Abstract
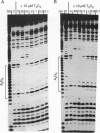
Molecular modeling has been used to predict that 2,6-disubstituted amidoanthraquinones, and not the 1,4 series, should preferentially interact with and stabilize triple-stranded DNA structures over duplex DNA. This is due to marked differences in the nature of chromophore-base stacking and groove accessibility for the two series. A DNA foot-printing method that monitors the extent of protection from DNase I cleavage on triplex formation has been used to examine the effects of a number of synthetic isomer compounds in the 1,4 and 2,6 series. The experimental results are in accord with the predicted behavior and confirm that the 1,4 series bind preferentially to double- rather than triple-stranded DNA, whereas the isomeric 2,6 derivatives markedly favor binding to triplex DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agbandje M., Jenkins T. C., McKenna R., Reszka A. P., Neidle S. Anthracene-9,10-diones as potential anticancer agents. Synthesis, DNA-binding, and biological studies on a series of 2,6-disubstituted derivatives. J Med Chem. 1992 Apr 17;35(8):1418–1429. doi: 10.1021/jm00086a010. [DOI] [PubMed] [Google Scholar]
- Cassidy S. A., Strekowski L., Wilson W. D., Fox K. R. Effect of a triplex-binding ligand on parallel and antiparallel DNA triple helices using short unmodified and acridine-linked oligonucleotides. Biochemistry. 1994 Dec 27;33(51):15338–15347. doi: 10.1021/bi00255a015. [DOI] [PubMed] [Google Scholar]
- Collier D. A., Neidle S. Synthesis, molecular modeling, DNA binding, and antitumor properties of some substituted amidoanthraquinones. J Med Chem. 1988 Apr;31(4):847–857. doi: 10.1021/jm00399a028. [DOI] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Degols G., Clarenc J. P., Lebleu B., Léonetti J. P. Reversible inhibition of gene expression by a psoralen functionalized triple helix forming oligonucleotide in intact cells. J Biol Chem. 1994 Jun 17;269(24):16933–16937. [PubMed] [Google Scholar]
- Durland R. H., Kessler D. J., Gunnell S., Duvic M., Pettitt B. M., Hogan M. E. Binding of triple helix forming oligonucleotides to sites in gene promoters. Biochemistry. 1991 Sep 24;30(38):9246–9255. doi: 10.1021/bi00102a017. [DOI] [PubMed] [Google Scholar]
- Duval-Valentin G., Thuong N. T., Hélène C. Specific inhibition of transcription by triple helix-forming oligonucleotides. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):504–508. doi: 10.1073/pnas.89.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparro F. P., Havre P. A., Olack G. A., Gunther E. J., Glazer P. M. Site-specific targeting of psoralen photoadducts with a triple helix-forming oligonucleotide: characterization of psoralen monoadduct and crosslink formation. Nucleic Acids Res. 1994 Jul 25;22(14):2845–2852. doi: 10.1093/nar/22.14.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev M., Praseuth D., Guieysse A. L., Robin P., Thuong N. T., Hélène C., Harel-Bellan A. Inhibition of gene expression by triple helix-directed DNA cross-linking at specific sites. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3501–3505. doi: 10.1073/pnas.90.8.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev M., Praseuth D., Robin P., Hemar A., Saison-Behmoaras T., Dautry-Varsat A., Thuong N. T., Hélène C., Harel-Bellan A. A triple helix-forming oligonucleotide-intercalator conjugate acts as a transcriptional repressor via inhibition of NF kappa B binding to interleukin-2 receptor alpha-regulatory sequence. J Biol Chem. 1992 Feb 15;267(5):3389–3395. [PubMed] [Google Scholar]
- Havre P. A., Gunther E. J., Gasparro F. P., Glazer P. M. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7879–7883. doi: 10.1073/pnas.90.16.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Latimer L. J., Hampel K. J. Coralyne binds tightly to both T.A.T- and C.G.C(+)-containing DNA triplexes. Biochemistry. 1993 Jun 1;32(21):5591–5597. doi: 10.1021/bi00072a014. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dervan P. B., Wold B. J. Kinetic analysis of oligodeoxyribonucleotide-directed triple-helix formation on DNA. Biochemistry. 1990 Sep 18;29(37):8820–8826. doi: 10.1021/bi00489a045. [DOI] [PubMed] [Google Scholar]
- Mayfield C., Ebbinghaus S., Gee J., Jones D., Rodu B., Squibb M., Miller D. Triplex formation by the human Ha-ras promoter inhibits Sp1 binding and in vitro transcription. J Biol Chem. 1994 Jul 8;269(27):18232–18238. [PubMed] [Google Scholar]
- Mergny J. L., Duval-Valentin G., Nguyen C. H., Perrouault L., Faucon B., Rougée M., Montenay-Garestier T., Bisagni E., Hélène C. Triple helix-specific ligands. Science. 1992 Jun 19;256(5064):1681–1684. doi: 10.1126/science.256.5064.1681. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Neidle S., Jenkins T. C. Molecular modeling to study DNA intercalation by anti-tumor drugs. Methods Enzymol. 1991;203:433–458. doi: 10.1016/0076-6879(91)03024-b. [DOI] [PubMed] [Google Scholar]
- Pilch D. S., Breslauer K. J. Ligand-induced formation of nucleic acid triple helices. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9332–9336. doi: 10.1073/pnas.91.20.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilch D. S., Waring M. J., Sun J. S., Rougée M., Nguyen C. H., Bisagni E., Garestier T., Hélène C. Characterization of a triple helix-specific ligand. BePI (3-methoxy-7H-8-methyl-11- [(3'-amino)propylamino]-benzo[e]pyrido[4,3-b]indole) intercalates into both double-helical and triple-helical DNA. J Mol Biol. 1993 Aug 5;232(3):926–946. doi: 10.1006/jmbi.1993.1440. [DOI] [PubMed] [Google Scholar]
- Plum G. E., Park Y. W., Singleton S. F., Dervan P. B., Breslauer K. J. Thermodynamic characterization of the stability and the melting behavior of a DNA triplex: a spectroscopic and calorimetric study. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9436–9440. doi: 10.1073/pnas.87.23.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Flint S. J., Kessler D. J., Hogan M. E. Evidence that a triplex-forming oligodeoxyribonucleotide binds to the c-myc promoter in HeLa cells, thereby reducing c-myc mRNA levels. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8227–8231. doi: 10.1073/pnas.88.18.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan I., Patel D. J. DNA triplexes: solution structures, hydration sites, energetics, interactions, and function. Biochemistry. 1994 Sep 27;33(38):11405–11416. doi: 10.1021/bi00204a001. [DOI] [PubMed] [Google Scholar]
- Rougée M., Faucon B., Mergny J. L., Barcelo F., Giovannangeli C., Garestier T., Hélène C. Kinetics and thermodynamics of triple-helix formation: effects of ionic strength and mismatches. Biochemistry. 1992 Sep 29;31(38):9269–9278. doi: 10.1021/bi00153a021. [DOI] [PubMed] [Google Scholar]
- Scaria P. V., Shafer R. H. Binding of ethidium bromide to a DNA triple helix. Evidence for intercalation. J Biol Chem. 1991 Mar 25;266(9):5417–5423. [PubMed] [Google Scholar]
- Shindo H., Torigoe H., Sarai A. Thermodynamic and kinetic studies of DNA triplex formation of an oligohomopyrimidine and a matched duplex by filter binding assay. Biochemistry. 1993 Aug 31;32(34):8963–8969. doi: 10.1021/bi00085a030. [DOI] [PubMed] [Google Scholar]
- Stonehouse T. J., Fox K. R. DNase I footprinting of triple helix formation at polypurine tracts by acridine-linked oligopyrimidines: stringency, structural changes and interaction with minor groove binding ligands. Biochim Biophys Acta. 1994 Aug 2;1218(3):322–330. doi: 10.1016/0167-4781(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanious F. A., Jenkins T. C., Neidle S., Wilson W. D. Substituent position dictates the intercalative DNA-binding mode for anthracene-9,10-dione antitumor drugs. Biochemistry. 1992 Nov 24;31(46):11632–11640. doi: 10.1021/bi00161a050. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Tanious F. A., Mizan S., Yao S., Kiselyov A. S., Zon G., Strekowski L. DNA triple-helix specific intercalators as antigene enhancers: unfused aromatic cations. Biochemistry. 1993 Oct 12;32(40):10614–10621. doi: 10.1021/bi00091a011. [DOI] [PubMed] [Google Scholar]
- Young S. L., Krawczyk S. H., Matteucci M. D., Toole J. J. Triple helix formation inhibits transcription elongation in vitro. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10023–10026. doi: 10.1073/pnas.88.22.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]