Abstract
Reptiles represent the crucial phylogenetic group as they were the ancestors of both birds and mammals hence very important to study. The objectives of the present study were to investigate the potential roles of testosterone in the innate immune responses and splenic lymphocyte proliferation in fresh water snake, Natrix piscator. Animals were mildly anesthetized and spleens were taken out to study the splenic macrophage phagocytosis, super oxide production and nitrite release using in vitro testosterone. Splenic lymphocytes were isolated by density gradient centrifugation and were studied for mitogen induced proliferation in presence of in vitro testosterone. Testosterone suppressed the phagocytosis and nitrite release in a concentration dependent manner. Biphasic suppressive effect of testosterone was observed in superoxide production as judged by reduction of nitroblue tetrazolium salt where salt reduction was suppressed at lower and higher concentrations of testosterone. Mitogen induced splenic lymphocyte proliferation was also suppressed by testosterone. By suppressing immune responses, testosterone may, therefore, act as a physiological mechanism regulating the relative amount of energy invested into either reproductive effort or immunocompetence.
Introduction
The immune system is a coordinated unit consisting of variety of cellular and humoral components that responds to foreign pathogens/factors, with the goal being to eliminate them and return to steady state that existed prior to detection of the same. Sexual dimorphism in immune functions is illustrated in various vertebrate taxa including human [1]–[6]. Field studies in mammals and birds have illustrated that the prevalence (i.e. proportions of the individual infected) and intensity (i.e. severity of the infection) of parasitic infections are often higher in male than female [7], [8]. Laboratory studies have demonstrated that males are more susceptible to infection than females in rodents, and this difference is related to the gonadal steroids [9]–[13]. Males generally exhibit lower immune responses than the female conspecifics [2], [8]. Specifically, the humoral immune responses (i.e., antibody production by B-cells) are typically elevated in females, as compared with males. Females of various species display higher IgM, IgG and IgA concentrations than males and are also better able to mount both primary and secondary antibody response to antigenic challenge than males [1], [14], [15].
Use of sex steroids or its receptor-antagonists or gonadectomy in animal models for studying the pathophysiology and immunoendocrinology has provided direct evidence for the role of sex steroids in immune modulation [16], [17]. Experimental evidences suggest that physiological concentrations of female sex hormones stimulate; while those of male sex hormones suppress the immune responses [18], [19]. Macrophages are considered one of the first surveillance cells of the immune system to encounter nonself elements [20], [21] and to remove them directly by phagocytosis or indirectly by cytotoxicity through release of reactive oxygen/nitrogen intermediates (ROI/RNI) [22], cytokines, such as Interlekin-1 (IL–1) [23], and tumor necrosis factor–α (TNF–α) [24]. The modulation of macrophage function by sex steroids has been the subject of intense investigation in mammals [25]–[28]. The in vivo and in vitro studies have demonstrated the differential effects of male and female sex steroids on macrophage activities. Physiological concentration of estrogen has been reported to stimulate phagocytosis, proliferative capacity, and tumor cell cytostasis of macrophages [25], [29]; whereas that of androgen, to suppress the cytotoxic activity of macrophages [28], [30]. Dose dependent suppressive effect of dihydrotestosterone (DHT) has been demonstrated on nitrite release by murine peritoneal macrophages [28]. However, Flynn (1986) [31] demonstrated that in vivo treatment of testosterone had no change of IL-1 production from murine peritoneal macrophages.
Reptiles, being phylogenetically important, become a pivotal group to study in order to provide significant insights into both the evolution and functioning of the immune system. Only two studies are available in reptiles by Mondal and Rai (1999,2001) [3], [32]. They have reported that sex steroids modulate phagocytosis and cytotoxic responses of splenic macrophages in the wall lizard. There is no report on this aspect in an ophidian, which have lacertilian lineage. Reptiles are generally long-lived, with an extended period of growth and maturation early in life. However, reptiles are unable to internally regulate their body temperature, and undergo strong seasonal change in behavior associated with environmental temperatures. Collectively, these characteristics may have profound effects on how reptiles partition energy resources to self-maintenance activities, including immune function. Hence, this study was performed in the fresh water snake, which is the first report of its kind. In this study, effects of in vitro testosterone on phagocytosis and cytotoxic responses of splenic macrophage as well as on mitogen induced splenic lymphocyte proliferation were studied.
Materials and Methods
Animals
Freshwater snakes, weighing 80–120g, were obtained from a local supplier who collected these animals in the suburbs of Varanasi (280 18′N; 830 01′E). As sexual dimorphism is evident, only males were used in this study during April and May, when animals were reproductively inactive [33]. Animals were brought to the unconditioned laboratory experiencing natural ambient environmental conditions (Max. Temp. 36–39 °C, Min. Temp.24–25 °C; photoperiod 12.5–13.20 h; Relative humidity 40–45%). Animals were housed in cages (size 50x30x30 cm). Each cage had wooden floor and frame with wire net sides, one side being window. Each cage had an earthen bowl (4 L capacity) filled with water to accommodate 4–5 snakes. Snakes were fed on small fishes once a week. Cages were cleaned, and bowl water was changed next day following feeding. Animals were acclimated to the laboratory conditions for two weeks, and experiments were performed. This study, involving use of snakes, was approved by Institutional Ethics Committee, Udai Pratap Autonomous College, Varanasi, India. The guidelines of the committee for the purpose of control and supervision of experiment on animals (CPCSEA), Ministry of Statistics & Programme Implementation, Government of India, were strictly followed in maintenance and sacrifice of animals.
Chemicals
MTT [3-(4, 5-dimethylthiozol-2-yl)-2, 5 diphenyl tetrazolium bromide], NBT (Nitroblue Tetrazolium salt), mitogens [PHA (Phytohemagglutinin), ConA (Concanavalin A) and LPS (Lipopolysaccharide)] and testosterone were purchased from Sigma Chemicals. Culture medium (RPMI-1640), lymphocyte separation medium (HiSep), L-glutamine, gentamycin, fetal bovine serum (FBS), dimethyl sulfoxide (DMSO), and other chemicals were purchased from Himedia Laboratories Pvt. Ltd. (India). The culture medium was supplemented with 1 µl ml–1 gentamycin, 10 µl ml–1 of 200 mM L-glutamine, 10 µl ml–1 anti-anti (Gibco) and 5% FBS and referred to as complete culture medium.
Preparation of macrophage monolayer
Snakes were given mild anesthesia of nembutal (sodium pentobarbital), spleen was taken out and then animals were sacrificed with nembutal overdose (100 mg/kg body weight). Care was taken to strictly follow the guidelines of Institutional Ethics Committee for experimentation on animals. Preparation of splenic macrophage monolayer and phagocytic assay were performed following the method of Mondal and Rai (1999,2001) [3], [32]. Briefly, spleen was excised under aseptic conditions and immediately macerated through a nylon strainer of pore size <100 µm into complete culture medium (2 ml per spleen) to get single cell suspension under a sterile laminar flow hood. Thereafter, the suspension was centrifuged at 600×g for 15 min and red blood corpuscles (RBCs) in pellet were lysed by hemolysate buffer. Cell viability, which exceeded 95%, was checked light microscopically through trypan blue exclusion test. Splenic cell suspension (200 µl) was flooded onto individual pre-washed and sterilized glass slides. Phagocytic macrophages were allowed to adhere by incubating the slides at 25°C in humidified CO2 atmosphere for 90 min. Non adherent cells were washed off with 0.2 M phosphate buffer saline (PBS; pH 7.2). The splenic macrophage monolayer was prepared in duplicate from each spleen. In the adherent cell population, more than 90% of the cells were macrophages as judged by their morphology.
Phagocytic assay
For phagocytic assay, the yeast cells were used as target. The yeast cell suspension was prepared by mixing 20 mg of commercial baker’s yeast (Saccharomyces cerevisae) in 10 ml of 0.2 M PBS. The suspension was kept at 80°C for 15 min. The cells were washed thrice in PBS and finally suspended in complete culture medium to get a concentration of 1x108 cells ml–1. The prepared macrophage monolayer, as above, was flooded with yeast cell suspension, and phagocytosis was allowed to proceed. Following 90 min incubation at 25°C in humidified CO2 atmosphere, the slides were rinsed thrice in PBS, fixed in methanol, stained with Giemsa, and examined under oil immersion. For each slide, a total of 100 macrophages were examined randomly without any predetermined sequence. The phagocytic index was determined by calculating the average number of yeast cells engulfed by single macrophage. The percent phagocytosis was calculated by counting the number of macrophages showing phagocytosis per 100 macrophages observed.
Dose-related effects of in vitro testosterone on phagocytosis
Splenic macrophages monolayer prepared on slides were exposed to five different concentrations (0.1, 1, 10, 50 and 100 ng ml–1) of testosterone. After 4 hour of incubation, the monolayers were washed three times to remove testosterone and phagocytosis was allowed to proceed.
NBT assay
NBT assay was performed following the methods of Berger and Slapnickova (2003) [34]. Splenocytes were counted and adjusted to 2x106 cells ml–1 in complete culture medium. Cell viability, checked through trypan blue exclusion test, exceeded 95%. Fifty microlitres of cell suspension (1x105 cells) was seeded with 50 µl of different concentrations of testosterone (final concentration of 10, 100 and 1000 ng ml–1) in wells of culture plate (96 well) and incubated for four hours in humidified CO2 atmosphere at 25°C, following which 50 µl of culture medium containing NBT (1 mg ml–1) was mixed. Well with Culture medium (150 ml) without cells served as blank. All assays were performed in triplicates for each spleen. Plate was then incubated for 2 h, centrifuged at 700×g, washed with PBS and fixed in 70% methanol. The formazan crystals, present inside the cells, were dissolved by mixing 20 µl of 0.1% triton X-100, 120 µl KOH (2 M) and 140 µl DMSO. Optical density was measured at 620 nm with the help of ELISA plate reader (Thermo Multiscan). Following blank subtraction, triplicates were averaged.
Nitrite assay
Nitrite content was measured by the method of Ding et al. (1988) [22]. Briefly, 100 µl of splenocytes (1x106 cells ml–1) was added in wells of culture plate (96 well). After two hours of incubation in CO2 atmosphere at 25°C, cells were washed with PBS. Fifty microlitres of fresh culture medium and 50 µl of different testosterone concentrations (final concentration of 10, 100 and 1000 ng ml−1) were added to each well, and then plates were incubated at for 24 h. Following incubation, plates were then centrifuged at 200×g, and supernatant was collected. Equal volume (50 µl) of supernatant (macrophage conditioned medium) and Griess reagent (1% sulfanilamide in 3N HCl and 0.1% naphthylenediamine dihydrochloride in distilled water) are mixed, and the optical density of solution was measured at 540nm with the help of ELISA plate reader (Thermo Multiscan). Culture medium without cells served as blank. All assays were performed in triplicates. Following blank subtraction, the triplicates were averaged. The standard curve of sodium nitrite was used to calculate the amount of nitrite in the conditioned medium. The concentration of nitrite is expressed in micromole (µM).
Splenic lymphocyte proliferation assay
Splenic single cell suspension prepared as above was treated with hemolysate buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2), washed with 0.2 M PBS (pH 7.2) thrice and resuspended in complete culture medium. Splenic lymphocytes were isolated by density gradient centrifugation using HiSep (Density 1.077 g ml–1). The cell suspension was overlaid on equal volume of HiSep and centrifuged at 400×g for 30 min with brakes off at 8°C. Following centrifugation, lymphocyte fraction at the interface between medium and HiSep was carefully aspirated, washed three times with PBS, counted and assessed for viability on a hemocytometer through trypan blue exclusion test. Viable cells (>95%) were adjusted to 2x106 cells ml–1 in complete culture medium.
Basal as well as mitogen-induced in vitro lymphocyte proliferation was assessed using colorimetric assay based on tetrazolium salt (MTT) following the method of Berridge et al. (2005) [35]. Stock solution of mitogen was made in 0.2 M PBS (pH 7.2) at a concentration of 1 mg ml–1. Further dilution was made in culture medium. Flat bottom 96 well culture plates were used. To study basal or spontaneous proliferation, 50 µl splenic lymphocyte suspension was seeded into well of culture plate along with 150 µl of mitogen-free culture medium. Additional well contained only 200 µl of culture medium and served as blank. To study mitogen induced proliferation, 50 µl of different mitogens (Con A, PHA and LPS; final concentration was 10 µg ml–1), 50 µl cell suspension (2x106 cells ml–1) and 100 µl of culture medium (total volume 200 µl) were seeded into well of culture plate. To study effect of in vitro testosterone, 50 µl of different mitogens, 50 µl cell suspension (2x106 cells ml–1) and 100 µl of culture medium containing different concentrations of testosterone (having final concentration of 10, 100 and 1000 ng ml–1) were added to each well. A set of wells that did not contain testosterone served as control. All samples were assayed in triplicates. Plates were incubated in humidified CO2 atmosphere at 25°C for 48 h. Following incubation, 20 µl of MTT reagent (5 mg ml–1) was added to each well, and plates were again incubated overnight in humidified CO2 atmosphere at 25°C. After incubation, the plates were centrifuged at 400×g for 10 min at 8°C. The supernatant was aspirated, and 100 µl of DMSO was added to each well to solubilize the formazan crystals. Absorbance was measured at 570 nm with the help of ELISA plate reader (Thermo Multiscan). Following blank subtraction, the triplicates are averaged.
Statistical Analysis
Each experiment was repeated thrice. Data are presented as mean ± SEM. Means were compared by Analysis of Variance (ANOVA) followed by Newman Keuls multiple-range test.
Result
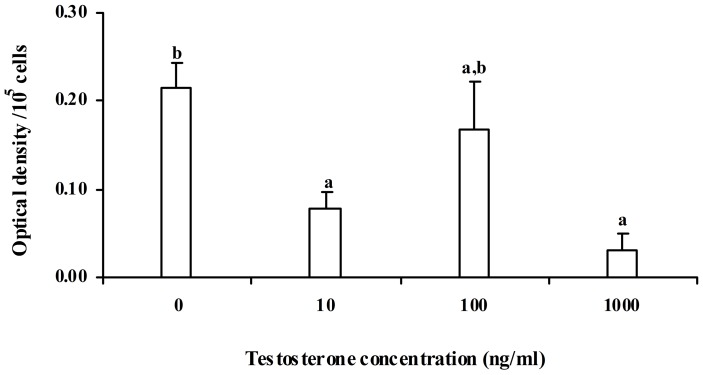
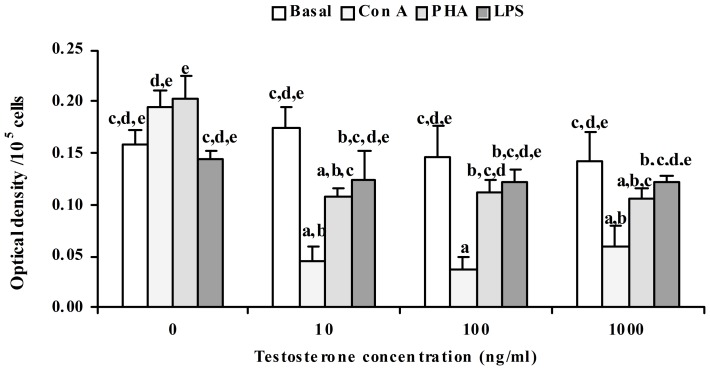
In vitro testosterone treatment significantly (p<0.05) decreased the percentage phagocytosis by splenic macrophages, but, there was no change in phagocytic index (Fig. 1). Further, it was observed that decrease in percentage phagocytosis was significantly correlated with the in vitro testosterone concentration. Maximum reduction of percentage phagocytosis was obtained at 100 ng ml–1 concentration of testosterone. Our pilot experiment showed that testosterone below 10 ng ml–1 concentrations was not sufficient to influence NBT reduction and nitrite release. Hence, higher concentrations of testosterone, 10, 100 and 1000 ng ml–1 were used. Effect of in vitro testosterone on NBT reduction was found to be differential, as NBT reduction was decreased at 10 ng ml–1, but was unaffected at 100 ng ml–1, and again decreased at 1000 ng ml–1. The decrease was maximum at 1000 ng ml−1 testosterone concentration (Fig. 2). Nitrite release was significantly (p<0.05) reduced by in vitro testosterone treatment in a concentration dependent manner (Fig. 3). When the macrophages were pre-incubated with testosterone receptor antagonist, cyproterone acetate (CPA), the decrease in Nitrite release was alleviated, as Nitrite release was comparable to that of control splenocytes incubated in medium alone. There was no change in basal proliferation of splenic lymphocytes in relation to in vitro testosterone treatment; while Con A and PHA induced proliferative response reduced significantly (p<0.05) by in vitro testosterone. The reduction in Con A induced proliferative response was more apparent than that PHA induced one. However, there was no change in LPS induced proliferative response (Fig. 4).
Figure 1. Effect of different concentrations of testosterone on splenic macrophage phagocytosis in the fresh water snake, Natrix piscator.
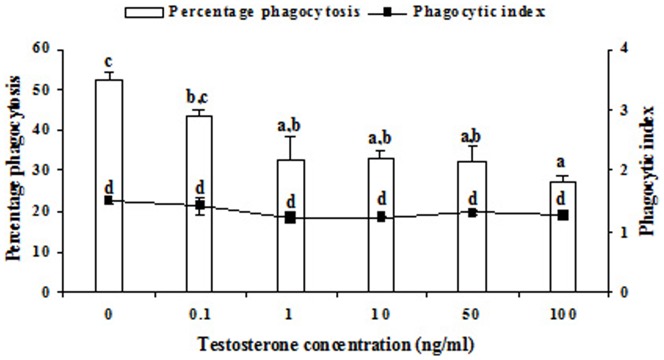
The error bars bearing the same superscript do not differ significantly (Newman–Keul’s multiple-range test, p<0.05).
Figure 2. Effect of different concentrations of testosterone on NBT reduction in Natrix piscator.
The error bars bearing the same superscript do not differ significantly (Newman–Keul’s multiple-range test, p<0.05).
Figure 3. Effect of different concentrations of testosterone (T 10 to 1000– Testosterone 10 to 1000ng ml−1) on nitrite release in Natrix piscator.

Effect of testosterone receptor antagonist cyproterone acetate (CPA, 100 ng ml−1) is also shown. The error bars bearing the same superscript do not differ significantly (Newman–Keul’s multiple-range test, p<0.05).
Figure 4. Effect of in vitro testosterone on mitogen induced splenocyte proliferation in Natrix piscator.
Mitogens: ConA – Concanavalin A, 10 µg ml–1; PHA – Phytohemagglutinin 10 µg ml–1; LPS – Lipopolysaccharide, 20 µg ml–1). The error bars bearing the same superscript do not differ significantly (Newman–Keul’s multiple-range test, p<0.05).
Discussion
The effect of gonadal steroids on lymphoid tissues is well known even before the importance of thymus in immune function had been recognized; researchers have noted thymic hypertrophy in response to gonadectomy [36]. The sex-related differences in immune capabilities are well established in mammals [37]. Females exhibit enhanced immunoreactivity compared to males as shown by higher immunoglobulin levels, better response to heteroantigens, and ability to combat infections [1], [14]. So far, the sex associated differences in immune parameters in reptiles are concerned, a few reports are available: in vivo administration of testosterone in either male or female lizard, Chalcides ocellatus, induces significant depletion of lymphoid elements, serum antibody titer to rat RBC and increase in skin allograft survival [38]. Decrease in the percentage of cortex, number of cortical and medullary lymphocytes in thymus and spleen of turtles, Mauremys caspica, have been found following testosterone administration [39], [40].
In the present investigation on cellular (splenocytes) immune responses, we found that the percentage phagocytosis was significantly decreased, when splenic macrophages were treated with testosterone, but no change occurred in the phagocytic index. Effect of testosterone was concentration dependent, and maximum reduction was obtained at 1000 ng ml−1 concentration. This finding corroborates to that of Mondal and Rai (1999, 2002) [3], [41], who reported that dihydrotestosterone had a suppressive effect, in a dose and duration dependent manner, on macrophage phagocytosis in wall lizard. Inhibition of macrophage phagocytosis was reported by in vitro testosterone in broiler chickens [42]. Implant of testosterone significantly reduced humoral immunity in both sexes of wild starling birds [43].
Reduction in nitrite release by snake splenic macrophages in a concentration dependent manner was found when treated with in vitro testosterone. Similarly, dose and time dependent sex steroids hormone regulation of nitrite release has been demonstrated by Mondal and Rai (2002) [41] in lizard’s splenic macrophages; but the results of present investigation differ from their study, as they have reported reduction at sex steroids concentrations ranging from 10−4 ng ml−1 to 104 ng ml−1; while we failed to find reduction in nitrite release at in vitro testosterone concentration below 10 ng ml−1: the reason might be that they have prestimulated macrophages by LPS before in vitro testosterone treatment.
In present study, effect of in vitro testosterone on NBT reduction was found to be differential, as NBT reduction was decreased at 10 ng ml–1 testosterone concentration, but was unaffected at 100 ng ml–1, and again decreased at 1000 ng ml–1. Such a biphasic effect of in vivo and in vitro estradiol on macrophage phagocytosis was reported in wall lizard [3]. The reduction of NBT is carried out by the superoxide anion (O2 –) produced by granulocytes and macrophages. NBT reduction test is a measure of activation of oxidative burst, which has a high reactive microbicidal effect [44] in response to antigenic stimulation. NBT reduction test in absence of antigenic stimulation is also an indirect measure of the intracellular hexosemonophosphate shunt activity [45]. In the present study, it has been demonstrated that reptilian splenic macrophages seem able to produce an increase amount of O2 – and testosterone could modulate the metabolic pathway mentioned above.
The reports on sex steroids-induced modulation of macrophage cytotoxic function in mammals present a very confusing picture. Ovariectomy in mice diminished IL-1 secretion, whereas castration had no effect [25], [26]. The castration also failed to influence the in vitro TNF-α release by rodent macrophages [30]. On the contrary, the castration in male mice enhanced IL-1 and TNF- α secretion by peritoneal macrophages [46]. Similarly, gonadectomy irrespective of sex has been reported to increase the nitrite release by murine peritoneal macrophages suggesting that the sex hormone deprivation results in an increase in amount of nitrite release [28]. Savita and Rai (1998) [28] also reported that in vitro treatment of estradiol, testosterone and progesterone markedly reduced the nitrite release from LPS-activated macrophages in a dose and time-dependent manner in mice. In other studies in mammals, in vitro testosterone treatment reduces the phagocytic capacity of macrophages and inhibits nitrite release by macrophages [27], [47]. The present investigation suggests that testosterone modulates nitrite production via a receptor-mediated system, as treatment with testosterone receptor antagonist, cyproterone acetate (CPA), interfered with the action of testosterone and considerably reduced the testosterone-induced suppression of nitrite release of splenocytes though Benten et al. (2002) [48] have demonstrated a membrane testosterone receptor on lymphocyte, enabling a nongenomic response to testosterone in T-cells.
Reports on testosterone and lymphocytes proliferation in vertebrate species present a gloomy picture, as the deprivation of male sex steroid through castration either has no effect on lymphocytes proliferation [49] or decreased proliferation in hamster [50] or increased the splenocyte proliferation in rodent [15], [17] as well as circulating testosterone level is not related to splenocyte proliferation in voles [51]. Marco et al. (2009) [52] reported no significant effect of testosterone on lymphocyte proliferation or cytokine secretion in mice. Al-Afaleq and Homeida (1998) [42] reported in vitro testosterone caused inhibition of lymphocyte proliferation in Broiler chickens. In the present study, T-cell mitogen (Con A and PHA) and B-cell mitogen (LPS) induced splenocyte proliferation was studied in relation in vitro testosterone treatment. Basal proliferation did not change when different concentrations of testosterone were used. T-cell mitogen induced proliferation was suppressed by different concentrations of in vitro testosterone, and the suppression was more apparent in Con A induced proliferation. In contrast, B-cell mitogen (LPS) induced proliferation did not change significantly. The present results suggest that T and B cells of this reptilian species respond differently to the male sex steroid. Present finding reveals testosterone as inhibitory to splenocytes’ immune responses in Natrix piscator. Further, it may be suggested that by suppressing immune responses, testosterone may, therefore, act as a physiological mechanism regulating the relative amount of energy invested into either reproductive effort or immunocompetence.
Acknowledgments
First author is indebted to Council of Scientific and Industrial Research, India for the award of Junior and Senior Research Fellowship.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data underlying the finding of this study are available within the paper.
Funding Statement
This work was supported by a financial grant (SR/SO/AS –15/2005) from the Department of Science and Technology, New Delhi, Govt. of India to Ramesh Singh. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Eidinger D, Garrett TJ (1972) Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med 136: 1098–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billingham RE (1986) Immunologic advantages and disadvantages of being a female. In D. A. Clarke and B. A. Croy (Eds.), Reproductive Immunology; Elsevier, New York pp. 109.
- 3. Mondal S, Rai U (1999) Sexual dimorphism in phagocytic activity of wall lizard’s splenic macrophages and its control by sex steroids. Gen Com Endocrinol 116: 291–298. [DOI] [PubMed] [Google Scholar]
- 4. Pawlak J, Brito V, Kuppers E, Beyer C (2005) Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res 138: 1–7. [DOI] [PubMed] [Google Scholar]
- 5. Rajput ZI, Song-hua HU, Arijo AG, Habib M, Khalid M (2005) Comparative study of anaplasma parasites in tick carrying buffaloes and cattle. J Zhejiang Univ SCI 6: 1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramzan M, Akhtar M, Muhammad F, Hussain I, Hiszczyńska-Sawicka E, et al. (2009) Seroprevalence of Toxoplasma gondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Trop Anim Health Prod 41(7): 1225–1229. [DOI] [PubMed] [Google Scholar]
- 7. Poulin R (1996) Helminth growth in vertebrate hosts: does host sex matter? Int J Parasitol 26: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 8. Zuk M, McKean KA (1996) Sex differences in parasite infections: Patterns and processes. Int J Parasitol 26: 1009–1024. [PubMed] [Google Scholar]
- 9. Alexander J, Stimson WH (1988) Sex hormones and the course of parasitic infection. Parasitol Today 4: 189–193. [Google Scholar]
- 10. Sano A, Miyaji M, Nishimura K (1992) Studies on the relationship between the estrous cycle of BALB/c mice and their resistance to Paracoccidioides brasiliensis infection. Mycopathologia 14: 63–87. [DOI] [PubMed] [Google Scholar]
- 11. Tiuria R, Horii Y, Makimura S, Ishikawa N, Tsuchiya K, et al. (1995) Effect of testosterone on the mucosal defense against intestinal helminths in Indian softfurred rats, Millardia meltada with reference to goblet and mast cell responses. Parasite Immunol 17: 479–484. [DOI] [PubMed] [Google Scholar]
- 12. Klein SL, Gamble HR, Nelson RJ (1999) Role of steroid hormones in Trichinella spiralis infection among voles. Am J Physiol Regul Integr Comp Physiol 277: R1362–R1367. [DOI] [PubMed] [Google Scholar]
- 13. Klein SL (2000) The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 24: 627–638. [DOI] [PubMed] [Google Scholar]
- 14. Butterworth M, McClellan B, Allansmith M (1967) Influence of sex on immunoglobulin levels. (Abstract). Nature 214: 1224–1225. [DOI] [PubMed] [Google Scholar]
- 15. Schuurs AHWM, Verheul HAM (1990) Effects of gender and sex steroids on the immune response. J Steroid Biochem 35: 157–172. [DOI] [PubMed] [Google Scholar]
- 16. Olsen NJ, Kovacs WJ (1996) Gonadal steroids and immunity. Endocr Rev 17: 369–384. [DOI] [PubMed] [Google Scholar]
- 17. Anja C, Michael TM, Samuel DT, Maria M, Susan M, et al. (2004) Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol 173: 6098–6108. [DOI] [PubMed] [Google Scholar]
- 18. Grossman CJ (1984) Regulation of the immune system by sex steroids. Endocr Rev 15: 435–55. [DOI] [PubMed] [Google Scholar]
- 19. Grossman CJ (1985) Interactions between gonadal steroids and the immune system. Science 227: 257–261. [DOI] [PubMed] [Google Scholar]
- 20.Adams DO, Hamilton TA (1992) Molecular basis of macrophage activation: diversity and its origins. In: Lewis CE, McGee JOD (eds) The Natural Immune System: The Macrophage. IRL Press, Oxford 75–114.
- 21. Keller R (1993) The macrophage response to infectious agents: Mechanisms of macrophage activation and tumor cell killing. Res Immunol 144: 271–273. [DOI] [PubMed] [Google Scholar]
- 22. Ding AH, Nathan CF, Stuehr DJ (1988) Release of reactive nitrogen intermediated and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol 141: 2407–2412. [PubMed] [Google Scholar]
- 23. Lachman LB, Dinarello CA, Llansa ND, Fidler IJ (1986) Natural and recombinant human interleukin 1-P is cytotoxic for human melanoma cells. J Immunol 136: 3098–3102. [PubMed] [Google Scholar]
- 24. Philip R, Epstein LB (1986) Tumor necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gammainterferon and interleukin-1. Nature 323: 86–89. [DOI] [PubMed] [Google Scholar]
- 25. Hu SK, Mitcho YL, Rath NC (1988) Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol 10: 247–252. [DOI] [PubMed] [Google Scholar]
- 26. Da Silva JA (1995) Sex hormones, glucocorticoids and autoimmunity: facts and hypotheses. Ann Rheum Dis 54: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chao TC, Van Alten PJ, Greager JA, Walter RJ (1995) Steroid sex hormones regulate the release of tumor necrosis factor by macrophages. Cell Immunol 160: 43–49. [DOI] [PubMed] [Google Scholar]
- 28. Savita, Rai U (1998) Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 119: 199–204. [DOI] [PubMed] [Google Scholar]
- 29. Boorman GA, Luster MI, Dean JH, Wilson RE (1980) The effect of adult exposure of diethylstilbesterol in the mouse on macrophage function and numbers. J Reticuloendothel Soc 28: 547–559. [PubMed] [Google Scholar]
- 30. Hughes TK, Fulep E, Juelich T, Smith EM, Stanton GL (1995) Modulation of immune responses by anabolic androgenic steroids. Int J Immunopharmacol 17: 857–863. [DOI] [PubMed] [Google Scholar]
- 31. Flynn A (1986) Expression of Ia and the production of interleukin-1 by peritoneal exudate macrophages activated in vivo by steroids. Life Sci 38: 2455–2460. [DOI] [PubMed] [Google Scholar]
- 32. Mondal S, Rai U (2001) In vitro effect of temperature on phagocytic and cytotoxic activities of splenic phagocytes of the wall lizard, Hemidactylus flaviviridis . Comp Biochem Physiol 129(a): 391–398. [DOI] [PubMed] [Google Scholar]
- 33. Haldar C, Pandey R (1989) Effect of pinealectomy on testicular cycle of Indian checkered water snake Natrix piscator. . Gen Comp Endocrinol 76: 214–222. [DOI] [PubMed] [Google Scholar]
- 34. Berger J, Slapnickova M (2003) Circadian structure of rat neutrophil phagocytosis. Comp Clin Pathol 12: 84–89. [Google Scholar]
- 35. Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol Annu Rev 11: 127–152. [DOI] [PubMed] [Google Scholar]
- 36.Hammar JA (1929) Die Menschenthymus in Gesundheit und Krankheit. Teil II. Das Organ unter anormalen Körperverhältnissen. Zugleich Grundlagen der Theorie der Thymusfunktion. Zeitschr mikrosk-anat Forsch Erg.-Bd 16.
- 37. Cannon JG, St Pierre BA (1997) Gender differences in host defense mechanisms. J Psychiat Res 31: 99–113. [DOI] [PubMed] [Google Scholar]
- 38. Saad AH, Khalek NA, Ridi R (1990) Blood testosterone level: A season-dependent factor regulating immune reactivity in lizards. Immunobiol 180: 184–194. [DOI] [PubMed] [Google Scholar]
- 39. Varas A, Saad AH, Torroba M, Zapata A (1991) Structural changes in the thymus gland of turtle following testosterone treatment. Thymus 17: 129–132. [PubMed] [Google Scholar]
- 40. Varas A, Torroba M, Zapata AG (1992) Changes in the thymus and spleen of the turtle Mauremis caspica after testosterone injection: A morphometric study. Dev Comp Immunol 16: 165–174. [DOI] [PubMed] [Google Scholar]
- 41. Mondal S, Rai U (2002) In vitro effect of sex steroids on cytotoxic activity of splenic macrophages in wall lizard (Hemidactylus flaviviridis). Gen Comp Endocrinol 125(2): 264–271. [DOI] [PubMed] [Google Scholar]
- 42. al-Afaleq AI, Homeida AM (1998) Effects of low doses of oestradiol, testosterone and dihydrotestosterone on the immune response of broiler chicks. Immunopharmacol Immunotoxicol 20: 315–327. [DOI] [PubMed] [Google Scholar]
- 43. Deborah LD, George EB, Deborah LD, Gregori FB (2000) Effects of testosterone on cell mediated and humoral immunity in non breeding adult European starlings. Behav Ecol 11: 654–662. [Google Scholar]
- 44. Bagasra O, Howeedy A, Kajdacsy-Balla A (1988) Macrophage function in chronic experimental alcoholism I. modulation of surface receptors and phagocytosis. Immunol 65: 405–409. [PMC free article] [PubMed] [Google Scholar]
- 45. Park BH, Fikrig SM, Smithwick EM (1968) Infection and nitroblue-tetrazolium reduction by neutrophils: A diagnostic aid. Lancet 2: 532–234. [DOI] [PubMed] [Google Scholar]
- 46. Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ (1995) Castration alters peripheral immune function in normal male mice. Immunology 84: 337–342. [PMC free article] [PubMed] [Google Scholar]
- 47. Straub RH, Cutolo M (2001) Involvement of the hypothalamic– pituitary–adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic pathogenetic role. Arthritis Rheum 44: 493–507. [DOI] [PubMed] [Google Scholar]
- 48. Benten WP, Stephan C, Wunderlich F (2002) B cells express intracellular but not surface receptors for testosterone and estradiol. Steroids 67(7): 647–654. [DOI] [PubMed] [Google Scholar]
- 49. Demas GE, Nelson RJ (1998) Photoperiod ambient temperature and food availability interact to affect reproductive and immune function in adult male deer mice (Peromyscus maniculatus). J Comp Physiol B 168: 419–426. [DOI] [PubMed] [Google Scholar]
- 50. Bilbo SD, Nelson RJ (2001) Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 280: R207–R213. [DOI] [PubMed] [Google Scholar]
- 51. Klein SL, Hairston JE, Devries AC, Nelson RJ (1997) Social environmentt and steroid hormones affect species and sex differences in immune function among voles. Horm Behav 32: 30–39. [DOI] [PubMed] [Google Scholar]
- 52. Marco AD, Karen N, Gloria S, Lorena L, Jesus R, et al. (2009) Immune sexual dimorphism: Effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J Steroid Biochem Mol Biol 113: 57–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data underlying the finding of this study are available within the paper.