Abstract
Objective
To investigate the protective effects and mechanisms of carbon monoxide-releasing molecule-2 (CORM-2) on barrier function of intestinal epithelial cells.
Materials and Methods
After pre-incubation with CORM-2 for 1 hour, cultured intestinal epithelial IEC-6 cells were stimulated with 50 µg/ml lipopolysaccharides (LPS). Cytokines levels in culture medium were detected using ELISA kits. Trans-epithelial electrical resistance (TER) of IEC-6 cell monolayers in Transwells were measured with a Millipore electric resistance system (ERS-2; Millipore) and calculated as Ω/cm2 at different time points after LPS treatment. The permeability changes were also measured using FITC-dextran. The levels of tight junction (TJ) proteins (occludin and ZO-1) and myosin light chain (MLC) phosphorylation were detected using Western blotting with specific antibodies. The subsequent structural changes of TJ were visualized using transmission electron microscopy (TEM).
Results
CORM-2 significantly reduced LPS-induced secretion of TNF-α and IL-1β. The LPS-induced decrease of TER and increase of permeability to FITC-dextran were inhibited by CORM-2 in a concentration dependent manner (P<0.05). LPS-induced reduction of tight junction proteins and increase of MLC phosphorylation were also attenuated. In LPS-treated cells, TEM showed diminished electron-dense material and interruption of TJ and desmosomes between the apical lateral margins of adjoining cells, which were prevented by CORM-2 treatment.
Conclusions
The present study demonstrates that CORM-2, as a novel CO-releasing molecule, has ability to protect the barrier function of LPS-stimulated intestinal epithelial cells. Inhibition of inflammatory cytokines release, restoration of TJ proteins and suppression of MLC phosphorylation are among the protective effects of CORM-2.
Introduction
Carbon monoxide (CO) was considered as a toxic gas for a long time due to its higher affinity for hemoglobin than oxygen. In recent years, some researchers have found that CO has additional functions in biological systems than was first thought. In mammalian cells, a small amount of endogenous CO is produced by heme oxygenase-1 (HO-1), a stress-inducible protein that catalyses conversion of heme into free Fe2+, CO, and biliverdin [1], [2]. An elevated level of CO was detected in exhaled air of critically ill patients as a result of HO-1 induction, which was considered to be a self-protective mechanism of an organism [3]. It has also been reported that induction of HO-1 or exogenous CO administration could improve the survival of septic mice [4], [5]. In a rat model of hepatic ischemia-reperfusion injury (I/Ri), exogenous CO attenuated the liver damage caused by I/Ri [6]. Other biological functions of CO include anti-inflammation, anti-thrombosis and vasodilatation [7]–[10]. Therefore, increasing attention has been focused on this diatomic gas as a potential messenger.
However, it is not easy to conduct experiments with CO, due to the formation of carboxyhemoglobin. Carbon monoxide-releasing molecules (CO-RMs) are a class of newly synthesized transition metal carbonyls which can release CO in a controlled manner without significantly altering the level of carboxyhemoglobin in animal models. CORM-2 is one of this type of compounds, which releases CO when it is dissolved in dimethyl sulfoxide (DMSO). In fact, CORM-2 has been widely used as a CO donor in a variety of experiments. In nerve-injured mice, CORM-2 administration reduced the neuropathic pain [11]. CROM-2 could also attenuate leukocytes infiltration in the renal tissue of thermally injured mice [12]. Moreover, CORM-2 could inhibit the migration of osteoarthritic synoviocytes [13].
Intestinal epithelial cells serve as the first line of defense against pathogens in the intestinal tract, playing an important role in maintenance of the gut barrier function. When permeability of intestinal epithelial cells increases, pathogens in the lumen will enter the systemic circulation, and subsequently contribute to the development of systemic inflammation. Kazuhiro Katada et al. showed that systemic administration of CORM-2 in mice attenuated ischemia/reperfusion-induced inflammation in the small intestine [14]. Moreover, other researchers reported that CORM-2 could suppress the inflammatory response induced by cytokines in Caco-2 cells, a human intestinal carcinoma cell line [15]. Thus, we hypothesized that CORM-2 may provide protective effects for the intestine when inflammation occurred. To our knowledge, there little is known about the effects of CO on the function of the intestinal barrier function. In this work, we chose IEC-6 cells, non-transformed intestinal epithelial cells, to form the cell monolayer. IEC-6 cells originally from the small intestinal crypt cells of the rat maintain the features of normal intestinal cells, such as tight junctions linking adjacent cells [16]. LPS, a component of the wall of Gram-negative bacteria, has been identified to possess the ability of interrupting the intestinal barrier [17] and has been used to mimic the conditions of inflammation and test therapeutic reagents [18].
The aim of this study is to examine whether CORM-2 can protect the barrier function of intestinal cell monolayer against inflammation. Furthermore, we have explored the possible mechanisms underlying this phenomenon. Our data indicate that COMR-2 can enhance the barrier function of the IEC-6 cell monolayer and CORM-2-derived CO is the effective factor. In addition, pro-inflammatory cytokines, TJ proteins, MLC phosphorylation, are all involved in the process.
Materials and Methods
Chemicals and Reagents
Anti-ZO-1, anti-β-actin (Santa Cruz Biotechnology), anti-phospho-myosin light chain (MLC) and anti-total-MLC (Cell Signaling), anti-occludin (Abcam) antibodies and peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson Immunologicals) were purchased from relevant manufacturers. CORM-2 and LPS (Escherichia coli 055:B5) were purchased from Sigma-Aldrich. CORM-2 was dissolved in DMSO and then diluted in culture media to achieve the required concentrations. Inactivated CORM-2 (iCORM-2) was prepared by incubating CORM-2 for 24 h at 37°C to liberate all CO.
Cell culture
IEC-6 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium with high glucose (DMEM, GibcoBRL) supplemented with 10% fetal bovine serum (FBS, GibcoBRL) and 10 µg/ml insulin (Sigma-Aldrich). Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. Cells were used at the 15th to 25th passage for all experiments.
Cytotoxicity assay
IEC-6 cells were seeded in 96-well culture plates at a concentration of 5×105 cells/ml 24 h prior to experiments. After different treatments, the cells were washed 3 times with phosphate-buffered saline (PBS). Ten µl of 5 mg/ml 3-(4, 5-dimethylthiazol-2-yl) -2,5 diphenyltetrazolium bromide (MTT) was added to each well and the contents were incubated for 4 h at 37°C. The media was removed and the formazan crystals inside the cells were dissolved in 200 µL of DMSO. The absorbance of each well was measured at 450 nm on a microplate reader.
Determination of trans-epithelial electrical resistance (TER) and permeability of the cell monolayer
TER values of IEC-6 cell monolayer were measured with a Millipore electric resistance system (ERS-2; Millipore), and calculated as Ω/cm2. The cells were seeded on inserts (0.4 µM pore size; Millipore) in 24-well transwell chambers. TER recorded in unseeded transwell inserts was subtracted from all values. Measurements were not started until the value reached 50 Ω/cm2. Trans-epithelial permeability for macromolecular tracers was measured with FITC-labeled Dextran (FD-40, Sigma). The cells were seeded on the inserts (0.4 µM pore size; Millipore) in a 12-well transwell chamber. After CORM-2 treatment, cells were stimulated with LPS for 24 h. Then the media in the bottom well was replaced with 1.5 mL DMEM, whilst media in the upper well was replaced with 0.5 mL DMEM containing FITC-Dextran at 10 mg/mL. After 1 h incubation, the amount of dextran presented in the bottom well was measured with a microplate reader.
Cytokine analysis
The culture medium of IEC-6 cells was collected,then centrifuged at 3000 rpm, for 10 min at 4°C. Cytokines levels in cell culture medium were measured using ELISA kits (TNF-α from R&D systems and IL-1β from RayBiotech), according to the manufacturer’s instructions. All standards and samples were run in duplicate.
Western blotting
Proteins were extracted from cultured cells using RIPA buffer and their concentrations were determined using a Bradford protein assay kit (BCA kit, Pierce Biotechnology). Equivalent protein samples were resolved on SDS-PAGE gels, then the proteins were transferred onto PVDF membrane, which was then blocked and probed with antibodies for occludin (1∶250), ZO-1 (1∶1000), β-actin (1∶1000), MLC (1∶1000), p-MLC (1∶1000) overnight at 4°C followed by incubation with HRP-conjugated secondary antibody (1∶5000) for 1 h at room temperature. Blots were visualized using an enhanced chemiluminescent kit (Thermo Fisher Scientific).
Transmission electron microscopy
Fully confluent cultured IEC-6 cells were washed and fixed with 4% (v/v) glutaraldehyde for 2 h and then post-fixed with 1% (w/v) osmium tetroxide. Thin sections were cut and stained with uranyl acetate and lead citrate. Images were taken with an H-600 (Hitachi, Japan) transmission electron microscope operated at 75 kV and images were captured digitally. Ultrastructural observations were made from multiple sites (10) of junctional complexes that were clearly identified. At least three images from each treatment group were analyzed by three people in a blinded fashion.
Data and Statistical Analysis
Data were presented as the mean±S.D and analyzed using the ANOVA test for comparisons between more than 2 groups. If a statistical significance was achieved, Student-Newman-Keuls test was used for comparison between 2 groups. Values of P<0.05 were considered statistically significant.
Results
Cytotoxic effects of CORM-2 on IEC-6 cells in vitro
Previous studies have shown that CORM-2 is not toxic to airway epithelial cells, but it has not been applied to intestinal IEC-6 cells. To test if CORM-2 is toxic for IEC-6 cells, we treated the cells with CORM-2 for 24 h in the absence or presence of LPS and found that CORM-2 alone (up to 100 µM) or in combination with LPS (50 µg/ml) was not toxic to IEC-6 cells (Fig. 1).
Figure 1. Effect of CORM-2 on cell viability.
IEC-6 cells were treated with CORM-2 in the absence or presence of LPS (/ml) for 24 h, and cell viability was assessed using the MTT assay. Results are the mean h, and cell viability was assessed using the MTT assay. Results are the mean±SD of 3 independent experiments. ANOVA test P>0.05.
CORM-2 attenuates the reduction of barrier function in LPS-treated IEC-6 cells
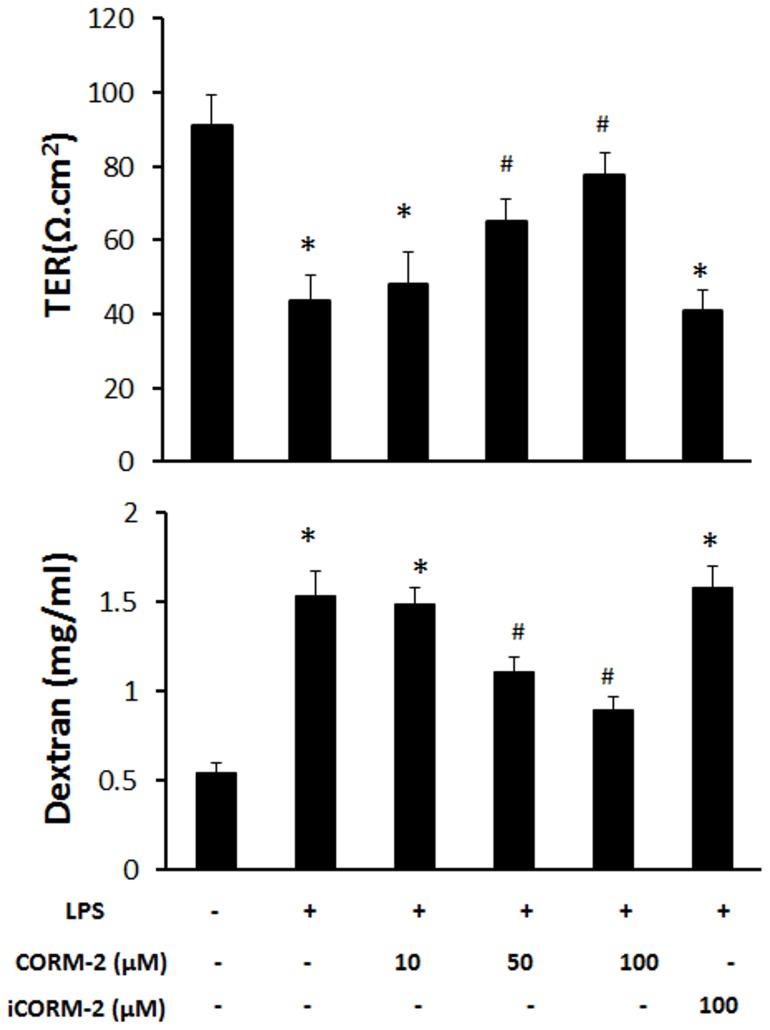
To examine whether CORM-2 could protect the barrier function of the cell monolayer, ICE-6 cells were plated in a transwell system and TER values and FITC-dextran permeations were measured. LPS decreased the TER value from 91.2±8.2 Ω/cm2 to 43.6±7.1 Ω/cm2. Addition of CORM-2 attenuated this reduction in a concentration-dependent manner, but inactivated CORM-2 (iCORM-2) failed to produce this effect (Fig. 2a). FITC-dextran was then used as a probe to investigate the permeability of the IEC-6 cell monolayer. As shown in Fig. 2b, CORM-2 decreased the LPS-induced dextran leakage from the upper to the lower compartment in the transwell system, whilst iCORM-2 did not. These results suggest that CO released from CORM-2 is able to improve the barrier function of the IEC-6 cells (Fig. 2).
Figure 2. Effect of CORM-2 on the permeability of LPS-treated IEC-6 cell monolayer.
When cells reached confluence in the transwell system, CORM-2 was added and incubated for 1 h. The cells were washed and treated with 50 h. The cells were washed and treated with 50 µg/ml LPS for 24 h. (a) Mean±SD of TER values from 3 independent experiments are shown. (b) Permeability of FITC-dextran across the cell monolayer indicated CORM-2 could decrease the LPS-induced increase. Results are the mean±SD of 3 independent experiments. *ANOVA test, p<0.05 as compared to control group, #p<0.05 as compared to LPS group.
CORM-2 inhibits release of TNF-α and IL-1β in LPS-treated IEC-6 cells
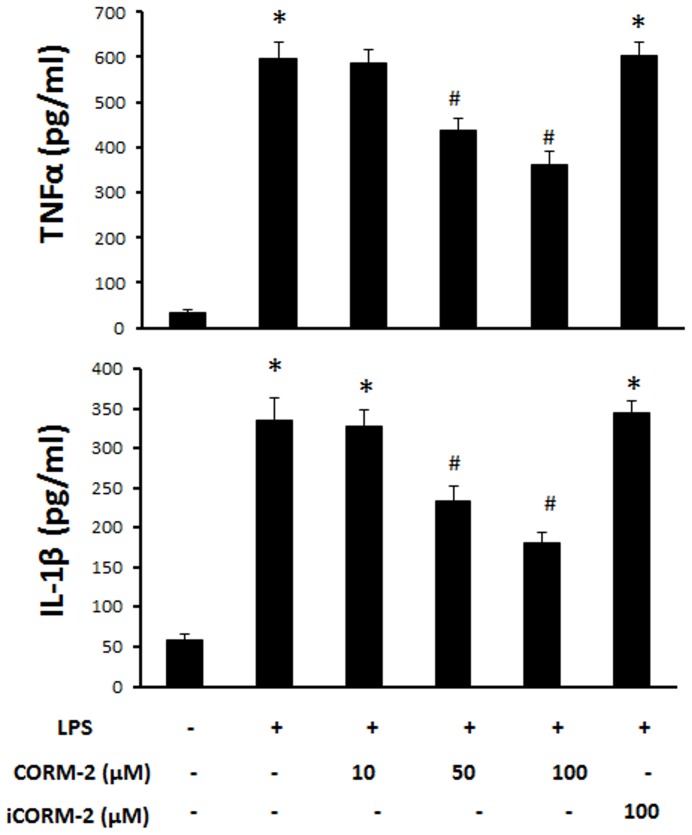
In order to explore the mechanism of COMR-2 protection of the barrier function of cell monolayer, we first investigated its effects on the release of proinflammatory cytokines from IEC-6 cells treated by LPS. Fig. 3a shows LPS significantly increased the release of TNF-α and addition of CORM-2 reduced the levels of TNF-α in the cell culture medium. Similarly, the increase in IL-1β was also attenuated by addition of CORM-2 whilst iCORM-2 showed no significant effect. This data indicates that CO released from CORM-2 can inhibit the secretion of LPS-induced proinflammatory cytokines (Fig. 3).
Figure 3. Effect of CORM-2 on the release of proinflammatory cytokines in LPS-treated IEC-6 cells.
IEC-6 cells were pretreated with CORM-2 for 1 h and cells were washed and stimulated with 50 h and cells were washed and stimulated with 50 µg/ml LPS for 24 h. TNF h. TNF-α (a) and IL-1β (b) in the culture medium were measure using ELISA kits. Mean±SD from 3 independent experiments are shown. *ANOVA test, P<0.05 when compared to control group (no treatment), #ANOVA test, P<0.05 when compared to LPS alone group.
CORM-2 ameliorates down-regulation of TJ proteins in LPS-treated IEC-6 cells
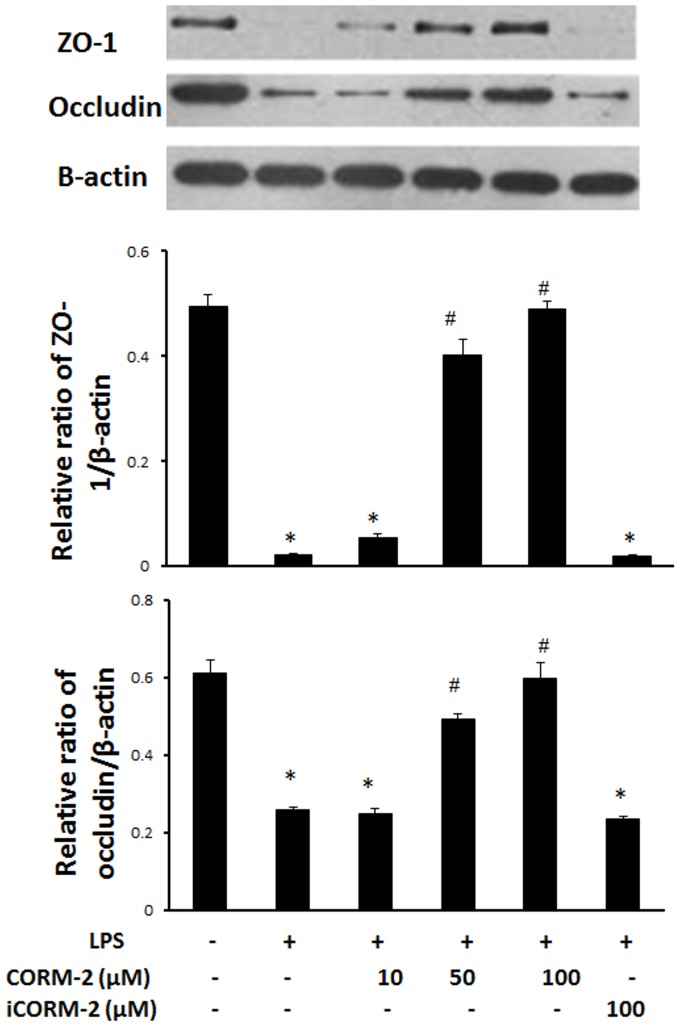
We investigated whether the tight junction proteins, occludin and ZO-1, were involved in the protective mechanisms induced by CORM-2. Treatment of IEC-6 cells with LPS resulted in a significant reduction in the TJ proteins, occludin and ZO-1. Western blots showed that CORM-2, but not iCORM-2, increased the expression of ZO-1 and occludin (Fig. 4).
Figure 4. Effect of CORM-2 on TJ protein expression in LPS-treated IEC-6 cells.
IEC-6 cells were pretreated with CORM-2 for 1 h and the cells were washed and stimulated with 50 h and the cells were washed and stimulated with 50 µg/ml LPS for 24 h. The cells were washed with PBS and harvested with RIPA buffer. The protein concentration was determined and proteins subjected to Western blotting (Methods). A typical image is shown (Upper panel). The relative ratios (Mean±SD) of ZO-1/β-actin (Middle panel) and occludin/β-actin (Lower panel) are calculated based on the densities of bands on Western blots from 3 independent experiments. *ANOVA test, P<0.05 when compared to control group (no treatment), #ANOVA test, P<0.05 when compared to LPS alone group.
CORM-2 suppresses myosin light chain (MLC) phosphorylation in LPS-treated IEC-6 cells
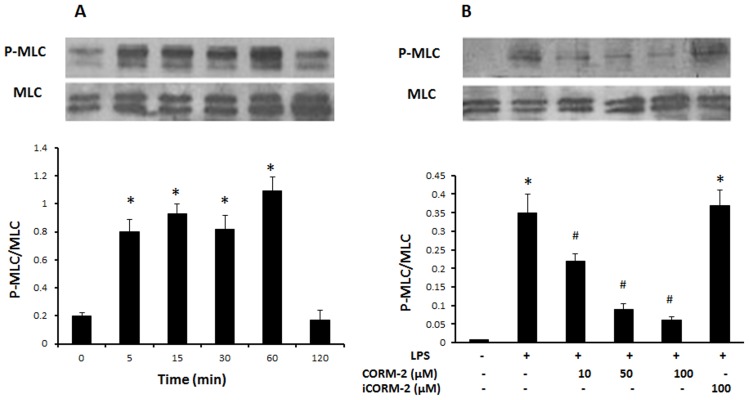
To investigate further the mechanism of CORM-2 in protecting the barrier function of the cell monolayer, the level of myosin light chain (MLC) phosphorylation in IEC-6 cells was examined. Fig. 5a shows that the basal levels of phosphorylated MLC were low in untreated IEC-6 cells. Treatment with 50 µg/ml LPS caused an obvious increase in MLC phosphorylation. Pre-incubation of CORM-2 but not iCORM-2 with IEC-6 cells significantly inhibited LPS-induced the phosphorylation of MLC (Fig. 5).
Figure 5. Effect of CORM-2 on the levels of MLC phosphorylation.
(A) IEC-6 cells were treated with 50 µg/ml LPS and harvested at different time points. MLC phosphorylation (p-MLC) was detected using phosphorylation-specific antibody. Sample loading was normalized using anti-MLC antibody. A typical blot is shown (Upper panel). (B) IEC-6 cells were pretreated with CORM-2 for 1 h followed by LPS (50 µg/ml) treatment for 1 h. A typical Western blot shows that CORM h. A typical Western blot shows that CORM-2 reduced LPS-enhanced MLC phosphorylation (Upper panel). The relative ratios (Mean±SD) of p-MLC/MLC are calculated based on the densities of bands on Western blots from 3 independent experiments (Lower panels of (A) and (B)). *ANOVA test, P<0.05 when compared to control group (no treatment), #ANOVA test, P<0.05 when compared to LPS alone group.
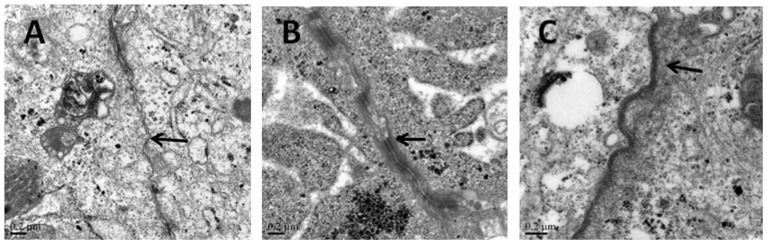
Changes of TJ in the transmission electron microscope
The ultrastructure of monolayers of IEC-6 cells was investigated by transmission electron microcopy (Fig. 6). The presence of electron-dense material in the intercellular space near the brush borders reflects the TJ. In cells without 50 µg/ml LPS treatment (control group Fig. 6a), the TJ and desmosomes displayed an intact structure. In cells treated with 50 µg/ml LPS (LPS-treated group Fig. 6b), the TJ and desmosomes were affected, this includes the loss of electron-dense material and the larger gap between adjoining cells. Conversely, pre-incubation with CORM-2 attenuated the LPS-induced disruption of TJ and desmosomes (Fig. 6c). These results demonstrate that CORM-2 reduced the distortion of the normal morphology of TJ complex induced by LPS.
Figure 6. Changes of TJ observed by Transmission Electron Microscopy.
Ultrastructure of TJs in a monolayer of cultured IEC-6 cells was observed by a transmission electron microscope. Panel (A) Normal control. (B) LPS–treated cells. The electron-dense materials were diminished in TJ and desmosomes. The space between adjoining cells was widened. (C) CORM-2 attenuated the LPS disruption of TJ and desmosomes. Arrows indicate the location of the TJs (Scale bar = 0.2 µm).
Discussion
The gastrointestinal tract functions not only as a digestive and absorptive organ, but also as a barrier against the pathogens in the lumen. A massive number of pathogenic bacteria colonize the gastrointestinal tract, and may cause systemic inflammation, such as sepsis, when they enter blood [19]. Sepsis, as a microvascular disease, may cause intestinal ischemia which further deteriorates the function of intestinal epithelial barrier [20], [21]. On the other hand, impaired barrier function promotes bacterial translocation and development of sepsis [22]. Therefore, it is crucial to protect the function of intestinal barrier in sepsis. In this study, we demonstrate for the first time that CORM-2 ameliorates the loss of barrier function of intestinal IEC-6 cells induced by LPS, by measuring the TER and permeability to a macromolecule, and the disruption to the TJ and desmosomes morphology observed under the TEM. Moreover, we observed that CORM-2 inhibits the secretion of proinflammatory cytokines. The TJ proteins, occludin and ZO-1, were restored by addition of CORM-2 and we also proved that CORM-2 could suppress the MLC phosphorylation in IEC-6 cells induced by LPS.
The effect of CORM-2 has also been investigated in Caco2 cells, a continuous cell line of heterogeneous human epithelial colorectal adenocarcinoma cells, and T84, a human colonic epithelial cell line [23], [24]. Both cell lines form polarized monolayer with much higher resistance tight junctions between adjacent cells and require much higher concentrations of LPS (800 µg/mL for Caco2 and 500 µg/mL for T84) to disrupt the barrier than that for IEC-6 cells (50 µg/mL) (Fig. S1 and S2). However, CORM-2 showed significant protective effect against high doses of LPS in term of permeability changes (Fig. S1 and S2). The TJ proteins, occludin, ZO-1 and claudin-1/4 in T84 cells, were restored by addition of CORM-2 (Fig. S3). These data have consolidated the findings with IEC-6 cells.
It is well known that HO-1 is not expressed under normal condition, but is up-regulated rapidly under various stimuli to enhance the production of endogenous CO, which is believed to be a defense mechanism for organisms [25]. It has been reported that induction of HO-1 can attenuate the damage of gastrointestinal mucosa [26]. Na Wang et al. have found that curcumin, a major active component of the food flavour turmeric, ameliorates hydrogen peroxide-induced barrier disruption of Caco-2 cells by up-regulating HO-1 [27]. Despite the fact that they identified that HO-1 was involved in the protective process, they did not investigate further which product of HO-1: Fe2+, CO, or biliverdin mediates this effect. In our study, we applied a CO-releasing molecule, CORM-2, to the monolayer of intestinal IEC-6 cells with inactivated CORM-2 (iCORM-2) as a control to demonstrate the effect of CO. The data obtained indicate that CO was the effective factor that protects the barrier function of intestinal epithelial cells. CORM-2 has been reported to have various effects on intestinal epithelial cells. Application of CORM-2 on duodenal mucosa in rats stimulates the release of HCO3 _, which protects the duodenal mucosa against acid [28]. CO can also modulate colonic ion transporters, such as Na+-K+-2Cl_ cotransporter, Cl_/HCO3_ exchanger, and K+ channels [29]. Here, we found that CO ameliorates LPS-induced loss of barrier function of the intestinal epithelial cell monolayer in vitro. Thus, CO may play important roles in protecting the integrity of the intestinal mucosa epithelia in vivo.
Proinflammatory cytokines, such as TNF-α and IL-1β, contribute substantially to the loss of the function of intestinal epithelial barrier. Recent studies have shown that TNF-α and IL-1β directly increased the permeability of intestinal epithelial tight junction [30]–[32]. TNF-α and IL-1β, as early-stage proinflammatory cytokines, are usually released at the start of inflammation. In this study, we observed that the release of TNF-α and IL-1β by IEC-6 cells was increased dramatically after challenge with LPS. Although it remains unknown whether the barrier loss caused by LPS is derived mainly from the direct effect of LPS or from the LPS-induced cytokines, TNF-α and IL-1β, however, at least they can account partly for the barrier loss of IEC-6 cells. Moreover, we found that CORM-2 has the ability to inhibit release of TNF-α and IL-1β from intestinal cells. Therefore, the inhibition of proinflammatory cytokines by CORM-2 is probably involved in protection on barrier function. In addition, it has been also reported that TNF-α and IL-1β induced barrier dysfunction requires activation of the NF-κB pathway [30], [32]. NF-κB is a transcription factor which controls gene expression of various proinflammatory cytokines. LPS stimulation can markedly activate the NF-κB pathway in IEC-6 cells [33]. Data from other investigators have demonstrated that CORM-2 can effectively inhibit activation of NF-κB pathway [34], [35]. Therefore, inhibition of the NF-κB pathway may be the mechanism by which CORM-2 down-regulates TNF-α and IL-1β. Since NF-κB, MAPK and MLCK are on the common pathways of LPS, TNFα and IL-1β, it is difficult to dissect the roles of LPS-induced TNFα and IL-1β in LPS-induced permeability changes. However, the resultant elevation of TNFα and IL-1β may cause a second wave of signaling and further increase the permeability of the cell monolayer. CORM-2 regulating NF-κB pathway will be expected to reduce the effects of LPS, TNFα and IL-1β. The latters will be investigated in our future studies.
Tight junctions seal the gap between adjacent epithelial cells, and prevent the passive diffusion of small molecules across the para-cellular space. Tight junction integrity plays a pivotal role in regulating the permeability of the cell monolayer. The tight junction complex consists of a series of tight junction proteins, including the trans-membrane protein occludin and intracellular plaque protein ZO-1 [36]. Previous studies have reported that LPS could down-regulate the expression of tight junction proteins and this is accompanied by an increase in permeability of the cell monolayer [37]. It has been confirmed by our study that LPS significantly down-regulates occludin, ZO-1 and claudin-1/4, and that CORM-2 can reverse this down-regulation. Our data suggest that CORM-2 may enhance the barrier function of the cell monolayer partly through up-regulating expression of tight junction proteins.
It has been demonstrated that the TJ complex associates tightly with the cytoskeleton in cells. When the cytoskeleton contracts, the TJ complex dissociates and the permeability of the cell monolayer is increased. Myosin light chain (MLC) is part of the cytoskeleton and its phosphorylation levels play a crucial role in maintenance of the function of epithelial barrier. Increase in MLC phosphorylation levels can induce the cytoskeleton to contract as well increasing the permeability of the cell monolayer [38], [39]. In our work, we observed a remarkable increase in phosphorylation levels of MLC after LPS challenge, indicating that the MLC pathway may take participate in the barrier dysfunction. Consistent with our results, an in vivo study also shows that LPS can increase MLC phosphorylation levels in the rat intestine and this is accompanied by disruption of the intestinal epithelial barrier [40]. In our work, we found that CORM-2 inhibited the phosphorylation of MLC. This data suggests that the MLC pathway plays a role in the CORM-2-protection of the cell monolayer barrier. However, there are two main upstream pathways, MLC and Rho kinase, which regulate the phosphorylation of MLC. Our work has not revealed which one is involved in the process [41] and this needs further investigation.
Conclusion
In summary, the present study demonstrates that CORM-2, as a novel CO-releasing molecule, possesses an ability to protect the barrier function of intestinal epithelial cells. Inhibition of inflammatory cytokines release, restoration of TJ proteins and suppression of MLC phosphorylation levels are among the protective effects of CORM-2. Further studies are required to reveal more detailed molecular mechanisms. Our study raises a possibility that CO-releasing molecules can be developed as agents for protecting gut barrier function in inflammatory diseases.
Supporting Information
Effect of CORM-2 on the permeability of LPS-treated Caco2 cell monolayers. Caco2 cells were grown for 21 days in transwells to achieve full differentiation. CORM-2 or inactivated CORM-2 (iCORM-2) (100 µM) was added and incubated for 1 h. The cells were then treated with 800 µg/mL LPS for 24 h. (A) Mean±SD of TER, with the UT value set 100% of 3 independent experiments are shown. (B) Permeability of FITC-dextran across the cell monolayer, with the UT value set as 100%. Results are the mean±SD of 3 independent experiments. (C) Permeability of HRP across the cell monolayer, with the value of untreated control (UT) set as 100%. Results are presented as mean±SD from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Effect of CORM-2 on the permeability of LPS-treated T84 cell monolayer. T84 cells were grown for 7 days in transwells. CORM-2 or inactivated CORM-2 (iCORM-2) (100 µM) was added and incubated for 1 h. The cells were then treated with 500 µg/mL LPS for 24 h. (A) Mean±SD of TER, with the UT value set 100% of 3 independent experiments are shown. (B) Permeability of FITC-dextran across the cell monolayer, with the UT value set as 100%. Results are the mean±SD of 3 independent experiments. (C) Permeability of HRP across the cell monolayer, with the value of untreated control (UT) set as 100%. Results are presented as mean±SD from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Effect of CORM-2 on TJ protein expression in LPS-treated T84 cells. T84 cells were pretreated with 100 µM CORM-2 or iCORM-2 for 1 h and the cells were then stimulated with 500 µg/mL LPS for 24 h. The cells were washed with PBS and lysed in clear lysis buffer. The protein concentration was determined and proteins subjected to Western blotting (Methods). (A) Representative Western blots are shown for claudin-1, claudin-4, occluding and ZO-1 with β-actin used as a loading control. The relative ratios (mean±SD) of claudin-1/β-actin (B), claudin-4/β-actin (C), occludin/β-actin (D) and ZO-1/β-actin (E) are calculated based on the densities of bands on Western blots from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Supporting Materials and Methods.
(DOCX)
Acknowledgments
Thanks to Professor Barry Campbell, Department of Gastroenterology, the University of Liverpool for providing the Caco2 and T84 cell lines as well as technical supports.
Funding Statement
This work was supported by National Natural Science Foundation of China (http://www.nsfc.gov.cn/publish/portal0/default.htm), No. 81171786, 81272148 and No. 81071546. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394. [PubMed] [Google Scholar]
- 2. Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554. [DOI] [PubMed] [Google Scholar]
- 3. Scharte M, Bone HG, Van Aken H, Meyer J (2000) Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun 267: 423–426. [DOI] [PubMed] [Google Scholar]
- 4. Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, et al. (2009) Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol 76: 173–182. [DOI] [PubMed] [Google Scholar]
- 5. Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, et al. (2009) Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther 329: 641–648. [DOI] [PubMed] [Google Scholar]
- 6. Wei Y, Chen P, de Bruyn M, Zhang W, Bremer E, et al. (2010) Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats. BMC Gastroenterol 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, et al. (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428. [DOI] [PubMed] [Google Scholar]
- 8. Cepinskas G, Katada K, Bihari A, Potter RF (2008) Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol 294: G184–191. [DOI] [PubMed] [Google Scholar]
- 9. Fei D, Meng X, Zhao M, Kang K, Tan G, et al. (2012) Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Transl Res 159: 99–109. [DOI] [PubMed] [Google Scholar]
- 10. Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, et al. (2004) Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol 142: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hervera A, Leanez S, Negrete R, Motterlini R, Pol O (2012) Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS One 7: e43693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun B, Sun Z, Jin Q, Chen X (2008) CO-releasing molecules (CORM-2)-liberated CO attenuates leukocytes infiltration in the renal tissue of thermally injured mice. Int J Biol Sci 4: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Arnandis I, Guillen MI, Gomar F, Castejon MA, Alcaraz MJ (2011) Control of cell migration and inflammatory mediators production by CORM-2 in osteoarthritic synoviocytes. PLoS One 6: e24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katada K, Bihari A, Mizuguchi S, Yoshida N, Yoshikawa T, et al. (2010) Carbon monoxide liberated from CO-releasing molecule (CORM-2) attenuates ischemia/reperfusion (I/R)-induced inflammation in the small intestine. Inflammation 33: 92–100. [DOI] [PubMed] [Google Scholar]
- 15. Megias J, Busserolles J, Alcaraz MJ (2007) The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol 150: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ (1979) Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura H, Sawada N, Tobioka H, Isomura H, Kokai Y, et al. (1997) Bacterial lipopolysaccharide reduced intestinal barrier function and altered localization of 7H6 antigen in IEC-6 rat intestinal crypt cells. J Cell Physiol 171: 284–290. [DOI] [PubMed] [Google Scholar]
- 18. Bogatcheva NV, Zemskova MA, Poirier C, Mirzapoiazova T, Kolosova I, et al. (2011) The suppression of myosin light chain (MLC) phosphorylation during the response to lipopolysaccharide (LPS): beneficial or detrimental to endothelial barrier? J Cell Physiol 226: 3132–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berg RD (1995) Bacterial translocation from the gastrointestinal tract. Trends Microbiol 3: 149–154. [DOI] [PubMed] [Google Scholar]
- 20. Ince C (2004) Microcirculation in distress: a new resuscitation end point? Crit Care Med 32: 1963–1964. [DOI] [PubMed] [Google Scholar]
- 21. Gosche JR, Garrison RN, Harris PD, Cryer HG (1990) Absorptive hyperemia restores intestinal blood flow during Escherichia coli sepsis in the rat. Arch Surg 125: 1573–1576. [DOI] [PubMed] [Google Scholar]
- 22. Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, et al. (1990) The process of microbial translocation. Ann Surg 212: 496–510 discussion 511–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749. [PubMed] [Google Scholar]
- 24. Donato RP, El-Merhibi A, Gundsambuu B, Mak KY, Formosa ER, et al. (2011) Studying permeability in a commonly used epithelial cell line: T84 intestinal epithelial cells. Methods Mol Biol 763: 115–137. [DOI] [PubMed] [Google Scholar]
- 25. Willis D, Moore AR, Willoughby DA (2000) Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol 190: 627–634. [DOI] [PubMed] [Google Scholar]
- 26. Becker JC, Grosser N, Boknik P, Schroder H, Domschke W, et al. (2003) Gastroprotection by vitamin C–a heme oxygenase-1-dependent mechanism? Biochem Biophys Res Commun 312: 507–512. [DOI] [PubMed] [Google Scholar]
- 27. Wang N, Wang G, Hao J, Ma J, Wang Y, et al. (2012) Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci 57: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 28. Takasuka H, Hayashi S, Koyama M, Yasuda M, Aihara E, et al. (2011) Carbon monoxide involved in modulating HCO3- secretion in rat duodenum. J Pharmacol Exp Ther 337: 293–300. [DOI] [PubMed] [Google Scholar]
- 29. Steidle J, Diener M (2011) Effects of carbon monoxide on ion transport across rat distal colon. Am J Physiol Gastrointest Liver Physiol 300: G207–216. [DOI] [PubMed] [Google Scholar]
- 30. Al-Sadi RM, Ma TY (2007) IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178: 4641–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Sadi R, Ye D, Dokladny K, Ma TY (2008) Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180: 5653–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, et al. (2004) TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 286: G367–376. [DOI] [PubMed] [Google Scholar]
- 33. De Plaen IG, Qu XW, Wang H, Tan XD, Wang L, et al. (2002) Endotoxin, but not platelet-activating factor, activates nuclear factor-kappaB and increases IkappaBalpha and IkappaBbeta turnover in enterocytes. Immunology 106: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee TS, Tsai HL, Chau LY (2003) Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Delta 12,14-prostaglandin J2. J Biol Chem 278: 19325–19330. [DOI] [PubMed] [Google Scholar]
- 35. Sun B, Zou X, Chen Y, Zhang P, Shi G (2008) Preconditioning of carbon monoxide releasing molecule-derived CO attenuates LPS-induced activation of HUVEC. Int J Biol Sci 4: 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–1228. [DOI] [PubMed] [Google Scholar]
- 37. Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, et al. (2011) The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci 46: 527–534. [DOI] [PubMed] [Google Scholar]
- 38. Ivanov AI (2008) Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 13: 6662–6681. [DOI] [PubMed] [Google Scholar]
- 39. Madara JL (1987) Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol 253: C171–175. [DOI] [PubMed] [Google Scholar]
- 40. Yuhan R, Koutsouris A, Savkovic SD, Hecht G (1997) Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113: 1873–1882. [DOI] [PubMed] [Google Scholar]
- 41. Guo Y, Ramachandran C, Satpathy M, Srinivas SP (2007) Histamine-induced myosin light chain phosphorylation breaks down the barrier integrity of cultured corneal epithelial cells. Pharm Res 24: 1824–1833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of CORM-2 on the permeability of LPS-treated Caco2 cell monolayers. Caco2 cells were grown for 21 days in transwells to achieve full differentiation. CORM-2 or inactivated CORM-2 (iCORM-2) (100 µM) was added and incubated for 1 h. The cells were then treated with 800 µg/mL LPS for 24 h. (A) Mean±SD of TER, with the UT value set 100% of 3 independent experiments are shown. (B) Permeability of FITC-dextran across the cell monolayer, with the UT value set as 100%. Results are the mean±SD of 3 independent experiments. (C) Permeability of HRP across the cell monolayer, with the value of untreated control (UT) set as 100%. Results are presented as mean±SD from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Effect of CORM-2 on the permeability of LPS-treated T84 cell monolayer. T84 cells were grown for 7 days in transwells. CORM-2 or inactivated CORM-2 (iCORM-2) (100 µM) was added and incubated for 1 h. The cells were then treated with 500 µg/mL LPS for 24 h. (A) Mean±SD of TER, with the UT value set 100% of 3 independent experiments are shown. (B) Permeability of FITC-dextran across the cell monolayer, with the UT value set as 100%. Results are the mean±SD of 3 independent experiments. (C) Permeability of HRP across the cell monolayer, with the value of untreated control (UT) set as 100%. Results are presented as mean±SD from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Effect of CORM-2 on TJ protein expression in LPS-treated T84 cells. T84 cells were pretreated with 100 µM CORM-2 or iCORM-2 for 1 h and the cells were then stimulated with 500 µg/mL LPS for 24 h. The cells were washed with PBS and lysed in clear lysis buffer. The protein concentration was determined and proteins subjected to Western blotting (Methods). (A) Representative Western blots are shown for claudin-1, claudin-4, occluding and ZO-1 with β-actin used as a loading control. The relative ratios (mean±SD) of claudin-1/β-actin (B), claudin-4/β-actin (C), occludin/β-actin (D) and ZO-1/β-actin (E) are calculated based on the densities of bands on Western blots from 3 independent experiments. ANOVA test, *p<0.05 as compared to untreated control (UT) group, # p<0.05 as compared to the LPS group.
(TIF)
Supporting Materials and Methods.
(DOCX)