Abstract
Background
In Africa, many areas are co-endemic for the two major Schistosoma species, S. mansoni and S. haematobium. Epidemiological studies have suggested that host immunological factors may play an important role in co-endemic areas. As yet, little is known about differences in host immune responses and possible immunological interactions between S. mansoni and S. haematobium in humans. The aim of this study was to analyze host cytokine responses to antigens from either species in a population from a co-endemic focus, and relate these to S. mansoni and S. haematobium infection.
Methodology
Whole blood cytokine responses were investigated in a population in the north of Senegal (n = 200). Blood was stimulated for 72 h with schistosomal egg and adult worm antigens of either Schistosoma species. IL-10, IL-5, IFN-γ, TNF-α, and IL-2 production was determined in culture supernatants. A multivariate (i.e. multi-response) approach was used to allow a joint analysis of all cytokines in relation to Schistosoma infection.
Principal Findings
Schistosoma haematobium egg and worm antigens induced higher cytokine production, suggesting that S. haematobium may be more immunogenic than S. mansoni. However, both infections were strongly associated with similar, modified Th2 cytokine profiles.
Conclusions/Significance
This study is the first to compare S. mansoni and S. haematobium cytokine responses in one population residing in a co-endemic area. These findings are in line with previous epidemiological studies that also suggested S. haematobium egg and worm stages to be more immunogenic than those of S. mansoni.
Author Summary
In the developing world, over 207 million people are infected with blood-dwelling parasitic Schistosoma worms. Schistosoma haematobium and S. mansoni are the most widespread species. In Africa, they often occur together in the same area, with many people carrying both species. Yet, little is known about the differences in immune response that the human host develops against these two species. It is also unknown whether the presence of one species may affect the immune response to the other. We here investigated 200 people from an area in the north of Senegal where both species occur. They were examined for Schistosoma infections, as well as for immune responses to the two species. We observed that both infections were characterized by very similar cytokine responses. However, S. haematobium antigens induced higher levels of cytokines than S. mansoni. This suggests that S. haematobium may give rise to stronger immune responses, and may help to explain differences between the two most important Schistosoma species regarding the occurrence of infection and morbidity.
Introduction
Schistosomiasis is a parasitic disease of major public health importance. Schistosoma mansoni and S. haematobium are the main human species. Both species are endemic in Africa, where their distributions show a great overlap [1]. Schistosomes are known to down-regulate host immune responses and to induce so-called modified Th2 responses. The exact phenotype of the induced response depends on a complex immunological ‘dialogue’ that involves cytokines and immune cells of Th2, but also Th1, Th17 and regulatory components of the immune system [2].
So far, little is known about differences in host immune responses to schistosomes and possible immunological interactions between S. mansoni and S. haematobium in humans. Yet, epidemiological studies have suggested that host immunological factors may play an important role in co-endemic areas. Interspecies differences in immunogenicity for example, may explain why infection-age curves and morbidity patterns differ between S. mansoni and S. haematobium. Also, immunological interspecies differences and/or immunological interactions between S. mansoni and S. haematobium may explain differences in morbidity levels between single and mixed Schistosoma infections. Cheever et al. reported a more pronounced reduction of S. haematobium than S. mansoni worm loads with age [3]. Similarly, in a mixed focus in northern Senegal, we found the age-infection curve of S. haematobium to decline more steeply after adolescence than that of S. mansoni [4], indicating that protective immunity against S. haematobium may develop more rapidly. In addition, we found that mixed S. mansoni and S. haematobium infection as compared with single S. haematobium infection tended to decrease the risk of S. haematobium-specific urinary tract pathology [5]. This appeared mainly due to ectopically excreted, possible hybrid eggs [6]. Others also found S. mansoni to affect S. haematobium-specific morbidity and vice versa [7], [8], indicating that the two infections may have different effects on the egg-induced immune responses that provoke morbidity.
The present study set out to compare Schistosoma-specific cytokine responses induced by S. mansoni and S. haematobium antigens, and to relate these to Schistosoma infection in a S. mansoni and S. haematobium co-endemic area. Schistosoma infection status (single and mixed) and infection intensities as well as Schistosoma-specific cytokine responses were determined in residents from a co-endemic focus in northern Senegal. A multivariate (i.e. multi-response) approach was used to allow a joint analysis of multiple cytokine responses (interleukin (IL)-10, IL-5, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and IL-2) [9].
Materials and Methods
Ethics statement
This study was part of a larger investigation on the epidemiology of schistosomiasis and innate immune responses (SCHISTOINIR) for which approval was obtained from the review board of the Institute of Tropical Medicine, the ethical committee of the Antwerp University Hospital and ‘Le Comité National d'Ethique de la Recherche en Santé’ in Dakar. Informed and written consent was obtained from all participants prior to inclusion into the study. For minors, informed and written consent was obtained from their legal guardians.
All community members were offered praziquantel (40 mg/kg) and mebendazole (500 mg) treatment after the study according to WHO guidelines [10].
Study area
This study was conducted in Ndieumeul and Diokhor Tack, two neighboring communities on the Nouk Pomo peninsula in Lake Guiers. Details on the study area have been described elsewhere [4], [5]. Between July 2009 and March 2010, parasitological data were collected from 857 individuals [4]. A random subsample of 200 subjects was followed up immunologically. These subjects were between 5 and 53 years of age. Individuals who had lived in an urban area in the 5 years preceding the study (n = 7), had taken praziquantel within the last year (n = 2), or had clinical signs of malaria (recruited upon recovery), and pregnant women (n = 18) were excluded from the immunological study.
Parasitology
Two feces and two urine samples were collected from each participant on consecutive days. Infection with Schistosoma spp. was determined quantitatively (by Kato-Katz and urine filtration), and infection with soil-transmitted helminths (STHs) Ascaris lumbricoides, Trichuris trichiura and hookworm, was assessed qualitatively (by Kato-Katz), as described elsewhere [4]. Aliquots of the first fecal samples were preserved in ethanol to confirm microscopy results by multiplex PCR (A. lumbricoides, hookworm and Strongyloides stercoralis) (n = 198) [11]. Infection with Plasmodium was determined by Giemsa-stained thick blood smears.
Whole blood culture
Five hours after venipuncture, heparinized blood was diluted 1∶4 in RPMI 1640 (Invitrogen) supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 1 mM pyruvate and 2 mM glutamate (all from Sigma). This mixture (200 µl sample volume) was incubated in 96-well round bottom plates (Nunc) at 37°C under 5% CO2 atmosphere for 72 h, together with one of four schistosomal water-soluble antigen preparations at a final concentration of 10 µg protein/ml:
Schistosoma egg antigen (SEA) derived from S. mansoni (SEAm);
SEA from S. haematobium (SEAh);
Adult worm antigen (AWA) from S. mansoni (AWAm); or
AWA from S. haematobium (AWAh).
Medium (see above) without stimulus was used as a negative control. After harvesting, supernatants were stored at −80°C. Schistosoma eggs and adult worms were isolated from either S. mansoni- or S. haematobium-infected golden hamsters. SEAm, SEAh, AWAm and AWAh were prepared from this material using identical procedures. In brief, eggs or worms were freeze-dried and then homogenized in phosphate-buffered saline (PBS) with 10% n-octyl-β-D-glucopyranoside. Subsequently, this mixture was sonicated, frozen, thawed and washed with PBS. The resulting pellet was dialyzed and filter-sterilized. While AWAm and AWAh batches were lipopolysaccharide (LPS)-free, SEAm and SEAh antigens contained equivalent amounts of LPS (final concentrations of 1–5 ng/ml).
Cytokine measurement
IL-10, IL-5, IFN-γ, TNF-α, and IL-2 in culture supernatants were analyzed simultaneously using custom Luminex cytokine kits (Invitrogen) according to the manufacturer's instructions. Samples with concentrations below the detection limit were assigned values corresponding to half of the lowest value detected. Lowest values detected were 0.063 pg/ml for IL-10, 0.044 pg/ml for IL-5, 0.090 pg/ml for IFN-γ, 0.051 pg/ml for TNF-α, and 0.063 pg/ml for IL-2.
Statistical analysis
Results were considered significant when the p-value was <0.05. The Pearson Chi-square test was used to determine the association between infection status on the one hand, and age and gender on the other. Nonparametric techniques were chosen because cytokine concentrations were not normally distributed. Univariate statistics were used to compare single antigen-induced responses within individuals (IBM SPSS 21.0). McNemar's tests were used to compare cytokine response frequencies between S. mansoni and S. haematobium antigen-induced responses within individuals (e.g. SEAm- versus SEAh-induced responses). Similarly, Wilcoxon Signed Rank tests were used to compare cytokine response levels between S. mansoni and S. haematobium antigen-induced responses within individuals. Multivariate (i.e. multi-response) statistics were used to collectively analyze multiple cytokine responses – i.e. cytokine profiles - in the study population, and to investigate interrelationships between these responses [9]. We chose the nonparametric technique nonmetric multidimensional scaling (nMDS; in R with the ‘Vegan’ package [12], [13]). This is a variant of the parametric principal component analysis (PCA), but with fewer assumptions about the nature of the data and the interrelationship of the variables [14]. This is important because cytokine response levels were not normally distributed, even after log-transformation. Also, levels of different cytokines typically correlate with one another. Upon computation of the cytokine profiles, associations between these cytokine profiles and Schistosoma infection were assessed. The approach is illustrated in Supporting Information S1. Before nMDS, cytokine concentrations in the negative control were subtracted from those in antigen-stimulated samples to obtain net cytokine responses. Negative values were set to zero. Net cytokine responses were normalized by log(base 10)-transformation after adding 1 pg/ml to allow for zeroes. Schistosoma infection intensities were normalized after adding half of the detection limit (i.e. 5 eggs per gram of feces and 0.5 eggs per 10 ml of urine for S. mansoni and S. haematobium, respectively). One nMDS was performed for each of the four Schistosoma-specific whole blood stimulations (either SEAm, SEAh, AWAm or AWAh) using the ‘metaMDS’ function [13]. Each nMDS was repeated several times to assess the robustness of the resulting pattern [14]. The Euclidean dissimilarity index was used [13], and cytokine profiles - i.e. the matrix of IL-10, IL-5, IFN-γ, TNF-α, and IL-2 - were plotted in three dimensions (3D) to adequately represent the variation in the data [14]. Afterwards, gradients of the separate cytokine responses, on which the nMDS was based, were fitted using the ‘envfit’ function [13]. The same function was used to fit infection intensities onto each 3D nMDS, and to statistically test associations of antigen-induced cytokine profiles with Schistosoma infection intensity or infection status, i.e. uninfected, single S. mansoni, single S. haematobium, versus mixed S. mansoni and S. haematobium infection. The ‘ordiellipse’ function was used to fit average group scores - with their 95% confidence intervals (CIs) - for different infection statuses [13]. In contrast to individual S. mansoni- and S. haematobium-induced cytokine responses which can be compared quantitatively within individuals as described above (univariate statistics), qualitative differences between S. mansoni- and S. haematobium-induced cytokine profiles could only be assessed visually by nMDS, not by formal statistical testing.
Results
Characteristics of the study population
The study population consisted of 88 males and 112 females with a median age of 16 (range 5–53) years. Malaria and STHs T. trichiura and hookworm were absent in this population, and A. lumbricoides and S. stercoralis rare (n = 3 and 2, respectively, with 100% concordance between microscopy and PCR). In contrast, 137 (69%) subjects were infected with S. mansoni, and 116 (58%) with S. haematobium. Sixty percent (95/158) of all Schistosoma infections were mixed S. mansoni and S. haematobium infections (Table 1). The distributions of S. mansoni and S. haematobium infections in the study population according to age and gender are shown in Table 2. Both Schistosoma infections peaked in adolescents (10 to 19 year-olds), but gender differences were not statistically significant. Epidemiological patterns of infection have been described in more detail elsewhere [4].
Table 1. Schistosoma infections in the study population.
| S. mansoni infection | S. haematobium infection | Prevalence (n) | Code for Infection Status In Figure 2 | |||
| Subjects | Feces | Urinea | Feces | Urine | ||
| Positive | 158 | |||||
| Single infections | 63 | |||||
| + | − | − | − | 42 | M (dark blue) | |
| − | − | − | + | 21 | H (light blue) | |
| Mixed infections | 95 | MH | ||||
| + | − | − | + | 81 | MH (pink) | |
| + | + | − | + | 13 | MH (yellow) | |
| − | + | − | + | 1 | MH (red) | |
| Negative | − | − | − | − | 42 | N (green) |
| Total | 136 | 14 | 0 | 116 | 200 | |
Table 2. Distribution of Schistosoma infection in the study population.
| n | S. mansoni infection | S. haematobium infection | |||
| Percentage of positives | p-value | Percentage of positives | p-value | ||
| Age (in years) | 0.001 | 0.001 | |||
| 5–9 | 51 | 58.8 | 66.7 | ||
| 10–19 | 59 | 88.1 | 72.9 | ||
| 20–39 | 55 | 58.2 | 49.1 | ||
| ≥40 | 35 | 65.7 | 34.3 | ||
| Gender | 0.32 | 0.20 | |||
| Male | 88 | 72.7 | 63.6 | ||
| Female | 112 | 65.2 | 53.6 | ||
General cytokine profiles
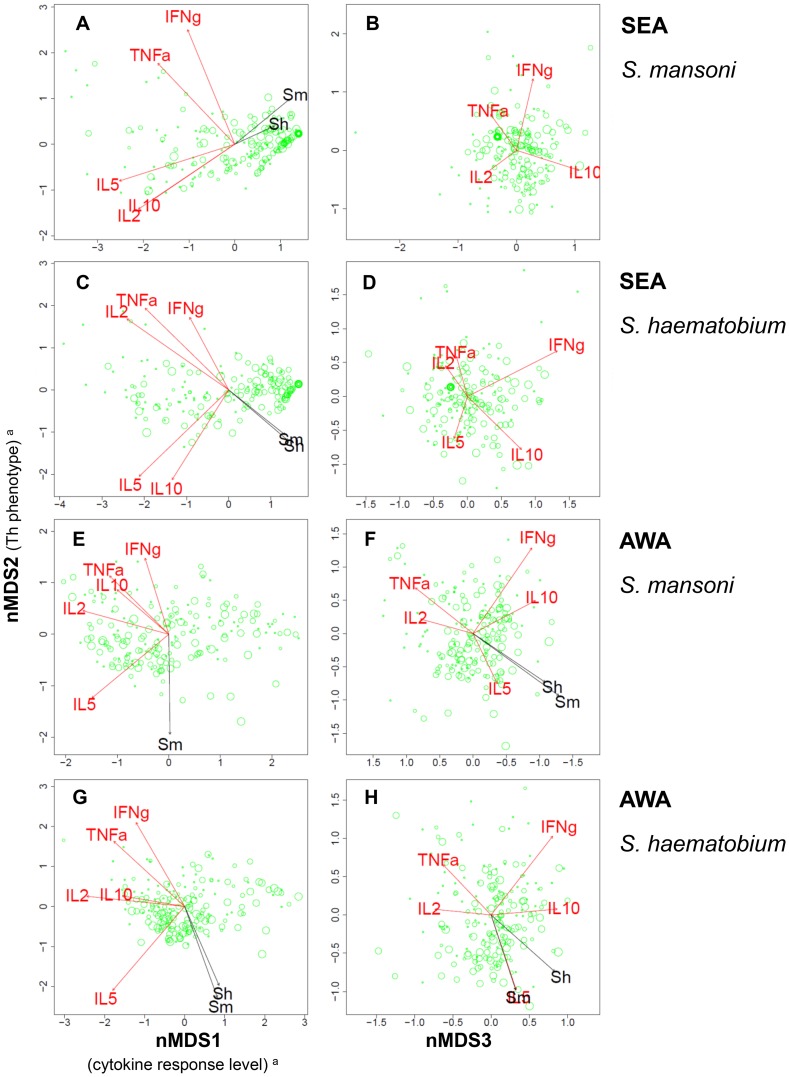
Insight into the different antigen-induced cytokine responses relative to one another was obtained by nMDS. Figure 1 and 2 show the variation in multivariate cytokine responses in the study population, with dots representing individuals. Distances between dots approximate inter-individual dissimilarities in cytokine responses with stress values (i.e. discrepancies) of 0.051 for SEAm, 0.041 for SEAh, 0.058 for AWAm, and 0.061 for AWAh. Red arrows indicate increasing gradients of IL-10, IL-5, IFN-γ, TNF-α and IL-2 responses, respectively. The level of a cytokine response increases in the direction of the corresponding arrow (see also Supporting Information S1). The length of a cytokine arrow indicates the goodness of fit of that arrow (or cytokine gradient).
Figure 1. Variation in Schistosoma antigen-induced cytokine responses in relation to Schistosoma infection intensity.
Each three-dimensional (3D) nMDS ordination is represented in two 2D planes (Supporting Information S1). Left and right panels represent the 1st and 2nd, and 2nd and 3rd dimensions, respectively. Panels A and B show the S. mansoni egg antigen (SEAm)-induced cytokine profile, Panels C and D that of S. haematobium SEA(h), Panels E and F that of S. mansoni adult worm antigens (AWAm), and Panels G and H show S. haematobium AWA(h)-induced cytokine profiles. Green dots represent individuals. Distances between dots approximate the rank order of dissimilarities in cytokine profiles between the respective individuals with stress values (i.e. discrepancies) of 0.051 for SEAm, 0.041 for SEAh, 0.058 for AWAm, and 0.061 for AWAh. Red arrows indicate linear gradients of normalized net cytokine responses on which the nMDS is based. Green dot sizes are proportional to individual values of normalized infection intensity of S. mansoni (for simplicity dots were only labelled with S. mansoni (not S. haematobium) infection intensity). Black arrows indicate linear gradients of post hoc fitted normalized infection intensity of S. mansoni (‘Sm’) and S. haematobium (‘Sh’). The length of the arrows is proportional to the goodness of fit onto the cytokine profile within one 2D plane, but lengths cannot be compared between cytokine and infection intensity arrows. Arrows are only depicted if their fit was significant at the level of p = 0.05 in 3D ordinations (see Table 4), as well as in the respective 2D planes. In Panel H, the arrows of IL-5 response and S. mansoni infection intensity are overlapping and their labels are therefore illegible. aThe biological a posteriori interpretation of nMDS1 (left x-axis) and nMDS2 (y-axis) were added between brackets on the axis labels, but nMDS3 (right x-axis) could not be interpreted.
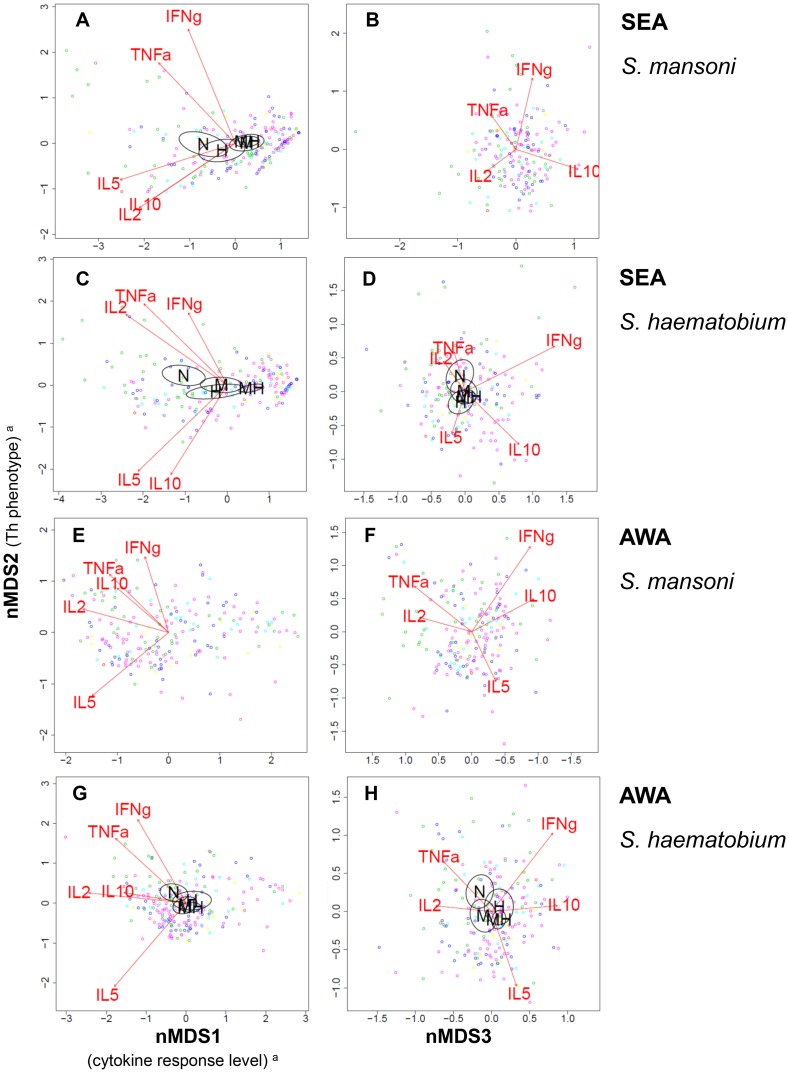
Figure 2. Variation in Schistosoma antigen-induced cytokine responses in relation to Schistosoma infection status.
Each three-dimensional (3D) nMDS ordination is represented in two 2D planes (Supporting Information S1) as in Figure 1: Left and right panels represent the 1st and 2nd, and 2nd and 3rd dimensions, respectively. Panels A and B show the S. mansoni egg antigen (SEAm)-induced cytokine profile, Panels C and D that of S. haematobium SEA(h), Panels E and F that of S. mansoni adult worm antigens (AWAm), and Panels G and H show S. haematobium AWA(h)-induced cytokine profiles. Dots represent individuals and distances between dots approximate the rank order of dissimilarities in cytokine profiles between the respective individuals with stress values (i.e. discrepancies) of 0.051 for SEAm, 0.041 for SEAh, 0.058 for AWAm, and 0.061 for AWAh. Red arrows indicate linear gradients of normalized net cytokine responses on which the nMDS is based. The length of the arrows is proportional to the goodness of fit onto the cytokine profile within one 2D plane, and arrows are only depicted if their fit was significant at the level of p = 0.05 in 3D ordinations (see Table 4), as well as in the respective 2D planes. Green dots represent uninfected individuals, dark blue those with single S. mansoni infections, light blue single S. haematobium, and the other colors indicate people with mixed infections: pink indicates mixed infections without ectopic egg elimination, yellow mixed infections with S. mansoni in feces as well as in urine and S. haematobium in urine, and red dots represent one individual with both S. mansoni and S. haematobium eggs in urine (possibly a hybrid species [4]–[6]; see also Table 1). Ellipsoids represent 95% confidence intervals for average group scores, for different infection statuses: uninfected (‘N’), single S. mansoni (‘M’), single S. haematobium (‘H’), versus mixed infection (‘MH’). Ellipsoids are drawn using the function ‘ordiellipse’, and only depicted if the fit of infection status onto the cytokine profile was significant at the level of p = 0.05 in 3D ordinations (see Table 4), as well as in the respective 2D planes. In Panel A and G, the labels for single S. mansoni (‘M’) and mixed infection (‘MH’) are overlapping. aThe biological a posteriori interpretation of nMDS1 (left x-axis) and nMDS2 (y-axis) were added between brackets on the axis labels, but nMDS3 (right x-axis) could not be interpreted.
The nMDS outcomes for the first axis (nMDS1) show that for each of the four antigen stimulations, all cytokine responses point to the left. Individuals plotted on the left produced consistently higher levels of all cytokines measured than those on the right. In other words, nMDS1 indicates a gradient of high (left) to low (right) cytokine responses. In analogy, the second axis (nMDS2), indicates a gradient of Th1-like (IFN-γ and TNF-α, top) to Th2-like (IL-5, bottom) phenotypes for each of the antigen stimulations. In contrast to SEA-induced IL-5, AWA-induced IL-5 was not accompanied by production of IL-10. IL-2 levels increased with Th1 cytokines, except for SEAm. The third axis (nMDS3) indicates a gradient of TNF-α and IL-2 (left) to IFN-γ and IL-10 (right).
In contrast to antigen-induced cytokines, spontaneously induced levels of cytokines in the control (medium only), did not show significant gradients, except for IL-5 on the third nMDS axis (stress = 0.11, data not shown).
Comparison between S. mansoni- and S. haematobium-induced cytokine responses and cytokine profiles
Figure 1 and 2 indicate that S. mansoni and S. haematobium antigens induced very similar cytokine profiles; cytokine profiles differed more between adult (AWA) and egg (SEA) life stages of the parasite than between the two Schistosoma species. Within individuals, S. haematobium-induced cytokine response levels were higher than those induced by S. mansoni (Table 3). This was statistically significant for all SEA- and AWA-induced cytokine responses that were measured, except for SEA-induced IFN-γ and IL-10.
Table 3. Levels of Schistosoma-induced cytokine responses in 72 h whole blood cultures (n = 200).
| Antigen | Species | Cytokine | Response (%) | Median Concentration in pg/ml (IQR)a | p-valueb | |
| SEA c | S. mansoni | |||||
| IL-10 | 92.0 | 12.7 | (5.2–32.4) | 0.874 | ||
| IL-5 | 78.5 | 3.7 | (1.0–19.0) | <0.001 | ||
| IFN-γ | 67.5 | 3.4 | (0.05–7.8) | 0.729 | ||
| TNF-α | 64.5 | 0.7 | (0.03–2.2) | 0.046 | ||
| IL-2 | 80.0 | 6.3 | (2.0–18.8) | <0.001 | ||
| S. haematobium | ||||||
| IL-10 | 90.5 | 13.1 | (4.7–32.2) | |||
| IL-5 | 77.0 | 5.2 | (0.9–47.4) | |||
| IFN-γ | 63.0 | 4.2 | (0.05–7.8) | |||
| TNF-α | 67.5 | 1.0 | (0.03–4.3) | |||
| IL-2 | 80.5 | 8.2 | (2.1–54.7) | |||
| AWA d | S. mansoni | |||||
| IL-10 | 98.5 | 25.7 | (13.2–48.2) | 0.008 | ||
| IL-5 | 94.5 | 69.3 | (11.8–201.2) | <0.001 | ||
| IFN-γ | 74.5 | 5.4 | (0.05–9.4) | 0.002 | ||
| TNF-α | 90.5 | 4.6 | (1.2–10.9) | <0.001 | ||
| IL-2 | 98.0 | 60.3 | (22.4–152.1) | <0.001 | ||
| S. haematobium | ||||||
| IL-10 | 99.0 | 30.0 | (17.0–50.4) | |||
| IL-5 | 96.0 | 108.6 | (25.9–237.9) | |||
| IFN-γ | 78.5 | 6.3 | (1.7–12.1) | |||
| TNF-α | 96.5 | 6.0 | (2.7–15.1) | |||
| IL-2 | 98.0 | 99.5 | (42.4–224.5) | |||
| None | ||||||
| IL-10 | 59.5 | 1.7 | (0.03–4.9) | |||
| IL-5 | 57.0 | 0.9 | (0.02–2.6) | |||
| IFN-γ | 58.0 | 2.2 | (0.05–5.8) | |||
| TNF-α | 63.5 | 0.4 | (0.03–1.5) | |||
| IL-2 | 45.5 | 0.03 | (0.03–2.9) | |||
Blood samples from one individual were divided into five and stimulated with Schistosoma antigens (SEAm, SEAh, AWAm, or AWAh), and with medium only (negative control; see Materials and Methods).
Crude cytokine levels are reported. IQR: Interquartile range (Tukey's hinges).
Wilcoxon Signed Rank test comparing S. mansoni- and S. haematobium-induced cytokine levels within individuals (either for SEA or AWA).
Schistosoma egg antigen.
Adult worm antigen.
Relation between cytokine profiles and Schistosoma infection intensity
Subsequently, we related the above-described variation in cytokine responses in the study population (i.e. plotted cytokine profiles) to infection intensity. Table 4 shows that all associations between Schistosoma antigen-induced cytokine profiles and infection intensity were statistically significant. In Figure 1, the direction of the black arrows represents the increasing gradients of S. mansoni and S. haematobium infection intensity, respectively (see also Supporting Information S1). On the first axis, which indicates cytokine response levels (see above), these arrows generally point into the opposite direction of cytokine responses. This indicates that people with elevated Schistosoma infection intensities are more likely to have lower cytokine responses, and vice versa. On the second axis, which indicates the Th1 versus Th2 response phenotype (see above), infection intensity generally increases with IL-5 and decreases with Th1 cytokines TNF-α, IFN-γ, and IL-2 (except for SEAm-induced IL-5 which decreases with increasing infection intensity). Briefly, as infection intensity increased, cytokine response levels decreased and the Th2 phenotype became more pronounced. The association between infection intensity and reduced cytokine responsiveness was more pronounced for SEA than for AWA stimulation. Schistosoma infection intensity increased with AWA-induced IL-5, but decreased with SEA-induced IL-5 levels, indicating that people with higher infection intensities produced more of a Th2-like response against AWA and more of a suppressive response (i.e. with low cytokine response levels) against SEA than people with lower infection intensities, and vice versa.
Table 4. Association between Schistosoma infection and Schistosoma antigen-induced cytokine profiles.
| Infection | Antigen-induced cytokine profile | |||
| SEAm | SEAh | AWAm | AWAh | |
| S. mansoni infection intensity | ||||
| R2 | 0.14 | 0.17 | 0.10 | 0.13 |
| p-value | 0.001 | 0.001 | 0.001 | 0.001 |
| S. haematobium infection intensity | ||||
| R2 | 0.05 | 0.18 | 0.07 | 0.15 |
| p-value | 0.02 | 0.001 | 0.003 | 0.001 |
| Infection Status | ||||
| R2 | 0.09 | 0.18 | 0.02 | 0.04 |
| p-value | 0.001 | 0.001 | 0.2 | 0.01 |
Figure 1 shows the fit of infection intensity and Figure 2 that of infection status (uninfected, single S. mansoni, single S. haematobium, versus mixed infections) onto each of the four Schistosoma antigen-induced cytokine profiles (either SEAm, SEAh, AWAm or AWAh), obtained by the ‘metaMDS’ and ‘envfit’ functions (see also Supporting Information S1) [12], [13]. Here, the goodness of these fits, i.e. squared correlation coefficients (R2), are shown. The statistical significance was assessed using permutation tests (n = 999), and presented p-values are approximations.
We did not observe differences in induced cytokine profiles between the two Schistosoma infections. Associations between cytokine profiles and infection intensity were comparable for S. mansoni and S. haematobium infections (Figure 1). Table 4 shows significant correlations between cytokine profiles and Schistosoma infection intensity for homologous combinations (i.e. infection intensity and antigen stimulation of the same species) as well as for heterologous combinations (i.e. infection intensity of one and antigen stimulation of the other species).
Relation between cytokine profiles and infection status (mixed versus single infections)
Schistosoma antigen-induced cytokine profiles were significantly associated with Schistosoma infection status, except upon stimulation with AWAm (Table 4). Figure 2 shows how antigen-induced cytokine profiles differed according to infection status (except for AWAm, which was not significantly associated with infection status), with 95% CI ellipsoids indicating the average nMDS scores per infection group: uninfected (‘N’), single S. mansoni (‘M’), single S. haematobium (‘H’), versus mixed (‘MH’) Schistosoma infection group. In analogy with Figure 1, uninfected individuals had higher cytokine responses than Schistosoma-infected subjects, and their cytokine profiles were skewed more towards the Th1 phenotype. On the whole, there was a gradient in cytokine profiles from uninfected individuals, to people with single and then mixed Schistosoma infections (Figure 2) and these profiles were in the same direction as the gradient of infection intensity (Figure 1). In other words, people with low cytokine responses of the Th2 phenotype tended to have both mixed and heavier infections, people with strong Th1 responses tended to be uninfected, and those with an intermediate cytokine profile tended to have both single and lighter Schistosoma infections.
For the SEAm-induced cytokine profile, there was a clear difference (i.e. separation between ellipsoids) between S. mansoni-infected individuals (with either single or mixed S. mansoni), and those without S. mansoni (no Schistosoma infection, or single S. haematobium infection; Figure 2A). There were no significant differences in this cytokine profile between single and mixed S. mansoni infections, or between uninfected individuals and those with single S. haematobium infections. This indicates that, in contrast to S. mansoni, S. haematobium infection status was not associated with SEAm-induced cytokine profiles. Schistosoma haematobium-induced cytokine profiles on the other hand, showed similar relationships with S. mansoni as well as with S. haematobium infection status. Cytokine profiles of people with single and mixed infections differed significantly from those of uninfected people, and cytokine profiles did not appear to differ between single S. mansoni and single S. haematobium infections.
Discussion
The objective of this study was to compare cytokine responses induced by S. mansoni and S. haematobium antigens, and to relate these to Schistosoma infection in a S. mansoni and S. haematobium co-endemic area. We showed that Schistosoma infection intensity was significantly associated with Schistosoma antigen-induced cytokine profiles and that it may explain up to 18% of the variation in cytokine responses observed in this population. As Schistosoma infection intensity increased, cytokine responses decreased and the Th2 phenotype became more pronounced. This was exemplified by relatively higher IL-5 (and IL-10) and relatively lower IFN-γ, TNF-α and IL-2 levels. Lightly infected and uninfected subjects on the other hand, had elevated cytokine responses, with a Th1 phenotype. These patterns are consistent with the modified Th2 response characteristic for schistosomiasis [2]. nMDS also indicated that the association between infection and the Th2 phenotype was more pronounced for AWA, while that between infection and (reduced) cytokine responsiveness was more pronounced for SEA. These observations fit with a previous study by Joseph et al. describing similar immunological differences between Schistosoma adult worm and egg life stages in a population from a S. mansoni mono-endemic area, using more conventional analyses [15].
Secondly, we demonstrated that increased Schistosoma infection intensity and mixed (as compared to single) infections were associated with similar, modified Th2, cytokine profiles. This is probably due to the fact that subjects with mixed infections were more likely to have higher infection intensities than those with single infections [4]. Also, similar, modified Th2, cytokine profiles were observed for both S. mansoni and S. haematobium infection intensity, whether blood was stimulated with antigens from the homo- or heterologous species. This may be indicative of immunological cross-reactivity between species. For S. mansoni-induced cytokine profiles however, this was unlikely, because profiles did not differ between single and mixed S. mansoni infection groups. While S. haematobium-induced cytokine profiles did differ between single and mixed S. haematobium infection groups, we could not determine whether these differences were due to mixed infection per se, or to higher S. haematobium infection intensity in mixed as compared to single infections. Other potentially confounding factors such as age may have been involved as well [4], and future studies should be performed to assess their respective roles in determining cytokine responses. To obtain more evidence on the existence of cross-reactivity between the two major human Schistosoma species, it is important to compare immune responses between different co- and mono-endemic areas, using different immunological parameters (e.g. cytokine, humoral and cytological data). To our knowledge, only one human study reported on functional S. mansoni – S. haematobium cross-reactivity. This study from 1974 reported lethal in vitro activity of sera from subjects infected with one species against schistosomula of the same but not of the other species [16]. Indeed, S. mansoni and S. haematobium may share few if any epitopes that are involved in protective immunity because they belong to genetically distinct groups. Potential cross-reactivity or the lack thereof merits further investigation as this may have important implications for our understanding of the epidemiology of schistosomiasis as well as for the development of an effective schistosomiasis vaccine.
The present study demonstrated that nMDS can be used successfully to analyze host cytokine responses collectively. In this way, it was possible to analyze cytokine responses in relation to one another, and in relation to Schistosoma infection. nMDS is a nonparametric, multivariate and visual method. It is a robust and powerful tool because it avoids problems of multiple statistical tests and violations of data assumptions [14]. Moreover, nMDS makes it easier to interpret complex data than traditional one-by-one graphs, tables, and tests. Here, we used this approach to study multivariate cytokine responses, but it can be used equally well to increase our understanding of other complex, multidimensional data, such as cytological and/or serological data (Durnez et al, unpublished data), as well as infection data on multiple co-endemic parasite species.
Additional analyses showed that, within individuals, S. haematobium antigens induced higher cytokine responses in 72 h whole blood cultures than those of S. mansoni. A very similar pattern was observed in parallel investigations in Ghana, in a population which was - in contrast to the Senegalese study population - first exposed to S. haematobium and then to both S. mansoni and S. haematobium, and with lower prevalences of S. mansoni and higher prevalences of S. haematobium (unpublished data, A.S. Amoah et al, and ref [4]). This suggests that this finding does not depend on the level of transmission or on exposure history, and that the two Schistosoma species may differ in their immunogenicity. This hypothesis is in line with observations from Van Remoortere et al. who found S. mansoni to induce mainly IgM antibodies – which are thought to inhibit protective host immune responses [17] – while S. haematobium induced both IgM and IgG antibodies against shared carbohydrate epitopes [18]. It is therefore tempting to speculate that lower cytokine response levels may prevent Ig class switching from IgM to IgG for these epitopes in S. mansoni infection, while stronger cytokine responses may promote class switching in S. haematobium infection. Alternatively, differences in their biochemical composition may underlie interspecies differences in both immunogenicity and humoral immune responses. These two immunological interspecies differences may also have contributed to earlier epidemiological findings. Several studies observed a steeper decline of the age-infection curve of S. haematobium as compared to S. mansoni after adolescence, indicating that protective immunity against S. haematobium might develop more rapidly [3], [4]. Secondly, higher levels of S. haematobium- as compared to S. mansoni-specific morbidity have been observed in co-endemic populations [5], [7], [8], suggesting that the immune responses provoked by S. haematobium eggs might be more pathogenic. It should be noted however, that other factors may also explain these two epidemiological observations. For example, S. mansoni and S. haematobium eggs accumulate in different organs, i.e. the liver and the urinary tract, respectively, and these differences in anatomical context may also explain the differences in the extent of morbidity between the two species. More research is necessary to investigate the abovementioned immunological interspecies differences and their implications for epidemiological patterns of infection and morbidity in more detail.
Conclusion
In conclusion, this is the first study to comprehensively investigate S. mansoni- and S. haematobium-induced cytokine responses in a S. mansoni and S. haematobium co-endemic area, and to relate these cytokine responses to Schistosoma infection. The present study demonstrates that nMDS can be used successfully as a tool for the joint analysis of multiple cytokine responses in relation to Schistosoma infection. We showed strong associations between Schistosoma infection and Schistosoma-induced cytokine profiles, and provided a first insight into potential differences and interactions between human S. mansoni and S. haematobium infections. This knowledge will contribute to an improved understanding of the mechanisms underlying Schistosoma infection and morbidity in co-endemic populations.
Supporting Information
Schematic representation of nonmetric multidimensional scaling.
(DOCX)
Acknowledgments
We gratefully thank the population of Ndieumeul and Diokhor Tack and the village chiefs, Daoure Mbaye and Daouda Pene, for their hospitality and participation in this study. This study would not have been possible without the field workers in Richard Toll, Abdoulaye Yague, Mankeur Diop, Moussa Wade and Ngary Sy, who assisted in the sample collection and microscopic analysis. We would also like to thank the medical and technical staff of the Health Centre in Richard Toll for their support, Yvonne Kruize for providing the immunologic stimuli and preparatory work, Rogier Achterberg, Mareen Datema and Churnalisa Doran for the cytokine measurements in Leiden, Pierre Legendre from the University of Montreal and Vincent Sluydts from Antwerp for their useful advice on multivariate analyses, as well as Lies Durnez from Antwerp for critically reviewing the statistical methods used. In addition, we would like to thank one of the reviewers who made important contributions to our manuscript in two Review rounds.
Funding Statement
This work was funded by the European Union's sixth framework programme (INCO-CT-2006-032405, http://cordis.europa.eu/fp6/) and the Flemish Inter-University Council (VLADOC to NB, http://www.vliruos.be/en/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 2. Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11: 375–388. [DOI] [PubMed] [Google Scholar]
- 3. Cheever AW, Kamel IA, Elwi AM, Mosimann JE, Danner R (1977) Schistosoma mansoni and S. haematobium infections in Egypt. II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg 26: 702–716. [DOI] [PubMed] [Google Scholar]
- 4. Meurs L, Mbow M, Vereecken K, Menten J, Mboup S, et al. (2012) Epidemiology of mixed Schistosoma mansoni and Schistosoma haematobium infections in northern Senegal. Int J Parasitol 42: 305–311. [DOI] [PubMed] [Google Scholar]
- 5. Meurs L, Mbow M, Vereecken K, Menten J, Mboup S, et al. (2012) Bladder Morbidity and Hepatic Fibrosis in Mixed Schistosoma haematobium and S. mansoni Infections: A Population-Wide Study in Northern Senegal. PLoS Negl Trop Dis 6: e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huyse T, Van den Broeck F, Hellemans B, Volckaert FA, Polman K (2013) Hybridisation between the two major African schistosome species of humans. Int J Parasitol 43: 687–689. [DOI] [PubMed] [Google Scholar]
- 7. Koukounari A, Donnelly CA, Sacko M, Keita AD, Landoure A, et al. (2010) The impact of single versus mixed schistosome species infections on liver, spleen and bladder morbidity within Malian children pre- and post-praziquantel treatment. BMC Infect Dis 10: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gouvras AN, Kariuki C, Koukounari A, Norton AJ, Lange CN, et al. (2013) The impact of single versus mixed Schistosoma haematobium and S. mansoni infections on morbidity profiles amongst school-children in Taveta, Kenya. Acta Trop 128: 309–317. [DOI] [PubMed] [Google Scholar]
- 9. Bourke CD, Nausch N, Rujeni N, Appleby LJ, Mitchell KM, et al. (2013) Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis 208: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO (2006) Preventive chemotherapy in human helminthiasis - Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers.
- 11. Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, et al. (2010) Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study). BMC Infect Dis 10: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org. [Google Scholar]
- 13.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, et al. (2013) Vegan: Community ecology package. R package, version 2.0-7; http://CRAN.R-project.org/package=vegan.
- 14.Clarke KR, Warwick RM (2001) Ordination of samples by multi-dimensional scaling (MDS). In: Cange in marine communities: An approach to statistical analysis and interpretation. Plymouth: Primer-E Ltd. [Google Scholar]
- 15. Joseph S, Jones FM, Kimani G, Mwatha JK, Kamau T, et al. (2004) Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect Immun 72: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith M, Webbe G (1974) Letter: Damage to schistosomula of Schistosoma haematobium in vitro by immune baboon and human sera and absence of cross-reaction with Schistosoma mansoni . Trans R Soc Trop Med Hyg 68: 70–71. [DOI] [PubMed] [Google Scholar]
- 17. Butterworth AE, Bensted-Smith R, Capron A, Capron M, Dalton PR, et al. (1987) Immunity in human schistosomiasis mansoni: prevention by blocking antibodies of the expression of immunity in young children. Parasitology 94 Pt 2: 281–300. [DOI] [PubMed] [Google Scholar]
- 18. van Remoortere A, van Dam GJ, Hokke CH, van den Eijnden DH, van Die, et al. (2001) Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect Immun 69: 2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of nonmetric multidimensional scaling.
(DOCX)