Abstract
From 2008 to 2012, the National Institutes of Health (NIH) Fogarty International Clinical Research Fellows Program (FICRF) provided 1-year mentored research training at low- and middle-income country sites for American and international post-doctoral health professionals. We examined the FICRF applicant pool, proposed research topics, selection process, and characteristics of enrollees to assess trends in global health research interest and factors associated with applicant competitiveness. The majority (58%) of 67 US and 57 international Fellows were women, and 83% of Fellows had medical degrees. Most applicants were in clinical fellowships (41%) or residencies (24%). More applicants proposing infectious disease projects were supported (59%) than applicants proposing non-communicable disease (NCD) projects (41%), although projects that combined both topic areas were most successful (69%). The numbers of applicants proposing research on NCDs and the numbers of these applicants awarded fellowships rose dramatically over time. Funding provided to the FICRF varied significantly among NIH Institutes and Centers and was strongly associated with the research topics awarded.
Introduction
Interest in global health engagement has increased substantially in recent years among students and trainees in the health professions.1–3 A variety of programs has been established to channel this interest into productive avenues and concrete skills, including research skills,4 and has generated discussion around ethics and best practices for global health engagement.5,6 The Fogarty International Clinical Research Scholars Program (FICRS) was initiated in 2003 by the National Institutes of Health (NIH) Fogarty International Center (FIC) as the FIC/Ellison Foundation Program.7 The FICRS Program was a 1-year mentored training experience that provided opportunities for US doctoral students in the health professions to participate in clinical research and gain hands-on experience at NIH-funded research centers across the globe from Bangladesh to Zambia by working alongside twinned counterpart Scholars from the host low- and middle-income countries (LMICs). The purpose of the program was to foster the next generation of clinical investigators and further international health research collaborations in LMICs (www.fogartyscholars.org).
Interest in FICRS was robust, which was reflected in numbers of applicants and competition for positions, most of which were for students from the United States and junior doctors in the LMICs.8 This interest, plus the conviction that LMICs research experiences may be equally strategic at more advanced stages of training, spurred the FIC to expand FICRS to create the Fogarty International Clinical Research Fellows (FICRFs) program in 2007. This program opened a similar opportunity to medical residents and fellows, scientists with PhD degrees engaged in health-related post-doctoral programs, and scientists in faculty or staff positions within 3 years of their last major training (e.g., registrar, residency, fellowship, or doctoral program). Like FICRS, FICRF provided 1-year clinical research training experiences in LMICs. Persons eligible for the program were either citizens or permanent residents of the United States or individuals from LMICs.
In 2008, the Fellows program launched its first cohort of Fellows. Through 2012, four FICRF cohorts engaged in international clinical research for 1–2 years, including 67 US and 57 international Fellows. The program was administered by the Fogarty International Clinical Research Scholars and Fellows (FICRS-F) Support Center at the Vanderbilt Institute for Global Health (VIGH) through an R24 grant (a Resource-Related Research Project) from the FIC.7,9 To assess trends in global health research interest and factors associated with competitiveness among post-doctoral trainees who sought concentrated global health research training opportunities, we examined the FICRF applicant pool, proposed research topics, selection process, and characteristics of enrollees.
Recruitment and Selection of Fellows
Recruitment of Fellows.
Recruitment efforts were conducted by the FICRS-F Support Center and initially focused on reaching mentors and advisors of potential applicants. Recruitment emails, US mailings, and website posts targeted key individuals from post-doctoral fellowship programs across numerous disciplines and institutions, contacts at NIH, FICRS external review committee members, FICRS training site principal investigators, institutes, centers, and departments of global health, historically black colleges and universities, and medical schools in Puerto Rico. Over the years, as the numbers of program alumni grew, the program's reputation grew by word of mouth and through presentations by alumni, visits to the Support Center website (www.fogartyscholars.org), alumni publications, and FIC newsletters reporting program outputs and sharing alumni narratives. The interdisciplinary nature of global health endeavors prompted interest from a broad range of graduates in the health sciences, including medicine, veterinary science, dentistry, osteopathic medicine, nursing, pharmacy, basic sciences, and behavioral sciences.
Existing FICRS sites and site directors, mentors, and partner institutions were offered to the first trainee cohort as training site options, but available sites were soon expanded to additional global health partners and networks. International sites not affiliated with the FICRS Program were required to submit applications to determine their capacity to mentor Fellows and support their proposed research projects. International sites were required to have a history of NIH or other US federal funding to accelerate ethics approvals (because review systems will have been set up in advance at such sites), nurture high-quality research and mentorship, and facilitate subcontracts with organizations already familiar with US government grants.
In the first year, competitive applications were open only to US applicants, and applicants who were selected as Fellows were twinned with counterpart LMIC Fellows selected by academic leaders from the host country. In years 2–4, the competitive application process was open to both US and international applicants. For this report, we consider first-year international Fellows as if they had been independent applicants to provide a coherent overall program summary.
Selection of Fellows.
Applications were posted and completed online using Research Electronic Data Capture (REDCap) tools hosted at Vanderbilt University (https://redcap.vanderbilt.edu). Required materials included a curriculum vitae and personal career statement, a research proposal developed in collaboration with US and international mentors, letters of support and brief biographical profiles from mentors, and a budget of anticipated personal and research expenses. The application requested that international travel or experience and language skills be indicated, although such experiences or skills were not required for eligibility. Fellow applicants were only eligible if they had doctoral degrees and were either in post-doctoral training positions or within 3 years of completion of their most recent training (e.g., fellowship, residency, internship, or PhD, MS, or MPH programs). US applicants could propose new training sites, but international applicants were required to be employed by or in training at a pre-approved LMICs research site.
Applications were first reviewed by Support Center staff to ensure that they were complete and that eligibility criteria were met (Round One). Applications were then reviewed by two global health-focused external reviewers from US- and LMICs-based academic institutions. Reviewers were asked to score applications on defined criteria (Table 1) from 1.0 (ideal candidate) to 5.0 (least competitive). Reviewers provided comments to justify their scores. Total scores were computed using the criterion weights shown in Table 1.
Table 1.
FICRF Program application review criteria and score weights
| Criterion | Percent of score |
|---|---|
| Candidate global research commitment | 20 |
| Originality and scientific merit of research proposal | 25 |
| Qualifications of candidate | 20 |
| Qualifications of mentors | 15 |
| Project feasibility | 10 |
| Project site | 10 |
The strongest applicants identified during the initial review were contacted to schedule interviews (generally by telephone or Skype) with a second set of reviewers, who convened in Nashville, Tennessee at VIGH to conduct the interviews over a 1.5-day period (Round Two). Interviewers were asked to evaluate the qualifications of the candidates, probe the scientific merit of proposals and the candidates' abilities to defend them, and the candidates' careers goals and relevance to global health research. Interviewers were asked to rate the applicants based on the same criteria and scale as in Round One.
FICRS Support Center staff at Vanderbilt promptly ranked the applicants based on scores from Rounds One and Two for discussion in a final selection meeting of interviewers while Round Two reviewers were still in Nashville. This ranking created a final rank list divided into three tiers based on scores and impressions shared by interviewers and Support Center staff. The program was consistently able to find funding for the top tier, regardless of their research topic areas. All tiers were presented to the FIC FICRS-F Program Officer, who sorted them by relevance to NIH Institutes and Centers' (ICs) research interests and presented them to IC representatives to request cofunding. Some candidates, for instance, proposed research topic areas of particular interest to specific ICs, and therefore, the Program Officer presented the candidates to these ICs for consideration for targeted funding. Other candidates were supported with core FIC R24-type parent grant funds; all funds were administered by the FICRS-F Support Center at VIGH. In 2009, the American Recovery and Reinvestment Act (ARRA) provided additional funds to support Fellows in 2010 (US Fellows only) and allow the Support Center to organize a 2010 symposium for alumni of both the Scholars and Fellows programs. Offers of acceptance into the program were determined by applicant ranking, research topic area, research site, and available funding streams.
Methods
We examined all complete applications submitted by US and international applicants during the 4 years of the FICRF Program (applying in 2007–2010 for deployment in 2008–2011). The applicants' demographics, academic disciplines, degrees earned, and proposed research topic areas were described by enrollment status (enrolled versus not enrolled), and comparisons of characteristics were made using χ2 and rank sum tests. We categorized research topic areas in major categories of infectious diseases, non-communicable diseases (NCDs), or infectious and NCDs (e.g., human papillomavirus [HPV] -related cervical cancer research, which we termed combination projects) as well as more detailed subtopics (e.g., we categorized HPV/cervical cancer research as related to human immunodeficiency virus [HIV] when appropriate and non-HIV sexually transmitted diseases and cancer). We produced histograms to summarize changes in the distributions of proposed research topic areas over time for applicants and enrollees. We also evaluated the proportions of research proposals that aligned with priorities of NIH ICs and compared them with actual support provided by the ICs. R software version 2.11.1 (www.r-project.org) was used for data analyses.
Results
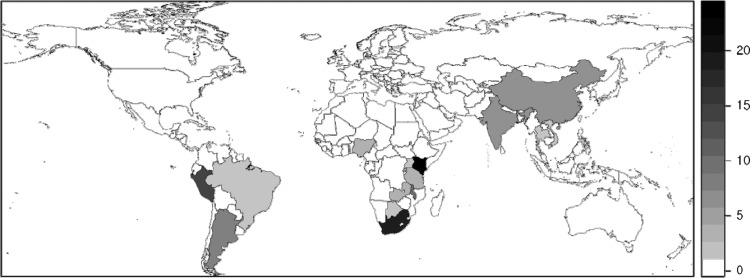
Among 231 complete applications submitted by 227 individuals, 117 (51%) applications were from US citizens or permanent residents, and 114 (49%) applications were from 22 LMICs (Tables 2 and 3). International applicants' countries of origin increased from 11 countries in the program's first year to 22 countries in its last year. At the time that they applied, applicants were located at 53 US, 42 LMIC, and 2 other international institutions or programs. They proposed to work in 27 site countries. Positions were offered to and accepted by 124 individuals (54% of applications), who subsequently worked in 22 LMICs in Africa, Latin America, the Caribbean, and Asia (Figure 1). Among these individuals were 67 US Fellows (57% of US applicants) and 57 international Fellows (50% of international applicants) from 32 US, 28 LMIC, and 1 other international institutions or programs in 15 countries other than the United States.
Table 2.
FICRF Program applicant characteristics and proposed research topics by enrollment status
| Enrolled | Not enrolled | Total | P value* | |
|---|---|---|---|---|
| N | 124 | 107 | 231 | |
| Year of application, n (%) | < 0.001 | |||
| 2008 | 33 (92) | 3 (8) | 36 (16) | |
| 2009 | 25 (58) | 18 (42) | 43 (19) | |
| 2010 | 32 (53) | 28 (47) | 60 (26) | |
| 2011 | 34 (37) | 58 (63) | 92 (40) | |
| Applicant type, n (%) | 0.3 | |||
| International | 57 (50) | 57 (50) | 114 (49) | |
| United States | 67 (57) | 50 (43) | 117 (51) | |
| Age (years), median (interquartile range) | 33 (32–36) | 33 (31–36) | 33 (31–36) | 0.6 |
| Sex, n (%) | 0.2 | |||
| Female | 74 (58) | 53 (42) | 127 (55) | |
| Male | 49 (48) | 53 (52) | 102 (45) | |
| Marital status, n (%) | 0.5 | |||
| Married/domestic partnership | 59 (64) | 33 (36) | 92 (58) | |
| Single | 38 (58) | 28 (42) | 66 (42) | |
| Degree, n (%)† | ||||
| MD | 81 (57) | 61 (43) | 142 (61) | 0.2 |
| DO | 0 (0) | 2 (100) | 2 (1) | 0.4 |
| MMed | 8 (67) | 4 (33) | 12 (5) | 0.5 |
| MBChB | 24 (50) | 24 (50) | 48 (21) | 0.7 |
| MPH/MSPH/MSHS | 20 (54) | 17 (46) | 37 (16) | 1.0 |
| MA/MPhil | 4 (67) | 2 (33) | 6 (3) | 0.8 |
| MS | 12 (46) | 14 (54) | 26 (11) | 0.5 |
| PharmD | 0 (0) | 3 (100) | 3 (1) | 0.2 |
| Current position (primary), n (%) | 0.2 | |||
| Employee | 16 (52) | 15 (48) | 31 (14) | |
| Faculty | 12 (41) | 17 (59) | 29 (13) | |
| Fellow | 59 (63) | 35 (37) | 94 (41) | |
| Resident | 24 (44) | 30 (56) | 54 (24) | |
| Scholar | 1 (50) | 1 (50) | 2 (1) | |
| Student | 10 (53) | 9 (47) | 19 (8) | |
| Major research topic, n (%) | 0.006 | |||
| Infectious diseases | 67 (59) | 46 (41) | 113 (49) | |
| NCDs | 35 (41) | 51 (59) | 86 (37) | |
| NCDs and infectious diseases | 22 (69) | 10 (31) | 32 (14) | |
| Research subtopic, n (%)† | ||||
| Basic science (genetics, molecular biology, or pathology) | 18 (49) | 19 (51) | 37 (16) | 0.6 |
| Cancer | 15 (68) | 7 (32) | 22 (10) | 0.2 |
| Cardiovascular diseases | 19 (56) | 15 (44) | 34 (15) | 0.9 |
| Dermatology | 4 (67) | 2 (33) | 6 (3) | 0.8 |
| Diabetes/kidney/metabolic | 5 (33) | 10 (67) | 15 (6) | 0.2 |
| Diarrheal diseases | 3 (43) | 4 (57) | 7 (3) | 0.8 |
| Environmental health | 3 (23) | 10 (77) | 13 (6) | 0.046 |
| Eye diseases | 6 (67) | 3 (33) | 9 (4) | 0.6 |
| Health behavior | 25 (54) | 21 (46) | 46 (20) | 1.0 |
| Health systems | 20 (41) | 29 (59) | 49 (21) | 0.06 |
| HIV/AIDS | 59 (69) | 26 (31) | 85 (37) | < 0.001 |
| Infectious diseases, not specified | 9 (43) | 12 (57) | 21 (9) | 0.4 |
| Maternal–child health | 15 (65) | 8 (35) | 23 (10) | 0.3 |
| Mental health | 12 (67) | 6 (33) | 18 (8) | 0.4 |
| Neurological diseases and stroke | 11 (58) | 8 (42) | 19 (8) | 0.9 |
| Non-HIV STDs | 16 (84) | 3 (16) | 19 (8) | 0.01 |
| Nutrition | 9 (56) | 7 (44) | 16 (7) | 1.0 |
| Parasitology | 13 (65) | 7 (35) | 20 (9) | 0.4 |
| Pulmonary diseases | 18 (51) | 17 (49) | 35 (15) | 0.9 |
| Substance abuse | 2 (50) | 2 (50) | 4 (2) | 1.0 |
| Surgery/trauma | 8 (40) | 12 (60) | 20 (9) | 0.3 |
| Tobacco | 1 (33) | 2 (67) | 3 (1) | 0.9 |
| Tuberculosis | 13 (48) | 14 (52) | 27 (12) | 0.7 |
| Special populations | ||||
| Adolescents | 3 (60) | 2 (40) | 5 (2) | 1.0 |
| Aging | 0 (0) | 2 (100) | 2 (1) | 0.4 |
| Animals | 3 (38) | 5 (62) | 8 (3) | 0.6 |
| Children | 29 (56) | 23 (44) | 52 (23) | 0.9 |
| Women | 23 (56) | 18 (44) | 41 (18) | 0.9 |
Percentages are computed using the number of applicants with a non-missing value. All applicants are summarized using column percentages, and Fellows and non-enrollees are summarized using row percentages (percent of applicants with corresponding characteristic).
χ2 and rank sum tests of association between accepted/enrolled and non-enrolled applicants. Fisher's exact test yielded the same results when expected cell counts fell below five.14
Percentages may sum to greater than 100%, because multiple categories may be relevant.
Table 3.
FICRF Program applicants' countries of citizenship by enrollment status
| Enrolled* | Not enrolled* | Total† | |
|---|---|---|---|
| n | 124 | 107 | 231 |
| Country, n (%) | |||
| Argentina | 6 (67) | 3 (33) | 9 (4) |
| Cameroon | 1 (100) | 0 (0) | 1 (0) |
| China | 5 (56) | 4 (44) | 9 (4) |
| Democratic Republic of the Congo | 1 (100) | 0 (0) | 1 (0) |
| Egypt | 0 (0) | 1 (100) | 1 (0) |
| Haiti | 0 (0) | 1 (100) | 1 (0) |
| India | 1 (17) | 5 (83) | 6 (3) |
| Jamaica | 1 (50) | 1 (50) | 2 (1) |
| Kenya | 11 (69) | 5 (31) | 16 (7) |
| Malawi | 2 (100) | 0 (0) | 2 (1) |
| Mexico | 0 (0) | 1 (100) | 1 (0) |
| Nigeria | 3 (30) | 7 (70) | 10 (4) |
| Peru | 8 (44) | 10 (56) | 18 (8) |
| Philippines | 0 (0) | 1 (100) | 1 (0) |
| Rwanda | 1 (100) | 0 (0) | 1 (0) |
| South Africa | 10 (62) | 6 (38) | 16 (7) |
| Tanzania | 1 (50) | 1 (50) | 2 (1) |
| Thailand | 1 (100) | 0 (0) | 1 (0) |
| United States | 67 (57) | 50 (43) | 117 (51) |
| Uganda | 0 (0) | 4 (100) | 4 (2) |
| Uruguay | 0 (0) | 1 (100) | 1 (0) |
| Zambia | 5 (71) | 2 (29) | 7 (3) |
| Zimbabwe | 0 (0) | 4 (100) | 4 (2) |
Percentages are computed using the numbers of applicants with non-missing values. Results are influenced by twinning of international Fellows with accepted US Fellows in the program's first year. Significance testing was not performed because of small cell numbers for many countries.
Percent of applicants from the respective country who were enrolled or not enrolled.
Percent of all applicants.
Figure 1.
Distribution of FICRFs across the globe in the FICRF Program from 2008 to 2011. Gray shading identifies the numbers of Fellows enrolled by site country.
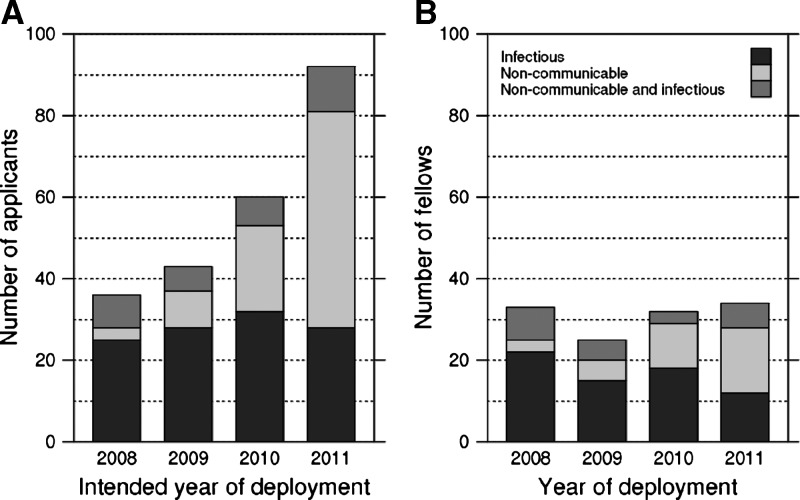
Numbers of both US and international applications more than doubled over 4 years (Figure 2A), and the program became more selective, dropping progressively from 92% enrollment in 2008 to 37% enrollment in 2011 (Figure 2B) (P < 0.001). The median age of applicants and Fellows was 33 years, with little difference between US and international applicants. Of applicants, 64% from the United States and 46% from LMICs were women; of enrolled Fellows, 58% from the United States and 62% from LMICs were women. One-half of US applicants, 68% of international applicants, and 64% of all enrolled Fellows were married or in domestic partnerships. A majority of applicants (61%) had MD degrees, and an additional 22% had other medical degrees (21% MBChB and 1% DO); equal proportions of US and LMIC applicants had medical degrees. More than one-half of applicants were in fellowship (41%) or residency (24%) positions. A minority were employees (14%) or faculty members (13%); almost all of them were LMICs applicants. The most common countries of study proposed by applicants were Peru (16%), South Africa (15%), and Kenya (15%); other countries were proposed by 7% or fewer applicants. All of the above characteristics were generally consistent over the 4 years of the program, and among them, only the year of application was associated with enrollment success (Table 2). Proposed site country was not associated with likelihood of applicant success. Institutions that submitted more applications yielded more total enrollees; 71 of 97 institutions submitted only one or two applications.
Figure 2.
FICRF (A) applicant pool and (B) Fellows supported by major topic area and year.
There was evidence that enrollment success differed by proposed major research topic (Table 2) (P = 0.006). Larger proportions of applicants proposing infectious disease (59%) or combination infectious/NCD projects (69%) were awarded and accepted Fellowships versus applicants proposing NCD projects (41%). Overall, applicants proposing research on HIV/acquired immunodeficiency syndrome (AIDS) were highly successful in enrolling (69%, P < 0.001) along with applicants proposing research on sexually transmitted diseases (STDs; 84%, P = 0.01). Applicants proposing research in environmental health were less likely to be offered positions (23%, P < 0.05). No other significant associations were detected between proposed topic and enrollment success, and there was little evidence for an interaction effect between year of application and major research topic on likelihood of program enrollment.
During the 4-year project period, the number of applicants proposing infectious disease projects remained steady, but the number proposing research on NCDs rose dramatically, reaching 70% by the fourth year (Figure 2A). Among Fellows enrolled, there was a commensurate increase in the number studying NCDs (Figure 2B). Although the number conducting only infectious disease research seems to have declined, this finding may be an artifact of changes in amounts and guidelines surrounding available funding and funding used in a different year from the year in which it was granted by the NIH ICs. Aside from infectious diseases, the most prevalent topics proposed by applicants were in health systems, health behavior, diseases of the heart and lungs, and basic sciences. More US than international applicants proposed research in health systems and surgery or trauma, but otherwise, US and international applicants displayed similar interests in topic categories.
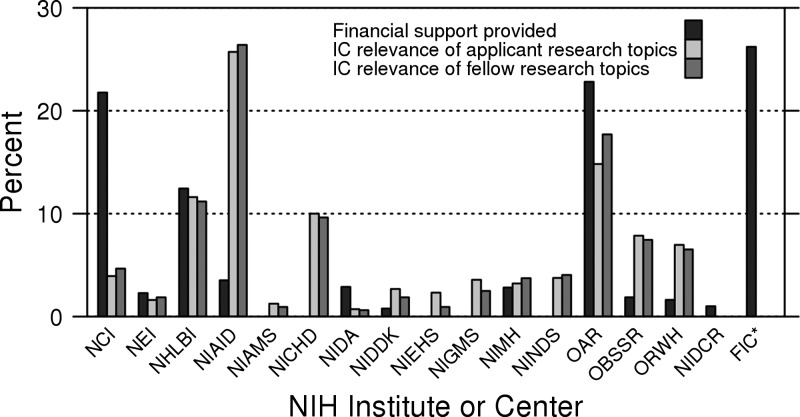
Qualitative review of the selection process and the comments of external reviewers also revealed that features associated with applicant success included the strength of the proposed research methodology, the feasibility of the proposed project within the 1-year timeline, the clarity of the applicant's career goals and plans to achieve those goals, and clear letters of support from the proposed mentors. Additionally, the availability of NIH IC-specific funding factored into acceptance and enrollment in complex ways. Figure 3 compares the proportions of research projects proposed by all applicants and awarded Fellows that were relevant to NIH ICs with the funding provided by those ICs. The largest number of applications was relevant to the National Institute of Allergy and Infectious Diseases (NIAID), but the NIAID provided support that was disproportionately lower than the number of relevant applicants. Many of these applications were also relevant to and supported by the Office of AIDS Research (OAR) in the Office of the NIH Director, which supplied substantial program funding. No support was received from some ICs with missions that were relevant to varying numbers of applications; although few applications were relevant to environmental health sciences (NIEHS), arthritis and musculoskeletal and skin diseases (NIAMS), general medical sciences (NIGMS), and neurological disorders and stroke (NINDS), a substantial number was relevant to child health and human development (NICHD). The converse was true for the National Cancer Institute (NCI), which supplied support that was disproportionately higher than applicant demand. Funds from FIC, which focuses on no specific disease category or special population, provided about 26% of overall funding (Figure 3), including support for many strong applicants (e.g., some who proposed infectious disease projects appropriate for NIAID) for whom IC-specific funds were not available.
Figure 3.
Support provided by NIH ICs compared with applicant and Fellow research topic interests in the FICRF Program from 2008 to 2011. Support provided (black bars) by NIH ICs for the FICRS and FICRF Programs (both pre-doctoral Scholars and post-doctoral Fellows) compared with topics of interest to enrolled Fellows (dark gray bars). NIH ICs to which the Fellow's research proposal has relevance are shown by the percentage of projects with relevance (light gray). Topics may be double counted when a research proposal has relevance to more than one IC. Amounts were normalized to total 100%. NEI = National Eye Institute; NHLBI = National Heart, Lung, and Blood Institute; NIDA = National Institute on Drug Abuse; NIMH = National Institute of Mental Health; ORWH = Office of Research on Women's Health; NICDR = National Institute of Dental and Craniofacial Research. *FIC indicates direct funding from the FIC. By definition, all applications were relevant to FIC.
Discussion
The NIH FICRF Program was an expansion of the pre-doctoral FICRS Program,7 which was in place for 4 years before the FIC converted both programs to a less centralized Global Health Program for Fellows and Scholars.10 Opportunities for post-doctoral research training experience offered by the FICRF Program received substantial interest among clinical and post-doctoral fellows from the United States and LMICs, growing with time as awareness of the program increased. Selectivity increased progressively through the 4 years, because applications nearly tripled, and the probability of a given applicant being awarded support decreased from 92% to 37%. The overall number of Fellows supported was largely stable over the 4 years of the program, reflecting NIH funding. Overall, roughly equal numbers of US and international candidates applied, and US applicants had only a slight edge in enrollment success.
Training positions in FICRF were accessed by individuals from 16 countries in addition to the United States, and Fellows were deployed to research sites in 22 LMICs that had NIH research funding, nearly all of which were affiliated or working closely with US-based partner institutions. No demographic characteristic was associated with significantly greater success in program enrollment. The majority of applicants and Fellows were women, and women experienced no selection disadvantage among either US or international applicant pools.
Applicants proposing research in infectious diseases, both with and without linkages to chronic diseases, such as cervical cancer, were more likely to attain fellowship positions than applicants proposing only NCD research topics. This result may have been related to the predecessor FICRS Program's initial linkages to the FIC's AIDS International Training and Research Program (AITRP), the historic causes of death in LMICs being predominantly infectious, and funding for global health research historically favoring infectious diseases, especially HIV/AIDS.
As the program matured, the numbers of chronic disease applications and awarded fellowships increased substantially in just 3 years, whereas infectious disease applications remained steady and infectious disease-related placements decreased. This result may reflect increasing recognition that non-communicable causes of death are rising in LMICs and that they, in fact, exceed communicable causes overall.11 NCD topics did not all have the same funding likelihood: cancer-related proposals were more likely to be funded, whereas proposals in diabetes, surgery, and trauma were less likely to be funded. This disparity reflects, in part, generous funding from NCI but very little funding provided by the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK) and the absence of NIH ICs focusing on non-cancer surgery and trauma. Proposals for research in mental health, maternal–child health, eye diseases, and projects focusing on women or children had a better than 50% chance at being awarded. Applications for research in cardiovascular or pulmonary diseases, tuberculosis, neurological diseases or stroke, health behavior, basic science, and nutrition were funded at rates close to 50%. Applications that addressed more than one topic were more likely to be funded than applications addressing single topics.
Future reports from the FICRS-F Support Center will examine outputs and outcomes of the FICRS-F Program. They will include publications, grants received, additional training and career positions attained by program alumni, and collaborative networks established. It is clear that substantial interest exists among clinical and other post-doctoral fellows in intensive year-long global health research training opportunities. In 2012, FICRF was decentralized from the single Support Center model to 20 institutions grouped into five consortia (the FIC Global Health Fellows Program)10 with autonomous selection processes that emphasize post-doctoral trainees. This change has enabled these 20 participating institutions to plan for trainees to work abroad over the lives of their grants, but it has restricted the likelihood of placement for Fellow applicants who are at institutions outside this pool. In addition, fewer foreign Fellows are accepted proportionately to the number of US Fellows in the new program, providing less incentive for clinical investigators to stay in their home nations to build their research careers.12,13
Substantial numbers of applicants can be anticipated for the new program, which is designed to jumpstart careers in global health. Although the clinical research topics proposed are likely to reflect the burdens of disease in LMICs, the distribution of future ICs funding will influence the topic areas supported and the backgrounds of new Fellows significantly, reflecting current NIH priorities for US research investments.
ACKNOWLEDGMENTS
The authors thank Drs. Roger Glass, Kenneth Bridbord, and Myat Htoo Razak at the FIC; the FICRS-F Site Principal Investigators and mentors; participating NIH Institute and Center directors and staff; and Sarah Schlachter, Anne-Gordon Smart, and Aditi Thite. Study data were collected and managed using REDCap tools hosted at Vanderbilt University (https://redcap.vanderbilt.edu).
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. D.C.H. had full access to all the data in the study and final responsibility for the decision to submit for publication.
Footnotes
Financial support: This work was supported by the NIH Office of the Director, FIC, OAR, NCI, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, NIAID, and NIH Office of Research on Women's Health through the FICRF Program at Vanderbilt-Association of American Medical Schools (Grant R24 TW007988). Additional support was received from the American Recovery and Reinvestment Act (http://recovery.nih.gov/) in 2010 and 2011 and the Vanderbilt Institute for Clinical and Translational Research (VICTR; Grant UL1TR000445 from National Center for Advancing Translational Sciences/NIH).
Authors' addresses: Douglas C. Heimburger, Catherine Lem Carothers, Meridith Blevins, and Sten H. Vermund, Vanderbilt University Institute for Global Health, Vanderbilt University, Nashville, TN, E-mails: douglas.heimburger@vanderbilt.edu, catherine.lem@vanderbilt.edu, meridith.blevins@vanderbilt.edu, and sten.vermund@vanderbilt.edu. Tokesha L. Warner, GrantSMART Office, Office of the Vice President for Research, University of Georgia, GA, E-mail: tlwarner@uga.edu. Yolanda Thomas, Accokeek, MD, E-mail: ythomas@ficrsconsultant.com. Pierce Gardner, Stony Brook University School of Medicine, Stony Brook, NY, E-mail: pgardner@stonybrookmedicine.edu. Aron Primack, Silver Spring, MD, E-mail: aprimack@rcn.co.
References
- 1.Panosian C, Coates TJ. The new medical “missionaries” – grooming the next generation of global health workers. N Engl J Med. 2006;354:1771–1773. doi: 10.1056/NEJMp068035. [DOI] [PubMed] [Google Scholar]
- 2.Shah SK, Nodell B, Montano SM, Behrens C, Zunt JR. Clinical research and global health: mentoring the next generation of health care students. Glob Public Health. 2011;6:234–246. doi: 10.1080/17441692.2010.494248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman DO, Gotuzzo E, Seas C, Legua P, Plier DA, Vermund SH, Casebeer LL. Educational programs to enhance medical expertise in tropical diseases: the Gorgas Course experience 1996–2001. Am J Trop Med Hyg. 2002;66:526–532. doi: 10.4269/ajtmh.2002.66.526. [DOI] [PubMed] [Google Scholar]
- 4.Drain PK, Holmes KK, Skeff KM, Hall TL, Gardner P. Global health training and international clinical rotations during residency: current status, needs, and opportunities. Acad Med. 2009;84:320–325. doi: 10.1097/ACM.0b013e3181970a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump JA, Sugarman J. Ethics and best practice guidelines for training experiences in global health. Am J Trop Med Hyg. 2010;83:1178–1182. doi: 10.4269/ajtmh.2010.10-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermund SH, Audet CM, Martin MH, Heimburger DC. Training programmes in global health. BMJ. 2010;341:c6860. doi: 10.1136/bmj.c6860. [DOI] [PubMed] [Google Scholar]
- 7.Heimburger DC, Lem C, Gardner P, Primack A, Warner TL, Vermund SH. Nurturing the global workforce in clinical research: the NIH Fogarty International Clinical Scholars and Fellows Program. Am J Trop Med Hyg. 2011;85:971–978. doi: 10.4269/ajtmh.2011.11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimburger DC, Warner TL, Carothers CL, Blevins M, Thomas Y, Gardner P, Primack A, Vermund SH. Recruiting trainees for a global health research workforce: the NIH Fogarty International Clinical Research Scholars Program selection process. Am J Trop Med Hyg. 2013;89:281–287. doi: 10.4269/ajtmh.12-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carothers CL, Heimburger DC, Schlachter S, Gardner P, Primack A, Warner TL, Vermund SH. Training programs within global networks: lessons learned in the Fogarty International Clinical Research Scholars and Fellows Program. Am J Trop Med Hyg. 2014;90:173–179. doi: 10.4269/ajtmh.12-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global Health Program for Fellows and Scholars http://www.fic.nih.gov/Programs/Pages/scholars-fellows-global-health.aspx Available at. Accessed March 25, 2014.
- 11.Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. 2013;369:1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 12.Mullan F, Frehywot S, Omaswa F, Sewankambo N, Talib Z, Chen C, Kiarie J, Kiguli-Malwadde E. The Medical Education Partnership Initiative: PEPFAR's effort to boost health worker education to strengthen health systems. Health Aff (Millwood) 2012;31:1561–1572. doi: 10.1377/hlthaff.2012.0219. [DOI] [PubMed] [Google Scholar]
- 13.Tankwanchi ABS, Özden Ç, Vermund SH. Physician emigration from Sub-Saharan Africa to the United States: analysis of the 2011 AMA Physician Masterfile. PLoS Med. 2013;10:e1001513. doi: 10.1371/journal.pmed.1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]