Abstract
Background
Stroke is a major cause of morbidity and mortality. We describe trends in the incidence, outcomes, and risk factors for stroke in the US Medicare population from 1988 to 2008.
Methods
We analyzed data from a 20% sample of hospitalized Medicare beneficiaries with a primary discharge diagnoses of ischemic (n=918,124) or hemorrhagic stroke (n=133,218). Stroke risk factors were determined from the National Health and Nutrition Examination Survey (years 1988–1994, 2001–2008) and medication uptake from the Medicare Current Beneficiary Survey (years 1992–2008). Primary outcomes were stroke incidence and 30-day mortality after stroke hospitalization.
Results
Ischemic stroke incidence fell from 927 per 100,000 in 1988 to 545 per 100,000 in 2008, and hemorrhagic stroke from 112 per 100,000 to 94 per 100,000. Risk adjusted 30-day mortality declined from 15.9% in 1988 to 12.7 % in 2008 for ischemic stroke and from 44.7% to 39.3% for hemorrhagic stroke. Although observed stroke rates declined, the Framingham stroke model actually predicted increased stroke risk (mean stroke score 8.3% in 1988–1994, 8.8% in 2005–2008). Statin use in the general population increased (4.0% in 1992, 41.4% in 2008) as did antihypertensive use (53.0% in 1992, 73.5% in 2008).
Conclusion
Incident strokes in the ≥65 years Medicare population fell by nearly 40% over the last 2 decades, a decline greater than expected based on the population’s stroke risk factors. Case-fatality from stroke also declined. Although causality cannot be proven, declining stroke rates paralleled increased use of statins and antihypertensive medications.
Keywords: stroke, ischemic stroke, hemorrhagic stroke, mortality, outcomes, statins, antihypertensive medications, risk, trends
Introduction
The burden of stroke in the United States (US) is enormous. With approximately 795,000 strokes occurring each year, stroke is the fourth leading cause of death in the US and a major source of morbidity and costs of care.1, 2 With the aging population, the absolute number of strokes in the US is likely to increase. However, stroke is a preventable disease. Several prevention strategies have been shown to be efficacious in clinical trial settings, in particular, modification of stroke risk factors such as hypertension and hyperlipidemia.1, 3, 4
Shedding light on trends in the burden of stroke among Medicare beneficiaries may provide important information for policy purposes, including describing the past and current scope of the condition, assessing the potential effect of stroke-prevention interventions on a national level, and identifying areas where resources can be targeted more specifically and effectively. The primary objective of this analysis was to examine two decade long trends from 1988 to 2008 in the incidence of, and subsequent short-term mortality from, stroke among individuals aged ≥ 65 years in the US. We then assessed factors that could explain changes in the incidence of ischemic stroke, the highest burden subtype among US elderly. We focused on trends in clinical risk factors for stroke, as well as trends in the uptake of medications that attenuate stroke risk. Rates and outcomes of stroke were determined separately for men and women due to increasing evidence that there are sex-related differences in stroke incidence, risk factors, treatment, and outcomes.
Methods
Data Sources, Stroke Incidence and Mortality
We used a 20% sample of Medicare Provider Analysis and Review (MedPAR) data to identify fee-for-service Medicare beneficiaries ≥65 years hospitalized for stroke between 1988 and 2008. Stroke hospitalizations were identified as primary discharge diagnoses of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 434.x and 436.x for ischemic stroke and 430.x and 431.x for hemorrhagic stroke. Only the first hospitalization for stroke during the time period was considered and was considered the index event date for each individual. Although these methods assume that the stroke event was incident, we lacked information about potential stroke events that may have occurred at ages younger than 65 years. We also searched for primary discharge diagnosis of transient ischemic attack (ICD-9-CM code 435.x) because advances in imaging techniques may have resulted in increasing numbers of transient ischemic attacks being coded as acute ischemic strokes over time.
Medicare denominator files were used to ascertain beneficiaries’ date of birth, sex, race (categorized as black, white, or other), enrollment status, region of residence (Midwest, Northeast, South, or West), and vital status (including date of death). We excluded patients residing outside the United States, (n=11,668, 1%) as well as patients enrolled in a Medicare health maintenance organization during the study period (n=146,493, 14%), as MedPAR data may not capture complete health care claims data for these individuals.
Information on clinical comorbidities were obtained using a two-year look-back window from the index admission date, excluding diagnoses obtained solely from the index admission. The comorbidities included in the Klabunde adaptation of the Charlson comorbidity index were used for risk adjustment.5, 6
Risk Factors and Preventive Medications
To examine possible explanations for reductions in stroke incidence, we calculated the predicted risk of stroke at three different time intervals using the Framingham stroke risk index, a validated instrument developed in the Framingham Heart Study cohort.7 We obtained data on the prevalence and physiologic level of stroke risk factors among patients from the Third National Health and Nutrition Examination Survey (NHANES III) aged 65 years or older with Medicare coverage using surveys conducted from 1988 through 1994, as well as from the continuous NHANES survey data from 2001 through 2008. The NHANES is a nationally-representative survey of the civilian, non-institutionalized population, which collects detailed data on medical conditions through direct patient interview and physiologic measurements through a mobile examination component.8 The prevalence of specific risk factors for stroke included self-reported coronary heart disease, congestive heart failure, diabetes, vascular disease, and hyperlipidemia. In addition, we obtained data on measured blood pressure, body mass index, total cholesterol, low density lipoprotein cholesterol levels, and self-reported smoking status.
A number of medications have been shown to both prevent strokes and to reduce the severity of strokes that do occur, including statins, antihypertensive agents, and antiplatelet medications.1, 9 We obtained rates of use of these medications among the 65 years and older Medicare population using the 1992–2008 Medicare Current Beneficiary Survey (MCBS). The MCBS is conducted on a representative sample of Medicare beneficiaries drawn from Medicare enrollment databases.10 Community-dwelling adults provide self-reported information on medication use, which is complemented with benefit statements, pharmacy receipts, and medication containers provided to the interviewers three times yearly, as well as Medicare Part D prescription databases for 2006–2008. We searched for use of antihypertensive medications (including ACE-inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, diuretics, and other antihypertensive agents), statins, and non-aspirin antiplatelet agents (clopidogrel and ticlopidine). Use of aspirin, because it is available without prescription, was not completely captured by the MCBS and was not included in the analysis.
Outcomes Measures
Primary outcomes included stroke incidence and 30-day mortality, stratified by stroke subtype (ischemic versus hemorrhagic). Secondary outcomes included predicted stroke risk applying the Framingham risk equation to NHANES risk factors, and rates of medication use from MCBS.
Data Analysis
Annual rates of hospitalization for first stroke were calculated for ischemic and hemorrhagic strokes among fee-for-service Medicare beneficiaries 65 years or older, and reported as number of events per 100,000 beneficiaries. Rates were standardized to the age and sex distribution of the year 2000 population and trends in the risk of stroke as a function of year were assessed using logistic regression, controlling for age. Changes in the demographic and clinical characteristics of stroke patients from 1988 to 2008 were examined separately for ischemic and hemorrhagic strokes. Analysis of variance was used for comparison of continuous variables and chi square tests for categorical variables.
Sex-specific mortality rates were ascertained at 30 days following the index stroke admission and are reported separately for ischemic and hemorrhagic strokes. Risk adjusted 30-day mortality rates were calculated using logistic regression, controlling for age, sex (except for sex-stratified analyses), race, region, and medical comorbidities.
We calculated a mean Framingham stroke risk score for NHANES respondents aged ≥ 65 years with Medicare coverage at three time intervals: 1988–1994, 2001–2004, and 2005–2008. The Framingham stroke risk score incorporates specific clinical risk factors (age, systolic blood pressure, antihypertensive treatment, diabetes mellitus, smoking status, cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy) to calculate a 10-year risk of stroke.7 Electrocardiographic data on left ventricular hypertrophy were available only for NHANES participants in years 1988–1994 so for subsequent years, left ventricular hypertrophy prevalence was estimated using data from the medical literature.11 Similarly, atrial fibrillation prevalence was obtained from a population-based study reporting atrial fibrillation rates for years 1992–2002.12 The prevalence of atrial fibrillation for earlier and later years was imputed assuming a linear trend, and we also ran the analysis assuming no change in atrial fibrillation rates. The 10-year Framingham stroke risk score was calculated for each individual in the NHANES, and risks were then averaged over each time interval and reported as mean and standard deviation for each of the three time periods.
Finally, we tested for changes in the self-reported use of specific classes of medications used to prevent stroke using data from the MCBS. Rates were calculated as the proportion of MCBS participants reporting use of specific medications divided by the total number of MCBS participants in that given year. Linear regression was used to test for changes in the rate of use by year.
Analyses were performed using the SAS 9.2 statistical package (SAS Institute, Cary, NC). Risk factor and medication trend analyses took into account the complex survey sample design of both the NHANES and MCBS surveys. Significance was set at P < 0.05 using two-sided tests for all analyses. This study was approved by the Institutional Review Board of the University of California, San Francisco.
Results
Trends in Stroke Incidence
We identified 1,051,342 incident strokes from 1988 to 2008 (representing 20% of Medicare claims) of which 918,124 (87.3%) were ischemic and 133,218 (12.7%) hemorrhagic strokes. Patients presenting with stroke frequently had concomitant risk factors, the most prevalent being hypertension, diabetes, and hyperlipidemia (Tables 1 and 2). A prior diagnosis of transient ischemic attack at age ≥ 65 was observed among 64,749 (7.1%) patients with ischemic stroke and 6,261 (4.7%) patients with hemorrhagic stroke.
Table 1.
Baseline Characteristics of Medicare Patients With a Ischemic Stroke Stratified by Sexa (1998–2008)
| No. (%) of Men b
|
No. (%) of Women b
|
|||||
|---|---|---|---|---|---|---|
| 1988–1994 (n=139,200) | 1995–2001 (n=124,905) | 2002–2008 (n=101,960) | 1988–1994 (n=202,302) | 1995–2001 (n= 194,732) | 2002–2008 (n=155,025) | |
| Age, y | ||||||
| 65–69 | 23,515 (16.9) | 19,237 (15.4) | 15,324 (15.0) | 21,262 (10.5) | 18,448 (9.5) | 13,940 (9.0) |
|
| ||||||
| 70–74 | 30,439 (21.9) | 25,831 (20.7) | 19,212 (18.8) | 31,438 (15.5) | 28,518 (14.6) | 20,425 (13.2) |
|
| ||||||
| 75–79 | 32,503 (23.4) | 28,779 (23.0) | 22,163 (21.7) | 40,936 (20.2) | 38,479 (19.8) | 28,158 (18.2) |
|
| ||||||
| 80–84 | 27,627 (19.9) | 25,660 (20.5) | 21,704 (21.3) | 44,819 (22.2) | 43,069 (22.1) | 34,843 (22.5) |
|
| ||||||
| ≥85 | 25,116 (18.0) | 25,398 (20.3) | 23,557 (23.1) | 63,847 (31.6) | 66,218 (34.0) | 57,659 (37.2) |
|
| ||||||
| Race | ||||||
| White | 118,762 (85.3) | 106,874 (85.6) | 85,775 (84.1) | 172,756 (85.4) | 164,980 (84.7) | 130,135 (83.9) |
|
| ||||||
| Black | 13,998 (10.1) | 13,232 (10.6) | 11,431 (11.2) | 22,682 (11.2) | 22,860 (11.7) | 18,257 (11.8) |
|
| ||||||
| Other | 6,440 (4.6) | 4,799 (3.8) | 4,754 (4.7) | 6,864 (3.4) | 6,892 (3.5) | 6,633 (4.3) |
|
| ||||||
| Region | ||||||
| Midwest | 36,631 (26.3) | 33,770 (27.0) | 26,682 (26.2) | 52,457 (25.9) | 51,359 (26.4) | 39,910 (25.7) |
|
| ||||||
| Northeast | 30,388 (21.8) | 24,598 (19.7) | 18,745 (18.4) | 44,963 (22.2) | 38,841 (20.0) | 29,602 (19.1) |
|
| ||||||
| South | 51,827 (37.2) | 50,021 (40.1) | 41,936 (41.1) | 76,446 (37.8) | 80,239 (41.2) | 64,849 (41.8) |
|
| ||||||
| West | 20,354 (14.6) | 16,516 (13.2) | 14,597 (14.3) | 28,436 (14.1) | 24,293 (12.5) | 20,664 (13.3) |
|
| ||||||
| Length of stay, median (25th–75th pct.), d | 7 (4–12) | 5 (3–9) | 4 (3–7) | 8 (5–13) | 5 (3–9) | 5 (3–7) |
|
| ||||||
| Discharge type | ||||||
| Home, self-care | 58,473 (42.0) | 45,848 (36.7) | 32,471 (31.9) | 69,584 (34.4) | 56,633 (29.1) | 36,450 (23.5) |
|
| ||||||
| Skilled nursing facility | 20,221 (14.5) | 25,029 (20.0) | 20,001 (19.6) | 41,385 (20.5) | 51,970 (26.7) | 41,501 (26.8) |
|
| ||||||
| Other type of inpatient facility | 29,096 (20.9) | 30,785 (24.7) | 30,147 (29.6) | 42,704 (21.1) | 47,974 (24.6) | 45,806 (29.6) |
|
| ||||||
| Dead | 15,766 (11.3) | 10,392 (8.3) | 6,489 (6.7) | 22,416 (11.1) | 16,100 (8.3) | 10,324 (6.7) |
|
| ||||||
| Other | 15,644 (11.2) | 12,851 (10.3) | 12,852 (12.6) | 26,213 (13.0) | 22,055 (11.3) | 20,944 (13.5) |
|
| ||||||
| Comorbid Conditions | ||||||
| Myocardial Infarction | 8,902 (6.4) | 11,924 (9.55) | 10,341 (10.14) | 9,841 (4.86) | 14,298 (7.34) | 12,414 (8.01) |
|
| ||||||
| Congestive heart failure | 17,711 (12.72) | 20,503 (16.41) | 18,147 (17.8) | 31,189 (15.42) | 37,717 (19.37) | 32,219 (20.78) |
|
| ||||||
| Hypertension | 26,107 (18.76) | 43,007 (34.43) | 48,069 (47.14) | 50,187 (24.81) | 82,922 (42.58) | 85,843 (55.37) |
|
| ||||||
| Diabetes | 18,133 (13.03) | 23,852 (19.1) | 24,055 (23.59) | 29,710 (14.69) | 37,735 (19.38) | 34,789 (22.44) |
|
| ||||||
| Atrial fibrillation | 14,638 (10.52) | 18,907 (15.14) | 18,132 (17.78) | 25,418 (12.56) | 33,042 (16.97) | 30,882 (19.92) |
|
| ||||||
| Vascular disease | 6,843 (4.92) | 8,714 (6.98) | 8,272 (8.11) | 67,86 (3.35) | 9,728 (5.00) | 9,171 (5.92) |
|
| ||||||
| Hyperlipidemia | 1,924 (1.38) | 9,464 (7.58) | 23,814 (23.36) | 39,59 (1.96) | 15,951 (8.19) | 35,247 (22.74) |
Characteristics are number(percentages) unless otherwise indicated. Data are from a 20% sample of Fee-For-Service Medicare Enrollees 65 years and older.
Comorbid conditions are defined using the Klabunde adaptation of the Charlson Score. Partial list of comorbid conditions presented here.
P value for trend < .001.
Percentages may not sum to 100 due to rounding.
Table 2.
Baseline Characteristics of Medicare Patients With a Hemorrhagic Stroke Stratified by Sex a (1998–2008)
| No. (%) of Men b
|
No. (%) of Women b
|
|||||
|---|---|---|---|---|---|---|
| 1988–1994 (n=17,734) | 1995–2001 (n=18,491) | 2002–2008 (n=17,423) | 1988–1994 (n=26,399) | 1995–2001 (n=28,042) | 2002–2008 (n=25,129) | |
| Age, y | ||||||
| 65–69 | 3,645 (20.6) | 3,134 (17.0) | 2,678 (15.4) | 3,848 (14.6) | 3,274 (11.7) | 2,640 (10.5) |
|
| ||||||
| 70–74 | 4,179 (23.6) | 4,129 (22.3) | 3,521 (20.2) | 4,944 (18.7) | 4,631 (16.5) | 3,757 (15.0) |
|
| ||||||
| 75–79 | 4,190 (23.6) | 4,399 (23.8) | 3,993 (22.9) | 5,588 (21.2) | 5,920 (21.1) | 5,017 (20.0) |
|
| ||||||
| 80–84 | 3,218 (18.2) | 3,634 (19.7) | 3,682 (21.1) | 5,554 (21.0) | 6,142 (21.9) | 5,745 (22.9) |
|
| ||||||
| ≥85 | 2,502 (14.1) | 3,195 (17.3) | 3,549 (20.4) | 6,465 (24.5) | 8,075 (28.8) | 7,970 (31.7) |
|
| ||||||
| Race | ||||||
| White | 15,081 (85.0) | 16,008 (86.6) | 14,872 (85.4) | 22,776 (86.3) | 24,116 (86.0) | 21,233 (84.5) |
|
| ||||||
| Black | 1,595 (9.0) | 1,545 (8.4) | 1,512 (8.7) | 2,504 (9.5) | 2,612 (9.3) | 2,386 (9.5) |
|
| ||||||
| Other | 1,058 (6.0) | 9,38 (5.1) | 1,039 (6.0) | 1,119 (4.2) | 1,314 (4.7) | 1,510 (6.0) |
|
| ||||||
| Region | ||||||
| Midwest | 4,483 (25.3) | 4,848 (26.2) | 4,318 (24.8) | 6,752 (25.6) | 7,351 (26.2) | 6,405 (25.5) |
|
| ||||||
| Northeast | 3,965 (22.4) | 3,772 (20.4) | 3,510 (20.2) | 5,985 (22.7) | 5,911 (21.1) | 4,993 (19.9) |
|
| ||||||
| South | 6,450 (36.4) | 7,099 (38.4) | 6,745 (38.7) | 9,680 (36.7) | 10,799 (38.5) | 9,836 (39.1) |
|
| ||||||
| West | 2,836 (16.0) | 2,772 (15.0) | 2,850 (16.4) | 3,982 (15.1) | 3,981 (14.2) | 3,895 (15.5) |
|
| ||||||
| Length of stay, median (25th–75th pct.), d | 7.5 (3–15) | 5(2–11) | 5 (2–10) | 8(3–15) | 5(2–11) | 5 (2–9) |
|
| ||||||
| Discharge type | ||||||
| Home, self-care | 3,934 (22.2) | 3,359 (18.2) | 2,679 (15.4) | 4,915 (18.6) | 3,920 (14.0) | 2,903 (11.5) |
|
| ||||||
| Skilled nursing facility | 2,275 (12.8) | 3,269 (17.7) | 2,838 (16.3) | 4,646 (17.6) | 6,291 (22.4) | 5,262 (20.9) |
|
| ||||||
| Other type of inpatient facility | 4,031 (22.7) | 4,741 (25.6) | 5,354 (30.7) | 5,374 (20.4) | 6,689 (23.9) | 7,464 (29.7) |
|
| ||||||
| Death | 6,516 (36.7) | 6,219 (33.6) | 5,510 (31.6) | 9,893 (37.5) | 9,645 (34.4) | 7,859 (31.3) |
|
| ||||||
| Other | 978 (5.5) | 903 (4.9) | 1,042 (6.0) | 1,571 (6.0) | 1,497 (5.3) | 1,641 (6.5) |
|
| ||||||
| Comorbid Conditions | ||||||
| Myocardial infarction | 645 (3.64) | 1,119 (6.05) | 1,335 (7.66) | 608 (2.3) | 1,144 (4.08) | 1,389 (5.53) |
|
| ||||||
| Congestive heart failure | 1,164 (6.56) | 1,901 (10.28) | 2,405 (13.8) | 1,967 (7.45) | 3,200 (11.41) | 3,619 (14.4) |
|
| ||||||
| Hypertension | 2,799 (15.78) | 5,293 (28.62) | 7,702 (44.21) | 4,865 (18.43) | 9,195 (32.79) | 12,111 (48.2) |
|
| ||||||
| Diabetes | 1,263 (7.12) | 2,199 (11.89) | 3,355 (19.26) | 1,899 (7.19) | 2,856 (10.18) | 3,929 (15.64) |
|
| ||||||
| Atrial fibrillation | 1,174 (6.62) | 2,318 (12.54) | 3,334 (19.14) | 1,644 (6.23) | 3,167 (11.29) | 4,015 (15.98) |
|
| ||||||
| Vascular disease | 607 (3.42) | 1,011 (5.47) | 1,242 (7.13) | 529 (2) | 933 (3.33) | 1,101 (4.38) |
|
| ||||||
| Hyperlipidemia | 196 (1.11) | 1,292 (6.99) | 4,111 (23.6) | 384 (1.45) | 1,842 (6.57) | 5,226 (20.8) |
Characteristics are number(percentages) unless otherwise indicated. Data are from a 20% sample of Fee-For-Service Medicare Enrollees 65 years and older. Comorbid conditions are defined using the Klabunde adaptation of the Charlson Score. Partial list of comorbid conditions are presented here. P value for trend < .001.
Percentages may not sum to 100 due to rounding.
The incidence of first stroke decreased significantly over time, from an age-adjusted rate of 1039 per 100,000 (95% confidence interval [CI], 1032, 1045) in 1988 to 639 per 100,000 (95% CI, 634, 644) in 2008. The decline was largely due to a marked reduction in the rate of ischemic stroke, which decreased overall from 927 per 100,000 (95% CI 923, 931) in 1988 to 545 per 100,000 (95% CI 542, 548) in 2008 (p<0.001). In men, the rates were 955 per 100,000 (95% CI, 948, 962) and 505 per 100,000 (95% CI, 500, 509); in women, the rates were 908 per 100,000 (95% CI, 902, 913) and 572 per 100,000 (95% CI, 568, 577) (p values <0.001, Figure 1).
Figure 1.
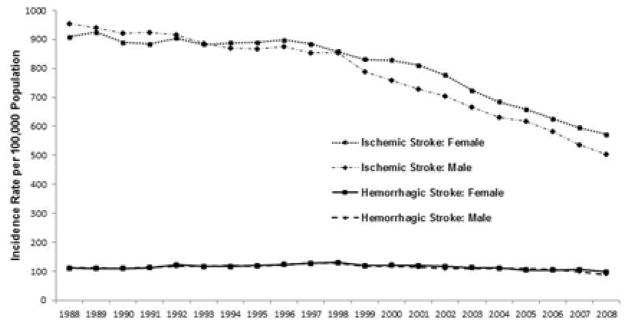
Age-Adjusted Incidence of Ischemic and Hemorrhagic Stroke in the US Medicare Population from 1988 to 2008
Rates of hemorrhagic stroke did not show as great a decline as ischemic stroke, falling from 112 per 100,000 (95% CI 110, 113) in 1988 to 94 per 100,000 (95% CI 93, 95) in 2008 (p<0.001) [112 per 100,000 (95% CI, 110, 115) and 88 per 100,000 (95% CI, 86, 90) in men and 111 per 100,000 (95% CI, 109, 113) and 98 per 100,000 (95% CI, 97, 100) in women (p<0.001 for both, Figure 1)]. Transient ischemic attack incidence declined significantly over the time period, from an age-adjusted rate of 417 per 100,000 (95% CI 414, 420) to 266 per 100,000 (95% CI 264, 268) [412 per 100,000 (95% CI, 407, 416) to 234 per 100,000 (95% CI, 231, 237) for men, and 421 per 100,000 (95% CI, 417, 424) to 288 per 100,000 (95% CI, 285, 291) for women (p<0.001 for both)].
Trends in Stroke Mortality
Crude 30-day mortality from ischemic stroke declined from 16.2% in 1988 to 15.2% in 2008 (p<0.001), as did risk-adjusted mortality: 15.9% in 1988 to 12.7% in 2008 (p<0.001). Among men, 30-day mortality from ischemic stroke fell from 16.3% to 11.2 % and among women from 15.6% to 13.6% (p< 0.001 for both, Figure 2).
Figure 2. Risk-adjusted 30-day Mortality Rates after Hospitalization for Ischemic or Hemorrhagic Stroke from 1988–2008 in a 20% Sample of Medicare Patients.
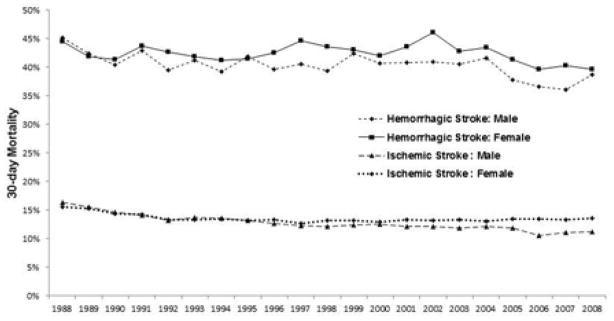
Adjusted for age, region, race, acute/prior myocardial infarction, congestive heart failure, vascular disease, pulmonary disease, dementia, paralysis, diabetes, renal disease, liver disease, ulcer disease, rheumatologic disease, and cancer.
Thirty-day mortality from hemorrhagic stroke also declined over time, albeit less so than ischemic stroke: crude mortality rates declined from 43.4% in 1988 to 42.1% in 2008 and risk-adjusted from 44.7% to 39.3% (p< 0.001 for both, Figure 2). Among men, 30-day mortality from hemorrhagic stroke declined from 45.2% to 38.8% and among women, from 44.4% to 39.6% (p < 0.001 for both, Figure 2).
Trends in Risk Factors and Predicted Incidence of Stroke
Table 3 presents the prevalence of individual stroke risk factors and the corresponding calculated Framingham stroke risk scores for the US population aged ≥ 65 years with Medicare coverage for over three time periods. While the prevalence of diabetes mellitus increased over time, other risk factors, such as cigarette smoking, measured systolic blood pressure, and total cholesterol values, declined. Using risk factor prevalence from NHANES to calculate Framingham stroke risk scores, the mean 10-year predicted stroke risk in the ≥ 65 population increased from 8.3% in 1988–1994 to 8.8 % in 2005–2008 (p=0.02). For men, predicted stroke risk decreased slightly from a mean Framingham score of 10.7% in 1988–1994 to 10.2% in 2005–2008 (p=0.06); for women, predicted stroke risk increased significantly from 6.5% in 1988–1994 to 7.7% in 2005–2008 (p<0.001). The mean predicted Framingham stroke risk when the prevalence of atrial fibrillation was assumed to be unchanged over the time period was 8.3% in 1988–1994 and 8.5% in 2005–2008 (p=0.38).
Table 3.
Prevalence of Stroke Risk Factors in NHANES Subjects Aged ≥ 65 years and Medicare Enrolled, Years 1988–1994, 2001–2004, and 2005–2008
|
|
|
|
||||
|---|---|---|---|---|---|---|
| 1988–1994 | 2001–2004 | 2005–2008 | ||||
| Men (n=2,301) | Women (n=2,649) | Men (n=1,148) | Women (n=1,293) | Men (n=1,003) | Women (n=1,024) | |
| Age, years (mean) | 73.1 | 74.3 | 73.9 | 74.8 | 73.5 | 74.1 |
|
| ||||||
| Diabetes mellitus (%) | 12.2 | 13.3 | 18.7 | 17.1 | 18.3 | 16.2 |
|
| ||||||
| Current smoker (%) | 15.1 | 10.9 | 9.7 | 6.4 | 8.4 | 9.1 |
|
| ||||||
| Cardiovascular disease etc. | 18.1 | 14.1 | 21.2 | 14.6 | 20.4 | 11.9 |
|
| ||||||
| Systolic blood pressure | 140 | 142 | 133 | 142 | 134 | 140 |
|
| ||||||
| Total cholesterol | 210 | 230 | 194 | 217 | 183 | 206 |
|
| ||||||
| HDL cholesterol | 46 | 56 | 48 | 61 | 50 | 61 |
|
| ||||||
| Left ventricular hypertrophy11 | 4.5 | 1.5 | 4.0 | 1.0 | 4.0 | 1.0 |
|
| ||||||
| Atrial fibrillation12 | 3.8 | 3.3 | 8.0 | 6.9 | 8.5 | 7.1 |
|
| ||||||
| Blood pressure medication | 29.1 | 39.6 | 48.0 | 54.3 | 51.2 | 58.6 |
|
| ||||||
| 10-year predicted Framingham stroke risk10 | ||||||
|
| ||||||
| Predicted risk of stroke | 10.7% | 6.5% | 10.4% | 7.7% | 10.2% | 7.7% |
|
| ||||||
| Standard deviation | 0.07 | 0.05 | 0.07 | 0.06 | 0.06 | 0.06 |
Trends in Medication Use
Figure 3 shows trends in the use of antihypertensives, statins, and non-aspirin antiplatelet agents by Medicare beneficiaries 65 years and older. Antihypertensive medication use increased significantly from 1992 to 2008 as did statin use (p<0.001 for both), which showed a marked increase particularly after 1996. We also observed increased prescription antiplatelet medication use over the time period, but the overall prevalence of antiplatelet use in the population was low.
Figure 3.
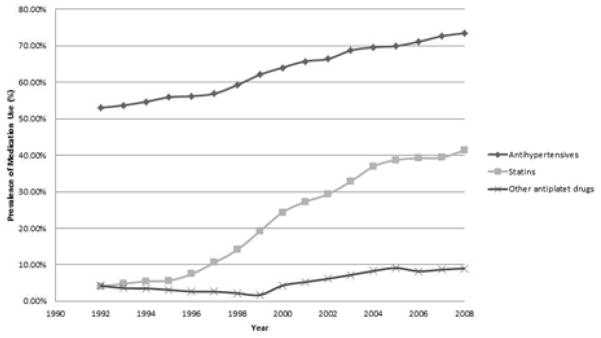
Prevalence of Medications Used to Prevent Stroke Among 138,821 Participants Aged ≥ 65 Years in the Medicare Current Beneficiary Survey (1992 – 2008)
Discussion
The rate of first stroke among patients aged 65 years or older declined by nearly 40% over the last two decades in the US Medicare population, a decline driven primarily by marked reductions in the incidence of ischemic stroke. Hemorrhagic strokes rates showed a much smaller decline, primarily among men. Short-term mortality from both ischemic and hemorrhagic strokes declined over time as well, although hemorrhagic strokes continue to be associated with very poor outcomes. The large decline in stroke rates was not matched by concomitant reductions in stroke risk factors that were part of the Framingham stroke risk score, but did parallel a substantial increase in the use of antihypertensive medications and statins in the general Medicare population.
Our study confirms earlier reports of declining stroke rates and was able to compare how the prevalence of stroke risk factors tracked with actual observed rates in stroke.13–17 Although some risk factors for stroke declined over time, such as reductions in rates of smoking, total cholesterol level, and systolic blood pressure, the magnitude of the observed reductions in stroke rate was greater than what might be estimated by the Framingham stroke score. It is possible that the Framingham stroke risk score does not validate well in our population. The rise in mean Framingham risk score in our study was primarily driven by an increase in the rates of diagnosed diabetes mellitus and the increased prevalence of antihypertensive medication use. Because criteria for diabetes mellitus diagnosis have changed over time, as have goal blood pressure targets, the calculated stroke risk score may not accurately reflect differences in actual stroke risk. The effect of risk factor changes may also not be detectable in the short-term. The Framingham model was developed in a cohort of well-educated, middle class individuals of predominantly European descent and may not reflect the baseline cardiovascular risk and racial/ethnic composition of the Medicare population we studied.
It is also possible that improved risk factor modification, such as more stringent blood pressure control and administration of statins, have significantly lowered the population stroke rate. Although our study design precludes us from making causal explanations, the decline in stroke rates occurred over period of significant uptake in the use of medications that attenuate stroke risk. Antihypertensive medications reduce the risk of stroke by ~32% and statins by ~21%.1, 3, 4 Stroke rates appeared to drop most sharply after year 1998, approximately when statin use became more prevalent. Use of prescription antiplatelet agents increased over the time period, but these agents were unlikely to be a major contributing factor to the reduction in stroke rates due to the weak effects of antiplatelets on primary stroke prevention and the low prevalence of use in our study.
Short-term stroke-related mortality from stroke has fallen significantly over time. Our analysis confirms the continuing and devastating effect of hemorrhagic stroke. Our analysis was unable to assess for causal factors influencing mortality rates; relatively few interventions have been shown to reduce stroke-related mortality. Timely administration of intravenous thrombolytics is associated with more favorable outcomes from ischemic stroke, but has not been shown to have significant effects on mortality9. In addition, the rate of thrombolytic administration continues to be low in the US.18
Our study has several limitations. Our report focuses on the aged ≥ 65 years US population and so is unable to assess trends in stroke rates and case-fatality in younger individuals. In addition, although it was likely that the majority of strokes were incident events, it is possible that some subjects had stroke events at ages <65 years that could not be captured by the Medicare database. Thus, our study can only comment on stroke rates occurring at ages 65 years and older. Stroke events were identified using administrative data, which is subject to misclassification and imperfect sensitivity and specificity. However, we note that the primary objective was to describe relative rather than absolute changes in incidence and mortality over time. We were not able to determine causality between medication use and stroke rates and outcomes and we lacked accurate assessments of non-prescription medications, in particular, aspirin. Although aspirin does not appear to have strong effects in the primary prevention of ischemic stroke, it may have small effects on reducing the risk of incident stroke in women.1 We obtained data from several databases, which had different means of data collection and ascertainment of risk factors. Unmeasured or unknown factors other than the risk factors and medications we examined in the analyses may have contributed to the observed reduction in stroke rate. We were also not able to ascertain whether the medications studied were specifically used for stroke risk reduction or for the secondary prevention of stroke. Atrial fibrillation and left ventricular hypertrophy were extrapolated from national estimates, although our findings did not change when we assumed that the prevalence of atrial fibrillation was unchanged over the time period. Because the actual prevalence of atrial fibrillation appears to be rising over time, it is unlikely that the observed declines in stroke rates were due to decreased population prevalence of atrial fibrillation.19
In conclusion, the rates of incident stroke have significantly fallen over the last 20 years in the US Medicare population, as has short-term stroke mortality. Antihypertensive medications and statin use rose significantly over the time period and might explain the observed decline in stroke rates. If true, then this illustrates how medical interventions have resulted in significant improvements in health on a population level.
Clinical Significance.
The rate of strokes in the United States has fallen by nearly 40% from years 1988 to 2008
Thirty-day mortality declined over time for both ischemic and hemorrhagic strokes
The decline in stroke rates paralleled increasing use of antihypertensive and statin medications and might explain the reduction in stroke rates. If true, this illustrates how medical interventions have resulted in significant improvements in health on a population level.
Footnotes
Author Contributions and Conflicts of Interest Disclosures
Margaret Fang: study design, data interpretation, drafting results, critical review. No conflicts of interest to disclose. Dr. Fang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Marcelo Coca Peraillon: data analysis and interpretation, drafting results, critical review. No conflicts of interest to disclose.
Kaushik Ghosh: data analysis and interpretation, drafting results, critical review. No conflicts of interest to disclose.
David Cutler: study design, data interpretation, critical review. No conflicts of interest to disclose.
Allison Rosen: study design, data interpretation, critical review. No conflicts of interest to disclose.
Financial Disclosure:
This work was supported by the National Institute on Aging (grants P01 AG031098 and K23 AG28978) and the National Center for Research Resources (grant UL1 RR031982). The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SL, Xu JQ, Kochanek KD. Deaths, preliminary data for 2010. National Vital Statistics Reports. 2012;60:1–68. [PubMed] [Google Scholar]
- 3.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents. JAMA: The Journal of the American Medical Association. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 4.Amarenco P, Labreuche J, Lavallée P, Touboul P-J. Statins in stroke prevention and carotid atherosclerosis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 7.Wolf P, D’Agostino R, Belanger A, Kannel W. Probability of stroke: A risk profile from the framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, National Center for Health Statistics. National health and nutrition examination survey 1988–1994, 1999–2000, 2001–2002, 2003–2004, 2005–2006, and 2007–2008 documentation files [Google Scholar]
- 9.Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 10.Adler GS. A profile of the medicare current beneficiary survey. Health Care Finance Rev. 1994;15:153–163. [PMC free article] [PubMed] [Google Scholar]
- 11.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA: The Journal of the American Medical Association. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 12.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population. Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 13.Ovbiagele B. Nationwide trends in in-hospital mortality among patients with stroke. Stroke. 2010;41:1748–1754. doi: 10.1161/STROKEAHA.110.585455. [DOI] [PubMed] [Google Scholar]
- 14.Gillum RF, Kwagyan J, Obisesan TO. Ethnic and geographic variation in stroke mortality trends. Stroke. 2011;42:3294–3296. doi: 10.1161/STROKEAHA.111.625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks. Stroke. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May D, Kittner S. Use of medicare claims data to estimate national trends in stroke incidence, 1985–1991. Stroke. 1994;25:2343–2347. doi: 10.1161/01.str.25.12.2343. [DOI] [PubMed] [Google Scholar]
- 17.Casper ML, Nwaise IA, Croft JB, Nilasena DS. Atlas of stroke hospitalizations among medicare beneficiaries. 2008 [Google Scholar]
- 18.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the united states. Journal of Hospital Medicine. 2010;5:406–409. doi: 10.1002/jhm.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the united states. The American Journal of Cardiology. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]


