-course points to mechanisms other than pure excitability changes and is consistent with the idea that different mechanisms may underlie the effect of tDCS applied during and before a task (for a review see Stagg & Nitsche, 2011). We speculate that the decline of the behavioral effect during stimulation is due to homeostatic mechanisms that compensate for the effects of tDCS by returning network activity to its baseline levels after a sustained increase in excitability (Iyer, Schleper, & Wassermann, 2003; Turrigiano, Leslie, Desai, Rutherford, & Nelson, 1998). This clearly has implications for the use of tDCS in a therapeutic setting.
If these homeostatic mechanisms are indeed triggered by tDCS, one might expect to see an aftereffect of opposite sign after tDCS offset. Instead, we found a behavioral effect with the same sign after tDCS offset (in both experiments). It is possible that our ability to resolve behavioral effects temporally is too coarse to see a negative aftereffect (especially because some homeostatic mechanisms operate on a scale of seconds; Benucci, Saleem, & Carandini, 2013). In addition, however, other mechanisms such as synaptic plasticity have been implicated in the aftereffects of tDCS (Liebetanz, Nitsche, Tergau, & Paulus, 2002; Nitsche, Fricke, et al., 2003; Nitsche et al., 2005), and these may mask any aftereffects of homeostatic regulation. Direct investigations at the cellular level are needed to resolve these issues of mechanism.
Clearly, uncertainty about the mode of action of tDCS limits the forcefulness with which we can draw conclusions from our experiments, and it is possible that some of our conclusions (and those of others) may have to be revisited once a better mechanistic understanding of tDCS has been developed. With that caveat, we continue the discussion based on the common, but simplifying, assumption that excitability is typically increased beneath an anode and decreased beneath a cathode (Nitsche & Paulus, 2000).
Motor control
We placed the electrodes at P3 and P4 to maximize the induced electric fields in the PPC, and to maximize behavioral effects by increasing excitability in one hemisphere and decreasing it in the other hemisphere. Even though recent studies support the focality of transcranial electrical stimulation to a particular brain region by showing specific behavioral and/or BOLD signal changes related to the stimulated area (Antal, Polania, Schmidt-Samoa, Dechent, & Paulus, 2011; Antal et al., 2004; Meinzer et al., 2012), we cannot eliminate the possibility of current spread to regions beyond the PPC (Wagner et al., 2007). Of particular relevance in this context is the possibility that current spread to motor cortex may have altered the subject's localization response (without changing their percept). However, if current spread to motor cortex were the sole cause of the behavioral effects, one would expect to see changes in reaction time (Gandiga, Hummel, & Cohen, 2006) or in the accuracy of the movements (Vines, Nair, & Schlaug, 2006). We found no evidence for this. Alternatively, if tDCS changed excitability in the motor region of the right hand, one may expect, for instance, that anodal stimulation over the left PPC would generate larger amplitude responses. Instead, we found an overall foveal bias regardless of centroid position. Therefore, we conclude that our effects are not simply due to changes in the motor response but reflect changes in perception driven by the modulation of the PPC.
Mechanisms underlying the behavioral effect
Our data show that tDCS of the PPC induced changes in perceived centroid position. Since the involvement of the PPC in spatial localization is complex, our stimulation protocol may have affected a number of neural mechanisms that affected the perceived centroid position. In our view a modulation of the mechanisms underlying attention is the most likely because the tDCS-induced mislocalization was similar to the mislocalization induced by exogenous attentional cues, but we also discuss alternative or additional explanations here.
The interhemispheric competition theory of attention (ICT) provides a useful framework to interpret our findings. The ICT states that homologous frontal and/or parietal cortical regions across hemispheres function as opponent processors through reciprocal inhibition (J. D. Cohen et al., 1994; Kinsbourne, 1977). An asymmetry of activation in these opponent processors drives the allocation of attention (Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990; Szczepanski & Kastner, 2013) such that the more activated hemisphere biases attention and thereby localization towards its contralateral visual field. This is consistent with findings in hemispatial neglect (M. S. Cohen & Bookheimer, 1994); a lesion of the right parietal cortex disinhibits the left parietal cortex, which results in increased attention to the right visual field, and therefore a mislocalization towards that visual field (as demonstrated in, for instance, a line bisection task; Bisiach, Bulgarelli, Sterzi, & Vallar, 1983). A number of previous stimulation studies also provide support for the ICT. For instance, disruption of the right PPC with transcranial magnetic stimulation induces leftward errors in line bisection (Brighina et al., 2002; Fierro et al., 2000) and anodal stimulation of the lesioned hemisphere in neglect patients (or cathodal stimulation of the nonlesioned hemisphere) reduces spatial deficits in a line bisection task (Ko, Han, Park, Seo, & Kim, 2008; Sparing et al., 2009). Our main findings (Figure 2, 3, 5, and 6) provide additional support in healthy observers by showing that dual (anodal/cathodal) stimulation of the PPC in the two hemispheres induced mislocalization towards the hemifield contralateral to the anode. In our experiments there was no statistically significant interaction between the location of the attentional cue and the effect of tDCS. In other words, tDCS' putative effect on the attentional opponency was additive. We note, however, that the ICT also predicts that mislocalization with rPPCa stimulation should have the opposite sign of the mislocalization induced with rPPCc relative to baseline (sham). This prediction was rejected by our findings (Figures 4 and 7). If tDCS indeed only generates an additive change in excitability (see above), this implies that the competition/interaction between the two hemispheres is not well described by a simple linear subtraction. Given the large number of parietal regions that are potentially involved in the allocation of attention, and the complexity of their interaction (Szczepanski & Kastner, 2013; Szczepanski et al., 2010), this may not be too surprising.
A second possible mechanism is that tDCS may have interfered with a preattentive visual representation of the dot stimuli in the PPC. For instance, altering the balance of activation between left and right PPCs may have boosted signal or reduced noise (Vicario, Martino, & Koch, 2013) in a lateralized manner, which could result in mislocalization.
Finally, neurons in the PPC are known to have eye-centered receptive fields (Hartmann, Bremmer, Albright, & Krekelberg, 2011), which are modulated by eye position (Andersen, Essick, & Siegel, 1985). Our recent work in the macaque monkey has demonstrated that these representations can account for a foveal bias (Morris, Bremmer, & Krekelberg, 2013), as well as mislocalization during eye movements (Morris et al., 2012). This implies that modulating the activity of the PPC by tDCS also modulates an internal eye position signal (but not eye position itself, as shown above). For instance if higher firing rates in the right PPC correspond to eye positions to the right of the midline, then increased excitability of the right PPC (rPPCa montage) would result in a rightward error in the eye position signal and therefore rightward mislocalization (Morris et al., 2012). This argument hinges on the assumption of a particular hemispheric bias in the eye position signal; such biases have been found in primary visual cortex (Durand, Trotter, & Celebrini, 2010), but not in parietal cortex of the macaque (Bremmer, Distler, & Hoffmann, 1997). A more quantitative assessment of the viability of this mechanism therefore requires more insight into the nature of eye position signals in the human PPC (Merriam, Gardner, Movshon, & Heeger, 2013).
Conclusions
Applying dual tDCS to the right and left PPC generated mislocalizations similar to those found after the presentation of an exogenous visual cue. This supports the causal involvement of the PPC in visual localization and suggests that the balance of activation between the hemispheres is a determining factor in localization. We also found a novel time course for tDCS-induced behavioral effects; there were short-term effects that dissipated while tDCS was still being applied, and aftereffects that arose after the offset of tDCS.
Acknowledgments
This work was supported by the Charles and Johanna Busch Memorial Fund at Rutgers, The State University of New Jersey, and by the Eye Institute of the National Institutes of Health, USA under award number R01 EY-017605.
Commercial relationships: none.
Corresponding author: Jessica M. Wright.
Email: jessica@vision.rutgers.edu.
Address: Center for Molecular and Behavioral Neuroscience, Rutgers University, Newark, NJ, USA.
Contributor Information
Jessica M. Wright, Email: jessica@vision.rutgers.edu.
Bart Krekelberg, Email: bart@vision.rutgers.edu.
References
- Andersen R. A., Essick G. K., Siegel R. M. (1985). Encoding of spatial location by posterior parietal neurons. Science, 230 (4724), 456–458, doi:10.1126/science.4048942 [DOI] [PubMed] [Google Scholar]
- Antal A., Polania R., Schmidt-Samoa C., Dechent P., Paulus W. (2011). Transcranial direct current stimulation over the primary motor cortex during fMRI. NeuroImage, 55 (2), 590–596, doi:10.1016/j.neuroimage.2010.11.085 [DOI] [PubMed] [Google Scholar]
- Antal A., Varga E. T., Nitsche M. A., Chadaide Z., Paulus W., Kovacs G., &, Vidnyanszky Z. (2004). Direct current stimulation over MT+/V5 modulates motion aftereffect in humans. Neuroreport , 15 (16), 2491–2494, doi:10.1097/00001756-200411150-00012 [DOI] [PubMed] [Google Scholar]
- Benucci A., Saleem A. B., Carandini M. (2013). Adaptation maintains population homeostasis in primary visual cortex. Nature Neuroscience, 16 (6), 724–729, doi:10.1038/nn.3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E., Bulgarelli C., Sterzi R., Vallar G. (1983). Line bisection and cognitive plasticity of unilateral neglect of space. Brain & Cognition, 2 (1), 32–38, doi:10.1016/0278-2626(83)90027-1 [DOI] [PubMed] [Google Scholar]
- Bocianski D., Müsseler J., Erlhagen W. (2010). Effects of attention on a relative mislocalization with successively presented stimuli. Vision Research, 50 (18), 1793–1802, doi:10.1016/j.visres.2010.05.036 [DOI] [PubMed] [Google Scholar]
- Bremmer F., Distler C., Hoffmann K. P. (1997). Eye position effects in monkey cortex. II. Pursuit- and fixation- related activity in posterior parietal areas LIP and 7A. Journal of Neurophysiology, 77 (2), 962–977 [DOI] [PubMed] [Google Scholar]
- Bridgeman B., Peery S., Anand S. (1997). Interaction of cognitive and sensorimotor maps of visual space. Perception & Psychophysics, 59 (3), 456–469, doi:10.3758/BF03211912 [DOI] [PubMed] [Google Scholar]
- Brighina F., Bisiach E., Piazza A., Oliveri M., La Bua V., Daniele O., &, Fierro B. (2002). Perceptual and response bias in visuospatial neglect due to frontal and parietal repetitive transcranial magnetic stimulation in normal subjects. Neuroreport, 13 (18), 2571–2575, doi:10.1097/01.wnr.0000052321.62862.7e [DOI] [PubMed] [Google Scholar]
- Cheal M., Lyon D. R. (1991). Central and peripheral precuing of forced-choice discrimination. Quarterly Journal of Experimental Psychology A: Human Experimental Psychology, 43 (4), 859–880, doi:10.1080/14640749108400960 [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Romero R. D., Servan-Schreiber D., Farah M. J. (1994). Mechanisms of spatial attention: The relation of macrostructure to microstructure in parietal neglect. Journal of Cognitive Neuroscience, 6 (4), 377–387, doi:10.1162/jocn.1994.6.4.377 [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Bookheimer S. Y. (1994). Localization of brain function using magnetic resonance imaging. Trends in Neurosciences, 17, 268–277, doi:10.1016/0166-2236(94)90055-8 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3 (3), 215–229, doi:10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Dambeck N., Sparing R., Meister I. G., Wienemann M., Weidemann J., Topper R., &, Boroojerdi B. (2006). Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Research, 1072 (1), 194–199, doi:10.1016/j.brainres.2005.05.075 [DOI] [PubMed] [Google Scholar]
- Datta A., Baker J. M., Bikson M., Fridriksson J. (2011). Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation, 4 (3), 169–174, doi:10.1016/j.brs.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Bansal V., Diaz J., Patel J., Reato D., Bikson M. (2009). Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation, 2 (4), 201–207, doi:10.1016/j.brs.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Zhou X., Su Y., Parra L. C., Bikson M. (2013). Validation of finite element model of transcranial electrical stimulation using scalp potentials: Implications for clinical dose. Journal of Neural Engineering, 10 (3), 5, doi:10.1088/1741-2560/10/3/036018 [DOI] [PubMed] [Google Scholar]
- Durand J. B., Trotter Y., Celebrini S. (2010). Privileged processing of the straight-ahead direction in primate area V1. Neuron, 66 (1), 126–137, doi:10.1016/j.neuron.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Fierro B., Brighina F., Oliveri M., Piazza A., La Bua V., Buffa D., &, Bisiach E. (2000). Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport, 11 (7), 1519–1521 [PubMed] [Google Scholar]
- Fink G. R., Marshall J. C., Shah N. J., Weiss P. H., Halligan P. W., Grosse-Ruyken M., …, Freund H. J. (2000). Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology, 54 (6), 1324–1331, doi:10.1212/WNL.54.6.1324 [DOI] [PubMed] [Google Scholar]
- Floel A., Suttorp W., Kohl O., Kurten J., Lohmann H., Breitenstein C., &, Knecht S. (2012). Non-invasive brain stimulation improves object-location learning in the elderly. Neurobiology of Aging, 33 (8), 1682–1689, doi:10.1016/j.neurobiolaging.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Fortenbaugh F. C., Robertson L. C. (2011). When here becomes there: Attentional distribution modulates foveal bias in peripheral localization. Attention, Perception & Psychophysics, 73 (3), 809–828, doi:10.3758/s13414-010-0075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga P. C., Hummel F. C., Cohen L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology, 117 (4), 845–850, doi:10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Giglia G., Mattaliano P., Puma A., Rizzo S., Fierro B., Brighina F. (2011). Neglect-like effects induced by tDCS modulation of posterior parietal cortices in healthy subjects. Brain Stimulation, 4 (4), 294–299, doi:10.1016/j.brs.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Hartmann T. S., Bremmer F., Albright T. D., Krekelberg B. (2011). Receptive field positions in area MT during slow eye movements. Journal of Neuroscience, 31 (29), 10437–10444, doi:10.1523/JNEUROSCI.5590-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Kowler E. (1991). Saccadic localization of eccentric forms. Journal of the Optical Society of America A: Optics & Image Science, 8 (2), 440–449, doi:10.1364/JOSAA.8.000440 [DOI] [PubMed] [Google Scholar]
- Herwig U., Satrapi P., Schonfeldt-Lecuona C. (2003). Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topography, 16 (2), 95–99, doi:10.1023/B:BRAT.0000006333.93597.9d [DOI] [PubMed] [Google Scholar]
- Hilgetag C. C., Theoret H., Pascual-Leone A. (2001). Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions' of human parietal cortex. Nature Neuroscience, 4 (9), 953–957, doi:10.1038/nn0901-953 [DOI] [PubMed] [Google Scholar]
- Iyer M. B., Mattu U., Grafman J., Lomarev M., Sato S., Wassermann E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology, 64 (5), 872–875 doi:10.1212/01.WNL.0000152986.07469.E9 [DOI] [PubMed] [Google Scholar]
- Iyer M. B., Schleper N., Wassermann E. M. (2003). Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. Journal of Neuroscience, 23 (34), 10867–10872, doi:10.1097/jcp.0b013e3181603f7c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell G., McCourt M. E. (2000). Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia, 38 (1), 93–110, doi:10.1016/s0028-3932(99)00045-7 [DOI] [PubMed] [Google Scholar]
- Kar K., Krekelberg B. (2012). Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. Journal of Neurophysiology, 108 (8), 2173–2178, doi:10.1152/jn.00505.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar K., Krekelberg B. (2013). Transcranial electrical stimulation mitigates motion adaption in V1, MT, and MST neurons of awake, behaving macaques. Poster session presented at the annual meeting of the Society for Neuroscience, November 9-13, San Diego, CA: [Google Scholar]
- Kar K., Krekelberg B. (2014). Transcranial alternating current stimulation attenuates visual motion adaptation. Journal of Neuroscience, 34 (21), 7334–7340, doi:10.1523/JNEUROSCI.5248-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M. (1977). Hemi-neglect and hemisphere rivalry. Advances in Neurology, 18, 41–49 [PubMed] [Google Scholar]
- Ko M. H., Han S. H., Park S. H., Seo J. H., Kim Y. H. (2008). Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neuroscience Letters, 448 (2), 171–174, doi:10.1016/j.neulet.2008.10.050 [DOI] [PubMed] [Google Scholar]
- Kosovicheva A. A., Fortenbaugh F. C., Robertson L. C. (2010). Where does attention go when it moves? Spatial properties and locus of the attentional repulsion effect. Journal of Vision, 10 (12): 5 1–13, http://www.journalofvision.org/content/10/12/33, doi:10.1167/10.12.33. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E., Blaser E. (1995). The accuracy and precision of saccades to small and large targets. Vision Research, 35 (12), 1741–1754, doi:10.1016/0042-6989(94)00255-K [DOI] [PubMed] [Google Scholar]
- Krekelberg B. (2001). The persistence of position. Vision Research, 41 (4), 529–539, doi:10.1016/S0042-6989(00)00281-9 [DOI] [PubMed] [Google Scholar]
- Krekelberg B., Lappe M. (2001). Neuronal latencies and the position of moving objects. Trends in Neurosciences, 24 (6), 335–339, doi:10.1016/s0166-2236(00)01795-1 [DOI] [PubMed] [Google Scholar]
- Liebetanz D., Nitsche M. A., Tergau F., Paulus W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125 (10), 2238–2247, doi:10.1093/brain/awf238 [DOI] [PubMed] [Google Scholar]
- Mateeff S., Gourevich A. (1983). Peripheral vision and perceived visual direction. Biological Cybernetics, 49 (2), 111–118, doi:10.1007/BF00320391 [DOI] [PubMed] [Google Scholar]
- Meinzer M., Antonenko D., Lindenberg R., Hetzer S., Ulm L., Avirame K., …, Floel A. (2012). Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. Journal of Neuroscience, 32 (5), 1859–1866, doi:10.1523/JNEUROSCI.4812-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam E. P., Gardner J. L., Movshon J. A., Heeger D. J. (2013). Modulation of visual responses by gaze direction in human visual cortex. Journal of Neuroscience, 33 (24), 9879–9889, doi:10.1523/JNEUROSCI.0500-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke D., Wrede A., Schulz-Schaeffer W., Taghizadeh-Waghefi A., Nitsche M. A., Rohde V., &, Liebetanz D. (2013). Cathodal transcranial direct current stimulation induces regional, long-lasting reductions of cortical blood flow in rats. Neurological Research, 35 (10), 1029–1037, doi:10.1179/1743132813Y.0000000248 [DOI] [PubMed] [Google Scholar]
- Moos K., Vossel S., Weidner R., Sparing R., Fink G. R. (2012). Modulation of top-down control of visual attention by cathodal tDCS over right IPS. Journal of Neuroscience, 32 (46), 16360–16368, doi:10.1523/JNEUROSCI.6233-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Bremmer F., Krekelberg B. (2013). Eye position signals in the dorsal visual system are accurate and precise on short time-scales. Journal of Neuroscience, 33 (30), 12395–12406, doi:10.1523/JNEUROSCI.0576-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Chambers C. D., Mattingley J. B. (2007). Parietal stimulation destabilizes spatial updating across saccadic eye movements. Proceedings of the National Academy of Sciences, USA, 104 (21), 9069–9074, doi:10.1073/pnas.0610508104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Kubischik M., Hoffmann K. P., Krekelberg B., Bremmer F. (2012). Dynamics of eye-position signals in the dorsal visual system. Current Biology, 22 (3), 173–179, doi:10.1016/j.cub.2011.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Liu C. C., Cropper S. J., Forte J. D., Krekelberg B., Mattingley J. B. (2010). Summation of visual motion across eye movements reflects a nonspatial decision mechanism. Journal of Neuroscience, 30 (29), 9821–9830, doi:10.1523/JNEUROSCI.1705-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H. J., Rabbitt P. M. (1989). Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception & Performance, 15 (2), 315–330, doi:10.1037/0096-1523.15.2.315 [DOI] [PubMed] [Google Scholar]
- Müsseler J., van der Heijden A. H. C., Mahmud S. H., Deubel H., Ertsey S. (1999). Relative mislocalization of briefly presented stimuli in the retinal periphery. Perception & Psychophysics, 61 (8), 1646–1661, doi:10.3758/BF03213124 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Cohen L. G., Wassermann E. M., Priori A., Lang N., Antal A., …, Pascual-Leone A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1 (3), 206–223, doi:10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Fricke K., Henschke U., Schlitterlau A., Liebetanz D., Lang N., …, Paulus W. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology, 553 (Pt 1), 293–301, doi:10.1113/jphysiol.2003.049916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M. A., Liebetanz D., Lang N., Antal A., Tergau F., Paulus W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology, 114 (11), 2220–2222, doi:10.1016/S1388-2457(03)00235-9 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Nitsche M. S., Klein C. C., Tergau F., Rothwell J. C., Paulus W. (2003). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114 (4), 600–604, doi:10.1016/S1388-2457(02)00412-1 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Paulus W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527, 633–639, doi:10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M. A., Paulus W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57 (10), 1899–1901 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Seeber A., Frommann K., Klein C. C., Rochford C., Nitsche M. S., …, Tergau F. (2005). Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. Journal of Physiology, 568 (1), 291–303, doi:10.1113/jphysiol.2005.092429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan J. K. (1984). Retinal versus extraretinal influences in flash localization during saccadic eye movements in the presence of a visible background. Perception & Psychophysics, 36 (1), 1–14, doi:10.3758/BF03206348 [DOI] [PubMed] [Google Scholar]
- Pourtois G., Vandermeeren Y., Olivier E., de Gelder B. (2001). Event-related TMS over the right posterior parietal cortex induces ipsilateral visuo-spatial interference. Neuroreport, 12 (11), 2369–2374 [DOI] [PubMed] [Google Scholar]
- Prinzmetal W., Amiri H., Allen K., Edwards T. (1998). Phenomenology of attention. I. Color, location, orientation, and spatial frequency. Journal of Experimental Psychology: Human Perception & Performance, 24 (1), 261–282, doi:10.1037//0096-1523.24.1.261 [Google Scholar]
- Radman T., Ramos R. L., Brumberg J. C., Bikson M. (2009). Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimulation, 2 (4), 215–228, doi:10.1016/j.brs.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Kinsbourne M., Moscovitch M. (1990). Hemispheric control of spatial attention. Brain & Cognition, 12 (2), 240–266, doi:10.1016/0278-2626(90)90018-J [DOI] [PubMed] [Google Scholar]
- Sack A. T., Hubl D., Prvulovic D., Formisano E., Jandl M., Zanella F. E., …, Linden D. E. (2002). The experimental combination of rTMS and fMRI reveals the functional relevance of parietal cortex for visuospatial functions. Brain Research: Cognitive Brain Research, 13 (1), 85–93, doi:10.1016/S0926-6410(01)00087-8 [DOI] [PubMed] [Google Scholar]
- Sparing R., Thimm M., Hesse M. D., Kust J., Karbe H., Fink G. R. (2009). Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain, 132 (Pt 11), 3011–3020, doi:10.1093/brain/awp154 [DOI] [PubMed] [Google Scholar]
- Stagg C. J., Nitsche M. A. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist, 17 (1), 37–53, doi:10.1177/1073858410386614 [DOI] [PubMed] [Google Scholar]
- Stork S., Musseler J., van der Heijden A. H. (2010). Perceptual judgment and saccadic behavior in a spatial distortion with briefly presented stimuli. Advances in Cognitive Psychology, 6, 1–14, doi:10.2478/v10053-008-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Cavanagh P. (1997). Focused attention distorts visual space: An attentional repulsion effect. Journal of Experimental Psychology: Human Perception & Performance, 23 (2), 443–463, doi:10.1037/0096-1523.23.2.443 [DOI] [PubMed] [Google Scholar]
- Szczepanski S. M., Kastner S. (2013). Shifting attentional priorities: Control of spatial attention through hemispheric competition. Journal of Neuroscience, 33 (12), 5411–5421, doi:10.1523/JNEUROSCI.4089-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski S. M., Konen C. S., Kastner S. (2010). Mechanisms of spatial attention control in frontal and parietal cortex. Journal of Neuroscience, 30 (1), 148–160, doi:10.1523/JNEUROSCI.3862-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsal Y., Bareket T. (1999). Effects of attention on localization of stimuli in the visual field. Psychonomic Bulletin & Review, 6 (2), 292–296, doi:10.3758/BF03212332 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature, 391 (6670), 892–896, doi:10.1038/36103 [DOI] [PubMed] [Google Scholar]
- van der Heijden A. H., van der Geest J. N., de Leeuw F., Krikke K., Musseler J. (1999). Sources of position-perception error for small isolated targets. Psychological Research, 62 (1), 20–35, doi:10.1007/s004260050037 [DOI] [PubMed] [Google Scholar]
- Vicario C. M., Martino D., Koch G. (2013). Temporal accuracy and variability in the left and right posterior parietal cortex. Journal of Neuroscience, 245, 121–128, doi:10.1016/j.neuroscience.2013.04.041 [DOI] [PubMed] [Google Scholar]
- Vines B. W., Nair D. G., Schlaug G. (2006). Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport, 17 (6), 671–674, doi:10.1097/00001756-200604240-00023 [DOI] [PubMed] [Google Scholar]
- Wagner T., Fregni F., Fecteau S., Grodzinsky A., Zahn M., Pascual-Leone A. (2007). Transcranial direct current stimulation: A computer-based human model study. Neuroimage, 35 (3), 1113–1124, doi:10.1016/j.neuroimage.2007.01.027 [DOI] [PubMed] [Google Scholar]
- Whitaker D., McGraw P. V., Levi D. M. (1997). The influence of adaptation on perceived visual location. Vision Research, 37 (16), 2207–2216, doi:10.1016/S0042-6989(97)00030-8 [DOI] [PubMed] [Google Scholar]
- Wright J. M., Morris A. P., Krekelberg B. (2011). Weighted integration of visual position information. Journal of Vision , 11 (14): 5 1–16, http://www.journalofvision.org/content/11/14/11, doi:10.1167/11.14.11. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]