Abstract
Helicobacter pylori is a well-known pathogen involved in the development of peptic ulcer, gastric adenocarcinoma and other forms of gastric cancer. Recently, there has been more considerable interest in strain-specific genes located in plasticity regions with great genetic variability. However, little is known about many of these genes. Studies suggested that certain genes in this region may play key roles in the pathogenesis of H. pylori-associated gastroduodenal diseases. JHP933, a conserved putative protein of unknown function, is encoded by the gene in plasticity region of H. pylori strain J99. Here we have determined the structure of JHP933. Our work demonstrates that JHP933 is a nucleotidyltransferase superfamily protein with a characteristic αβαβαβα topology. A superposition demonstrates overall structural homology of the JHP933 N-terminal fragment with lincosamide antibiotic adenylyltransferase LinA and identifies a possible substrate-binding cleft of JHP933. Furthermore, through structural comparison with LinA and LinB, we pinpoint conservative active site residues which may contribute to divalent ion coordination and substrate binding.
Introduction
Helicobacter pylori is one of the most widespread bacterial pathogens of humans, which infects approximately 50% of the world's population. H. pylori infection induces chronic gastric inflammation progressing to a variety of diseases ranging in severity from mild gastritis to peptic ulcers and some forms of gastric cancer [1], [2].
The complex pathology for various clinical outcomes has not been fully elucidated. It has been proposed that genetic variability may underlie the host adaptation differences of various H. pylori strains, which is reflected in distinct disease severities [3], [4]. Genome sequence comparisons in first fully sequenced H. pylori strains J99 and 26695 revealed plasticity zones in which nearly half the strain-specific genes of H. pylori are located [5]. With more complete genome sequence of H. pylori strains determined, the comparative analyses indicated that most strain-specific genes are preferentially localized to either plasticity regions or potential genome rearrangement sites [6]. Recently, there has been considerable interest in the strain-specific genes found in these plasticity regions. Studies have suggested that some genes are associated with the pathogenesis of H. pylori related diseases [7]–[9]. However, little is known about the function of many of the genes within the plasticity regions; thus, further studies are necessary to elucidate their roles in pathogenesis.
Many previous studies have focused on the plasticity region genes in H. pylori strain J99 (jhp914–jhp961) [10]. As studied, jhp947 is significantly associated with duodenal ulcer and gastric cancer; therefore jhp947 could be a good candidate marker for gastroduodenal diseases [7]. Another pathogenicity associated gene in the plasticity regions is dupA (jhp917–jhp918), which encodes homologues of the VirB4 ATPase and is involved in both an increased risk for duodenal ulcer and reduced risk for gastric cancer [11]. Type IV secretion systems (T4SS) play important roles in DNA transfer contributing to bacterial genetic variability. Tfs3 and tfs4 have been successively identified and characterized as T4SS apparatus located in two different plasticity zones of H. pylori [6], [9], [12], [13].
Jhp933 is one of the genes located in the plasticity region in J99 [14]. Analysis of H. pylori strains including strains 26695, J99 and HPAG1, jhp933 has a prevalence rate of 51% [12]. The examination of plasticity region open reading frames (ORFs) in a small sample of gastritis and peptic ulcer patients revealed that the jhp933 gene was found with a prevalence rate of 23.8% (5 of 21 patients) and 28.6% (4 of 14 patients), respectively [15].
The molecular details regarding the function of JHP933 are unknown due to the lack of sequence similarity with other well-characterized proteins. A BLAST search revealed that this protein is well conserved in some Helicobacter [Table S1 and Fig. S1] and closely related species. A conserved domain search indicated that JHP933 might be classified into the nucleotidyltransferase (NTase) superfamily, which constitutes a highly diverse superfamily of proteins with various important biological functions; including chromatin remodeling, RNA polyadenylation, RNA editing, DNA repairing, protein activity regulation, and antibiotic resistance [16]–[18]. Therefore, the specific biological function of JHP933 remains to be elucidated.
Here we have determined the crystal structure of JHP933 and revealed that JHP933 possesses a characteristic nucleotidyltransferase superfamily protein fold with a distinct, but conserved, active site. This structural description should contribute significantly to further uncovering the role of JHP933 in H. pylori pathogenesis.
Materials and Methods
Protein expression, purification, and crystallization
The gene encoding the full-length JHP933 from Helicobacter pylori strain J99 (NP_223650, 267 amino acids) was cloned into the modified pET15b vector (Novagen) and over-expressed as selenomethionyl protein in the E. coli strain BL21(DE3) using methionine pathway inhibition at 293 K. Bacterial cells were lysed by ultrasonication on ice in a buffer containing 50 mM Tris (pH 8.0), 300 mM NaCl, 5 mM β-mercaptoethanol, 0.1% Triton-X100 and 5% glycerol. Soluble N-terminally decahistidine-tagged JHP933 was bound to nickel-sepharose affinity resin. The eluted protein was further purified with size exclusion chromatography at 25 mM Tris (pH 8.0), 200 mM NaCl, 5 mM β-mercaptoethanol, 5% glycerol. The N-terminal histidine tag was removed by cleavage with TEV protease. Purified JHP933 was concentrated to 12 mg/mL without buffer exchange. SDS polyacrylamide gel electrophoresis of purified protein showed one major band at an approximate molecular weight of about 31 kDa, indicating pure full-length protein. Crystals were obtained with the sitting drop vapour-diffusion method at 293 K with 2 µL of protein mixed with 2 µL of a mother liquid solution containing 32% PEG4K, 0.1 M Potassium Sodium tartrate at 0.1 M HEPES (pH 7.5) buffer. Crystals were flash-frozen in liquid nitrogen with a mother liquid containing 25% PEG400 as cryoprotectant.
Data collection, structure determination and refinement
The selenomethionyl single wavelength anomalous dispersion (SAD) dataset for JHP933 were collected at a wavelength of 0.9792 Å at 100 K on the BL17U1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF) to a diffraction limit of 2.1 Å. Diffraction images and the anomalous data set were processed and scaled with HKL2000 [19]. SAD data processing statistics are summarized in Table 1. The locations of 6 selenium atoms were determined and an initial model built using the AutoSolve program of the Phenix suite [20]. The model was manually rebuilt with Coot [21] and further refined in Phenix. The final model contains residues 11–243, with refinement statistics summarized in Table 1. The Ramachandran statistics were calculated with Procheck [22]. Structure superimpositions were complemented by CCP4 LSQ superposion [23]. Figures were produced with Pymol (www.pymol.org). Multiple sequence alignments were generated manually or by using ESPript [24].
Table 1. Data collection and refinement statistics.
| Data collection | SeMet |
| Space group | P 62 |
| Cell parameters | |
| a, b, c (Å) | 90.06, 90.06, 70.87 |
| α, β, γ (°) | 90, 90, 120 |
| Resolution (Å) | 2.1 |
| Rmerge (%) | 0.143 (0.756) |
| I/ơI | 18.04 (2.40) |
| Completeness (%) | 99.7 (100) |
| Redundancy | 7.5 (7.4) |
| Wilson B-factor (Å2) | 36.33 |
| Refinement | |
| Resolution (Å) | 2.100–38.005 |
| No. reflections | 18941 (1829) |
| Rwork/Rfree (%) | 20.43/23.08 |
| No. atoms | |
| Protein | 1867 |
| Water | 76 |
| R.m.s.d bonds (Å) | 0.008 |
| R.m.s.d angles (°) | 1.137 |
| Ramachandran plot | |
| Favored (%) | 96.96 |
| Allowed (%) | 3.04 |
| Outliers (%) | 0.00 |
| Rotamer outliers (%) | 0.00 |
Numbers in parentheses refer to the highest-resolution shell.
Accession Numbers
Coordinates and structure factors have been deposited in the Protein Data Bank with accession number 4O8S.
Results and Discussion
The gene encoding the full-length JHP933 from H. pylori strain J99 was subcloned from genomic DNA, and the recombinant protein expressed as selenomethionyl protein in E. coli and purified using standard methods. Diffracting protein crystals were obtained and SAD diffraction data was used to solve the structure in the space group P62. The final crystal structure of JHP933, containing residues 11–243, was refined at a resolution of 2.1 Å with a Rwork and Rfree of 20.43% and 23.08%, respectively.
The overall structure of JHP933 consists of two domains: an N-terminal core domain and a C-terminal tail domain [Fig. 1]. The N-terminal core domain covers residues 11–170 and contains 5 α-helices (α1–α5) and 7 β-strands (β1–β7). A 310 helix (η1) connects β-strands β5 and β6. The C-terminal tail domain is formed by α-helices α6–α7 followed by an extended α-helix (α8). The two domains are connected by another 310 helix (η2) between α-helices α5 and α6.
Figure 1. Overall structure of JHP933.
Ribbon diagram of the JHP933 structure, N-terminal core domain is colored in lime and C-terminal tail domain in cyan. α-helices are labelled with α, β-strands are labelled with β, and 310 helices are labelled with η.
The N-terminal core domain of JHP933 has an αβαβαβα topology formed by α1-β1-α2-β2-α3-β5-α4, which is coincident with the common α/β-fold structure of nucleotidyltansferase (NTase) fold proteins (Fig. S2) [16]. For most NTase fold proteins, the core structure is usually decorated with various additional structural elements. In the JHP933 structure, the N-terminal core domain contains a seven-stranded, mixed β-sheet flanked by 4 α-helices, β1 and β2 forming antiparallel β-sheet, β2 and β5 forming parallel β-sheet, β5 forming antiparallel β-sheet with additional β-strands β3 and β4, and a stranded small β-sheet β6–β7 making a big turn linked to α-helices α4–α5 [Fig. 1 and S2].
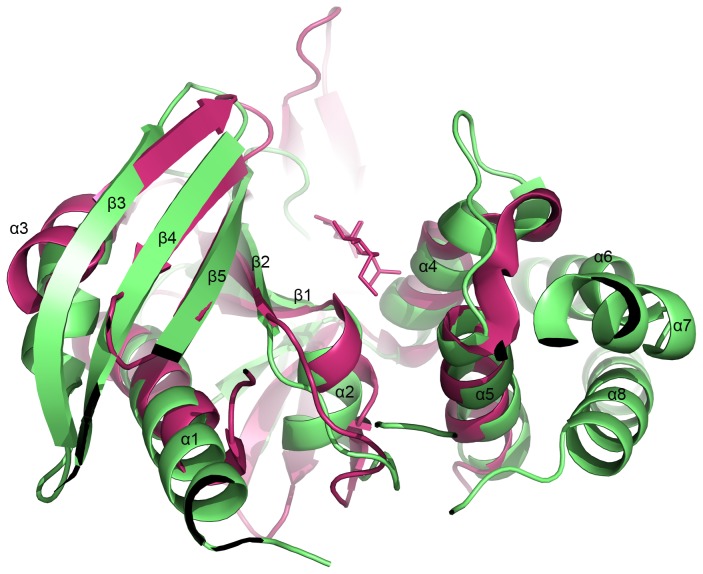
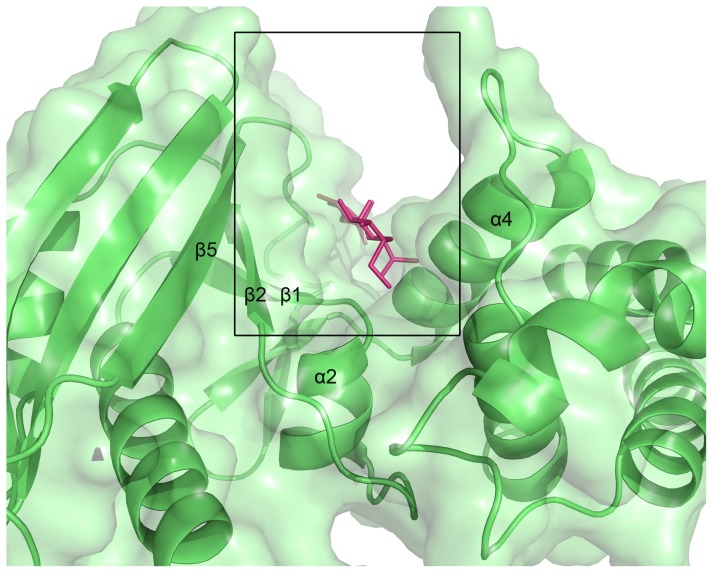
A Dali search for structural homology identified lincosamide antibiotic adenylyltransferase LinA as the closest related structure with a Z-score of 9.6. LinA (pdb code: 4E8J) shares 16% sequence identity with JHP933 and superimposes with a Cα root-mean-square deviation (rmsd) of 2.7 Å over the N-terminal domain [Fig. 2 and 3]. The superposition of these two structures demonstrates a surprisingly high overall homology of the core structural elements including β-stands β1–β5 and the flanking α-helices α1–α4 in the N-terminal domain. The structural homology is highest in the core structure while significant differences can be seen in the addition of accessory structural elements and the loops which connect core elements [Fig. 2]. By comparison to the active site of LinA complex structure, a conservative large cleft is identified as a possible active site for substrate binding at the N-terminal core domain of JHP933. This substrate-binding cleft is surrounded mainly by β-strands β1, β2, β5 and α-helices α4, α2 with a size of around 13×20×20 Å [Fig. 4]. As LinA is a member of NTase superfamily, this high structural similarity further indicates that JHP933 should belong to the same superfamily.
Figure 2. The superposition of JHP933 and LinA/Lincomycin complex (4E8J) structures.
Ribbon diagram of JHP933/LinA, with JHP933 is colored in lime and LinA in magenta, and substrate lincomycin of LinA is shown in ball-and-stick representation.
Figure 3. Sequence and secondary structure comparison of JHP933 with structurally related LinA.
The secondary structures of JHP93 (top row) are labeled in lime and LinA from S. haemolyticu (bottom row) in magenta. The conserved active site motifs involved in catalysis ([DE]h[DE]h, h[DE]h) and substrate binding (hG) of NTase superfamily are shadowed in gray.
Figure 4. Putative substrate binding site of JHP933.
Ribbon diagram and surface representation of JHP933 are colored in lime, the modelled substrate lincomycin of the superimposed LinA/lincomycin complex is shown in ball-and-stick representation and colored in magenta (LinA protein not shown).
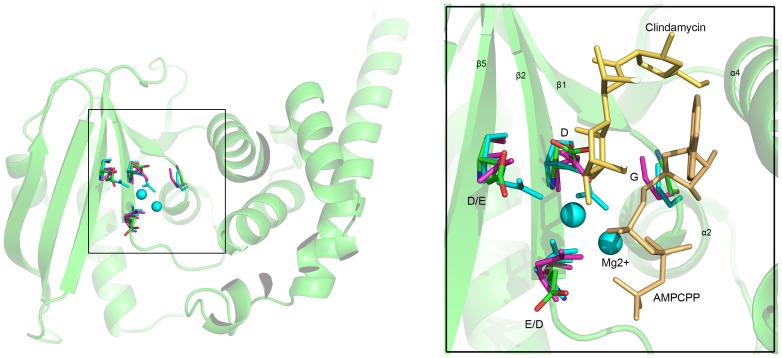
Through sequence analyses of distinct members of NTase superfamily, a common sequence motif of active site residues has been noted: h[G/S], [D/E]h[D/E]h and h[D/E]h (h indicates a hydrophobic amino acid) [17]. The corresponding residues are G39, D55hD57 and E113 in JHP933; with G39 at the connection of β1 and α2, D55 and D57 located on β2, and E113 is placed on β5 structurally adjacent to β2 [Fig. 5]. To further clarify the active site and molecular mechanism for JHP933 substrate binding, we compared the structure of JHP933 N-terminal fragment with the LinA/lincomycin complex, in addition to another NTase fold protein LinB complexed with Mg2+, AMPCPP and clindamycin (pdb code: 3JZ0) [Fig. S3] [25]. The structural superpositions reveal that not only is the fold conserved but also position of catalytic residues. According to the superimposed structure of JHP933, the sites of G39, D55/D57 and E113 are strictly conserved 3-dimensionally [Fig. 5]. Conservation of the catalytic residues likely indicates a similar mechanism of action. Therefore, with reference to the structural conservation of these NTase superfamily proteins, the conserved G39 should play a crucial role in binding of substrates, and D55/D57 and E113 likely are involved in the coordination of divalent ions such as Mg2+, which chelates the phosphates of a nucleoside triphosphate substrate and plays a crucial role in activation of the second substrate's hydroxyl group [16], [25]. However, residues responsible for second substrate binding of LinB or LinA are not conserved in JHP933, likely reflecting differences in identity and structure of second substrates. However, the overall structure clearly confirms that JHP933 belongs to the NTase superfamily with the characteristic structural features well maintained.
Figure 5. Active site conservation and substrate binding of JHP933, LinA and LinB.
The C atoms of active site residues are shown in ball-and-stick representation and distinctively colored: lime for JHP933, magenta for LinA (4E8J), and cyan for LinB (3JZ0). The substrate Mg2+ ions, as cyan spheres, AMPCPP and clindamycin, in yellow, are from LinB complex structure.
In summary, the crystal structure of JHP933 of H. pylori strain J99 described here presents precise evidence to confirm JHP933 as a member of the nucleotidyltransferase superfamily. The structural information demonstrates that JHP933 conserves the overall fold of NTase superfamily proteins with an αβαβαβα topology and catalytic residues for substrate binding within conservative active site. Interestingly, from this large superfamily, we can observe that the proteins take a common core conformation though they display little sequence similarity and play diverse physiological roles.
Most NTase fold proteins can transfer nucleoside monophosphate (NMP) from substrate nucleoside triphosphate (NTP) to the hydroxyl group of their second substrate, such as a small molecule, nucleic acid or protein [17]. It is also worth noting that the overall fold of JHP933 and LinA is highly similar, which leads us to consider a role for JHP933 in lincosamide antibiotic resistance. A study of primary clindamycin resistance reported a prevalence rate of 13.1% in H. pylori strains from dyspeptic patients [26]. To date, mutations in the 23S rRNA gene are a clinically reported mechanism of resistance to lincosamide antibiotics in H. pylori [27]. However, for many bacteria, producing enzymes to modify antibiotics is a common mechanism of resistance for a number of classes of antibiotics. Given its structural similarity to LinA, it is possible that JHP933 may represent a, yet unobserved, mechanism of resistance; using the nucleotidyl transfer to modify antibiotics and inhibit their activity. However, this hypothesis needs further investigation as the putative substrate for JHP933 remains unknown. For a thorough understanding of JHP933's role in pathogenesis of H. pylori related diseases, this structural model represents a critical step in the description of JHP933 function.
Supporting Information
A sequence alignment of JHP933 from strain J99 and the 20 closest orthologs (corresponding accession number see Table S1) found in other H. pylori.
(TIF)
Comparison of secondary structures of JHP933 and other nucleotidyltransferase fold proteins. JHP933 structure (top row) noting secondary structure elements and additional domains aligned with some representative NTase fold proteins of known structure (inside the frame and marked with pdb code, UniProtKB ID, and source organism). JHP933's secondary structure elements and the positions of conserved active site motifs involved in substrate binding (hG) and catalysis ([DE]h[DE]h, h[DE]h) are marked.
(TIF)
The sequence alignment for NTase superfamily core fragment of JHP933, LinA (UniProtKB ID: P06107, from S. haemolyticu) and LinB (UniProtKB ID: Q9WVY4, from Enterococcus faecium) from the top row to the bottom row. The secondary structural elements of JHP933 are illustrated.
(TIF)
A BLAST search of JHP933 (marked with accession number) in fully sequenced H. pylori genomes.
(DOCX)
Acknowledgments
The authors thank staff at the beamline BL17U1 at Shanghai Synchrotron Radiation Facility (SSRF) for support in diffraction data collection.
Funding Statement
The National Nature Science Foundation of China (31270790, 81271784), Key Project of Fujian Province (2012Y0070), the Nature Science Foundation of Fujian Province (2013J01151, 2012J01130), Key Project of Science Research in Fujian Medical University (09ZD018), National Thousand Talents Program of China, and Scientific Research Starting Foundation for Returned Overseas Chinese Scholars of State Human Resource Ministry of China for support on this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R (1999) Helicobacter pylori virulence and genetic geography. Science 284: 1328–1333. [DOI] [PubMed] [Google Scholar]
- 2. Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, et al. (2008) Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res 68: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atherton JC, Peek RM Jr, Tham KT, Cover TL, Blaser MJ (1997) Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112: 92–99. [DOI] [PubMed] [Google Scholar]
- 4. Blaser MJ (1997) Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet 349: 1020–1022. [DOI] [PubMed] [Google Scholar]
- 5. Alm RA, Trust TJ (1999) Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J Mol Med (Berl) 77: 834–846. [DOI] [PubMed] [Google Scholar]
- 6. Fischer W, Windhager L, Rohrer S, Zeiller M, Karnholz A, et al. (2010) Strain-specific genes of Helicobacter pylori: genome evolution driven by a novel type IV secretion system and genomic island transfer. Nucleic Acids Res 38: 6089–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos A, Queiroz DM, Menard A, Marais A, Rocha GA, et al. (2003) New pathogenicity marker found in the plasticity region of the Helicobacter pylori genome. J Clin Microbiol 41: 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jonge R, Kuipers EJ, Langeveld SC, Loffeld RJ, Stoof J, et al. (2004) The Helicobacter pylori plasticity region locus jhp0947–jhp0949 is associated with duodenal ulcer disease and interleukin-12 production in monocyte cells. FEMS Immunol Med Microbiol 41: 161–167. [DOI] [PubMed] [Google Scholar]
- 9. Kersulyte D, Velapatino B, Mukhopadhyay AK, Cahuayme L, Bussalleu A, et al. (2003) Cluster of type IV secretion genes in Helicobacter pylori's plasticity zone. J Bacteriol 185: 3764–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alm RA, Ling LS, Moir DT, King BL, Brown ED, et al. (1999) Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397: 176–180. [DOI] [PubMed] [Google Scholar]
- 11. Lu H, Hsu PI, Graham DY, Yamaoka Y (2005) Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128: 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaoka Y (2008) Roles of the plasticity regions of Helicobacter pylori in gastroduodenal pathogenesis. J Med Microbiol 57: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez-Gonzalez E, Backert S (2014) DNA transfer in the gastric pathogen Helicobacter pylori. Journal of Gastroenterology 49: 594–604. [DOI] [PubMed] [Google Scholar]
- 14. Occhialini A, Marais A, Alm R, Garcia F, Sierra R, et al. (2000) Distribution of open reading frames of plasticity region of strain J99 in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect Immun 68: 6240–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salih BA, Abasiyanik MF, Ahmed N (2007) A preliminary study on the genetic profile of cag pathogenicity-island and other virulent gene loci of Helicobacter pylori strains from Turkey. Infect Genet Evol 7: 509–512. [DOI] [PubMed] [Google Scholar]
- 16. Kuchta K, Knizewski L, Wyrwicz LS, Rychlewski L, Ginalski K (2009) Comprehensive classification of nucleotidyltransferase fold proteins: identification of novel families and their representatives in human. Nucleic Acids Research 37: 7701–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aravind L, Koonin EV (1999) DNA polymerase beta-like nucleotidyltransferase superfamily: identification of three new families, classification and evolutionary history. Nucleic Acids Research 27: 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogozin IB, Aravind L, Koonin EV (2003) Differential action of natural selection on the N and C-terminal domains of 2′-5′ oligoadenylate synthetases and the potential nuclease function of the C-terminal domain. J Mol Biol 326: 1449–1461. [DOI] [PubMed] [Google Scholar]
- 19. Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A 276: 307–326. [DOI] [PubMed] [Google Scholar]
- 20. Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, et al. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallographica Section D-Biological Crystallography 58: 1948–1954. [DOI] [PubMed] [Google Scholar]
- 21. Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallographica Section D-Biological Crystallography 60: 2126–2132. [DOI] [PubMed] [Google Scholar]
- 22. Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck - a Program to Check the Stereochemical Quality of Protein Structures. Journal of Applied Crystallography 26: 283–291. [Google Scholar]
- 23. Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gouet P, Courcelle E, Stuart DI, Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15: 305–308. [DOI] [PubMed] [Google Scholar]
- 25. Morar M, Bhullar K, Hughes DW, Junop M, Wright GD (2009) Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure 17: 1649–1659. [DOI] [PubMed] [Google Scholar]
- 26. Toro C, Garcia-Samaniego J, Carbo J, Iniguez A, Alarcon T, et al. (2001) [Prevalence of primary Helicobacter pylori resistance to eight antimicrobial agents in a hospital in Madrid]. Rev Esp Quimioter 14: 172–176. [PubMed] [Google Scholar]
- 27. Wang G, Taylor DE (1998) Site-specific mutations in the 23S rRNA gene of Helicobacter pylori confer two types of resistance to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob Agents Chemother 42: 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A sequence alignment of JHP933 from strain J99 and the 20 closest orthologs (corresponding accession number see Table S1) found in other H. pylori.
(TIF)
Comparison of secondary structures of JHP933 and other nucleotidyltransferase fold proteins. JHP933 structure (top row) noting secondary structure elements and additional domains aligned with some representative NTase fold proteins of known structure (inside the frame and marked with pdb code, UniProtKB ID, and source organism). JHP933's secondary structure elements and the positions of conserved active site motifs involved in substrate binding (hG) and catalysis ([DE]h[DE]h, h[DE]h) are marked.
(TIF)
The sequence alignment for NTase superfamily core fragment of JHP933, LinA (UniProtKB ID: P06107, from S. haemolyticu) and LinB (UniProtKB ID: Q9WVY4, from Enterococcus faecium) from the top row to the bottom row. The secondary structural elements of JHP933 are illustrated.
(TIF)
A BLAST search of JHP933 (marked with accession number) in fully sequenced H. pylori genomes.
(DOCX)