Abstract
During 2012, four north-central Texas counties experienced high West Nile virus (WNV) disease incidence. Aerial insecticide spraying was conducted in two counties. To evaluate the effect of spraying on WNV disease, we calculated incidence rate ratios (IRRs) in treated and untreated areas by comparing incidence before and after spraying; for unsprayed areas, before and after periods were defined by using dates from a corresponding sprayed area. In treated areas, WNV neuroinvasive disease incidence before and after spraying was 7.31/100,000 persons and 0.28/100,000 persons, respectively; the IRR was 26.42 (95% confidence interval [CI]: 12.42–56.20). In untreated areas, the before and after incidence was 4.80/100,000 persons and 0.45/100,000 persons, respectively; the IRR was 10.57 (95% CI: 6.11–18.28). The ratio of IRRs was 2.50 (95% CI: 0.98–6.35). Disease incidence decreased in both areas, but the relative change was greater in aerial-sprayed areas.
Introduction
West Nile virus (WNV) is an arthropod-borne virus in the family Flaviviridae. It is maintained in nature in a transmission cycle involving mosquitoes and amplifying vertebrate hosts, primarily birds. The WNV is transmitted to humans through the bite of an infected mosquito. Approximately 80% of WNV infections are asymptomatic; the majority of symptomatic persons experience an acute systemic febrile illness that often includes headache, myalgia, or arthralgia.1 Less than 1% of infected persons experience neuroinvasive disease, which typically manifests as encephalitis, meningitis, or poliomyelitis-like acute flaccid paralysis (AFP).2 The WNV transmission is seasonal. The majority of patients have onset of illness during July–September, and disease incidence usually peaks during August.1 Localized disease outbreaks often occur.
During 2012, Texas experienced a substantial outbreak of WNV disease. The reported WNV neuroinvasive disease incidence was 3.24/100,000 persons, at least 1.6 times higher than any previous year since WNV disease cases were first reported in Texas during 2002.3 Among the 844 neuroinvasive disease patients, 356 (42%) resided in four adjacent north-central Texas counties as follows: Collin, Dallas, Denton, and Tarrant. In response to the outbreak in these four counties, the local health departments and mosquito control districts increased surveillance and control activities. Efforts to reduce the abundance of WNV-infected mosquitoes and risk for WNV transmission to humans included insecticide application to mosquito larval habitats (larviciding) and application of insecticide to kill adult mosquitoes by truck (ground-based spraying). After extensive consultation, aerial adulticide spraying was also initiated in areas of Dallas County during mid-August and Denton County during late August. This was the first time aerial insecticide spraying had been used for WNV control in north-central Texas, and limited data regarding the effect of aerial spraying on WNV disease are available. We evaluated the effect of aerial insecticide spraying on the incidence of human WNV disease in north-central Texas.
Methods
Setting.
We performed the evaluation in four counties in north-central Texas, including Collin County (0.8 million persons; ∼2,290 square kilometers [km2]), Dallas County (2.4 million persons; ∼2,350 km2), Denton County (0.7 million persons, ∼2,470 km2), and Tarrant County (1.8 million persons; ∼2,340 km2).
Human WNV disease data.
We defined a human WNV disease case according to the National Notifiable Diseases Surveillance System case definition (i.e., a person with a clinically compatible illness and laboratory evidence of WNV disease)4; each patient was a resident of one of the four counties, and had illness onset during January 1–December 31, 2012. Cases were classified as neuroinvasive (e.g., meningitis, encephalitis, or AFP) or nonneuroinvasive. Data were obtained from the Texas Department of State Health Services (DSHS). Incidence was calculated by using the U.S. Census Bureau 2010 midyear population estimates.5
Adult mosquito control measures.
Dates and locations of aerial and ground-based application of insecticides targeting adult mosquitoes were requested from municipalities and other jurisdictions in the four counties. We also conducted an Internet search for additional maps and ground spray data that were not provided. For aerial spray data, insecticide applications that occurred in a predefined area over multiple days were classified as a single aerial spray event. Locations of aerial and ground-based insecticide applications were mapped by using ArcMap 10.0 (Esri, Redlands, CA).
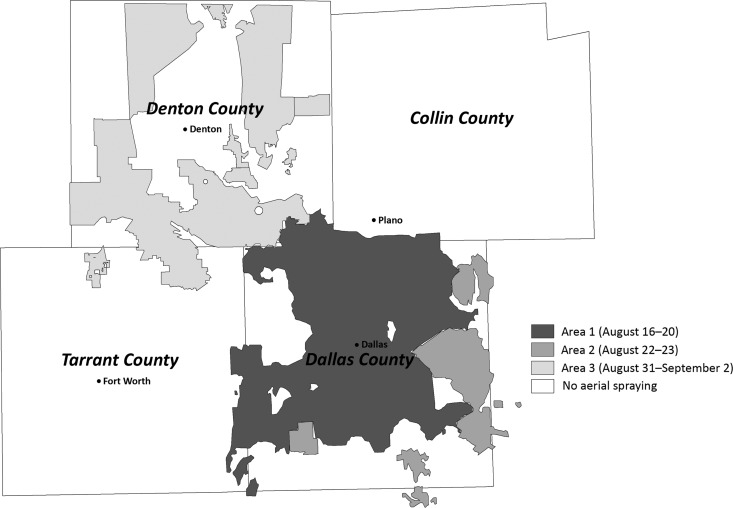
Three aerial spray events were conducted in north-central Texas during 2012, covering ∼2,893 (31%) of the 9,476 total square kilometers in the four counties (Figure 1). The first occurred during August 16–20 and covered 1,448 km2 primarily in northern and central Dallas County (Area 1). The event was interrupted because of weather conditions and was conducted during 5 nights; the full area was covered once by August 19. The second spray event occurred during August 22–23 and covered 303 km2 primarily in eastern and southern Dallas County (Area 2); the full area was covered once on August 22. The final aerial spray event occurred during August 31–September 2 and covered 1,142 km2 of Denton County (Area 3); the full area was covered once by September 1.
Figure 1.
Location of Area 1, Area 2, and Area 3 aerial spray events in north-central Texas, 2012.
Because ground-based applications were numerous, varied in size, and were poorly documented, mapping of these activities was limited to the > 400 spray events that occurred during the 3 weeks before and after aerial spraying. Data were aggregated and the total area sprayed each week was calculated (Figure 1).
Defining cut-off dates.
We assigned cut-off dates to define periods before and after aerial insecticide applications. In aerial-sprayed areas, we defined the cut-off as the earliest date by which the entire treated area was covered by aerial spraying, regardless of additional applications (i.e., August 19 for Area 1, August 22 for Area 2, and September 1 for Area 3). In each of the areas, additional applications covered areas previously sprayed.
In unsprayed areas, the cut-off dates were selected from the three sprayed areas. In Denton County, the cut-off date used in the county's sprayed area (September 1) was also assigned to the unsprayed area. For the untreated areas in Dallas, Collin, and Tarrant Counties, no single cut-off date could be assigned from an adjoining area because two spray events occurred in Dallas County and no aerial spray events were focused in Collin or Tarrant Counties. Therefore, for the unsprayed areas in these three counties, the cut-off date yielding the most conservative conclusions about the effectiveness of spraying was chosen. The date was selected by using a computer model that simulated possible courses of the outbreak and varied effectiveness of spraying. Nine scenarios were considered, incorporating three different patterns of disease rates over time (constant rate, decreasing rate, or increasing then decreasing rate), and three different levels of spraying effectiveness (not effective, reduces WNV neuroinvasive disease incidence by 25%, or reduces incidence by 50%); for each scenario, 5,000 data sets were generated. With each data set, we compared WNV neuroinvasive disease incidence before and after spraying by using each of the three possible cut-off dates (i.e., August 19, August 22, or September 1). As a result of this analysis, August 19 was determined to be the most conservative cut-off date for the untreated areas of Dallas, Collin, and Tarrant Counties.
Categorizing disease cases.
The WNV neuroinvasive and nonneuroinvasive disease patients were categorized as residing within or outside an aerial-sprayed area. Patients without a known home street address were excluded. To account for the WNV disease incubation period, we subtracted the average incubation period of 7 days from each patient's symptom onset date to establish an estimated date of infection. This date was then used to categorize patients as being infected before or after the relevant cut-off date for their place of residence.
Assessment of data consistency and homogeneity.
To assess consistency in detection and reporting of cases and determine if the effect on both neuroinvasive and nonneuroinvasive WNV disease cases should be evaluated, we calculated the proportions of WNV disease cases that were classified as nonneuroinvasive disease in aerial-sprayed and unsprayed areas before and after the cut-off dates and compared them by using the Mantel-Haenszel χ2 test.
To determine if we could combine the results from each treated area, and also combine results from each untreated area, we calculated odds ratios for being a neuroinvasive or nonneuroinvasive disease patient before and after the cut-off dates in each of the three treated areas and in each of the four untreated areas, and compared results from each area by using Woolf's test for homogeneity.
Effect analysis.
To evaluate the effect of aerial spraying on human WNV disease, we calculated incidence rates (IR)/100,000 persons for illnesses in the treated and untreated areas before and after the cut-off dates. For post treatment calculations, the denominator was determined by subtracting the number of persons who had been infected before the cut-off date from the relevant population figure. To determine the change in IR from before the cut-off date to after the cut-off date, we calculated IR ratios (IRR) for the treated and untreated areas (e.g., IR in the treated area before spraying/IR in the treated area after spraying). Finally, to compare the change in IRs between treated and untreated areas, we calculated a ratio of IRRs (i.e., IRR in the treated area/IRR in the untreated area). We calculated 95% confidence intervals (CIs) for the IRRs and the ratio of IRRs.
To estimate the number of cases prevented by spraying, we calculated the expected number of cases in the absence of spraying by applying the change in IR from the untreated area to the treated area. Actual cases were subtracted from expected cases to determine the number of prevented cases. A 95% prediction interval was calculated. In consideration of the potential variability in the incubation period for WNV disease, we conducted a sensitivity analysis by using minimum and maximum incubation periods of 2 and 14 days.2 To account for the possible effect of ground-based insecticide applications in the aerially treated and untreated areas, we calculated the square kilometers and proportion of the total area covered by ground-based spraying in each area ∼3 weeks before and after the cut-off dates, and compared them by fitting a log-linear model to a three-way array formed by the aerial spraying, ground spraying, and timing classifications. For all analyses, a two-sided P < 0.05 was considered statistically significant.
The evaluation did not meet the definition of human subjects research under 45 CFR 46.102(d). Therefore, institutional review board review was not required.
Results
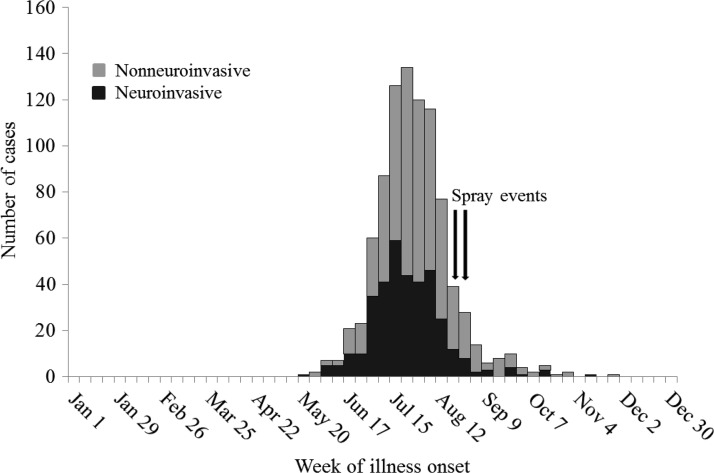
A total of 902 cases of WNV disease were reported to Texas DSHS from the four counties during 2012, including 356 (39%) classified as neuroinvasive and 546 (61%) classified as nonneuroinvasive disease. The WNV disease cases occurred during May 25–November 25, and 757 (84%) of the cases had illness onset before the first aerial spraying, which started on August 16 (Figure 2). Among the 902 cases, two (0.2%) patients with neuroinvasive disease had unknown street addresses and were excluded from the analysis.
Figure 2.
West Nile virus disease cases in Collin, Dallas, Denton, and Tarrant Counties, Texas, by week of illness onset and clinical syndrome, 2012.
Data consistency and homogeneity.
Among the 900 WNV disease patients with a known address, 495 (55%) patients resided in areas that underwent aerial spraying, and 405 (45%) resided in areas that were not aerially sprayed. In the aerially sprayed areas, the proportion of cases classified as nonneuroinvasive disease increased from 60% (277 of 462) before spraying to 79% (26 of 33) after the aerial spraying (P = 0.03). In the untreated areas, the proportion of nonneuroinvasive disease cases was similar before (60%; 218 of 366) and after (64%; 25 of 39) the cut-off dates (P = 0.6). Given the possible increase in the diagnosis of nonneuroinvasive disease in the treated areas after aerial spraying, the evaluation was limited to neuroinvasive disease cases.
Among the 354 neuroinvasive disease cases, 192 (54%) were reported among residents of aerial-sprayed areas, and 162 (46%) were reported among residents of unsprayed areas. Woolf's test for homogeneity indicated that odds ratios for the three aerially treated areas were similar (P = 0.3), and four untreated areas were similar (P = 0.7), indicating these areas could be combined and evaluated as one treated and one untreated group.
Effect of aerial spraying on WNV neuroinvasive disease.
In treated areas, neuroinvasive disease IR before spraying was 7.31/100,000 persons, and after spraying decreased to 0.28/100,000 (Table 1). In untreated areas, the IR was 4.80/100,000 persons before the cut-off date and decreased to 0.45/100,000 after. The IRR was 26.42 (95% CI: 12.42–56.20) for the treated area, and was 10.57 (95% CI: 6.11–18.28) for the untreated area. The ratio of these IRRs was 2.50 (95% CI: 0.98–6.35), indicating that the decrease in neuroinvasive disease IR was an estimated 2.5 times greater in the treated area. Ten cases (95% prediction interval: 3–35) of WNV neuroinvasive disease might have been prevented by the intervention.
Table 1.
West Nile virus neuroinvasive disease cases, incidence rates/100,000 persons, and incidence rate ratios before and after aerial spraying in treated and untreated areas—Collin, Dallas, Denton, and Tarrant Counties, Texas, 2012
| Area | Before aerial spraying | After aerial spraying | IRR* | (95% CI) | Ratio of IRRs† | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Population | IR | Cases | Population | IR | |||||
| Treated | 185 | 2,530,019 | 7.31 | 7 | 2,529,557 | 0.28 | 26.42 | (12.42−56.20) | 2.50 | (0.98−6.35) |
| Untreated | 148 | 3,085,121 | 4.80 | 14 | 3,084,755 | 0.45 | 10.57 | (6.11−18.28) | ||
IR before cut-off date/IR after cut-off date.
IRR in treated areas/IRR in untreated areas.
IR = Incidence rate; IRR = incidence rate ratio; CI = confidence interval.
In the sensitivity analysis, by using a minimum incubation period of 2 days, the ratio of IRRs was 2.13 (95% CI: 0.95–4.80); by using a maximum incubation period of 14 days, the ratio of IRRs was 1.40 (95% CI: 0.46–4.25).
Ground-based insecticide applications.
In the combined aerial sprayed areas, the proportion covered by ground-based spraying was similar during the 3 weeks before (10%; 298 of 2,893 km2) and after (9%; 256 of 2,893 km2) the aerial applications (Table 2). However, in the combined areas where aerial spraying was not performed, the proportion covered by ground-based insecticide applications increased from 5% (334 of 6,583 km2) during the 3 weeks before the cut-off dates to 11% (738 of 6,583 km2) covered after the cut-off dates. The change in proportion of area covered before and after the cut-off dates was significantly greater in the unsprayed area (P < 0.001).
Table 2.
Area covered with ground-based insecticide spraying during the 3 weeks before and after the aerial spray applications—Collin, Dallas, Denton, and Tarrant Counties, Texas, 2012
| Total area | Area covered with ground-based spraying before aerial applications | Area covered with ground-based spraying after aerial applications | |
|---|---|---|---|
| Square kilometers | Square kilometers (%) | Square kilometers (%) | |
| Aerial sprayed areas | |||
| Area 1 | 1,448 | 254 (18) | 75 (5) |
| Area 2 | 303 | 41 (14) | 160 (53) |
| Area 3 | 1,142 | 3 (< 1) | 21 (2) |
| Total | 2,893 | 298 (10)* | 256 (9)* |
| Aerial unsprayed areas | |||
| Collin County | 2,266 | 80 (4) | 127 (6) |
| Dallas County | 756 | 213 (28) | 572 (76) |
| Denton County | 1,393 | 31 (2) | 10 (1) |
| Tarrant County | 2,168 | 10 (1) | 29 (1) |
| Total | 6,583 | 334 (5)† | 738 (11)† |
P = 0.3 for the difference in the proportions covered before and after aerial spraying.
P < 0.001 for the difference in the proportions covered before and after aerial spraying.
Discussion
Aerial spraying measures implemented for WNV control were associated with a reduction in WNV neuroinvasive disease. As expected, given that aerial spray events occurred relatively late during the outbreak, disease incidence decreased during the after-spray period in both treated and untreated areas. However, the relative change was greater in aerial-sprayed areas. Although the lower bound of the confidence interval was 0.98, the result nonetheless suggested a greater decrease in treated areas.
On the basis of previous studies, for every reported case of WNV neuroinvasive disease, there are an estimated 30–70 nonneuroinvasive disease cases.6,7 We estimated 10 cases of neuroinvasive disease were prevented in the aerial sprayed areas; therefore, ∼300–700 nonneuroinvasive disease cases might have been prevented. An even higher effect might have been expected if aerial spraying had been conducted earlier during the outbreak.
Previous studies have shown that aerial spraying can reduce the abundance and WNV infection rates of Culex mosquitoes.8–10 An evaluation of the effect of aerial spraying during an outbreak in Dallas, Texas, of St. Louis encephalitis virus, an arbovirus closely related to WNV, reported reductions in mosquito density and St. Louis encephalitis virus infection rates among mosquitoes after spraying.11 Data regarding the effect of aerial spraying on human WNV disease are limited. However, one previous study that compared WNV disease incidence between treated and untreated areas during a substantial WNV disease outbreak in Sacramento County, California, showed that aerial mosquito adulticiding reduced human WNV disease cases.12 Our findings support this conclusion and provide additional information because the California study evaluated the effect of spraying in areas where the primary WNV vectors are Culex pipiens and Culex tarsalis; our evaluation was conducted in counties where Culex quinquefasciatus is the main WNV vector.8,13,14
Aerial spraying can treat large areas more rapidly than ground-based spraying, and using aircraft allows access to areas inaccessible by roads. Aerial spraying can be particularly valuable for controlling WNV vectors such as Cx. quinquefasciatus, because multiple, closely spaced treatments are often required.15 Assessing the effect of ground-based insecticide applications and whether ground-spraying was a confounding factor in this analysis was difficult. However, on the basis of available data, the proportion of the aerially treated area covered by ground-based spraying was similar before and after the aerial applications. In contrast, in the combined areas where aerial spraying was not performed, the proportion covered by ground-based insecticide applications more than doubled between the 3 weeks before and after the cut-off dates. These data indicate that it is unlikely that ground-based spraying accounted for the markedly greater decline in neuroinvasive disease incidence in aerially treated versus untreated areas. In addition, < 12% of land was covered by ground-based spraying in either aerially treated or untreated areas during the pre- or postspray periods. Although the percentage of land area covered might not equate directly with the percentage of the population residing in ground-sprayed areas, it is likely a limited proportion. A more accurate assessment of ground-based spraying's effect was not possible because data regarding dates and locations of ground-based insecticide applications were often incomplete or difficult to interpret, and we could not take into account the type and application rate of the insecticide products.
Human and mosquito WNV surveillance programs are important for monitoring WNV activity, directing vector control efforts, and enabling a timely response to outbreaks. Outbreak response should comprise community education and awareness activities and enhanced vector control activities. Integrated vector management programs should include source reduction and larval control activities complemented by the timely use of ground or aerially applied insecticides, as needed.16
There are several limitations of this analysis. As a result of apparent changes in the detection of nonneuroinvasive disease cases after the aerial spraying, the analysis was limited to neuroinvasive cases. However, detection and reporting of WNV neuroinvasive disease cases is believed to be more consistent than for nonneuroinvasive disease because of the considerable morbidity associated with neuroinvasive disease cases.2 The higher proportion of nonneuroinvasive disease cases reported in the treated areas after aerial spraying might have resulted from increased awareness after media publicity of the outbreak, and more visits to health care providers or increased laboratory testing for persons with febrile illness. Viremic blood donors identified through routine screening of the blood supply can be a useful and possibly unbiased measure of human WNV infections, but the number of viremic blood donors in the four-county area was too low to be useful for this analysis.
In analyzing the human disease data, we assumed that WNV infections were acquired at the residential address. Primary WNV vectors bite most actively from dusk to dawn when people are more likely to be at their places of residence, but infections could have been acquired elsewhere.16 We also assumed that aerial spraying's effect occurred within the boundaries defined by the aerial spray geographical data, but certain variability in insecticide application on the edges of the spray zone might have occurred. These factors might have resulted in misclassification of cases as occurring in treated or untreated areas. Finally, aerial spray events occurred during multiple days and at different times in each area. We used conservative cut-off dates to define the before and after periods and applied them to seemingly comparable nonsprayed areas. However, factors other than the aerial insecticide applications might have accounted for the changes in disease incidence before and after spraying and for the greater decline observed in treated versus untreated areas (e.g., differences in diagnosis rates or case classification, weather patterns, other mosquito control efforts, varying effects of spraying in different ecological areas, or increased vigilance with personal protective measures in aerial sprayed areas).
The results of our evaluation provide data that show the possible effect of aerial spraying on human WNV neuroinvasive disease during a WNV disease outbreak. Further studies would be useful to evaluate the possible confounding effect of ground-spraying and the potential effect of earlier implementation of aerial spraying during a WNV outbreak.
ACKNOWLEDGMENTS
We thank Nicole Evert, Jim Schuermann, Dawn Hesalroad, Mary D'Anton, Scott Sawlis, Wendy Chung, Peggy Wittie, Muriel Marshall, Misty Brown, Anita Kurian, Kayleen Tomason, Bing Burton, Juan Rodriguez, Jennifer Ochieng, Michael Wheeler, Nancy Arnett, and Mei-chien Fucci for assistance with data gathering. We also thank Tim J. Hawkins and Tracy Haywood for GIS support.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Duke J. Ruktanonchai and Satish K. Pillai, Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: DRuktanonchai@cdc.gov and SPillai@cdc.gov. Shelley Stonecipher and James Zoretic, Health Service Region 2/3, Texas Department of State Health Services, Arlington, TX, E-mails: Shelley.Stonecipher@dshs.state.tx.us and James.Zoretic@dshs.state.tx.us. Nicole Lindsey, Janet McAllister, Kalanthe Horiuchi, Mark Delorey, Brad J. Biggerstaff, Roger Nasci, Marc Fischer, and Susan L. Hills, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: NPLindsey@cdc.gov, JMcAllister@cdc.gov, KHoriuchi@cdc.gov, MDelorey@cdc.gov, BBiggerstaff@cdc.gov, RNasci@cdc.gov, Mfischer@cdc.gov, and Shills@cdc.gov. Tom Sidwa, Zoonosis Control Branch, Texas Department of State Health Services, Austin, TX, E-mail: Tom.Sidwa@dshs.state.tx.us.
References
- 1.Centers for Disease Control and Prevention (CDC) Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 2.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) West Nile virus disease and other arboviral diseases—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:513–517. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Arboviral neuroinvasive and non-neuroinvasive diseases. Nationally notifiable diseases and conditions and current case definitions. Atlanta, GA: CDC; 2011. http://www.cdc.gov/nndss/script/ConditionSummary.aspx?CondID=17. Available at. Accessed January 17, 2014. [Google Scholar]
- 5.U.S. Census Bureau Census. 2010. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml Available at. Accessed January 6, 2014.
- 6.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell G. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 7.Busch MP, Wright DJ, Custer B, Tobler LH, Stramer SL, Kleinman SH, Prince HE, Bianco C, Foster G, Petersen LR, Nemo G, Glynn SA. West Nile virus infections projected from blood donor screening data, United States, 2003. Emerg Infect Dis. 2006;12:395–402. doi: 10.3201/eid1203.051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elnaiem DE, Kelley K, Wright S, Laffey R, Yoshimura G, Reed M, Goodman G, Thiemann T, Reimer L, Reisen WK, Brown D. Impact of aerial spraying of pyrethrin insecticide on Culex pipiens and Culex tarsalis (Diptera: Culicidae) abundance and West Nile virus infection rates in an urban/suburban area of Sacramento County, California. J Med Entomol. 2008;45:751–757. doi: 10.1603/0022-2585(2008)45[751:ioasop]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Mount GA, Biery TL, Haile DG. A review of ultralow-volume aerial sprays of insecticide for mosquito control. J Am Mosq Control Assoc. 1996;12:601–618. [PubMed] [Google Scholar]
- 10.Macedo PA, Schleier JJ, 3rd, Reed M, Kelley K, Goodman GW, Brown DA, Peterson RK. Evaluation of efficacy and human health risk of aerial ultra-low volume applications of pyrethrins and piperonyl butoxide for adult mosquito management in response to West Nile virus activity in Sacramento County, California. J Am Mosq Control Assoc. 2010;26:57–66. doi: 10.2987/09-5961.1. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins CC, Hollinger FB, Johnson RF, Dewlett HJ, Newhouse VF, Chamberlain RW. The epidemiology of St. Louis encephalitis in Dallas, Texas, 1966. Am J Epidemiol. 1975;102:1–15. doi: 10.1093/oxfordjournals.aje.a112128. [DOI] [PubMed] [Google Scholar]
- 12.Carney RM, Husted S, Jean C, Glaser C, Kramer V. Efficacy of aerial spraying of mosquito adulticide in reducing incidence of West Nile virus, California, 2005. Emerg Infect Dis. 2008;14:747–754. doi: 10.3201/eid1405.071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geraghty EM, Margolis HG, Kjemtrup A, Reisen W, Franks P. Correlation between aerial insecticide spraying to interrupt West Nile virus transmission and emergency department visits in Sacramento County, California. Public Health Rep. 2013;128:221–230. doi: 10.1177/003335491312800312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung WM, Buseman CM, Joyner SN, Hughes SM, Fomby TB, Luby JP, Haley RW. The 2012 West Nile encephalitis epidemic in Dallas, Texas. JAMA. 2013;310:297–307. doi: 10.1001/jama.2013.8267. [DOI] [PubMed] [Google Scholar]
- 15.Andis MD, Sackett SR, Carroll MK, Bordes ES. Strategies for the emergency control of arboviral epidemics in New Orleans. J Am Mosq Control Assoc. 1987;3:125–130. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. 2013. http://www.cdc.gov/westnile/resources/pdfs/wnvGuidelines.pdf Available at. Accessed January 6, 2014.