Abstract
The combination of negatively-charged membranes and angiotensin I-converting enzyme inhibitors (ACEi) evokes hypersensitivity reactions (HSR) during hemodialysis and bradykinin (BK)-related peptides have been hypothesized as being responsible for these complications. In this study, we tested the effects of neutralizing the membrane electronegativity (zeta potential) of polyacrylonitrile AN69 membranes by coating a polyethyleneimine layer (AN69-ST membranes) over the generation of kinins induced by blood contact with synthetic membranes. We used minidialyzers with AN69 or AN69-ST membranes in an ex vivo model of plasma and we showed that plasma dialysis with AN69 membranes led to significant BK and des-Arg9-BK release, which was potentiated by ACEi. This kinin formation was dramatically decreased by AN69-ST membranes, even in the presence of an ACEi, and kinin recovery in the dialysates was also significantly lower with these membranes. High molecular weight kininogen and factor XII detection by immunoblotting of the protein layer coating both membranes corroborated the results: binding of these proteins and contact system activation on AN69-ST membranes were reduced. This ex vivo experimental model applied to the plasma, dialysate and dialysis membrane could be used for the characterization of the kinin-forming capacity of any biomaterial potentially used in vivo in combination with drugs which modulate the pharmacological activity of kinins.
Keywords: Haemodialysis membrane, Biocompatibility, Electroactive polymer, Hypersensitivity
1. Introduction
Although rare in the 1980s, the incidence of hypersensitivity reactions (HSR) in hemodialysis increased significantly during the 1990s due to the widespread use of angiotensin I-converting enzyme inhibitors (ACEi). HSR were encountered more frequently in hemodialyzed patients with electro-negatively-charged polyacrylonitrile membranes [1]. The pathogenesis of these reactions was not well elucidated until the demonstration of triggering factors, such as an acidic milieu and the generation of kinins by blood contact with synthetic membranes. Other postulates, such as backfiltration of endotoxin-contaminated dialysates [2] and hypersensitivity to ethylene oxide [3] or isocyanate [4], were ruled out. The pivotal role of bradykinin (BK) was suggested and convincingly demonstrated: BK accumulates in the blood of dialyzed patients as the consequence of contact activation of the intrinsic coagulation pathway during the first minutes of hemodialysis [5–7].
BK is a nonapeptide released from high molecular weight kininogen (HK) by plasma kallikrein during contact system activation in plasma. BK exerts its pharmacological activity by stimulating B2 receptors before being metabolized by different peptidases, named kininases. BK is mainly metabolized by 3 Zn2+ metallopeptidases in human plasma. Angiotensin I-converting enzyme (ACE, kininase II) is the main degrading pathway, accounting for 75% of plasma kininase activity. Aminopeptidase P (APP) is the second inactivating pathway of BK, although carboxypeptidase N transforms BK into an active metabolite des-Arg9-BK which stimulates B1 receptors before to be inactivated by APP and ACE [8].
We have hypothesized that these HSR are of a multifactorial nature. Previously we have studied the importance of the pharmacologic, metabolic and genetic factors in HSR occurrence [9,10]. However, and until now, we did not define the biomaterial role in these pathophysiological complications.
In this study, taking an ex vivo experimental approach, we define the effect of dialysis membrane zeta potential on kinetic profile of kinin (BK and des-Arg9-BK) generation and accumulation in dialyzed plasma. We show that kinin accumulation is potentiated by ACEi, blocking the main BK-degrading pathway. For this reason, human plasmas were dialyzed through minidialysers equipped either with electronegative AN69 or with the AN69-ST membranes whose surface was rendered neutral by coating with a layer of polyethyleneimine. Our investigations at the plasma level were completed by kinin quantification in the dialysates and the characterization of contact system proteins (factor XII and HK) in the protein layer (“protein cake”) coating the synthetic membranes.
2. Materials and methods
2.1. Reagents
The ACEi enalaprilat was obtained from Merck Frosst Canada (Kirkland, QC, Canada). NaCl and sodium dodecyl sulfate (SDS) were from Bioshop Canada Inc. (Burlington, ON, Canada). Anti-HK antibodies were produced by our lab [11]; anti-factor XII and anti-albumin were purchased from Abcam Inc. (Cambridge, MA, USA). Reagents of analytical grade were procured from Fisher Scientific (St-Laurent, QC, Canada) unless specified otherwise.
2.2. Plasma collection
The study was conducted according to French regulations and was approved by the Université de Montréal Ethics Committee. Written informed consent was obtained from all participants. One hundred mL of total venous blood were collected into propylene tubes containing 0.1 mol/L sodium citrate as anticoagulant (1 volume of sodium citrate to 9 volumes of blood) from 18 dialyzed patients (9 men and 9 women) between January 2005 and September 2006. After centrifugation (22 °C, 15 min, 2500g), the plasma was decanted and stored at −80 °C until ex vivo experiments. We have shown previously that this blood processing avoids the artefactual in vitro activation of plasma contact system [8].
The plasma samples were screened for APP and ACE activities according to methods described previously [12]. The results are expressed in units/mL (μmol of substrate hydrolyzed/mL/min).
2.3. Dialysers
Hollow-fibre AN69 and AN69-ST minimodule dialysers were synthesized by Gambro-Hospal France on a 1/10 scale. These dialysers, of a similar industrial design as that used for dialyzed patients, were built as small-scale models of existing clinical dialysis systems to enable membrane type evaluation. The number of fibres (n = 256) corresponded to an effective surface area of 405 cm2. In their final configuration (hollow-fibre), this material could be tested under hemodynamic conditions similar to those employed in clinical practice. For the ST type, the membrane’s inner surface was treated with high molecular weight polyethyleneimine according to the process stipulated for Nephral ST dialysers. Minimodules are sterilized by gamma radiation before deployment [13].
2.4. Ex vivo plasma circulation
Before use, the internal surfaces of AN69 and AN69-ST minidialysers were first rinsed with 50 mL of NaCl 9 g/L at a flow rate of 1 mL/min.
Citrated plasmas preincubated with enalaprilat at a concentration which totally inhibits ACE activity (final concentration: 130 nM) were dialyzed at 37 °C through AN69 and AN69-ST membranes, respectively. Plasma was pumped at a flow rate of 1.0 mL/min through the dialyser before being collected in 0.5 mL fraction of dialyzed plasma each 0.5 min for a dialysis period of 20 min. Dialyzed plasma fractions were collected in propylene tubes in 2.0 mL ethanol. After incubation at 4 °C and centrifugation, the ethanolic extracts were evaporated to dryness in a Speed Vac system (SC 210A, Savant Instruments, Inc., Holbrook, NY, USA) before immunoreactive kinin quantification.
The same citrated, preincubated or not with enalaprilat, plasma samples were dialyzed through AN69 membranes only under the conditions described above.
Plasma effluents (n = 3) corresponding to the fraction starting at 8 and 20 min of dialysis with AN69 membranes in the presence of an ACEi as described above. The contact system of each plasma sample was then activated in vitro by glass beads [8]. Activated plasmas aliquots were tested at different time intervals (0–60 min) and were constrained to ethanolic extraction, as described above before BK quantification. BK concentrations released during glass-bead activation were measured to design the metabolic profile and to calculate the AUC of BK released during the 60-min activation period [8]. This AUC corresponded to the residual kinin-forming capacity of HK, which was not activated during passage through negatively-charged AN69 membranes [14].
2.5. Assessment of kinin
A pool of plasma (n = 3) preincubated with enalaprilat was dialyzed with AN69 and AN69-ST membranes as described above. Dialysis was stopped at 3 different times: 6, 8 and 20 min after the beginning of ex vivo dialysis.
At these 3 times, both membranes dialysates were treated with ethanol, as described above, to investigate the plasma effluent before the quantification of immunoreactive kinins in triplicate.
2.6. Assessment of contact system activation
The internal compartment of each minidialyser was filled with SDS 2% (3 mL) and incubated for 2 h at room temperature with agitation. The eluted proteins were collected, quantified and characterized by immunoblotting.
2.7. Quantitation of BK and des-Arg9-BK
Residues of evaporated ethanolic extracts were resuspended in 50 mM Tris/HCl buffer, pH 7.4, containing 100 mM NaCl and 0.05% Tween-20. After resuspension, BK and des-Arg9-BK were quantified by 2 specific competitive enzyme immunoassays, as described previously [15,16]. These methods have been validated and their analytical performances were reported.
Residues of evaporated ethanolic extracts containing immunoreactive BK and des-Arg9-BK were dissolved in 200 μl of 5 mM KH2PO4, pH 3.0, and 25% acetonitrile with 1.0% H3PO4. HPLC separation was undertaken in an Agilent 1100 Series system (Agilent Technologies Canada, Mississauga, ON, Canada) with a 2-sulfoethyl aspartamide column (PolySULFOETHYL A™, The Nest Group, Inc., Southborough, MA, USA), using a linear gradient of KCl (0–300 mM) in 5 mM KH2PO4, and 25% acetonitrile (v/v), pH 3.0, for 30 min. Samples were collected at a flow rate of 1 mL/min. Different eluted 1 mL fractions were evaporated before the quantification of immunoreactive BK and des-Arg9-BK [14]. Retention time of each immunoreactive fraction was compared to this of standard peptides.
2.8. Total proteins
Total protein in the dialysates and protein cakes was quantified by Bicinchoninic Acid Protein Assay Kit, as suggested in the manufacturer’s protocol (Pierce, Rockford, IL, USA).
2.9. Immunoblotting
Protein cakes dissolved in 2% SDS were concentrated 10 times in Microcon centrifugal filter devices (YM 50, Millipore Corporation, Bedford, MA, USA). Samples were resolved by SDS-PAGE (10% polyacrylamide gel electrophoresis) before transfer on nitrocellulose membranes (Bio-Rad, Mississauga, ON, Canada), which were incubated overnight with primary antibodies raised against HK (1/10,000), factor XII (1/1000) and albumin (1/1000), followed by 1-h incubation with appropriate horseradish peroxydase-conjugated antibody (Bio-Rad). Protein was quantified with Chemi Glow West Kits (Alpha Innotech Corporation, San Leandro, CA, USA) according to the manufacturer’s instructions.
2.10. Statistical analysis
For ex vivo dialysis, the quantity of BK and des-Arg9-BK released was studied using the area under the kinetic profiles in different experimental conditions. The AUC for BK and des-Arg9-BK was calculated by summing the area of the different quadrilaterals obtained by joining kinin concentrations measured at consecutive times. AUC means were compared between the 3-dialysis conditions (AN69 membrane alone, AN69 and AN69-ST membranes in the presence of an ACEi) by 2-way analysis of variance.
To evaluate the residual kinin-forming capacity of dialyzed plasma, the quantity of BK and des-Arg9-BK released during activation by glass beads in vitro was calculated by a similar approach.
3. Results
ACE and APP activities, measured in 18 dialyzed patient plasmas, were, respectively, equal to 49 ± 16 and 188 ± 172 units/mL.
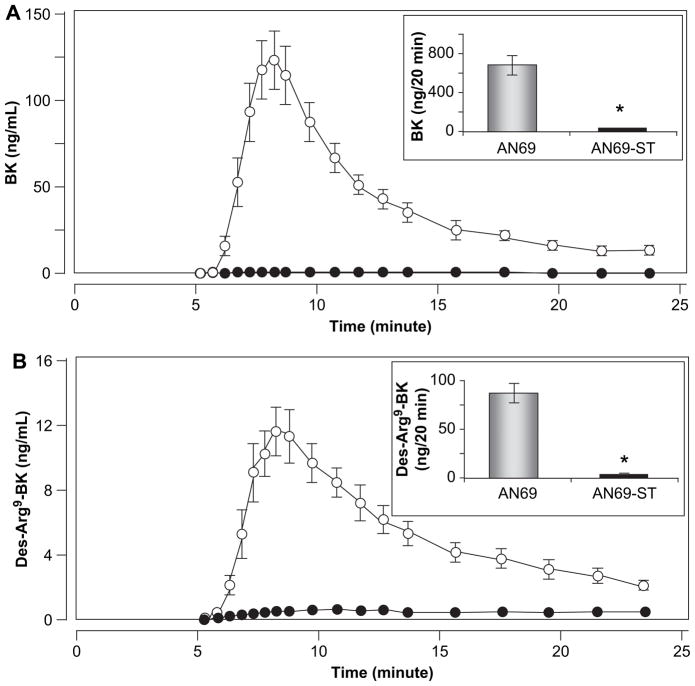
Fig. 1 depicts the kinetic profile of BK and des-Arg9-BK released from dialyzed plasma (n = 18) in the presence of an ACEi, through AN69 and AN69-ST membranes. Peak concentration was observed for BK (mean value: 123.0 ± 65.4 ng/mL) and des-Arg9-BK (mean value: 11.6 ± 5.8 ng/mL) after 8 min of dialysis through AN69 membranes, whereas contact with AN69-ST membranes did not increase kinin generation, which was maintained at the background level.
Fig. 1.
Mean kinetic profiles (n = 18) of BK (A) and des-Arg9-BK (B) obtained when human plasma was dialyzed through AN69 (○) and AN69-ST (●) membranes in the presence of an ACEi. In each case, the upper right panel shows representative AUCs corresponding to both elution profiles. Error bars correspond to standard deviation of the mean difference; if values are small, bars are missing. *p < 0.001.
Mean values of the area under the curves (AUC), which represent the total amounts of BK (699 ± 363.9 ng) and des-Arg9-BK (86.3 ± 50.7 ng) measured during the first 20 min of dialysis for AN69 membranes, were significantly higher than those observed for AN69-ST membranes (respectively, 7.5 ± 6.1 ng and 6.4 ± 3.5 ng/20 min, p < 0.001).
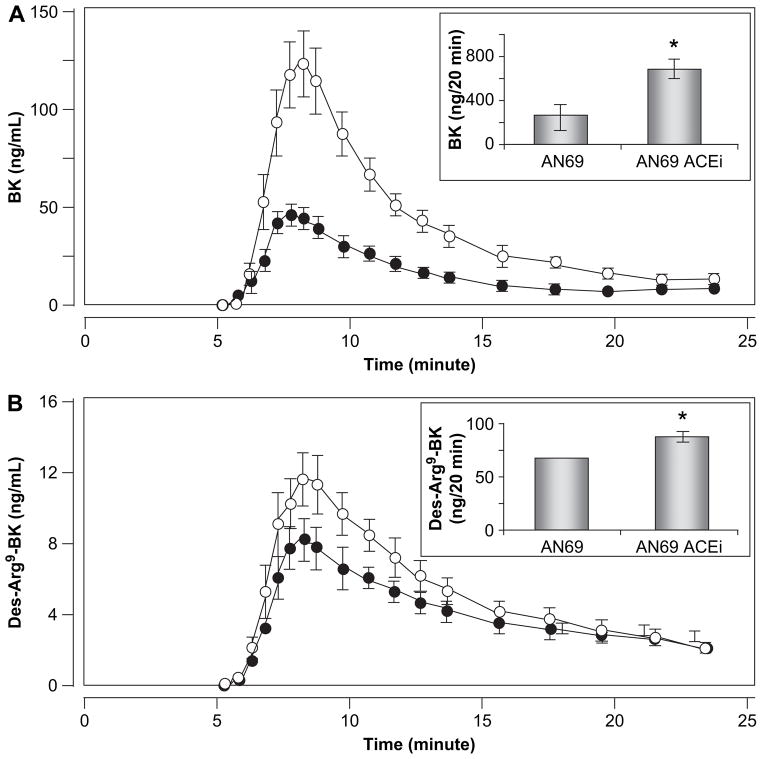
As illustrated in Fig. 2, dialysis runs of the same 18 plasma samples through AN69 membranes alone led to a significant release of BK (AUC: 269.5 ± 182.3 ng/20 min) with peak concentration at 8 min. However, preincubation of plasma with an ACEi had a marked potentiating effect on BK accumulation during the study period (AUC: 699.0 ± 363.9 ng/ 20 min; p < 0.001), without any peak time modifications. ACE inhibition had a similar effect on the kinetic profiles of des-Arg9-BK. In this case, preincubation of plasmas with enalaprilat increased des-Arg9-BK accumulation from 65.7 ± 40.9 ng/20 min to 86.3 ± 50.7 ng/20 min ( p < 0.001).
Fig. 2.
Mean kinetic profile (n = 18) of BK (A) and des-Arg9-BK (B) obtained when human plasma was dialyzed through AN69 in the absence (●) or presence (○) of an ACEi. In each case, the upper right panel shows representative AUCs corresponding to both elution profiles. Error bars correspond to standard deviation of the mean difference; if values are small, bars are missing. *p < 0.001.
The amounts of BK released by glass beads during in vitro activation of plasma effluents, collected at 8 and 20 min of ex vivo dialysis. The mean AUC, which represented the residual kinin-forming capacity (4551.4 ± 1239.7 ng/60 min) of 6 effluents collected at 20 min of ex vivo dialysis, was significantly higher than that obtained for effluents collected at 8 min (2246.0 ± 980.8 ng/60 min, p < 0.001).
Table 1 gives the dialysate concentrations (ng/mL) of immunoreactive BK and des-Arg9-BK resulting from dialysis sessions with both kinds of membranes. As with the kinetic profiles in plasma, concentrations of immunoreactive kinins in AN69-ST dialysates were significantly lower than in AN69 dialysates throughout the dialysis sessions. For AN69 dialysates, BK and des-Arg9-BK at 8 min, respectively, equalled 2.0 and 7.8% of peak concentrations in plasma.
Table 1.
Kinin (BK and des-Arg9-BK) concentrations (ng/mL) in AN69 and AN69-ST dialysates collected after 6, 8 and 20 min of plasma dialysis
| AN69-ST
|
AN69
|
|||
|---|---|---|---|---|
| BK (ng/mL) | Des-Arg9-BK (ng/mL) | BK (ng/mL) | Des-Arg9-BK (ng/mL) | |
| 6 min | 0.01 | 0.04 | 0.04 | 0.03 |
| 8 min | 0.02 | 0.06 | 0.50 | 0.32 |
| 20 min | 0.05 | 0.07 | 0.74 | 0.14 |
Fig. 3 presents the comparative immunoreactive patterns of native HK and factor XII detected in the protein layer coating both membranes, at 6, 8 and 20 min of dialysis. HK and factor XII bindings were lower on AN69-ST membranes than on AN69 membranes. Activated factor XII, as measured by immunoreactive factor XII heavy chain, could also be detected throughout the dialysis period, for both kinds of membranes. However, the strongest activation of factor XII was observed for AN69 membranes.
Fig. 3.
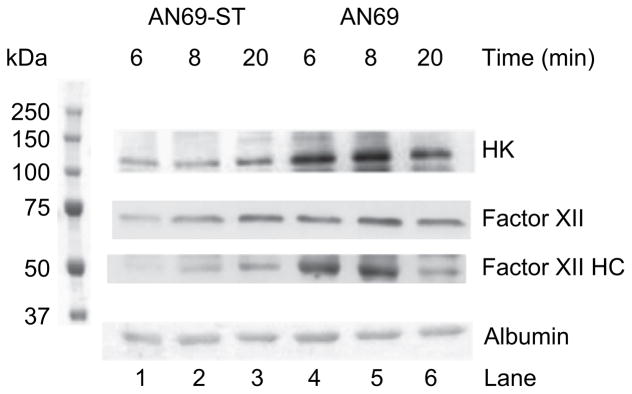
Qualitative analysis with immunoblots of HK, factor XII and albumin on 35 μg of protein cakes obtained after plasma dialysis on AN69-ST and AN69 membranes for 6, 8 or 20 min. Lanes 1, 2 and 3 correspond to the protein cake eluted respectively after 6, 8 and 20 min of plasma dialysis with the AN69-ST membrane. Lanes 4, 5 and 6 correspond to the same times for the AN69 membrane.
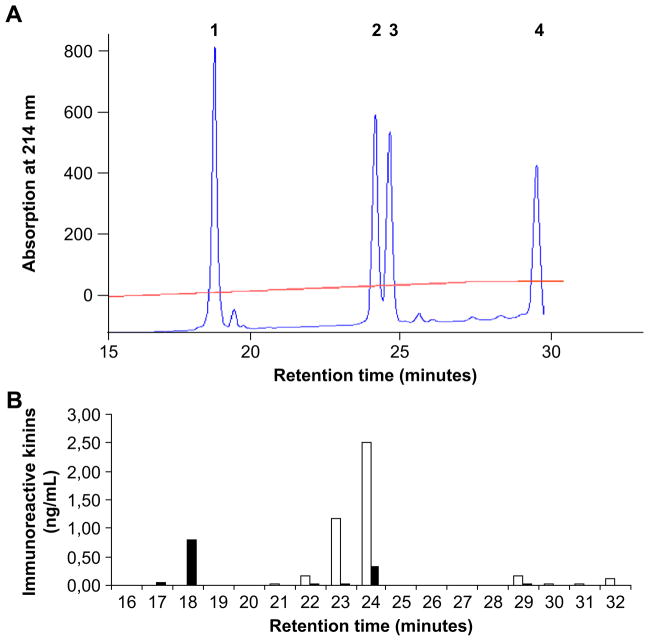
Fig. 4A charts the elution profiles of standard kinins after HPLC according to their retention times: des-Arg9-BK = 18.6 min; BK = 24.1 min; Lys-des-Arg9-BK = 24.7 min; and Lys-BK = 29.8 min. Fig. 4B illustrates the immunoreactivity profiles after HPLC separation of kinins isolated from dialyzed plasma collected at peak concentration (8 min). Only immunoreactivity corresponding to the elution time of des-Arg9-BK (18.6 min) and BK (24.1 min) could be detected. No significant immunoreactivity could be found for elution times corresponding to Lys-BK and Lys-des-Arg10-BK.
Fig. 4.
Typical elution profiles of immunoreactive kinins after HPLC. (A) UV absorbance (wave length 314 nm) of standard peptides: #1, des-Arg9-BK (retention time: 18.6 min); #2, BK (retention time: 24.1 min); #3, Lys-des-Arg9-BK (retention time: 24.7 min) and #4, Lys-BK (retention time: 29.8 min). (B) Immunogram of immunoreactive kinins in plasma after dialysis through an AN69 membrane in the presence of an ACEi (■ des-Arg9-BK; □ BK).
4. Discussion
Using an ex vivo experimental model, we showed, for the first time, that polyethyleneimine-coated AN69 membranes, i.e. AN69-ST membranes, suppressed the kinetic release of BK and its active metabolite des-Arg9-BK from plasma substrates, even in the presence of total ACE inhibition during a 20 min ex vivo dialysis, a time period compatible with HSR episodes occurrence in hemodialyzed patients [10]. This result contrasted with the opposite effect induced by basic polyacrylonitrile AN69 membranes which, in the same experimental conditions, stimulated kinin release and accumulation. This study completes former investigations which have reported but not characterized the influence of membrane zeta potential on contact system activation [17,18]. However, until now none has used an ex vivo approach which mimics what could happen in vivo during the HSR episode. Moreover, the present study is the first to investigate at the plasma, dialysate and dialysis membrane level the influence of zeta potential on contact system activation, using a multianalytical approach. It is also the first to define the effect of the combination of both factors which have been found to be responsible for an increased incidence of HSR: dialysis membranes and ACEi.
Both BK and des-Arg9-BK concentrations peaked early during the ex vivo dialysis session with AN69 membranes, at times compatible with the occurrence of HSR episodes in hemodialyzed patients [10]. Although plasma contact with AN69 membranes alone already led to significant kinin release, peak concentration and the total amount of both BK and des-Arg9-BK were clearly potentiated by inhibiting ACE, the main kininase in human plasma. The BK and des-Arg9-BK quantities released during dialysis were much more significant than those responsible for experimental inflammation [19]. Analysis of the descending part of the kinetic profiles revealed a higher kinin-forming capacity of dialyzed plasma at 20 min, compared to that measured at 8 min. In other words, the lower concentrations of immunoreactive BK and des-Arg9-BK found at 20 min were not due to enhanced degradation but to lesser generation from lower activation of plasma HK. These results may explain why in this experimental approach we could not obtain evidence of an effect of APP level on BK accumulation, in contrast to those we published earlier using continuous plasma contact with glass beads in the presence of an ACEi [8,10].
Both immunoreactive kinins were quantified by specific antibodies directed against the COOH-terminal part of both peptides responsible for B2 or B1 pharmacological activity. To delineate NH2-terminal residues (Lys or Arg), we undertook physicochemical characterization by HPLC. Such an approach allowed us to confirm that activation of the contact system was the sole mechanism leading to the generation of kinins in this ex vivo model and that immunoreactive peptides are native kinins.
We clearly demonstrated that coating AN69 membranes with polyethyleneimine, which significantly decreased AN69 membranes zeta potential, suppressed the kinin kinetic release in the presence of ACEi. In other words, the zeta potential of AN69-ST membranes (between −5 and −15 mV), in contrast with that of AN69 membranes (around −70 mV), significantly reduced the amount of kinins generated during 20 min of ex vivo dialysis.
The kinin concentration of dialysates not only corroborates but also completes the significance of plasma kinin levels, generated by plasma contact with both membranes. For the first time, we report the presence of immunoreactive kinins in dialysates collected after exposure to AN69 membranes. These concentrations, although lower than their plasma counterparts, allow the calculation of concentration ratios, which would not be possible for AN69-ST membranes due to the low levels of both BK and des-Arg9-BK. This observation confirms that the low kinetic profile of both kinins for AN69-ST membranes is not attributable to higher diffusion of kinins in the dialysates, but rather to the absence of their release from HK.
We demonstrated that binding of immunoreactive HK on AN69 membranes after 6 and 8 min of plasma dialysis was reduced on AN69-ST membranes in the same dialysis periods. These results were completed by immunodetection of native factor XII and its product of activation, factor XII heavy chain. At 8 min of the dialysis runs with AN69 membranes, factor XII activation combined with HK accumulation contributed to kinin release. At 20 min, the decreased binding of both HK and factor XII heavy chain to the membranes corresponded to the lack of kinin release at this timepoint. These results confirm and extend earlier observations that the coating of AN69 membranes suppresses the binding of radiolabeled HK [17,18].
Our study, however, has some limitations. We do not know, at the present time, the role of patient vascular bed endothelium in vasoactive kinins inactivation in vivo, mainly in presence of an ACEi. This ex vivo study has, however, a clinical relevance, as we have previously shown that HSR episodes are associated with a decrease of the kinin-forming capacity of dialylized patient plasma [20]. We have also shown that the activation of the contact system by dextran sulphate in rabbit is associated with a significant and acute decrease of blood pressure, one of the symptoms of HSR in dialyzed patient [21].
5. Conclusions
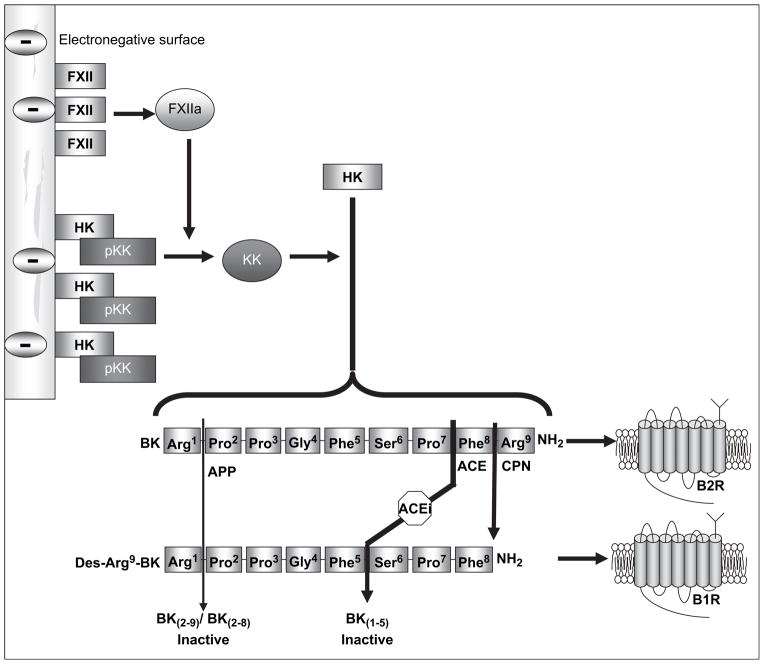
This study must be reviewed in the context of our former hypothesis, summarized in Fig. 5, on the multifactorial nature of HSR. Here, we explored the roles of dialysis membranes and their zeta potential, mainly in the presence of ACEi in the triggering of kinins, and mediators of HSR. These results show the importance of dialysis membrane electronegativity in stimulating kinin generation potentiated by ACEi. Neutralizing the electronegative AN69 membrane surface, as with AN69-ST membranes, decreased kinins generation in plasma and dialysate triggered by HK binding and activation at the membrane level. The experimental approach of this ex vivo study could be used in the future to test and modulate the kinin-forming capacity of some biomaterials to be used in combination with ACEi in other medical fields as transfusion, apheresis or extracorporeal circulations.
Fig. 5.
Hypothesis of HSR pathophysiology with negatively-charged membranes.
Acknowledgments
We thank Gambro-Hospal France for logistical support, Dr P. Clavel and S. Lavaux (ARPDD, Reims, France) for blood sampling. We are grateful to Professor Jean-Christophe Leroux for constructive comments during the preparation of this manuscript. The work of AA is funded by the Canadian Institutes of Health Research (Grant MOP-14077) and the National Institutes of Health (NIH Grant 1-R01-HL079184).
References
- 1.Schaefer RM, Schaefer L, Horl WH. Anaphylactoid reactions during hemodialysis. Clin Nephrol. 1994;42(Suppl 1):S44–7. [PubMed] [Google Scholar]
- 2.Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, et al. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991;338:1423–4. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- 3.Bommer J, Wilhelms OH, Barth HP, Schindele H, Ritz E. Anaphylactoid reactions in dialysis patients: role of ethylene-oxide. Lancet. 1985;2:1382–5. doi: 10.1016/s0140-6736(85)92554-1. [DOI] [PubMed] [Google Scholar]
- 4.Chanard J, Lavaud S, Lavaud F, Toupance O, Kochman S. IgE antibodies to isocyanates in hemodialyzed patients. ASAIO Trans. 1987;33:551–3. [PubMed] [Google Scholar]
- 5.Fink E, Lemke HD, Verresen L, Shimamoto K. Kinin generation by hemodialysis membranes as a possible cause of anaphylactoid reactions. Braz J Med Biol Res. 1994;27:1975–83. [PubMed] [Google Scholar]
- 6.Schulman G, Hakim R, Arias R, Silverberg M, Kaplan AP, Arbeit L. Bradykinin generation by dialysis membranes: possible role in anaphylactic reaction. J Am Soc Nephrol. 1993;3:1563–9. doi: 10.1681/ASN.V391563. [DOI] [PubMed] [Google Scholar]
- 7.Verresen L, Fink E, Lemke HD, Vanrenterghem Y. Bradykinin is a mediator of anaphylactoid reactions during hemodialysis with AN69 membranes. Kidney Int. 1994;45:1497–503. doi: 10.1038/ki.1994.195. [DOI] [PubMed] [Google Scholar]
- 8.Cyr M, Lepage Y, Blais C, Jr, Gervais N, Cugno M, Rouleau JL, et al. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am J Physiol Heart Circ Physiol. 2001;281:H275–83. doi: 10.1152/ajpheart.2001.281.1.H275. [DOI] [PubMed] [Google Scholar]
- 9.Duan QL, Nikpoor B, Dube MP, Molinaro G, Meijer IA, Dion P, et al. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–26. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molinaro G, Duan QL, Chagnon M, Moreau ME, Simon P, Clavel P, et al. Kinin-dependent hypersensitivity reactions in hemodialysis: metabolic and genetic factors. Kidney Int. 2006;70:1823–31. doi: 10.1038/sj.ki.5001873. [DOI] [PubMed] [Google Scholar]
- 11.Adam A, Albert A, Calay G, Closset J, Damas J, Franchimont P. Human kininogens of low and high molecular mass: quantification by radioimmunoassay and determination of reference values. Clin Chem. 1985;31:423–6. [PubMed] [Google Scholar]
- 12.Molinaro G, Carmona AK, Juliano MA, Juliano L, Malitskaya E, Yessine MA, et al. Human recombinant membrane-bound aminopeptidase P: production of a soluble form and characterization using novel, internally quenched fluorescent substrates. Biochem J. 2005;385:389–97. doi: 10.1042/BJ20040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvihill J, Cazenave JP, Mazzucotelli JP, Crost T, Collier C, Renaux JL, et al. Minimodule dialyser for quantitative ex vivo evaluation of membrane haemocompatibility in humans: comparison of acrylonitrile copolymer, cuprophan and polysulphone hollow fibres. Biomaterials. 1992;13:527–36. doi: 10.1016/0142-9612(92)90104-v. [DOI] [PubMed] [Google Scholar]
- 14.Moreau ME, Thibault L, Desormeaux A, Chagnon M, Lemieux R, Robillard P, et al. Generation of kinins during preparation and storage of whole blood-derived platelet concentrates. Transfusion. 2007;47:410–20. doi: 10.1111/j.1537-2995.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 15.Decarie A, Drapeau G, Closset J, Couture R, Adam A. Development of digoxigenin-labeled peptide: application to chemiluminoenzyme immunoassay of bradykinin in inflamed tissues. Peptides. 1994;15:511–8. doi: 10.1016/0196-9781(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 16.Raymond P, Drapeau G, Raut R, Audet R, Marceau F, Ong H, et al. Quantification of des-Arg9-bradykinin using a chemiluminescence enzyme immunoassay: application to its kinetic profile during plasma activation. J Immunol Methods. 1995;180:247–57. doi: 10.1016/0022-1759(94)00320-v. [DOI] [PubMed] [Google Scholar]
- 17.Chanard J, Lavaud S, Randoux C, Rieu P. New insights in dialysis membrane biocompatibility: relevance of adsorption properties and heparin binding. Nephrol Dial Transplant. 2003;18:252–7. doi: 10.1093/ndt/18.2.252. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Valette P, Mausset AL, Dejardin P. High molecular weight kininogen adsorption on hemodialysis membranes: influence of pH and relationship with contact phase activation of blood plasma. Influence of pre-treatment with poly(ethyleneimine) Int J Artif Organs. 2000;23:20–6. [PubMed] [Google Scholar]
- 19.Decarie A, Adam A, Couture R. Effects of captopril and Icatibant on bradykinin (BK) and des [Arg9] BK in carrageenan-induced edema. Peptides. 1996;17:1009–15. doi: 10.1016/0196-9781(96)00145-3. [DOI] [PubMed] [Google Scholar]
- 20.Adam A. Ecogenetic aspects of acute side effects caused by angiotensin converting enzyme inhibitors. Bull Mem Acad R Med Belg. 2004;159:307–13. discussion: 314–5. [PubMed] [Google Scholar]
- 21.Sabourin T, Guay K, Houle S, Bouthillier J, Bachvarov DR, Adam A, et al. Absence of ligand-induced regulation of kinin receptor expression in the rabbit. Br J Pharmacol. 2001;133:1154–62. doi: 10.1038/sj.bjp.0704158. [DOI] [PMC free article] [PubMed] [Google Scholar]