Abstract
Prostate cancer is the leading cancer-related cause of death for men in the USA. Prostate cancer risk is significantly lower in Asian countries compared with the USA, which has prompted interest in the potential chemo-preventive action of soyand green teathat are more predominant in Asian diets. It has been proposed that chronic inflammation is a major risk factor of prostate cancer, acting as both an initiator and promoter. Specifically, the nuclear factor-kappa B (NF-κB) pathway has been implicated as an important mediator between chronic inflammation, cell proliferation and prostate cancer. Dietary factors that inhibit inflammation and NF-κB may serve as effective chemo-preventive agents. Recent studies have demonstrated that soy and green tea have anti-inflammatory properties, and may have the potential to block the inflammatory response during cancer progression. This minireview discusses the relationship between chronic inflammation and prostate cancer, emphasizing on the significance of NF-κB, and further explores the anti-inflammatory effects of soy and green tea. Finally, we propose that dietary strategies that incorporate these bioactive food components as whole foods may be a more effective means to target pathways that contribute to prostate cancer development.
Keywords: soy, green tea, inflammation, prostate cancer, nuclear factor-kappa B
Introduction
Prostate cancer is the most common type of cancer found in American men, accounting for 10% of male cancer-related deaths.1 Risk factors of prostate cancer include age, family history, ethnicity and hormonal status, but there is compelling evidence to suggest that environmental factors, such as diet and lifestyle, play more important roles in prostate carcinogenesis than genetic predisposition.2 The incidence of latent prostatic lesions in men appears uniform across Asian and Western countries, but prostate cancer outcomes and mortality rates are considerably higher in Western countries.3 Moreover, prostate cancer rates have been increasing gradually in major industrialized cities in Asia. For example, between 1978 and 1997, prostate cancer incidence has risen to more than double in Singapore.4 It has been postulated that westernization and specifically increased consumption of ‘Western diets’ could be contributing factors to the increased prostate cancer rate in these Asian cities. Some of the popular foods in Asian diet, such as soy and green tea, have been investigated for their anticancer properties, including their abilities to target inflammatory pathways as means to attenuate cancer development. The goal of this literature review is to examine the interactions of green tea, soy and inflammation in prostate cancer development.
Chronic inflammation, nuclear factor-kappa B and prostate cancer
The connection between chronic inflammation and cancer was first noted by Virchow in 1863. He hypothesized that a combination of some classes of irritants, tissue injuries and associated inflammation enhanced cell proliferation and contributed to the origin of cancer. Chronic inflammation has been estimated to contribute to as much as one-third of all cancers,5 acting as both initiators and/or promoters in the carcinogenesis process. Cells that mediate inflammatory responses are major contributors of in vivo production of reactive oxygen species (ROS). Polymorphonuclear leukocytes (PMNs, which include neutrophils, eosinophils and basophils) and mononuclear phagocytes (monocytes and macrophages) produce superoxide, nitric oxide, hydrogen peroxide and hypochloric acid in attempts to defend against pathogens. High concentrations and the persistent presence of these ROS are mutagenic and can lead to tissue damage. Furthermore, damaged tissues generate proliferation signals for cell growth and repair. Both increased ROS and cell proliferation contribute to the initiation and promotion of carcinogenesis, and in combination they provide an optimal microenvironment for tumorigenesis.
Inflammation is implicated as a major risk factor for prostate cancer. Population studies have found an increased relative risk of prostate cancer in men with prior histories of prostatitis (inflammation of the prostate).6 Benign prostatic hyperplasia (BPH), which is a condition that often precedes and coexists with prostate cancer, demonstrates signs of inflammatory response. Specifically, almost all human BPH specimens showed inflammatory infiltration and high expression of pro-inflammatory cytokines, including interleukin-17 (IL-17) that promotes stromal growth and chronic inflammation.7 Interestingly, inflammatory pathways including the cyclooxygenase-2 (COX-2) and nuclear factor kappa B (NF-κB) are over-expressed in human prostate adenocarcinomas compared with normal prostate tissues,8–10 and targeting these inflammatory pathways have shown promise as an intervention strategy for prostate cancer. Regular intakes of non-steroidal anti-inflammatory drugs (NSAIDs) appear to reduce prostate cancer growth by decreasing inflammation in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model, and there is a correlation between long-term usage of NSAIDs and lower prostate cancer risk.11,12 The NF-κB pathway has gained much interest in recent years because of its influence on many cellular responses that contribute to carcinogenesis, including regulation of cell cycle, cell proliferation, apoptosis and inflammation. The deregulation of NF-κB pathway increases cell proliferation by up-regulating antiapoptotic protein, BcL2,13,14 promoting angiogenesis,15 and inhibiting immune surveillance via production of immunosuppressive interleukins, such as IL-10.16 Thus, inflammation plays a major role in cancer etiology and may provide selective growth advantages for cancer cells. Targeting aberrant NF-κB activation in cancer cells may restore deregulated cellular functions by inducing apoptosis and by decreasing uncontrolled cell proliferation and chronic inflammation; thus strategies to mitigate NF-κB have important preventive or therapeutic values against cancer.
Constitutive activation of NF-κB is responsible for numerous diseases.17 Potential toxicity associated with NF-κB blockage leads to liver apoptosis,18 but the first link implicating NF-κB as a possible target for chemoprevention was based on the recognition that c-Rel, which encodes an NF-κB subunit, is the cellular homolog of the v-Rel onco-gene. Upon stimulation, IkB kinase (IKK) is activated. Activated IKK phosphorylates IkB that is bound to NF-κB and targets IkB for poly-ubiquitination and degradation. Free NF-κB homodimers and heterodimers then translocate into the nucleus and transactivate genes involved in inflammatory responses and cell proliferation.19,20 NF-κB activity is negatively self-regulated by transcriptional up-regulation of its inhibitor, IκBα. Initially, it was believed that IκBα solely prevents nuclear localization of activated NF-κB, but recent studies suggest that IκBα also directly interacts with DNA-bound NF-κB. IκBα dissembles NF-κB from DNA by changing conformation of p65 subunits, and channels NF-κB to the cytoplasm via its nuclear export signal.21 Furthermore, IκBs are controlled by upstream kinases, the IKKs via phosphorylation. The complexity of NF-κB pathway suggests that total blockage of the NF-κB in healthy cells is not ideal, but the multiple levels of regulation in the NF-κB pathway provide many possible targets for chemoprevention in cancer cells.
Over-expression of NF-κB promotes tumor growth through activations of genes responsible for inflammation, apoptosis and cell proliferation. For instance, NF-κB controls expressions of cyclin D1, which influences cell growth and differentiation.21,22 NF-κB also amplifies inflammatory signals, including COX-2 and pro-inflammatory cytokines, such as IL-6, by acting as a transcriptional activator.23 Constitutive activation of IL-6 by NF-κB has been implicated in prostate cancer progression.24 Furthermore, NF-κB intimately interacts with the homeostasis of steroid hormones. Androgen receptor (AR) expression antagonizes NF-κB activity. Prostate cancer cell lines without endogenous AR expression, such as malignant PC3 cells, have constitutive NF-κB activity, whereas cell lines that express AR (LnCap cells) have low basal NF-κB activity.25 Upon the loss of AR expression and androgen dependency, deregulation of NF-κB becomes a major promoting factor for transformation of cancer cells to malignancy and poor prognosis. Many in vitro studies have shown that targeting NF-κB was effective in inducing cell death in prostate cancer cells. Blocking NF-κB activity in PC3 cells by transfection of mutant IκBα suppresses angiogenesis and cell invasion.26 Strategies utilizing IκBα-super repressors or inhibitors of NF-κB activity to sensitize prostate epithelial cells to chemotherapy have been deemed promising.27,28 Even though there are limited in vivo studies investigating the impact of NF-κB in prostate cancer, these studies suggest that NF-κB is a crucial mediator of tumorigenesis in several cancer models. Deletion of IKKβ is associated with an increase in epithelial apoptosis during tumor promotion and a decrease in tumor incidence in a colitis-associated cancer mouse model.29 Tumor necrosis factor α (TNF-α)-induced NF-κB activation does not affect tumor initiation, but is essential for the promotion of hepatitis-induced hepatocellular carcinoma in mice.30
Overall, both pharmacological and genetic strategies have provided strong support for the rationale of using agents that target inflammatory pathways as a cancer chemoprevention strategy. Importantly, many factors found in the diet also can target these pathways and possibly exert anti-inflammatory and cancer preventive properties.
Diet and prostate cancer
Human migration studies emphasize the importance of diet and lifestyle on prostate cancer development. For instance, first-generation Asian migrants (high soy and tea intake population) have a lower incidence of prostate and mammary cancers than subsequent generations of Asian Americans.31 Incidences of prostate cancer in Chinese (24 per 100,000), Japanese (29.6 per 100,000) and Filipino (56.8 per 100,000) men born in China, Japan and the Philippines were about half of those born in the USA (44.4, 42.2 and 111.3 per 100,000, respectively).2 These observations have prompted studies to investigate and compare dietary components in the Asian diet, such as soy and green tea that may have cancer preventive properties. A typical soy-rich Japanese diet consists of 25–100 mg soy isoflavones/d (1 serving of traditional fermented soy food contains about 25 mg soy isoflavones), while the typical American diet contains about 1–3 mg soy isoflavones/d.32 The green tea consumption in Asian countries averages ~360–480 mL/d, whereas only 8% of the American population regularly consumes ~180 mL of green tea per day.33 The different dietary intake levels of soy and green tea are hypothesized to contribute moderately to differences in prostate cancer outcomes between the two populations.
Various mechanisms for the anticarcinogenic properties of soy or green tea have been proposed. For instance, soy isoflavones, classified as phytoestrogens, act as both estrogen agonists and antagonists by differentially binding to estrogen receptor α or β34,35 and/or altering enzymes involved in hormone metabolism.34–36 Soy isoflavones induce G2/M arrest and p21 expression in androgen-independent PC3 prostate cancer cells37 and may act through hormone-independent pathways that target cell cycle or apoptotic mechanisms. Genistein is a known inhibitor of protein tyrosine kinase,38 topoisomerase II,39 and up-regulates p21 in various cancer cells.40–42 Green tea polyphenols, especially EGCG, exerts anticarcinogenic effects at multiple stages of carcinogenesis. EGCG is a strong antioxidant and effective inhibitor for carcinogen activation. EGCG induces cell cycle arrest, apoptosis and attenuates tumor promotion.43 EGCG suppresses growth of human gastric cancer xenografts in mice through the inhibition of tumor invasion and metastasis by regulating expression of matrix metalloproteinases and vascular endothelial growth factor production.43,44 The role of EGCG in prostate cancer prevention and treatment are summarized by Stuart et al.45 Among the various mechanisms, we favor the likelihood that inflammation, especially as dependent on the NF-κB pathway, to be a target of inhibition by soy and green tea. Whether dietary soy or green tea has significant impacts on chronic inflammation and NF-κB in vivo is still unclear and is an important area of future research. If increasing the consumption of soy or green tea may prevent prostate cancer development, these dietary recommendations would provide a more cost-effective and a safer chemo-preventive strategy than using pharmacological agents.
Green tea and prostate cancer
Green tea (Camellia sinensis) is one of the most widely consumed beverages, especially in Asia. It is different from black or Oolong tea by methods of processing. Green tea is prepared by pan-frying or steaming fresh leaves to heat inactivate oxidative enzymes followed by drying, whereas Oolong and black tea are prepared by additional steps of fermentation.
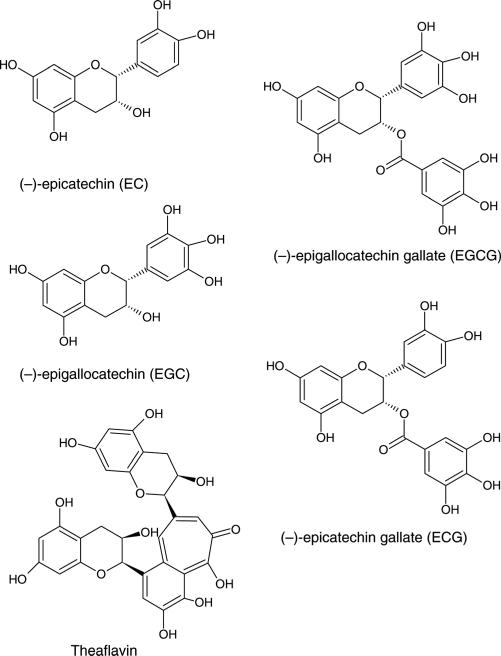
The bioactive components of green tea include catechins, epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG) and epicatechin (EC) and theaflavins (Figure 1). Catechins are especially concentrated in green tea, accounting for 30–40% of its dry weight.46 During fermentation, most of the catechins are converted to oligomeric theaflavins and polymeric thearubigins in black tea.47
Figure 1.
Chemical structures of primary green tea constituents
Epidemiological studies suggest protective properties of green tea against the development of stomach,48 lung,49 pancreatic and colorectal,50 and breast cancer.51 Most studies ascribe the beneficial effects of green tea to the most abundant catechin in green tea, EGCG. EGCG exerts anticarcinogenic effects at multiple stages of the carcinogen-esis pathway. Noticeably, EGCG is a strong antioxidant and effective inhibitor of carcinogen activation. EGCG induces phase-II detoxifying enzymes, such as glutathione peroxidase and quinone reductase, cell cycle arrest and apoptosis. Moreover, the inhibitory effects of EGCG on inflammation and tumor promotion are well established.52 In particular, EGCG appears to target NF-κB as a key mechanism of action. EGCG exerts pro-apoptotic effects through inhibition of NF-κB in vitro by inducing kinase (NIK) and subsequent activation of the IKK/NIK signaling complex in human lung cancer cells.53 In JB6 mouse epidermal cells, EGCG decreased phosphorylated-IκBα; hence blocking the degradation of NF-κB inhibitor proteins,54 and increased the ratio of the pro-apoptotic protein Bax to antiapoptotic protein BcL-2.13 Importantly, the pro-apoptotic effects of EGCG appeared to specifically target tumor cells because while EGCG significantly decreased NF-κB activation in epidermoid carcinoma (A431) cells, normal human epidermal keratinocytes were unaffected.55 EGCG suppressed abnormal over-production of pro-inflammatory mediators in mouse macrophage cells (RAW2647)56 and in vivo, EGCG supplementation decreased downstream markers in the NF-κB pathway, such as oncogene cyclin D, COX-2 and prostaglandin E2 production and reduced N-nitrosomethylbenzylamine-induced esophageal tumors.22 One possible mechanism by which EGCG inhibits NF-κB may be the attenuation of p-ERK activation and subsequent mitigation of NF-κB and COX-2 in vitro.57
EGCG appears to attenuate NF-κB at different levels along the pathway in vitro. However, there are limited studies investigating the effects of green tea or green tea polyphenols on inflammation and prostate carcinogenesis in vivo. Most studies have focused on the effects of green tea on tumorigenesis. For example, one study showed that oral infusion of green tea polyphenols at human physiologically achievable concentrations inhibited prostate cancer development and distant-site metastasis in the TRAMP model.58 They also showed that GTP infusion decreased IGF-1/IGFBP-3 ratio, a predictor in prostate cancer progression.58–61 These findings suggested that a green tea polyphenol mixture inhibited growth signaling, progression and invasion of malignant tumors. Another study using male athymic nude mice inoculated with human prostate cancer cells lines showed that EGCG supplementation significantly attenuated growth of both androgen dependent and independent tumors.62,63 Nevertheless, these studies had focused on progression and metastatic potentials of established prostate tumors, not the processes contributing to the initiation and promotion of prostate cancer, such as chronic inflammation. The complexity of hormonal signaling and balance in the prostate further complicated the story and rendered it very difficult to pinpoint a specific target for intervention. For example, the effects of green tea on prostate hormone homeostasis, inflammation and NF-κB in vivo are still not clearly defined and are an important area for further research.
Soy and prostate cancer
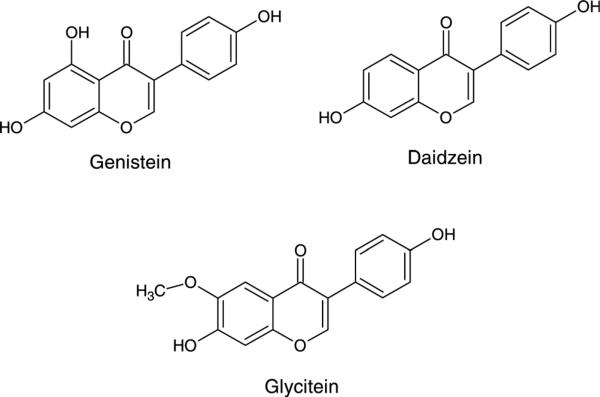
Soy foods have been the subject of considerable investigation since the 1960s, largely due to the potential health effects of soy isoflavones. Soybeans are the only food to contain nutritionally relevant amounts of isoflavones. Soy isoflavones are classified as phytoestrogens, and have been suggested to modulate endogenous hormone homeostasis because their phenolic ring structures resemble estradiol and can bind to estrogen receptors, acting as either an estrogen agonist or antagonist.56,57 The primary isoflavones in soybeans are genistein (4′5,7-trihydroxyisoflavone), daidzein (4′,7-dihydroxyisoflavone) and glycitein (4′,7-dihydroxy-6-methoxyisoflavone) (Figure 2), and their respective β-glycosides, genistin, daidzin and glycitin. Isoflavones are phytoalexins, substances formed by the host plant tissue in response to environmental stimuli and possess properties that enhance the survival of the soybean. Therefore, isoflavone concentrations vary depending on environmental conditions; normally isoflavone levels increase with increasing environmental stresses. Nevertheless, soybeans contain ~1.2–3.3 mg of isoflavones/g dry weight. Soy isoflavones exist predominantly as glycosides in soybeans, but fermentation and processing significantly alter the aglycone contents of soy products.32
Figure 2.
Chemical structures of primary soy isoflavones
Epidemiological studies suggest that consumption of soy foods is associated with lower risk of prostate cancer.64 The plasma and serum phytoestrogen concentrations in Japanese men are at least 10-fold higher than their Caucasian counterparts in the UK.36,65 The protective properties of soy against prostate cancer are not only apparent for the low-intake populations; even among Japanese men with relatively high soy intake compared with Caucasians, higher soy consumption was associated with a decrease in localized prostate cancer.66 Such observations led to hypotheses that soy or components in soy exerts anticancer effects, and most have attributed these effects to soy isoflavones, especially genistein.52 The anticarcinogenic effects of genistein are summarized in several reviews.67,68 Soy constituents modulate several steroidogenic enzymes important for hormone homeostasis, such as 5α-reductase,69 aromatase,36 17β-hydroxysteroid oxidoreductase35 and cytochrome P450.34 Furthermore, dietary genistein decreases AR expression,70,71 thus limiting the contribution of AR deregulation in prostate carcinogenesis. Daily consumption of soy grits decreases prostate specific antigen (PSA) levels and the free/total PSA ratio in prostate cancer patients,72 and total and free testosterone concentrations are also inversely correlated with soy intake in Japanese men.73 Soy isoflavones also act through hormone-independent pathways. Genistein is a known inhibitor of protein tyrosine kinase38 and topoisomerase II,39 and inhibits angiogenesis and cancer cell proliferation.74–77 In prostate cancer, soy isoflavones induce cell cycle arrest in prostate cancer cells.37 Soy constituents, especially isoflavones, appear to target different stages of the carcinogenesis pathway.
Previous studies have looked at anti-inflammatory effects of soy in different inflammatory diseases and organ systems. Soy protein and isoflavones have been suggested to act as antioxidants and exert beneficial effects on vascular reactivity.78 Genistein regulates immune function in mice by modulating humoral and cell-mediated immunity.79 Obese Zucker rats fed soy proteins enriched with isoflavones showed significant lower plasma TNF-α and IL-1β levels and protections against lipid oxidation in the liver.80 Soy decreased UVB-induced COX2 expression and skin damage in mice,81 and decreased NF-κB, nitric oxide and prostaglandin E2 pathways in neuronal and immune cells.82,83 In vitro, genistein inhibits constitutive NF-κB/Ap-1 activities in breast cancer cells84 and prostate cancer PC3 cells. Similar to EGCG, inhibitory effects are cancer cell-specific because non-tumorigenic prostate epithelial cells, CRL2221, are not affected by genistein.85,86 This ability to target cancerous cells is highly desirable for chemo-preventive agents. In vivo, Yatkin et al.87,88 found that dietary soy (7.61% extracted and toasted soy protein and 4.25% soy oil) decreased inflammatory foci in the prostate and marginally decreased development of obstructive voiding in Noble rats. In contrast to other established effects of soy, the anti-inflammatory effects of soy constituents in prostate carcinogenesis are relatively understudied.
Our lab has also utilized the Noble rat model to study the combined effects of dietary soy and green tea on inflammation and prostate cancer development. Eighty to 100% of Noble rats develop hormone-induced prostate intraepithelial neoplasia (PIN) after 36 weeks of testosterone and estradiol treatments, and PIN lesions in the Noble rats were useful intermediate endpoints in assessing efficacy of chemo-preventive agents.87 The prostates in the Noble rats are also characterized by inflammation prior to tumor development.89 The slow progression and distinctive stages of disease progression observed in this model mimics the human condition, and the model can be a useful tool for understanding the intricate relationships among chronic inflammation, hormonal microenvironment, prostate carcinogenesis and the efficacies of cancer-preventive agents in attenuating these processes. Using this model, we have found that dietary supplementation of both green tea and soy effectively inhibited NF-κB activation, decreased inflammation and decreased hyperplasia incidence in the prostate of hormone-treated Noble rats. The combination of soy and green tea suppressed NF-κB p50 binding activity and protein levels via induction of IκBα. Soy and green tea also decreased prostate inflammatory infiltration, increased Bax/BcL2 ratio and decreased protein expression of TNF-α, IL-6 and IL1-β compared with control. Soy and tea attenuated prostate malignancy by decreasing prostate hyperplasia. These effects were not apparent in groups treated with soy or tea alone (unpublished data). More in vivo studies are needed to establish their anti-inflammatory and antioxidative properties in the prostate, but both tea and soy appear to interfere with cellular signaling pathways that modulate inflammation and cell proliferation, deeming it a promising chemo-preventive agent against prostate cancer.
‘Whole food’ approaches for cancer prevention
More studies in recent years have started to take a ‘holistic’ instead of a ‘reductionist’ approach in searching for effective disease-preventive agents. Consuming a food instead of a supplement containing high levels of a specific bioactive component from that food may be more effective in preventing disease. This approach has become a more appealing and economic consideration for disease prevention. Different components of a whole food may work synergistically together, or form specific food matrix effects that provide greater benefits, which are omitted when using only a single component of whole food. In addition, high levels of a specific individual bioactive agent may also elicit toxicity.
Many in vitro studies with soy isoflavones and green tea polyphenols have used concentrations of soy isoflavones and green tea polyphenols to inhibit cell growth in prostate cancer cells that are beyond physiological levels and higher than attainable from diet (50–100 μmol/L soy isoflavones; ≥100 μmol/L EGCG).84,85,90–93 High levels of dietary genistein and daidzein had a stimulatory effect on MCF-7 breast cancer tumor growth in ovariectomized athymic mice.94,95 There is also emerging evidence that suggests that acute administration of high levels of soy isoflavones had no protective effects against other hormonal cancers such as breast cancer in postmenopausal women.96–98 Therefore, the timing and dosing of soy isoflavone supplementation may be a critical determining factor for its beneficial effects. Similarly, EGCG at high concentrations may also elicit adverse effects. High levels of EGCG were shown to produce hydrogen peroxide, and its pro-oxidant activity inhibited gap junction communication in rat liver cells, which was linked to carcinogenesis.99,100
On the contrary, lower dosages of a combination of bio-active compounds as a whole food may eliminate the possible toxicity and induce additional/synergistic effects among them. Previous studies have demonstrated that dietary lycopene, one of the bioactive components of tomatoes, is effective in reducing prostate cancer. Interestingly, a diet supplemented with whole tomato product (dried tomato powder) was more effective in reducing prostate cancer mortality than dietary supplementation with equivalent levels of lycopene alone.101 These results indicated that other compounds in tomatoes, such as folate, vitamin C, β-carotene or phenolic compounds, may enhance the beneficial effects of lycopene. In addition, combinations of soy isoflavones appear to be more effective in inhibiting prostate cancer cell growth than individual soy isoflavones, genistein or daidzein treatments alone.102 Results from our in vitro studies found that soy extract, containing soy isoflavones and other bioactive compounds, induced significantly higher percentage of cells undergoing apoptosis than genistein or daidzein alone at equal concentrations. Soy extract did not induce cell cycle arrest or apoptosis in non-cancerous BPH-1 cells, whereas both genistein and daidzein induced apoptosis.103 These findings further suggest that individual isoflavones may have cytotoxicity in non-cancerous cells, and supported the whole-food based approach to prostate cancer prevention.
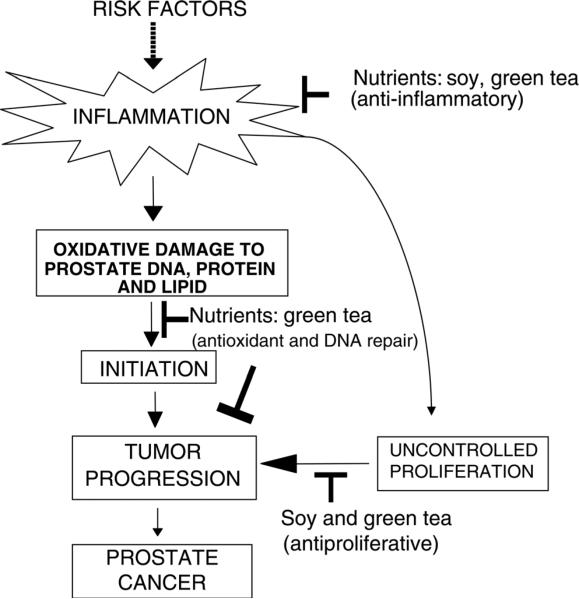
Whole foods contain multiple antioxidants and nutrients that may target various points of the carcinogenesis pathway. For example, soy contains several compounds besides isoflavones that have been suggested to have anticarcinogenic effects, such as saponins and protease inhibitors,104–106 and green tea contains vitamin C and other phytochemicals. These compounds may target different mechanisms that become deregulated during the development of cancer, such that some nutrients act as antioxidants while others target cell proliferation or angiogenesis (Figure 3). Furthermore, combination of different foods can provide novel chemo-preventive strategies. Zhou et al.107 showed that combination of soy phytochemical concentrates and green tea reduced angiogenesis, IGF-1 and estrogen-dependent breast and prostate tumorigenicity in mice.108 Using the Noble rat model, we demonstrated that dietary soy and green tea in combination decreased prostate inflammation and precancerous lesions via attenuation of NF-κB and downstream apoptotic pathways in vivo.109 Soy or green tea alone did not demonstrate similar effects, suggesting that the interactions between soy and green tea provided additional benefits against prostate cancer. This study suggests that interactions among several foods may potentiate the activities of any single supplement. Furthermore, the most effective strategy for the prevention of prostate cancer may involve enhancing the entire antioxidant/anti-inflammation/antiproliferative network. Whole foods are more efficacious than single nutrient supplements because they contain an array of nutrients and phytochemicals that can target a wide range of biological functions and cellular pathways that are deregulated during cancer development. Furthermore, little is known about the metabolism of these phytochemicals and how they influence each other in vivo. It is possible that the nutrient bioavailability and metabolism interactions between phytochemicals within the food matrix are important for prostate cancer prevention. The precise mechanisms by which combining foods provide additional beneficial effects still need to be elucidated. In addition to targeting multiple pathways, combination of foods may provide additional beneficial effects by improving bioavailability of active compounds. Very few studies attempt to undertake the daunting task of understanding synergism and interactions among nutrients and phytochemicals, but studies focusing on these subjects will be crucial for establishing dietary recommendations in the future.
Figure 3.
Schematic of the whole-food approach with soy and green tea
Conclusion
Diet and nutrition contribute to prevention of most chronic diseases and are a major focus of prostate cancer research. In the USA, prostate cancer is the most prevalent non-cutaneous cancer in men and one of the leading causes of cancer-related deaths. In contrast, men from Asian countries have a 10–15-fold lower rate of prostate cancer than US men. Migration studies further emphasize the impact of diet and lifestyle. Moreover, there is a gradual increase of prostate cancer rate in major industrialized cities in China, reflecting that greater extent of westernization and adaptation of western diet is associated with increased prostate cancer risk.4 These observations have prompted studies in recent years to investigate and compare different dietary components in the Asian diet that may have cancer preventive properties such as soy and green tea. Studies support using soy and green tea as possible chemo-preventive strategies against prostate inflammation and carcinogenesis. Apoptosis and inflammatory pathways, such as the NF-κB pathway, are key pathways of regulation by soy and green tea in attenuation of prostate carcinogenesis. More studies focusing on in vivo food matrix or food combinations are required for public dietary recommendations, but increasing both soy and green tea consumption may have the potential to help reduce prostate cancer incidence and health-care cost in the USA.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health (NIH) grant CA107693, Oregon Agriculture Experiment Station (OR00735) and the Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210).
Footnotes
Author contributions: All authors (AH, TMB and EH) participated in the design, writing and editing of the manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA: A Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161:152–5. [PubMed] [Google Scholar]
- 3.Muir CS, Nectoux J, Staszewski J. The epidemiology of prostatic cancer. Geographical distribution and time-trends. Acta Oncol (Stockholm, Sweden) 1991;30:133–40. doi: 10.3109/02841869109092336. [DOI] [PubMed] [Google Scholar]
- 4.Sim HG, Cheng CW. Changing demography of prostate cancer in Asia. Eur J Cancer. 2005;41:834–45. doi: 10.1016/j.ejca.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Ames BN, Wakimoto P. Are vitamin and mineral deficiencies a major cancer risk? Nat Rev. 2002;2:694–704. doi: 10.1038/nrc886. [DOI] [PubMed] [Google Scholar]
- 6.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, Nelson WG. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–81. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 7.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–16. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, Mizokami A. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–8. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–96. [PubMed] [Google Scholar]
- 11.Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10:7727–37. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- 12.Narayanan BA, Narayanan NK, Pttman B, Reddy BS. Adenocarcina of the mouse prostate growth inhibition by celecoxib: downregulation of transcription factors involved in COX-2 inhibition. Prostate. 2006;66:257–65. doi: 10.1002/pros.20331. [DOI] [PubMed] [Google Scholar]
- 13.Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001;159:387–97. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–6. [PubMed] [Google Scholar]
- 15.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. [PubMed] [Google Scholar]
- 17.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 20.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–17. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 21.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li ZG, Shimada Y, Sato F, Maeda M, Itami A, Kaganoi J, Komoto I, Kawabe A, Imamura M. Inhibitory effects of epigallocatechin-3-gallate on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in F344 rats. Int J Oncol. 2002;21:1275–83. [PubMed] [Google Scholar]
- 23.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–8. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 24.Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–15. [PubMed] [Google Scholar]
- 25.Suh J, Payvandi F, Edelstein LC, Amenta PS, Zong WX, Gelinas C, Rabson AB. Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate. 2002;52:183–200. doi: 10.1002/pros.10082. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 27.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol Med. 2000;28:1317–27. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 28.Kwon O, Kim KA, Kim SO, Ha R, Oh WK, Kim MS, Kim HS, Kim GD, Kim JW, Jung M, Kim CH, Ahn JS, Kim BY. NF-kappaB inhibition increases chemosensitivity to trichostatin A-induced cell death of Ki-Ras-transformed human prostate epithelial cells. Carcinogenesis. 2006;27:2258–68. doi: 10.1093/carcin/bgl097. [DOI] [PubMed] [Google Scholar]
- 29.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 31.Dixon RA. Phytoestrogens. Annu Rev Plant Biol. 2004;55:225–61. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- 32.Gilani GS, Anderson JB. Phytoestrogens and Health. AOCS Press; Champaign, IL: 2002. p. 660. [Google Scholar]
- 33.Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71:1705S–7S. doi: 10.1093/ajcn/71.6.1705S. discussion 1708S–9S. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Wang HW, Su HY, Hao NJ. The structure–activity relationships of flavonoids as inhibitors of cytochrome P-450 enzymes in rat liver microsomes and the mutagenicity of 2-amino-3-methyl-imidazo[4,5-f]quinoline. Mutagenesis. 1994;9:101–6. doi: 10.1093/mutage/9.2.101. [DOI] [PubMed] [Google Scholar]
- 35.Makela S, Poutanen M, Kostian ML, Lehtimaki N, Strauss L, Santti R, Vihko R. Inhibition of 17beta-hydroxysteroid oxidoreductase by flavonoids in breast and prostate cancer cells. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY) 1998;217:310–6. doi: 10.3181/00379727-217-44237. [DOI] [PubMed] [Google Scholar]
- 36.Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT, Jr, Vickery LE. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;44:147–53. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- 37.Handayani R, Rice L, Cui Y, Medrano TA, Samedi VG, Baker HV, Szabo NJ, Shiverick KT. Soy isoflavones alter expression of genes associated with cancer progression, including interleukin-8, in androgen-independent PC-3 human prostate cancer cells. J Nutr. 2006;136:75–82. doi: 10.1093/jn/136.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 39.Schmidt F, Knobbe CB, Frank B, Wolburg H, Weller M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep. 2008;19:1061–6. [PubMed] [Google Scholar]
- 40.Choi YH, Lee WH, Park KY, Zhang L. p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin B1 and G2/M arrest by the isoflavone genistein in human prostate carcinoma cells. Jpn J Cancer Res. 2000;91:164–73. doi: 10.1111/j.1349-7006.2000.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lian F, Li Y, Bhuiyan M, Sarkar FH. p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer. 1999;33:125–31. doi: 10.1207/S15327914NC330202. [DOI] [PubMed] [Google Scholar]
- 42.Shao ZM, Alpaugh ML, Fontana JA, Barsky SH. Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis. J Cell Biochem. 1998;69:44–54. [PubMed] [Google Scholar]
- 43.Na HK, Surh YJ. Intracellular signaling network as a prime chemopreventive target of (−)-epigallocatechin gallate. Mol Nutr Food Res. 2006;50:152–9. doi: 10.1002/mnfr.200500154. [DOI] [PubMed] [Google Scholar]
- 44.Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, Cai SR, Ma JP, Zhang CH. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–9. doi: 10.3748/wjg.v13.i8.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart EC, Scandlyn MJ, Rosengren RJ. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006;79:2329–36. doi: 10.1016/j.lfs.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 46.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 47.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 48.Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Kuroishi T, Tominaga S. Tea and coffee consumption and the risk of digestive tract cancers: data from a comparative case-referent study in Japan. Cancer Causes Control. 1998;9:209–16. doi: 10.1023/a:1008890529261. [DOI] [PubMed] [Google Scholar]
- 49.Fujiki H, Suganuma M, Okabe S, Sueoka N, Komori A, Sueoka E, Kozu T, Tada Y, Suga K, Imai K, Nakachi K. Cancer inhibition by green tea. MutatRes. 1998;402:307–10. doi: 10.1016/s0027-5107(97)00310-2. [DOI] [PubMed] [Google Scholar]
- 50.Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, Blot WJ, Fraumeni JF., Jr Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997;70:255–8. doi: 10.1002/(sici)1097-0215(19970127)70:3<255::aid-ijc1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 51.Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–61. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Okabe S, Fujimoto N, Sueoka N, Suganuma M, Fujiki H. Modulation of gene expression by (−)-epigallocatechin gallate in PC-9 cells using a cDNA expression array. Biol Pharmaceut Bull. 2001;24:883–6. doi: 10.1248/bpb.24.883. [DOI] [PubMed] [Google Scholar]
- 54.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (−)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–90. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000;376:338–46. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 56.Park JW, Choi YJ, Suh SI, Kwon TK. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7 cells. Biochem Biophys Res Commun. 2001;286:721–5. doi: 10.1006/bbrc.2001.5415. [DOI] [PubMed] [Google Scholar]
- 57.Kundu JK, Na HK, Chun KS, Kim YK, Lee SJ, Lee SS, Lee OS, Sim YC, Surh YJ. Inhibition of phorbol ester-induced COX-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J Nutr. 2003;133:3805S–10S. doi: 10.1093/jn/133.11.3805S. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science (New York, NY) 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 60.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Nat Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 61.Zumkeller W. IGFs and IGFBPs: surrogate markers for diagnosis and surveillance of tumour growth? Mol Pathol. 2001;54:285–8. doi: 10.1136/mp.54.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96:239–43. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- 63.Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chemico-Biol Interactions. 2008;171:89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int J Cancer. 2005;117:667–9. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- 65.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–71. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 66.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16:538–45. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 67.Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91:513–31. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 68.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–57. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 69.Evans BA, Griffiths K, Morton MS. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J Endocrinol. 1995;147:295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- 70.Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol Cell Endocrinol. 2002;186:89–99. doi: 10.1016/s0303-7207(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton-Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Isoflavone-rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor-beta expression or serum hormonal profiles in men at high risk of prostate cancer. J Nutr. 2007;137:1769–75. doi: 10.1093/jn/137.7.1769. [DOI] [PubMed] [Google Scholar]
- 72.Dalais FS, Meliala A, Wattanapenpaiboon N, Frydenberg M, Suter DA, Thomson WK, Wahlqvist ML. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64:510–5. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Nagata C, Inaba S, Kawakami N, Kakizoe T, Shimizu H. Inverse association of soy product intake with serum androgen and estrogen concentrations in Japanese men. Nutr Cancer. 2000;36:14–8. doi: 10.1207/S15327914NC3601_3. [DOI] [PubMed] [Google Scholar]
- 74.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–4. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–7S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- 76.Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–31. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- 77.Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Matsumoto K, Nishino H, Aoike A. Genistein arrests cell cycle progression at G2–M. Cancer Res. 1993;53:1328–31. [PubMed] [Google Scholar]
- 78.Siow RC, Li FY, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radical Biol Med. 2007;42:909–25. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Cooke PS, Selvaraj V, Yellayi S. Genistein, estrogen receptors, and the acquired immune response. J Nutr. 2006;136:704–8. doi: 10.1093/jn/136.3.704. [DOI] [PubMed] [Google Scholar]
- 80.Gudbrandsen OA, Wergedahl H, Berge RK. A casein diet added isoflavone-enriched soy protein favorably affects biomarkers of steatohepatitis in obese Zucker rats. Nutrition (Burbank, Los Angeles County, CA) 2009;25:574–80. doi: 10.1016/j.nut.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Chen N, Scarpa R, Zhang L, Seiberg M, Lin CB. Nondenatured soy extracts reduce UVB-induced skin damage via multiple mechanisms. Photochem Photobiol. 2008;84:1551–9. doi: 10.1111/j.1751-1097.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 82.Valsecchi AE, Franchi S, Panerai AE, Sacerdote P, Trovato AE, Colleoni M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: anti-inflammatory and antioxidant activity. J Neurochem. 2008;107:230–40. doi: 10.1111/j.1471-4159.2008.05614.x. [DOI] [PubMed] [Google Scholar]
- 83.Dia VP, Berhow MA, Gonzalez De Mejia E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56:11707–17. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- 84.Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol. 2004;25:1389–95. [PubMed] [Google Scholar]
- 85.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35:167–74. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–77. [PubMed] [Google Scholar]
- 87.Christov KT, Moon RC, Lantvit DD, Boone CW, Steele VE, Lubet RA, Kelloff GJ, Pezzuto JM. 9-cis-retinoic acid but not 4-(hydroxyphenyl) retinamide inhibits prostate intraepithelial neoplasia in Noble rats. Cancer Res. 2002;62:5178–82. [PubMed] [Google Scholar]
- 88.Yatkin E, Streng T, Kauppila ML, Bernoulli J, Saarinen N, Santti R. The soy effect in the disease models of nonbacterial prostatitis and obstructive voiding. Exp Biol Med (Maywood) 2007;232:674–81. [PubMed] [Google Scholar]
- 89.Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Nat Cancer Inst. 1988;80:1045–53. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- 90.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–9. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 91.Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 92.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–22. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 93.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–67. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 94.Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–63. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 95.Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008;29:2162–8. doi: 10.1093/carcin/bgn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–57. doi: 10.1093/carcin/bgh178. [DOI] [PubMed] [Google Scholar]
- 97.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:3095S–108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 98.Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–7. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 99.Kang NJ, Lee KM, Kim JH, Lee BK, Kwon JY, Lee KW, Lee HJ. Inhibition of gap junctional intercellular communication by the green tea polyphenol (−)-epigallocatechin gallate in normal rat liver epithelial cells. J Agric Food Chem. 2008;56:10422–7. doi: 10.1021/jf801981w. [DOI] [PubMed] [Google Scholar]
- 100.Trosko JE, Chang CC. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemotherapy of cancer. Mutat Res. 2001;480–481:219–29. doi: 10.1016/s0027-5107(01)00181-6. [DOI] [PubMed] [Google Scholar]
- 101.Campbell JK, Canene-Adams K, Lindshield BL, Boileau TW, Clinton SK, Erdman JW., Jr Tomato phytochemicals and prostate cancer risk. J Nutr. 2004;134:3486S–92S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- 102.Hedlund TE, van Bokhoven A, Johannes WU, Nordeen SK, Ogden LG. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2006;66:557–66. doi: 10.1002/pros.20380. [DOI] [PubMed] [Google Scholar]
- 103.Hsu A, Bray TM, Ho E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp Biol Med. 2010;235:90–97. doi: 10.1258/ebm.2009.009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fournier DB, Erdman JW, Jr, Gordon GB. Soy, its components, and cancer prevention: a review of the in vitro, animal, and human data. Cancer Epidemiol Biomarkers Prev. 1998;7:1055–65. [PubMed] [Google Scholar]
- 105.Kerwin SM. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr Med Chem. 2004;4:263–72. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 106.McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer. 2007;57:184–93. doi: 10.1080/01635580701277478. [DOI] [PubMed] [Google Scholar]
- 107.Zhou JR, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516–21. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu A, Bruno RS, Lohe CV, Dashwood RH, Bray TM, Ho E. Effects of soy and tea on hormone-induced prostate cancer in Noble rat model. J Nutr Biochem. 2010 doi: 10.1016/j.jnutbio.2010.04.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]